Abstract
Transcranial direct current stimulation (tDCS) was paired with eye tracking to elucidate contributions of frontal, temporoparietal and anterior temporal cortex to early visual search patterns during picture naming (e.g., rapid visual scanning to diagnostic semantic features). Neurotypical adults named line drawings of objects prior to and following tDCS in three separate sessions, each employing a unique electrode montage. The gaze data revealed montage by stimulation (pre/post) interaction effects characterized by longer initial visual fixations (mean difference =89 ms; Cohen’s d = .8) and cumulative fixation durations (mean difference =98 ms; Cohen’s d =.9) on key semantic features (e.g., the head of an animal) after cathodal frontotemporal stimulation relative to the pre-stimulation baseline. We interpret these findings as reflecting a tDCS-induced modulation of semantic contributions of the anterior temporal lobe(s) to top-down influences on object recognition. Further, we discuss implications for the optimization of tDCS for the treatment of anomia in aphasia.
Keywords: semantic cognition, picture naming, anterior temporal lobe, tDCS, eye tracking
1. Introduction
Visual confrontation naming tests are sensitive to impairments not only in language but also among sensorimotor processes, memory and executive functioning. Indeed, despite seeming an effortless and routine ability, it draws upon numerous complex cognitive processes. At the very least, picture naming involves stimulus-based perceptual analysis that leads to visual object recognition, activation of conceptual knowledge and then lexical access, followed by a range of form encoding processes that ultimately result in articulation of the correct verbal output. Longstanding basic research questions have included whether confrontation naming can be decomposed into a discrete set of component processes that engage distinct cortical networks and can be selectively modulated or impaired. In what follows, we briefly discuss some neuropsychological observations relevant to these questions before describing an original multimodal study using transcranial direct current stimulation (tDCS) and eye tracking to explore the contribution of different brain regions to the processes underpinning visual confrontation naming.
Confrontation naming impairment (i.e., anomia) can arise as a result of damage to a wide range of cortical regions (Baldo, Arevalo, Patterson & Dronkers, 2013; DeLeon et al., 2007). Anomia is common in numerous post-stroke aphasia syndromes that impact the middle cerebral artery distribution, including classical perisylvian language areas such as left inferior frontal gyrus (IFG; approximately Broca’s area) and/or the left temporoparietal cortices (e.g., Wernicke’s area). Naming impairments are also a core diagnostic feature of some neurodegenerative conditions such as Alzheimer’s disease and frontotemporal lobar degeneration (FTLD). The origins of anomia particularly in semantic dementia (a variant of FTLD, sometimes referred to as semantic variant primary progressive aphasia) have been strongly linked to anterior temporal lobe pathology (e.g., Lambon Ralph et al. 2001).
It is well established that the qualitative nature of the naming impairment (as evaluated by error type, for example) differs by lesion site. For example, patients with temporoparietal damage exhibit word-finding difficulties and often produce visual semantic naming errors (e.g., ‘ball’ for ‘apple’), whereas phonemic naming errors are more consistent in frontal lobe pathologies (Reilly, Peelle, Antonucci, & Grossman, 2011; Reilly, Rodriguez, Peelle, & Grossman, 2011). People who experience frontal and/or temporoparietal damage are often amenable to cueing techniques (e.g., provision of the initial phoneme of the target word), suggesting an underlying impairment in ‘access’ to the correct lexical targets (Jefferies & Lambon Ralph, 2006), which is consistent with multi-method evidence of a more general role of these regions in the controlled selection/retrieval of lexical-semantic information (Badre & Wagner, 2007; Devlin, Matthews & Rushworth, 2003; Thompson-Schill, D’Esposito, Aguirre & Farah, 1997; Whitney, Kirk, O’Sullivan, Lambon Ralph & Jefferies, 2011). In contrast, damage to the ATL in semantic dementia, for example, is associated with anomia dominated by a preponderance of omission and superordinate errors (e.g., ‘bird’ for ‘swan’; Jefferies & Lambon Ralph, 2006; Migliaccio et al., 2016). This, and related neuropsychological observations, have contributed significantly to the ATL being attributed with a crucial role in mapping between conceptual knowledge and phonological form in tasks such as picture naming and reading aloud (Binney et al., 2016; Mesulam et al., 2013; Schwartz et al., 2009; Woollams et al., 2007). In fact, the study of semantic dementia more generally has led to the genesis of a hypothesis that the ATL is a core substrate for supramodal conceptual knowledge representations (e.g., Lambon Ralph, Jefferies, Patterson & Rogers, 2017; Reilly, Peelle, Garcia & Crutch, 2016) for which there is now converging evidence from functional neuroimaging, direct intracranial recording, and transcranial magnetic stimulation (TMS) studies among neurotypical adults (e.g., Binney, Embleton, Jefferies, Parker & Lambon Ralph, 2010; Chan et al., 2011; Pobric, Jefferies & Lambon Ralph, 2007; Shimotake et al., 2014; Vandenberghe, Price, Wise, Josephs & Frackowiak, 1996).
Past basic research into the neural basis of confrontation naming has relied heavily upon correlational analyses, including associations of behavior with lesion distributions or regional activations. In contrast, non-invasive brain stimulation offers an alternative mode of investigation with the potential advantage of yielding causal inference (Pobric, Jefferies & Lambon Ralph, 2007). tDCS involves the application of constant low intensity electrical current to the cortex via two or more electrodes strategically positioned in a montage over the scalp. The mechanism of tDCS involves perturbing excitability of underlying neuronal assemblies via hyperpolarization or depolarization of resting membrane potentials (Stagg & Nitsche, 2011) and there is evidence to suggest that tDCS holds promise as a means for modulating language processing and learning (Price, McAdams, Grossman & Hamilton, 2015). While a coarse spatial resolution limits its utility as a fine grained brain-behavior localization technique, the continued neuroscientific appeal of tDCS relates to its translational potential as a neurorehabilitative tool, particularly given its portability and low relative cost (Baker, Rorden, & Fridriksson, 2010; Cappon, Jahanshahi & Bisiacchi, 2016; Sanders, Cloutman & Woollams, 2016). Indeed, there is considerable interest in tDCS for aphasia intervention, particularly as an adjuvant to speech-language therapy, where it might be used to promote or guide neuroplasticity and thereby facilitate learning and recovery (Holland & Crinion, 2012; Tippett, Hillis, & Tsapkini, 2015). However, research into how tDCS can be optimally configured (e.g., dose, electrode positioning) to modulate performance on clinically-relevant language measures remains at an early stage.
