Abstract
Background
Powassan virus (POWV) is a tick-borne flavivirus that causes rare, but often severe, disease in humans. POWV neuroinvasive disease was added to the U.S. nationally notifiable disease list in 2001 and non-neuroinvasive disease was added in 2004. The only previous review of the epidemiology of POWV disease in the United States based on cases reported to the Centers for Disease Control and Prevention (CDC) covered the period from 1999 through 2005.
Methods
We describe the epidemiology and clinical features of laboratory-confirmed POWV disease cases reported to CDC from 2006 through 2016.
Results
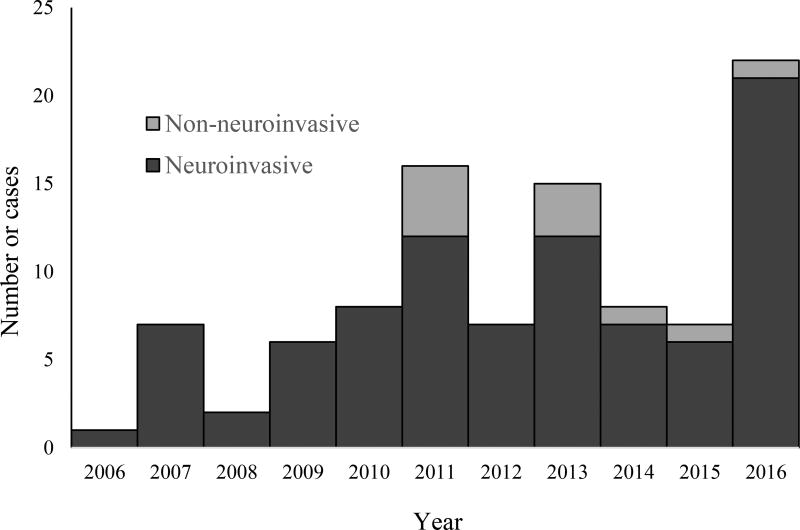
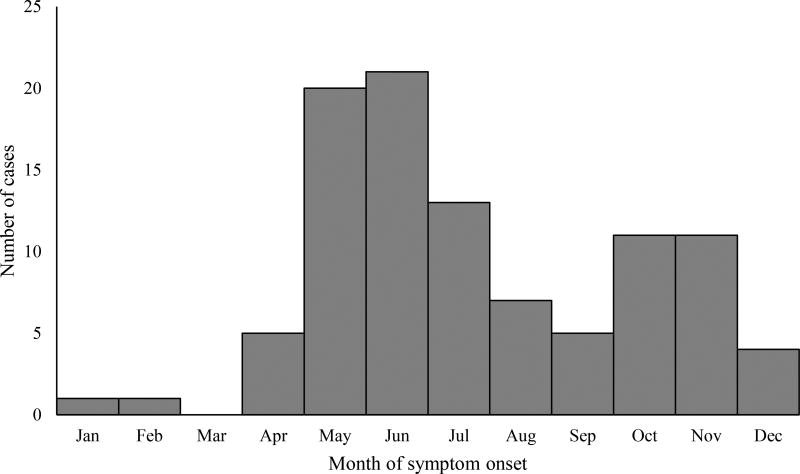
There were 99 cases of POWV disease reported during the 11-year period, including 89 neuroinvasive and 10 non-neuroinvasive disease cases. There was a median of 7 cases per year (range: 1–22), with the highest numbers of cases reported in 2011 (n=16), 2013 (n=15) and 2016 (n=22). Cases occurred throughout the year but peaked in May and June. Cases were reported primarily from northeastern and north-central states. Overall, 72 (73%) cases were in males and the median age was 62 years (range: 3 months – 87 years). Of the 11 (11%) cases who died, all were aged >50 years. The average annual incidence of neuroinvasive POWV disease was 0.0025 cases per 100,000 persons.
Conclusions
POWV disease can be a severe disease and has been diagnosed with increased frequency in recent years. However, this might reflect increased disease awareness, improved test availability, and enhanced surveillance efforts. Clinicians should consider POWV disease in patients presenting with acute encephalitis or aseptic meningitis who are resident in, or have traveled to, an appropriate geographic region.
Keywords: Powassan, encephalitis, arboviral disease, United States
Introduction
Powassan virus (POWV) is a tick-borne flavivirus that causes rare, but often severe, disease in humans. The most common clinical presentation is encephalitis, although other neurological presentations (e.g. aseptic meningitis) have been observed (CDC 2001, Hinten 2008). Milder febrile illness also can occur and serological data suggest POWV infections can be subclinical (McLean 1960, Hinten 2008). Among published cases of neurological POWV disease, physical or cognitive sequelae have been reported in up to 70% (El Khoury 2013, Hinten 2008) and 10–15% were fatal in the acute stage (Johnson 2010, Artsob 1988). There is no vaccine to prevent or antiviral medication to treat POWV disease.