In the present study, our primary focus was assessing whether alternate tDCS electrode configurations (referred to as montages) could differentially modulate visual confrontation naming in neurotypical adults. These questions are fundamental to the building of foundations for the translation of tDCS to aphasia rehabilitation. A small set of studies have used tDCS to probe the contribution of frontal, temporoparietal and anterior temporal cortex to naming, but in separate investigations. tDCS targeting the left frontal cortex (e.g., Fertonani, Rosini, Cotelli, Rossini, & Miniussi, 2010; Holland et al., 2011) and left temporoparietal cortex (e.g., Fiori et al., 2011; Sparing, Dafotakis, Meister, Thirugnanasambandam, & Fink, 2008) is reported to modulate object naming, and tDCS targeting the ATL is reported to modulate proper name retrieval for familiar faces and landmarks (Ross, McCoy, Coslett, Olson, & Wolk, 2011; Ross, McCoy, Wolk, Coslett, & Olson, 2010), but all these studies vary not only by site, but stimuli, task and other stimulation parameters and thus represent a complex set of results to interpret together. As far as we are aware, there are only five prior studies that directly compare montages (Malyutina and den Ouden, 2015; Meinzer, Yetim, McMahon, & de Zubicaray, 2016; Pisoni, Papagno, & Cattaneo, 2012; Pisoni, Vernice, Iasevoli, Cattaneo, & Papagno, 2015; Westwood, Olsen, Miall, Nappo, & Romani, 2017). Pisoni et al. (2012) contrasted the effect of anodal tDCS targeting the left frontal and left temporoparietal cortex, and reported enhanced naming performance associated with the former and detrimental effects of the latter. Malyutina and den Ouden (2015) reported enhanced naming performance following cathodal tDCS targeting either the left frontal or temporoparietal cortex. Similarly, Meinzer, et al. (2016) observed facilitation of naming following left frontal and following left posterior temporal anodal tDCS, as compared to sham stimulation. In contrast, a more recent study found no evidence of effects of either left frontal or posterior temporal anodal stimulation (Westwood et al., 2017). The fifth comparative study by Pisoni et al. (2015) was the only to assess the effects of ATL stimulation. They observed diminished naming performance following anodal stimulation but no effect of cathodal ATL stimulation. Anodal IFG stimulation, on the other hand, enhanced performance. The present study is the first to attempt to systematically compare the effect of bilateral stimulation of the ATL, the frontal and the temporoparietal cortex on basic level confrontation naming. We used a fully counter-balanced factorial within-subjects design within neurotypical adults. Further, we repeated the experiment with two independent participant samples and using opposite configuration of tDCS polarity. This was to systematically compare the effects of anodal and cathodal stimulation and their potential to produce excitatory effects versus inhibitory effects, respectively (Nitsche & Paulus, 2000; Nitsche et al., 2007). While on the basis of the abovementioned studies, we hypothesized that stimulation of all these regions would elicit some effect on picture naming (but not a control task), we also predicted that there would be difference in the magnitude or quality of the effect, as we shall discuss below. The effects were assessed via response latencies, as is typical, but our investigation focused primarily on additional eye tracking measures that potentially improve sensitivity for detecting perturbations in behavior.
We predicted that altering cortical excitability of different components of the network underpinning naming would modulate performance in a qualitatively different way reflecting a) their representational or executive contribution, and b) whether this contribution is particularly important in early or late stages of the naming process. While some early models have proposed a discrete and serial transition between each stage of processing (e.g., Levelt, 1989), others argue the necessity of recurrent ‘interactivity’ between stages (Dell, Schwartz, Martin, Saffran & Gagnon, 1997) and highlight the importance of ‘top down’ influences, particularly at the level of visual object recognition (Humphreys, Riddoch & Price, 1997). Indeed, over the last two decades it has become increasingly clear that, in both the auditory and visual domains, higher-order processes (e.g., attention and expectation bias) influence lower-level perceptual interpretations (Gilbert & Li, 2013; Tervaniemi et al., 2009; Trapp & Bar, 2015). A number of recent studies suggest that prior conceptual knowledge, in particular, has a significant impact on visual object processing (Chiou & Lambon Ralph, 2016; Clarke, Taylor, Devereux, Randall & Tyler, 2013; Panichello, Cheung, & Bar, 2012). There is, however, limited understanding regarding which brain structures are responsible for these semantic modulations, with evidence for both frontal and anterior temporal involvement (Chiou & Lambon Ralph, 2016). The present study sought to use a novel pairing of transcranial direct current stimulation (tDCS) and eye tracking measures to explore these questions. In particular, we hypothesized that stimulation-based modulation of early top-down influences on object recognition would be evident in visual search patterns; since not all aspects of a picture are equally informative for identification, increased conceptual top-down input will bias visual search patterns towards key semantic features (e.g., the head, in case of animals). Consistent with this notion, gaze patterns of healthy subjects during object naming have been shown to differentiate according to semantic category (i.e., whether it is an animal or a tool) with more attention paid to features with the highest salience/diagnostic value for that category (Bauer, Hung, Grossman, & Reilly, 2015; Kovic, Plunkett, & Westermann, 2009a, 2009b). In line with our previous assertions that the ATL is key to the representation of conceptual information (Binney, Hoffman & Lambon Ralph, 2016; Reilly et al., 2016), we predicted that tDCS targeting this region would have the greatest effect on gaze patterns during picture naming reflecting a perturbation of top-down conceptual influences on object recognition.
2. Material and methods
We employed a multi-session within-subject design wherein participants were stimulated using three different electrode montages in sessions spaced one week apart. One participant sample received anodal stimulation over the target regions, whereas the other received cathodal stimulation. These key regions were the frontal, the ATL and inferior parietal/posterior temporal cortices and they were differentially targeted by each of the three montages (see Section 2.2). The order of stimulation sessions was fully counterbalanced across participants, and participants were blinded to the anatomical stimulation target(s). In each session, we employed an offline tDCS protocol; participants named a well-normed set of line drawings prior to and immediately following stimulation, and as we monitored their eye movements and oral naming response latencies. Participants also read 6-digit numbers aloud as an active control for general arousal and speech production in the context of low-level semantic demands.
2.1. Participants
Participants included neurotypical young adults (N=24, mean age=21.2 years, range=18–30) distributed equally in the anodal (n=12, 1 male) and cathodal (3 males) conditions. All participants were native English speakers with normal or corrected-to-normal vision and hearing, as confirmed through threshold Snellen (vision) and pure tone Audiometric (hearing) screening. Participants were by self-report free of a history of neurological disorders. Participants were right-handed with the exception of one individual in the cathodal tDCS condition who self-reported as ambidextrous. All participants provided informed consent and were provided nominal compensation in accord with the institutional review board of Temple University.
2.2. tDCS parameters
We conducted brain stimulation using a Soterix 1×1 tDCS device coupled to a passive splitter system (Soterix Medical, model no. PS1224B). For one channel, the electrical current (2 mA) was split across two ‘target’ electrodes placed on homologous lateral regions of the cortex (thus approximately 1 mA at each). A single large, distal ‘return’ electrode was positioned over an anterior or at posterior midline region. The two lateral ‘target’ electrodes we encased in 5cm2 saline-soaked sponges while a larger (5 × 7 cm) sponge was used for the midline ‘return’ electrode. Current density is attenuated as a function of the surface area of the sponge; thus, the purpose of the larger sponges at the midline electrode was to diffuse the current and lessen any potential localized effects of stimulation (DaSilva, Volz, Bikson, & Fregni, 2011; Nitsche et al., 2007). We standardized electrode positioning using a customized 10/20 MCN-system elasticated placement cap (http://easycap.de).
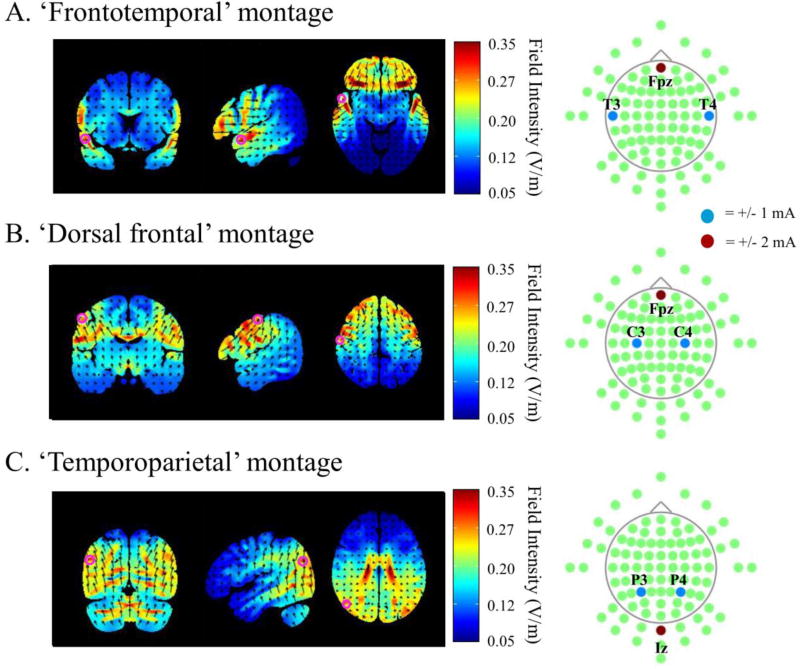
Details of the three montages are given in Table 1. When targeting the ATL, the left and right hemisphere lateral electrodes were positioned over locations T3 and T4 of the international 10/20 positioning system for EEG. The ‘return’ electrode was placed over the orbital midline (Fpz) with the intention of creating a symmetrical distribution of current flow across the hemispheres as well as keeping the flow to anterior (as opposed to posterior) temporal lobe cortex. The resulting current flow was estimated with HD-Explore™ software (Soterix Medical) which uses a finite-element-method approach to model electrical field intensities throughout the brain (Datta et al, 20131). This estimation is displayed in Figure 1A. The limited spatial focality of conventional ‘pad’ tDCS is clearly evident in Figure 1. Indeed, the T3/T4/Fpz montage results in a current flow that implicates not only the lateral ATL, but much of the temporal lobe and ventrolateral and ventromedial frontal cortices, bilaterally. The peaks (see Figure 1A) appear around the anterior superior temporal cortex and the frontal operculum. For this reason, from here on in, we refer to this montage as the ‘frontotemporal’ montage. To attempt to disentangle the effects of anterior temporal and ventral frontal stimulation, we used HD-explore™ to tailor a further montage that results in a current flow that implicates the same frontal cortices but not, or at least much less, the anterior temporal cortices. This ‘dorsal frontal’ montage (as it shall be referred to here on in) involved placing the left and right lateral electrodes over the C3 and C4 locations of the 10/20 system, and the ‘return’ electrode, once again over Fpz. The model of the resultant current flow is displayed in Figure 1B, where dorsolateral and ventral frontal regions are estimated to receive a much greater dosage than the ATL, and the ATL dosage is substantially lower than it is in the frontotemporal montage. To quantify this estimated difference in ATL stimulation, in Table 1 we provide a value for the field intensity modelled at the superior ATL in the case of each montage. This was extracted using a Montreal Neurological Institute (MNI) coordinate associated with expressive semantic processing in the study of Geranmayeh, Leech & Wise (2014; −54, +8, −10). On the basis of these estimates, we reasoned that a modulation of behavior that occurs following stimulation with the frontotemporal but not the dorsal frontal montage could reasonably be interpreted to differential stimulation of the ATL (although this is, of course, not the only possible interpretation and the limited spatial focality must remain close to mind). Finally, to target the temporoparietal (inferior parietal and posterior temporal) cortex, the left and right lateral electrodes were positioned over the P3 and P4 locations of the 10/20 system, which approximately correspond to the angular gyrus. The ‘return’ electrode was placed over the inion (Iz). The estimated current flow is presented in Figure 1C where not only is inferior parietal cortex implicated but also posterior temporal and occipital cortex, as well as the cerebellum. For the sake of brevity, however, we shall only refer to this as the ‘temporoparietal’ montage. For completeness, in Table, 1 we also provide values for the estimated field intensity at MNI coordinates approximately underlying the P3/P4 (−48, −68, +28; Seghier, Fagan & Price, 2010) and C3/C4 (+/−57, −13, 48; Vitali et al., 2002) electrodes, in the context of each of the three montages.
Table 1.
Electrode Configurations and estimated resultant intensity at target regions
| Montage | Electrode Configuration MCN 10/20 system |
Field intensity at lateral ATL (+/−54, 8, −10) |
Field intensity at AG (+/−48, −68, 28) |
Field intensity in dorsal motor cortex (+/−57, −13, 48) |
|
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| Frontotemporal | Anodal | T3 (+1 mA), T4 (+1 mA), Fpz (−2 mA) | 0.28 V/m |
0.05 V/m |
0.16 V/m |
| Cathodal | T3 (−1 mA), T4 (−1 mA), Fpz (+2 mA) | 0.28 V/m | 0.05 V/m | 0.16 V/m | |
| Temporoparietal | Anodal | P3 (+1 mA), P4 (+1 mA), Iz (−2 mA) | 0.09 V/m | 0.27 V/m | 0.12 V/m |
| Cathodal | P3 (−1 mA), P4 (−1 mA), Iz (+2 mA) | 0.09 V/m | 0.27 V/m | 0.12 V/m | |
| Dorsal frontal | Anodal | C3 (+1 mA), C4 (+1 mA), Fpz (−2 mA) | 0.15 V/m | 0.13 V/m | 0.24 V/m |
| Cathodal | C3 (−1 mA), C4 (−1 mA), Fpz (+2 mA) | 0.15 V/m | 0.13 V/m | 0.24 V/m |
Field intensity values estimated using HD-Explore™ software (Soterix Medical) and averaged across hemispheres. Cortical coordinates given in Montreal Neurological Institute (MNI) space. ATL = Anterior temporal lobe; AG = Angular gyrus; MCN = Modified Combinatorial Nomenclature.
Figure 1.
Electrode configurations displayed in the MCN 10/20 system (right) and the resulting distribution of field intensities as modeled using HD-Explore™ 3.1 software (left; Soterix Medical, New York, NY). Electrode configurations were the same in the cathodal and anodal stimulation conditions aside from the reversal of electrode polarities. Pink circles on brain sections approximately mark cortical targets (see main text and Table 1 for further details).
2.3. Materials and Procedures
Stimuli included black-and-white line drawings (n=120) from the Snodgrass and Vanderwart (1980) picture series that elicited >80% name agreement in the original norming study. Stimuli were quasi-randomly assigned to six separate sets (n=20 pictures each) with these sets roughly matched on the number of living/non-living items (χ2 = 0.34, p >.05), name agreement, image agreement, image familiarity, and visual complexity (F =0.91, p>0.05, Wilk’s Λ =.85, partial η2 = .04; see supplementary Table S1 for means for each block). To ensure the absence of systematic differences in difficulty between the sets, we had eleven volunteers (a separate sample to that of the tDCS experiment) name the items within all six while we recorded their response latencies (further details are found in the below section describing the Area of Interest construction). A one-way repeated-measures ANOVA confirmed no significant differences between sets in mean naming latency [F(5, 114) = .58]. For the number reading task, 120 random 6-digit numbers (e.g., 105, 996 – ‘one hundred and five thousand nine hundred and ninety six’) were generated and randomly assigned to six sets of twenty. Numbers appeared in a black font set against a white background. All stimuli were scaled to approximately 5.2 × 5.2 inches (500 × 500 pixels; 1280 × 1024 screen resolution; 17” monitor). Each participant was presented with each set of pictures/numbers only once, and with allocation to each of the three sessions and the pre/post stimulation testing epochs counterbalanced across individuals using a balanced Latin Squares approach. This allocation ensured that across the 12 individuals in the anodal/cathodal condition each set of 20 items occurred in each testing epoch an equal number of times. As such, pre-post stimulation and between-session effects are effectively disentangled from any remaining differences between the stimulus sets. Stimuli within a set were presented in a fixed pseudorandom order.