Humans are infected with POWV by the bite of an infected tick. POWV is transmitted primarily by ticks in the genus Ixodes, including Ixodes cookei, Ixodes marxi, and Ixodes scapularis. (Main 1979, Ebel 2010). Ix. cookei and Ix. marxi rarely bite humans, however Ix. scapularis often bites humans (Ebel 2010). There are two lineages of POWV: POWV lineage 1 and POWV lineage 2 (deer tick virus). Although the two lineages have distinct enzootic cycles, they are closely related and are clinically and serologically indistinguishable (El Khoury 2013, Hermance 2017). POWV lineage 1 appears to be maintained in enzootic transmission cycles between Ix. cookei and groundhogs and skunks, or Ix. marxi and squirrels. The main enzootic cycle for POWV lineage 2 is considered to be between Ix. scapularis and white-footed mice (Ebel 2010). Human cases have been reported primarily from eastern Canada, the northeastern United States, and more recently from the north-central United States (Deibel 1979, Hinten 2008).
In the United States, the first reported human case of POWV disease occurred in 1970 (Goldfield, 1973) and 10 more laboratory-confirmed cases were reported in the literature through 1998 (Artsob 1988, Embil 1983, CDC 1995). POWV neuroinvasive disease was formally added to the list of nationally notifiable diseases in 2001 and non-neuroinvasive POWV disease was added in 2004. A previous review described the epidemiology of POWV disease in the United States based on nine cases reported to the Centers for Disease Control and Prevention (CDC) from 1999 through 2005 (Hinten, 2008). In this review, we describe the epidemiology and clinical characteristics of POWV disease cases reported from 2006 through 2016.
Materials and Methods
Data source and case definitions
Since 2003, POWV disease cases have been reportable to CDC through ArboNET, the electronic passive surveillance system for nationally notifiable arboviral diseases. State health departments report cases meeting the arboviral disease case definition (CDC 2015). We identified probable and confirmed cases of POWV disease reported to ArboNET from 2006 through 2016.
The standard surveillance case definition for arboviral diseases, including POWV disease, includes clinical and laboratory components (CDC 2015). A clinically compatible case of neuroinvasive disease is defined as a case with encephalitis, meningitis, acute flaccid paralysis, or other acute signs of central or peripheral neurologic dysfunction. Other cases with fever and without neuroinvasive disease are defined as non-neuroinvasive disease cases.
A confirmed POWV disease case must meet at least one of the following laboratory criteria: 1) detection of POWV or antigen or nucleic acid in tissue, blood, cerebrospinal fluid (CSF) or other bodily fluid; 2) a fourfold or greater change in POWV quantitative antibody titers between acute and convalescent serum samples; 3) detection of POWV immunoglobulin M (IgM) with confirmatory POWV neutralizing antibodies; or 4) detection of POWV IgM in CSF without IgM to other endemic arboviruses. A probable case has detection of POWV IgM in the serum or CSF with no other testing performed (CDC 2015). POWV lineage data are not collected in ArboNET.
Data analysis
We reviewed data on age, sex, place of residence, month and year of illness onset, clinical syndrome, hospitalization, and mortality. Categorical variables were presented as counts and proportions, and continuous variables as medians and ranges. Incidence rates were calculated only for neuroinvasive POWV disease cases, as detection and reporting of neuroinvasive disease cases is assumed to be more consistent and complete than that of non-neuroinvasive disease cases because of the substantial associated morbidity. U.S. Census Bureau population estimates were used for incidence calculations (United States Census Bureau). Annual neuroinvasive POWV disease incidence was calculated using national population estimates and state incidence using the average state population for 2006 through 2016.
We analyzed the data using Excel version 2010 (Microsoft, Redmond, WA) and R version 3.2.1 (Vienna, Austria). Maps were made using ArcGIS 10.3 (ESRI, Redlands, CA).
Results
There were 99 cases of POWV disease reported to ArboNET from 2006 through 2016, including 89 neuroinvasive and 10 non-neuroinvasive disease cases. Among the 99 cases, 90 were confirmed and 9 were probable. There was a median of 7 cases per year (range: 1–22), with the highest number of cases reported in 2011 (n=16), 2013 (n=15) and 2016 (n=22) (Figure 1). Among the 99 POWV disease cases, 72 (73%) were male and the median age was 62 years (range: 3 months – 87 years) (Table 1). Cases occurred in all months except March, with a peak in May and June (Figure 2). The most commonly reported clinical syndrome was encephalitis (72%), followed by meningitis (16%) and febrile illness (10%) (Table 1). Eighty-nine (90%) cases were hospitalized. Of the 11 (11%) cases who died, all were aged >50 years and presented with neuroinvasive disease.