At the beginning of each session, participants were familiarized with the tasks, receiving instructions to name the stimuli as quickly and as accurately as possible, and given six practice trials of picture naming and number reading to complete. Subsequently, participants were fitted with the tDCS electrode montage which would remain in place until the end of the session. A single set of 20 picture naming trials and then a single set of 20 number reading trials were administered prior to and also following tDCS (this task order was fixed across participants and pre/post stimulation testing epochs). The procedure included four other language/cognitive tests that were subject to separate analyses not reported here (but see Binney et al., 2018). The order of the administration of these tests relative to the naming/reading tests was fully counter-balanced across participants, but fixed across pre/post stimulation testing epochs. tDCS was delivered for 20mins (including 30s fade-in and fade-out phases) with the participant in a state of rest (i.e., with no concurrent task). The session was concluded with a self-paced survey which required 10-point scale ratings of intensity of sensations experienced during tDCS (e.g., pain, itchiness, burning, heat, and fatigue).
Trials were presented via Experiment Center Software (Sensorimotoric Instruments, Inc, Boston, MA). Each trial began with the presentation of an attention fixation cross at the top and center of the screen. This fixation acted as a gaze trigger that automatically advanced after a cumulative dwell time of 1500ms (i.e., aggregate saccade and fixation durations). Picture stimuli appeared for 3000ms (3500ms for number stimuli) accompanied by a brief 250Hz pure tone that enabled offline calculations of naming latencies (using Audacity 2.03 software). A blank screen followed for 500ms before the next trial began. The tone and verbal responses were recorded via a TASCAM DR-40 digital recorder.
2.4. Eye tracking
We tracked gaze dynamics using a remote infrared eyetracker with a sampling rate of 120Hz and a spatial precision of 0.01° (RED-M; Sensorimotoric Instruments, Inc.). Participants were seated and positioned on a chinrest 60 cm away from the infrared eyebar and monitor. We performed a 5-point calibration and validation procedure prior to each set of 20 picture/number stimuli with minimum thresholds of 0.5° of visual angle on both the X- and Y-axes.
2.5. Area of interest (AOI) generation for eye tracking data
Prior to conducting the tDCS experiment, we conducted a normative study with the aim of defining visual areas of interest (AOIs) within each picture stimulus (e.g., flat tail of a beaver). Participants (N= 11) included neurotypical young adults not enrolled in the tDCS study (2 males; mean age 22.2). Each participant named all picture stimuli during two counterbalanced sessions. We post-processed eye tracking data using BeGaze analysis software (Sensorimotor Instruments, Inc.). First, gaze data were averaged across participants and temporally windowed between 100 ms and 724 ms (the grand mean response latency). We then examined ‘dwell time’ (i.e., cumulative time spent in fixation and saccades within part of a stimulus) to identify the region of the picture that received the most visual attention, a proxy metric for semantic informativeness of a particular stimulus feature (Blair, Watson, Walshe, & Maj, 2009; Henderson & Hollingworth, 1999). We demarcated feature AOIs by resampling pixel-wise cumulative gaze data and superimposing over the stimulus in a 12 × 12 gridded ‘attention’ map. Then, if the most gazed upon cell within each matrix clearly contained a single stimulus feature, we manually centered a 95 × 95 pixel AOI over this feature. In other cases (e.g., the cell contained more than one identifiable feature), a Gaussian smoothed heat map was additionally used to determine the feature that would anchor the AOI and in particularly ambiguous cases (e.g., visual scan paths distributed across multiple cells), a consensus agreement was sought from two experimenters. The final AOIs selected for each stimulus are listed in Table S2.
2.6. Statistical Analyses
Data from the ‘cathodal’ participant sample and the ‘anodal’ sample were analyzed separately to avoid entangling what may be subtle effects of tDCS with individual differences. We also analyzed the data obtained from the picture naming task and number naming control task separately. Therefore, all statistical treatment involved a 2-way repeated-measures analysis of variance (ANOVA) with ‘Montage’ as a 3-level within-subject factor and ‘stimulation’ (pre- versus post-stimulation) as the second within-subject factor. The effects of interest here was the interaction effects which would indicate a differential effect of tDCS on performance according to the cortical regions targeted. Post hoc paired sample t-tests were employed to further explore significant interaction effects. Significant main effects of ‘stimulation’ (pre/post) are reported to address concerns regarding practice or fatigue effects.
We examined the effects of tDCS on picture naming via two gaze-tracking measures, and response latencies. The first gaze measure, the duration of the first fixation within the AOI, might reflect how engaging/informative that feature is (Poole & Ball, 2006). The second gaze measure, the sum of the durations of all fixations within the AOI, is thought to reflect the attention allocated to the key feature compared to other parts of the picture (Poole & Ball, 2006). We did not directly analyze durations of fixations on the object but outside of the AOI, as the presence of such fixations was inconsistent. To avoid oversampling, we windowed eye movement data from between 0ms and the previously estimated grand mean picture naming latency (i.e., 724ms post-stimulus onset). For all analyses, we eliminated trials corresponding to incorrect responses, fillers, false starts, self-corrections and latency outliers (−2 > z > 2). We evaluated multi-digit number reading exclusively via response latencies.
3. Results
3.1. Cathodal Stimulation
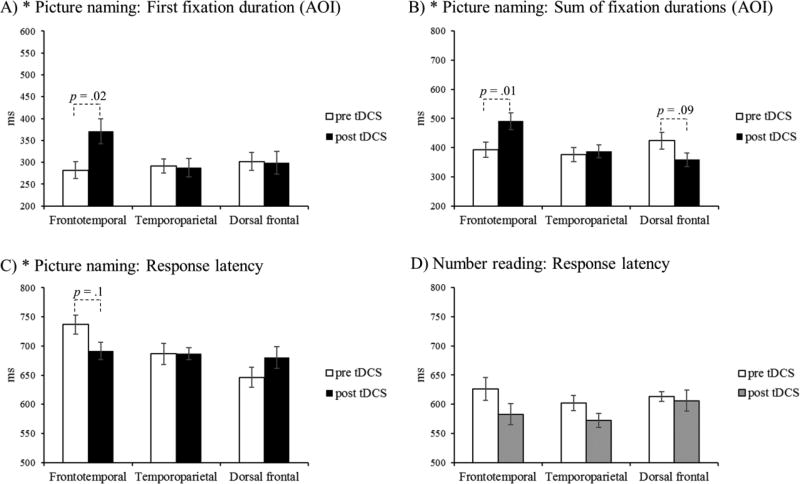
The analysis of gaze data revealed a significant Montage by Cathodal Stimulation (pre/post) interaction effect on the duration of the first visual fixation over pre-defined AOIs in picture naming [F(2, 22) = 3.83, p = .04, partial η2 = .26). Pairwise comparisons revealed a significant site-specific increase in the first visual fixation duration following frontotemporal stimulation [t(11) = 2.74, p = .02; Cohen’s d = .80; mean difference = 89 ms]. Changes in first fixation duration were not apparent in the context of temporoparietal [t(11) = . 15, p = .88] or dorsal frontal stimulation [t(11) = . 11, p = .92] (see Figure 2, panel A).
Figure 2.
Effect of cathodal frontotemporal, temporoparietal or dorsal frontal tDCS on patterns of eye movements during picture naming (panels A–B), and on response latencies in picture naming and number reading (panels C–D). Gaze measures included the duration of the first fixation on a stimulus area of interest (AOI; panel A) and the sum of durations of all fixations on the area of interest (panel B). Each bar represents mean durations/latencies and the corresponding standard error adjusted for within-subject comparisons (O’Brien & Cousineau, 2014).* denotes a significant interaction between stimulation site and pre/post stimulation (p ≤ .05). p-values for pairwise comparisons are shown where the interaction effect was significant and the pairwise test revealed a p-value equal to or less than 0.1.
Similarly, we observed a significant Montage by Cathodal Stimulation (pre/post) interaction effect on the cumulative fixation time within AOIs [F(2, 22) = 7.54, p < .01, partial η2 = .41]. This interaction was such that stimulation with the frontotemporal montage resulted in longer cumulative dwell times within key AOIs [t(11) = 3.22, p = .01; Cohen’s d = .93; mean difference = 98 ms] while there was no change following temporoparietal stimulation [t(11) = .29, p = .77]. There was a decrease in AOI dwell times following dorsal frontal stimulation but it was not statistically significant [t(11) = 1.89, p = .09; Cohen’s d = .53; mean difference = 65 ms; see Figure 2, panel B]. Following these observations, we performed a post hoc analysis to determine whether we might also see evidence of visual search modulations in the latencies from stimulus presentation onset to the first AOI fixation. Speeded latencies, for example, might reflect more efficient detection of key object features. However, neither the interaction (p = .84) nor the main effect of ‘stimulation’ (p = 0.95) were significant.
The analysis of picture naming latencies revealed a borderline significant Montage by Cathodal Stimulation (pre/post) interaction [F(2, 22) = 3.37, p = .05, partial η2 = .23]. This appeared to reflect reduced latencies following tDCS with the frontotemporal montage (mean difference = 45 ms; Cohen’s d = .52) although this difference was not statistically significant [t(11) = 1.82, p = .10]. There was no indication of a speeding or slowing of picture naming latencies following either temporoparietal [t(11) = .02, p = .98] or dorsal frontal stimulation [t(11) = 1.51, p = . 16; see Figure 2, panel C]. We observed generalized speeding of latencies in the number reading task [F(1, 11) = 5.41, p = .04, partial η2 = .33; mean difference = 27 ms; see Figure 2, Panel D], that likely reflected practice effects (there was not a significant interaction effect).
3.2. Anodal Stimulation
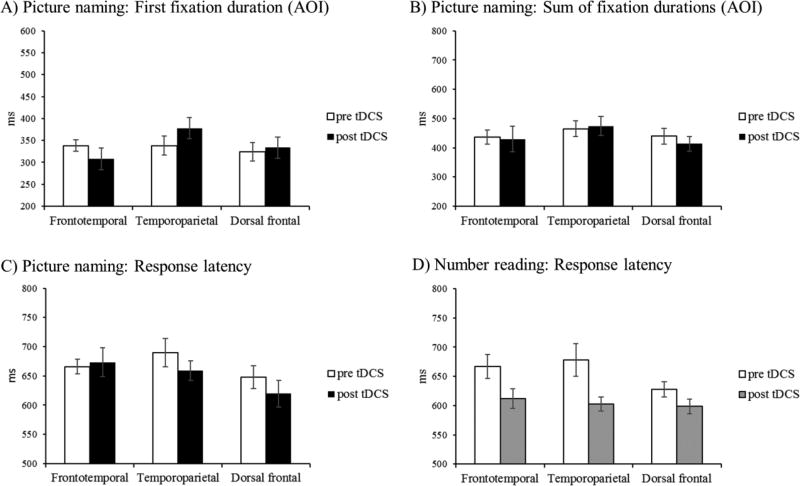
There were no significant main effects nor interaction effects of anodal stimulation on eye gaze metrics or picture naming latencies [all p>.05; see Figure 3]. As in the case cathodal tDCS condition, there was a non-site specific speeding of number reading latencies in the anodal condition [F(1, 11) = 12.81, p < .01, partial η2 = .54; mean difference = 53 ms].
Figure 3.
Effect of anodal frontotemporal, temporoparietal or dorsal frontal tDCS on patterns of eye movements during picture naming (panels A–B), and on response latencies in picture naming and number reading (panels C–D). Gaze measures included the duration of the first fixation on a stimulus area of interest (AOI; panel A) and the sum of durations of all fixations on the area of interest (panel B). Each bar represents mean durations/latencies and the corresponding standard error adjusted for within-subject comparisons (O’Brien & Cousineau, 2014). * denotes a significant interaction between stimulation site and pre/post stimulation (p ≤ .05). p-values for pairwise comparisons are shown where the interaction effect was significant and the pairwise test revealed a p-value equal to or less than 0.1.
3.3. Tolerability of Stimulation
Mean ratings of sensations associated with each tDCS montage are displayed in the supplementary information (Table S3). These ratings were summed to create composite measures of tDCS-induced sensation (max 120) which were treated with a one-way repeated measures ANOVA to examine whether sensation differed as a function of the stimulation target (irrespective of whether it was anodal or cathodal stimulation). One subject was excluded due to having not completed all surveys. There was a significant effect of stimulation site [F(2, 44) = 4.22, p = .02, partial η2 = . 16] reflecting greater sensation experienced during frontotemporal [t (22) = 4.00, p <0.01] and dorsal frontal [t(22) = 2.05, p= 0.05] stimulation as compared to temporoparietal stimulation, which likely relates to the midline electrode being placed on the forehead in these two anterior montages. There was no difference between these montages (p = 0.65). On the basis of these observations, and particularly given the low ratings in general, we interpret the following task results as site-specific neuromodulatory effects and reject the possibility that they were non-specific effects related to differential tolerability of montages.
4. Discussion
In a classic eyetracking study, Yarbus (1967, pg. 174) demonstrated significant variability in the dynamics of visual search for the same portrait (Repin’s Unexpected Visitor) under different task cues. Scan paths, saccade amplitudes, and fixation patterns all differed when participants estimated ages of the people in the portrait vs. recalling their spatial positions. These findings empirically confirmed a phenomenon of intense interest today at the intersection of semantics and vision research (see Barsalou, 2017). Namely, it is widely contended that lower level visuoperceptual processes are guided by task goals and semantic expectancies (Belke, Humphreys, Watson, Meyer & Telling, 2008; Chiou & Lambon Ralph, 2016; Daalman, Verkooijen, Derks, Aleman & Sommer, 2012; Trapp & Barr 2015; but see Firestone & Scholl, 2016). Yet, the nature of such top-down (conceptually driven) perceptual processes and their neural substrates remains controversial.
In the present study, we explored the contribution(s) of frontal, temporoparietal and anterior temporal brain regions to visual confrontation naming. We paired tDCS with eye-tracking both to improve sensitivity for detecting neural perturbations and also to test the hypothesis that modulations of top-down conceptual influences on picture naming processes would be evident in visual search patterns. Analysis of eye gaze data revealed that bilateral cathodal frontotemporal stimulation, but not bilateral dorsal frontal or bilateral temporoparietal stimulation, modulated the spatial distribution of stimulus fixations during picture naming. Specifically, participants exhibited longer dwell times over key semantic features (e.g., the head of an animal) post tDCS relative to the pre-stimulation baseline. This was coupled with a trend for speeded naming latencies, suggesting that the cognitive effect of stimulation was facilitative in nature (but see below). The use of a control task, the fact that the effect was limited to one of three stimulation montages and also that it was polarity-dependent rules out non-specific effects of frontotemporal tDCS. Below, we discuss our interpretation of these findings as reflecting a contribution of anterior temporal cortices, in particular, to conceptual top-down influences on visual object recognition. Furthermore, we consider the implications of the study for applications of tDCS in rehabilitation of aphasia.