Figure 1.
Powassan virus disease cases by year of onset and clinical syndrome, United States, 2006–2016
Table 1.
Characteristics of Powassan virus disease cases reported to ArboNET, United States, 2006–2016
| Characteristic | (N=99) No. (%) |
|
|---|---|---|
| Age group (years) | ||
| 0–9 | 8 (8) | |
| 10–19 | 3 (3) | |
| 20–29 | 3 (3) | |
| 30–39 | 6 (6) | |
| 40–49 | 7 (7) | |
| 50–59 | 13 (13) | |
| 60–69 | 29 (29) | |
| 70–79 | 22 (22) | |
| ≥80 | 8 (8) | |
| Male | 72 (73) | |
| Clinical syndrome | ||
| Neuroinvasive | 89 (90) | |
| Encephalitis | 71 (72) | |
| Meningitis | 16 (16) | |
| Other neurologic* | 2 (2) | |
| Non-neuroinvasive | 10 (10) | |
| Febrile illness | 10 (10) | |
| Hospitalized | 89 (90) | |
| Death | 11 (11) | |
Presentation for one patient was with ataxia, fever, rash, fatigue, and myalgia, and for one patient was headache, tremors, hemiparesis, and altered mental status
Figure 2.
Powassan virus disease cases by month of illness onset, United States, 2006–2016
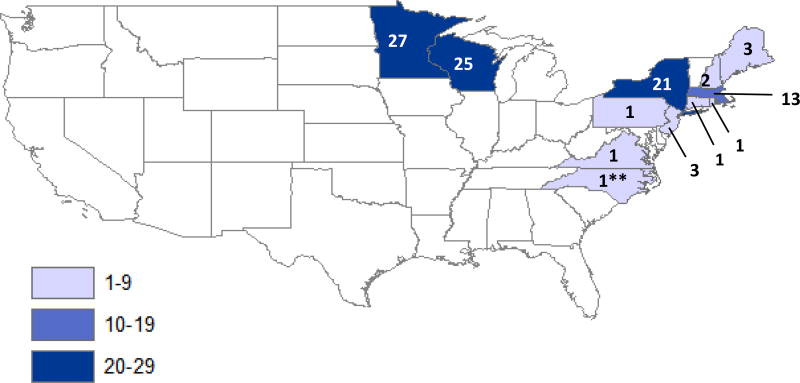
Cases were reported from 56 counties in 12 states (Figure 3). During the 11-year period, 5 states without previously reported cases of POWV disease reported cases for the first time: Connecticut, Minnesota, New Hampshire, Rhode Island, and Virginia. North Carolina also reported a first case, but it was acquired out of state.
Figure 3.
Powassan virus disease cases by state of residence, United States, 2006–2016.*
*There were no cases in residents of Alaska or Hawaii.
**North Carolina case reported as acquired out of state.
For the 11-year period from 2006 through 2016, the average annual incidence of neuroinvasive POWV disease was 0.0025 cases per 100,000 persons (range: 0.0003–0.0059 cases per 100,000). The states with the highest average annual incidence of POWV disease were Minnesota (0.468 per 100,000), Wisconsin (0.333), Maine (0.226), and Massachusetts (0.197).
Discussion
During the 11-year period from 2006 through 2016, a total of 99 cases of POWV disease were reported to ArboNET, mostly from northeastern and north-central U.S. states. While there was substantial year-to-year variability, higher incidence of reported disease occurred more frequently in recent years, including in 2011, 2013, and 2016. Most cases presented with neuroinvasive disease and the majority required hospitalization.
Prior to 2006, only 20 cases of POWV disease in the United States had been reported in the literature, including nine published cases reported to CDC from 1999 to 2005. An increase in testing availability as well as improved awareness of the disease have likely resulted in increased diagnosis and reporting of POWV disease during the last decade. Enhanced surveillance in Minnesota and Wisconsin may have contributed to the large number of cases reported from those states (Johnson 2010, Neitzel 2013). Beginning in 2003 in Wisconsin and 2008 in Minnesota, there were efforts to improve awareness and knowledge of providers and the public, and both states are now able to conduct POWV testing at state level at their public health laboratories. The apparent increase in disease incidence also might be related to the spread of POWV into new areas, with several states without previously published or reported cases of POWV disease reporting cases for the first time.
Similar to previously published U.S. cases, there was a higher burden of disease in persons aged ≥50 years, and the majority of cases were in males (Artsob 1988, Embil 1983, CDC 1995). The reason for the preponderance of male cases is unknown, but might reflect different behavioral patterns, differential tick exposure, the presence of underlying medical conditions that might be risk factors for the development of neuroinvasive disease after POWV infection, or other biological factors. A similar pattern of higher neuroinvasive disease incidence among males is also observed with several mosquito-borne flavivirus infections (Lindsey 2010, Kari 2006).