The demonstration of montage-specific effects of brain stimulation on behavior attest to the necessity of a region (or regions) for the task/process at hand. To our knowledge, this is the first study to use both non-invasive brain stimulation and eye tracking and provide evidence for a causal role of the ATL in visual search during picture naming. This is in line with cognitive models that posit the ATL as a key representational substrate for semantic knowledge (e.g., Lambon Ralph et al., 2017; Reilly et al., 2016). Its contribution to visual object naming, in particular, has been demonstrated within a set of convergent multi-method studies in patients and healthy adults (Mesulam et al., 2013; Pobric, Jefferies, & Lambon Ralph, 2007; Price, Devlin, Moore, Morton, & Laird, 2005; Schwartz et al., 2009; Tomaszewki Farias, Harrington, Broomand, & Seyal, 2005) but only a small number of studies specifically addressed the possibility of top-down contributions of the region in addition to more established feedforward lexical-semantic processes (e.g., Clarke et al., 2013). A recent transcranial magnetic theta-burst stimulation study suggests the ATL plays an important role in providing top-down support in visual object recognition (Chiou & Lambon Ralph, 2016). The results of the present study support this hypothesis specifically in the context of confrontation naming and further suggests that this support includes guiding visual search (e.g., scanning to semantic features that are key to recognition).
Coarse spatial precision and current flow ambiguities in conventional tDCS, however, limit strong localization claims. In the present study, the frontotemporal montage implicates a large swathe of bilateral cortex, including the ATL, the IFG and orbitofrontal cortex (OFC; Figure 1A). The OFC in particular has also been posited as a major contributor of top-down influences in object recognition (Panichello, Cheung, & Bar, 2012; Trapp & Bar, 2015). Further, the effects may not be attributable to any singular region but instead to a stimulation-induced increase in functional connectivity amongst a network of regions (Luft, Pereda, Banissy, & Bhattacharya, 2014). Therefore, it remains for future research to utilize techniques that afford greater spatial precision to systematically evaluate the contribution of different candidate sources for the generation of semantically-based top-down influences. Neurostimulation, in combination with functional imaging that could capture brain remote/network effects, offers a particularly promising approach. Research to identify the optimal montage configurations for modulating naming processes with tDCS, however, has particular clinical translational value.
To our knowledge, very few studies have directly contrasted the capacity for tDCS targeting alternate sets brain regions to modulate naming performance in healthy adults. This is surprising given the growing interest in tDCS as a therapeutic tool for aphasia, applied either in isolation or as an adjuvant to speech-language therapy (Holland & Crinion, 2012; Tippett, Hillis, & Tsapkini, 2015), and the fact that naming impairment is the single most common and one of the most debilitating features of aphasia. The hope is that tDCS has potential to guide neuroplasticity in recovery and thereby facilitate (re)learning during behavioral therapy. It has gained particular attention due to its portability and cost-effectiveness relative to other neuromodulatory techniques like TMS. However, research into how tDCS can be optimally configured to effectively target the functionally relevant neural circuits remains at a nascent stage and there are even greater gaps in our understanding of how these protocols should be adapted when the integrity of these circuits is compromised. Our results suggest that in the context of rehabilitation of naming impairments, tDCS could be most efficacious when applied to the bilateral frontotemporal cortices, with particular emphasis on electrode placement over the ATL.
The effect of frontotemporal cathodal tDCS on the gaze measures was coupled with a trend for speeded naming latencies. This suggests that the gaze modulation reflects facilitative effects of stimulation. However, compared to the other sessions, there was an inexplicably slow baseline mean naming response in this montage condition, and for this reason, this particular result should be treated with caution. Indeed, we cannot rule out the possibility that the modulations of gaze reflect negative effects of stimulation on cogniion (e.g., the longer dwell times might reflect a greater effort in analyzing object features). Recall that we predicted that cathodal stimulation would have negative effects. This was in line with the seminal studies by Priori and colleagues and, later, Nitsche and colleagues that directly associated neural excitation and inhibition of sensorimotor systems with the anodal and cathodal electrodes respectively (Nitsche & Paulus, 2000; Priori, Berardelli, Rona Accornero & Manfredi, 1998). It is important to note, however, that a more complex picture has emerged in the context of stimulating higher-order cognitive systems (Garnett, Malyutina, Datta, & den Ouden, 2015; Jacobson, Koslowsky, & Lavidor, 2012). For example, in the context of language processing, there are only a few reports of effective cathodal stimulation (see Monti et al., 2013). Further, and of particular relevance to the present findings, there are a growing number of tDCS investigations of higher cognition that report facilitatory effects of cathodal tDCS (Moos, Vossel, Weidner, Sparing, & Fink, 2012; Nozari, Woodard, & Thompson-Schill, 2014; Pirulli, Fertonani, & Miniussi, 2014; Weiss & Lavidor, 2012). What could be driving this more complex pattern is unclear. There are a number of potential physiological factors under investigation including site-to-site variation in the orientation of neurons relative to the electric field, the neural activation state of cell assemblies at time of stimulation (e.g., whether they are engaged by the experimental task or another demanding task), duration and intensity of stimulation, and many others (Garnett et al., 2015; Gill, Shah-Basak & Hamilton, 2015; Nozari et al., 2014). Whether the direction of the effects seen in the present study is attributable to certain elements of our tDCS protocol must be addressed by future studies that systematically and orthogonally vary these factors. It is also important to be mindful of disentangling inferences regarding the effects of stimulation at the level of cognition/behavior from those taking at the neurophysiological level. For example, positive behavioral effects could conceivably reflect either excitation of a region or inhibition of a region that itself actively inhibits certain processes/behaviors (Chrysikou et al., 2013).
Finally, many of our tDCS parameters (e.g., using bilateral montages) differed to those of the prior studies of naming (Malyutina and den Ouden, 2015; Pisoni, et al., 2012, 2015). Future research should also, therefore, determine whether the discrepancies in outcome, such as the null effects we observed in the dorsal frontal and temporoparietal conditions, can be attributed to one or more of these parameters, and/or issues such as the statistical power required to detect subtle effects.
5. Conclusions
To summarize, the present study found that bilateral frontotemporal cathodal tDCS, but not temporoparietal nor dorsal frontal stimulation, modulates visual search patterns during picture naming. We interpret these findings as consistent with a hypothesis that conceptual top-down influences on object recognition originate from a bilateral anterior temporal semantic representational system. Our results might also be used to inform clinical translational research concerning therapeutic applications of tDCS in acquired language impairment. In particular, they suggest that treatments targeting naming might optimize gains by applying tDCS to the bilateral frontotemporal cortices with particular emphasis on electrode placement over the ATL. Yet, as we have highlighted above, while there is considerable promise regarding tDCS in treatment of aphasia, there is much left to be done regarding discovery of the optimal procedures for safe and efficacious application.
Supplementary Material
Statement of significance to the neurobiology of language.
Our results suggest that tDCS targeting the anterior temporal cortex could be used to modulate early top-down conceptual influences on object recognition during confrontation naming. In turn, this suggests that treatments targeting naming impairments might optimize gains by applying tDCS with emphasis on electrode placement over this region.
Highlights.
tDCS was paired with eye tracking to elucidate neural contributions to picture naming.
Neurotypical adults named line drawings prior to and following tDCS
tDCS targeted frontotemporal, dorsal frontal or temporoparietal cortex.
Frontotemporal stimulation resulted in longer visual fixations on key semantic features.
We conclude that anterior temporal tDCS modulates visual search during naming.
Acknowledgments
This research was funded by US Public Health Service Grant R01 DC013063 (Reilly)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
While this and previous papers by the same authors provide evidence for the validity of such models, the current flow and associated field intensities discussed in the present study should, in our opinion, only be considered rough estimates because the head model is not representative of our sample, nor does the model account for the specific apparatus and stimulation parameters employed.
Disclosure: The authors declare no potential conflicts of interest.
References
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Arevalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: Evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49(3):658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. What does semantic tiling of the cortex tell us about semantics? Neuropsychologia. 2017;105:18–38. doi: 10.1016/j.neuropsychologia.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Bauer A, Hung J, Grossman M, Reilly J. Eyetracking Reveals Aberrant Visual Search During Confrontation Naming of Alzheimer’s Disease and Primary Progressive Aphasia; Paper presented at the Academy of Aphasia 53rd Annual Meeting; Tuscon, AZ, USA. 2015. [Google Scholar]
- Belke E, Humphreys GW, Watson DG, Meyer AS, Telling AL. Top-down effects of semantic knowledge in visual search are modulated by cognitive but not perceptual load. Perception & Psychophysics. 2008;70(8):1444–1458. doi: 10.3758/pp.70.8.1444. [DOI] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJM, Lambon Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic sementia. Cerebral Cortex. 2010;20(11):2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Binney RJ, et al. Reading words and other people: A comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex. 2016;82:147–163. doi: 10.1016/j.cortex.2016.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Hoffman P, Lambon Ralph MA. Mapping the multiple graded contributions of the anterior temporal lobe representational hub to abstract and social concepts: evidence from distortion-corrected fMRI. Cerebral Cortex. 2016;26(11):4227–4241. doi: 10.1093/cercor/bhw260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Zuckerman BM, Waller HN, Hung J, Ashaie SA, Reilly J. Cathodal tDCS of the bilateral anterior temporal lobes facilitates semantically-driven verbal fluency. Neuropsychologia. 2018;111:62–71. doi: 10.1016/j.neuropsychologia.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair MR, Watson MR, Walshe RC, Maj F. Extremely selective attention: eye-tracking studies of the dynamic allocation of attention to stimulus features in categorization. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(5):1196–1206. doi: 10.1037/a0016272. [DOI] [PubMed] [Google Scholar]
- Cappon D, Jahanshahi M, Bisiacchi P. Value and efficacy of transcranial direct current stimulation in the cognitive rehabilitation: a critical review since 2000. Frontiers in neuroscience. 2016:10. doi: 10.3389/fnins.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AM, Baker JM, Eskandar E, Schomer D, Ulbert I, Marinkovic K, Halgren E. First-pass selectivity for semantic categories in human anteroventral temporal lobe. Journal of Neuroscience. 2011;31(49):18119–18129. doi: 10.1523/JNEUROSCI.3122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou R, Lambon Ralph MA. The anterior temporal cortex is a primary semantic source of top-down influences on object recognition. Cortex. 2016;79:75–86. doi: 10.1016/j.cortex.2016.03.007. doi: http://dx.doi.org/10.1016/j.cortex.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Hamilton RH, Coslett HB, Datta A, Bikson M, Thompson-Schill SL. Noninvasive transcranial direct current stimulation over the left prefrontal cortex facilitates cognitive flexibility in tool use. Cognitive Neuroscience. 2013;4(2):81–89. doi: 10.1080/17588928.2013.768221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Taylor KI, Devereux B, Randall B, Tyler LK. From Perception to Conception: How Meaningful Objects Are Processed over Time. Cerebral Cortex. 2013;23(1):187–197. doi: 10.1093/cercor/bhs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daalman K, Verkooijen S, Derks EM, Aleman A, Sommer IE. The influence of semantic top-down processing in auditory verbal hallucinations. Schizophrenia Resesearch. 2012;139(1–3):82–86. doi: 10.1016/j.schres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. Journal of Visualized Experiments. 2011;(51):2744. doi: 10.3791/2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Zhou X, Su Y, Parra LC, Bikson M. Validation of finite element model of transcranial electrical stimulation using scalp potentials: implications for clinical dose. Journal of neural engineering. 2013;10(3):036018. doi: 10.1088/1741-2560/10/3/036018. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Hillis AE. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130(Pt 5):1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, Gagnon DA. Lexical access in aphasic and nonaphasic speakers. Psychological Review. 1997;104(4):801–838. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C. Naming facilitation induced by transcranial direct current stimulation. Behavioural brain research. 2010;208(2):311–318. doi: 10.1016/j.bbr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, Marangolo P. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience. 2011;23(9):2309–2323. doi: 10.1162/jocn.2010.21579. [DOI] [PubMed] [Google Scholar]
- Firestone C, Scholl BJ. Cognition does not affect perception: Evaluating the evidence for “top-down” effects. Behavioral and Brain Sciences. 2016;39:e229. doi: 10.1017/S0140525×15000965. [DOI] [PubMed] [Google Scholar]
- Garnett EO, Malyutina S, Datta A, den Ouden DB. On the use of the terms anodal and cathodal in high-definition transcranial direct current stimulation: A Technical note. Neuromodulation. 2015;18(8):705–713. doi: 10.1111/ner.12320. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Leech R, Wise RJ. Semantic retrieval during overt picture description: Left anterior temporal or the parietal lobe? Neuropsychologia. 2014;76:125–135. doi: 10.1016/j.neuropsychologia.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W. Top-down influences on visual processing. Nature Reviews Neuroscience. 2013;14(5):350–363. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Shah-Basak PP, Hamilton R. It’s the thought that counts: examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain stimulation. 2015;8(2):253–259. doi: 10.1016/j.brs.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Hollingworth A. High-level scene perception. Annual Review of Psychology. 1999;50(1):243–271. doi: 10.1146/annurev.psych.50.1.243. [DOI] [PubMed] [Google Scholar]
- Holland R, Crinion J. Can tDCS enhance treatment of aphasia after stroke? Aphasiology. 2012;26(9):1169–1191. doi: 10.1080/02687038.2011.616925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ, Price CJ. Top-down processes in object identification: evidence from experimental psychology, neuropsychology and functional anatomy. Philosophical Transactions of the Royal Society B: Biological Sciences. 1997;352(1358):1275–1282. doi: 10.1098/rstb.1997.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research. 2012;216(1):1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129(Pt 8):2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Kovic V, Plunkett K, Westermann G. Eye-tracking study of animate objects. Psihologija. 2009a;42(3):307–327. doi: 10.2298/PSI0903307K. [DOI] [Google Scholar]
- Kovic V, Plunkett K, Westermann G. Eye-tracking study of inanimate objects. Psihologija. 2009b;42(4):417–436. doi: 10.2298/PSI0904417K. [DOI] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nature Reviews Neuroscience. 2017;18(1):42–55. doi: 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Speaking: From intention to articulation. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Luft CD, Pereda E, Banissy MJ, Bhattacharya J. Best of both worlds: promise of combining brain stimulation and brain connectome. Frontiers in Systems Neuroscience. 2014;8:132. doi: 10.3389/fnsys.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyutina S, den Ouden D. High-Definition tDCS of noun and verb retrieval in naming and lexical decision. NeuroRegulation. 2015;2(3):111–125. [Google Scholar]
- Meinzer M, Yetim O, McMahon K, de Zubicaray G. Brain mechanisms of semantic interference in spoken word production: An anodal transcranial direct current stimulation (atDCS) study. Brain and Language. 2016;157–158:72–80. doi: 10.1016/j.bandl.2016.04.003. https://doi.org/10.1016/j.bandl.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136(Pt 2):601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Boutet C, Valabregue R, Ferrieux S, Nogues M, Lehéricy S, Teichmann M. The brain network of naming: A Lesson from primary progressive aphasia. PLoS One. 2016;11(2):e0148707. doi: 10.1371/journal.pone.0148707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti A, Ferrucci R, Fumagalli M, Mameli F, Cogiamanian F, Ardolino G, Priori A. Transcranial direct current stimulation (tDCS) and language. Journal of Neurology, Neurosurgery, & Psychiatry. 2013;84(8):832–842. doi: 10.1136/jnnp-2012-302825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos K, Vossel S, Weidner R, Sparing R, Fink GR. Modulation of top-down control of visual attention by cathodal tDCS over right IPS. Journal of Neuroscience. 2012;32(46):16360–16368. doi: 10.1523/jneurosci.6233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karaköse T, Antal A, Liebetanz D, Lang N, Paulus W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. Journal of Neurophysiology. 2007;97(4):3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- Nozari N, Woodard K, Thompson-Schill SL. Consequences of cathodal stimulation for behavior: when does it help and when does it hurt performance? PLoS One. 2014;9(1):e84338. doi: 10.1371/journal.pone.0084338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien F, Cousineau D. Representing error bars in within-subject designs in typical software packages. The Quantitative Methods for Psychology. 2014;10(1):56–67. [Google Scholar]
- Panichello MF, Cheung OS, Bar M. Predictive feedback and conscious visual experience. Frontiers in Psychology. 2012;3:620. doi: 10.3389/fpsyg.2012.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirulli C, Fertonani A, Miniussi C. Is neural hyperpolarization by cathodal stimulation always detrimental at the behavioral level? Frontiers in Behavioral Neuroscience. 2014;8:226. doi: 10.3389/fnbeh.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni A, Papagno C, Cattaneo Z. Neural correlates of the semantic interference effect: New evidence from transcranial direct current stimulation. Neuroscience. 2012;223:56–67. doi: 10.1016/j.neuroscience.2012.07.046. doi: http://dx.doi.org/10.1016/j.neuroscience.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Pisoni A, Vernice M, Iasevoli L, Cattaneo Z, Papagno C. Guess who? Investigating the proper name processing network by means of tDCS. Neuropsychologia. 2015;66:267–278. doi: 10.1016/j.neuropsychologia.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A, Ball LJ. Eye Tracking in Human Computer Interaction and Usability Research: Current Status and Future Prospects. In: Ghaoui C, editor. Encyclopedia of Human Computer Interaction. Pennsylvania: Idea Group, Inc; 2006. [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR. Meta-analyses of object naming: effect of baseline. Human Brain Mapping. 2005;25(1):70–82. doi: 10.1002/hbm.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AR, McAdams H, Grossman M, Hamilton RH. A meta-analysis of transcranial direct current stimulation studies examining the reliability of effects on language measures. Brain stimulation. 2015;8(6):1093–1100. doi: 10.1016/j.brs.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Reilly J, Peelle JE, Antonucci SM, Grossman M. Anomia as a marker of distinct semantic memory impairments in Alzheimer’s disease and semantic dementia. Neuropsychology. 2011;25(4):413–426. doi: 10.1037/a0022738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Rodriguez AD, Peelle JE, Grossman M. Frontal lobe damage impairs process and content in semantic memory: evidence from category-specific effects in progressive non-fluent aphasia. Cortex. 2011;47(6):645–658. doi: 10.1016/j.cortex.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Peelle JE, Garcia A, Crutch SJ. Linking somatic and symbolic representation in semantic memory: the dynamic multilevel reactivation framework. Psychonomic Bulletin & Review. 2016;23(4):1002–1014. doi: 10.3758/s13423-015-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, McCoy D, Wolk DA, Coslett HB, Olson IR. Improved proper name recall by electrical stimulation of the anterior temporal lobes. Neuropsychologia. 2010;48(12):3671–3674. doi: 10.1016/j.neuropsychologia.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Ross LA, McCoy D, Coslett HB, Olson IR, Wolk DA. Improved proper name recall in aging after electrical stimulation of the anterior temporal lobes. Frontiers in Aging Neuroscience. 2011;3:16. doi: 10.3389/fnagi.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandars M, Cloutman L, Woollams AM. Taking sides: An integrative review of the impact of laterality and polarity on efficacy of therapeutic transcranial direct current stimulation for anomia in chronic poststroke aphasia. Neural plasticity. 2016 doi: 10.1155/2016/8428256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132(12):3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. Journal of Neuroscience. 2010;30(50):16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotake A, Matsumoto R, Ueno T, Kunieda T, Saito S, Hoffman P, Lambon Ralph MA. Direct exploration of the role of the ventral anterior temporal lobe in semantic memory: Cortical stimulation and local field potential evidence from subdural grid electrodes. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sparing R, Dafotakis M, Meister IG, Thirugnanasambandam N, Fink GR. Enhancing language performance with non-invasive brain stimulation--a transcranial direct current stimulation study in healthy humans. Neuropsychologia. 2008;46(1):261–268. doi: 10.1016/j.neuropsychologia.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. The Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Kruck S, De Baene W, Schroger E, Alter K, Friederici AD. Top-down modulation of auditory processing: effects of sound context, musical expertise and attentional focus. European Journal of Neuroscience. 2009;30(8):1636–1642. doi: 10.1111/j.1460-9568.2009.06955.x. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippett DC, Hillis AE, Tsapkini K. Treatment of Primary Progressive Aphasia. Current treatment Options in Neurology. 2015;17(8):362–362. doi: 10.1007/s11940-015-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewki Farias S, Harrington G, Broomand C, Seyal M. Differences in Functional MR Imaging Activation Patterns Associated with Confrontation Naming and Responsive Naming. American Journal of Neuroradiology. 2005;26(10):2492–2499. [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Bar M. Prediction, context, and competition in visual recognition. Annals of the New York Academy of Sciences. 2015;1339:190–198. doi: 10.1111/nyas.12680. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383(6597):254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vitali P, Avanzini G, Caposio L, Fallica E, Grigoletti L, Maccagnano E, Villani F. Cortical location of 10–20 system electrodes on normalized cortical MRI surfaces. International Journal of Bioelectromagnetism. 2002;4(2):147–148. [Google Scholar]
- Weiss M, Lavidor M. When less is more: evidence for a facilitative cathodal tDCS effect in attentional abilities. Journal of Cognitive Neuroscience. 2012;24(9):1826–1833. doi: 10.1162/jocn_a_00248. [DOI] [PubMed] [Google Scholar]
- Westwood SJ, Olsen A, Miall C, Nappo R, Romani C. Limits to tDCS effects in language: Failure to modulate word production in healthy participants with frontal or temporal tDCS. Cortex. 2017;86:64–82. doi: 10.1016/j.cortex.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex. 2011;21(5):1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams AM, Ralph MA, Plaut DC, Patterson K. SD-squared: on the association between semantic dementia and surface dyslexia. Psychological Review. 2007;114(2):316–339. doi: 10.1037/0033-295x.114.2.316. [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye movements and vision. New York, NY USA: Plenum Press; 1967. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.