Although the majority of cases had illness onset in the late spring through early summer, cases occurred in all months except March, suggesting some year-round risk for exposure to POWV. This is similar to many other tick-borne diseases, but is different from the risk period for most mosquito-borne arboviral diseases (Biggs 2016, Schwartz 2017, Reimann 2008). A late summer and early fall peak in incidence occurs for most mosquito-borne arboviral diseases, and in most areas risk is relatively limited during the first half of the year. The extended risk period for POWV disease reflects tick activity well before and after the peak mosquito season.
The mortality rate of 11% is consistent with previously reported rates (Johnson 2010, Artsob 1988). One published case series suggested a higher case fatality rate when patients were followed up after discharge; among 14 patients, one (7%) patient died in hospital and an additional 4 (29%) died within approximately 8 months of discharge (El Khoury 2013). Among survivors, high rates of neurological sequelae and frequent need for prolonged rehabilitation have been reported (El Khoury 2013, Hinten 2008, Piantadosi 2016). While POWV disease can be severe, these high rates are likely due, in part, to testing and surveillance bias, with the diagnosis primarily considered in persons with encephalitis and severe disease for whom another diagnosis is not identified.
There are several limitations to our analysis. ArboNET is a passive surveillance system which relies on patients presenting for medical care, clinicians considering the diagnosis and submitting samples for diagnostic testing, and laboratory-confirmed cases being reported. Patients with less severe disease might not seek care, local variation in physician disease awareness is likely, and until recently testing was only available in a limited number of state public health laboratories and at CDC’s Arboviral Diseases Branch. Therefore, cases reported to ArboNET likely underestimate true disease incidence. Conversely, ArboNET does not require information about clinical symptoms or laboratory findings, and as such cases cannot be evaluated to confirm they meet the national case definition.
Reducing exposure to ticks is the best defense against POWV disease. Further education should be provided to the public about measures to prevent POWV and other tick-borne infections. These include avoiding direct contact with ticks by avoiding brushy and wooded areas and walking in the center of trails; repelling ticks on skin and clothing by using approved tick repellents, wearing long sleeves and pants, and treating clothing and gear with permethrin; and examining gear and pets which can bring ticks into the home (https://www.cdc.gov/ticks/avoid/on_people.html). As soon as possible after spending time outdoors, efforts should be made to find and remove ticks from the body by doing thorough tick checks and showering to remove any nonattached ticks. Rapid identification is important, as transmission of POWV can occur as soon as 15 minutes after tick attachment (Ebel, 2004). Finally, dry clothing should be placed in a tumble dryer on high heat to kill any remaining ticks.
Conclusions
The findings described here suggest that POWV disease can be a severe disease and has been diagnosed with increased frequency in recent years, although this might reflect increased disease awareness, improved test availability, and enhanced surveillance efforts. Clinicians should consider POWV disease in patients presenting with acute encephalitis or aseptic meningitis who are resident in, or have traveled to, an appropriate geographic region, particularly those with a history of tick bite or when more common etiologies have been ruled out. A high index of suspicion is needed for diagnosis because the clinical features of POWV disease resemble those of other arboviral infections. The extended season for possible exposure should be kept in mind. Clinicians should contact their local or state health department to request testing for POWV disease. Laboratory-confirmed cases should be reported through state and local health departments to CDC.
Acknowledgments
We would like to thank Jennifer Lehman and the vector-borne disease surveillance coordinators in local and state health departments for their assistance. We would also like to thank Dr Erin Staples for her review of the manuscript.
Footnotes
Author disclosure statement
The authors have no financial or personal conflicts to declare. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Elisabeth R. Krow-Lucal, Email: ekrowlucal@cdc.gov.
Nicole P. Lindsey, Email: nplindsey@cdc.gov.
Marc Fischer, Email: mfischer@cdc.gov.
Susan L. Hills, Email: shills@cdc.gov.
References
- Artsob H. Powassan encephalitis. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. IV. Boca Raton, Florida: CRC Press, Inc; 1988. pp. 29–49. [Google Scholar]
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR Recomm Rep. 2016;65:1–44. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- CDC. Arboviral disease--United States, 1994. MMWR Morb Mortal Wkly Rep. 1995;44:641–644. [PubMed] [Google Scholar]
- CDC. Outbreak of Powassan encephalitis--Maine and Vermont, 1999–2001. MMWR Morb Mortal Wkly Rep. 2001;50:761–764. [PubMed] [Google Scholar]
- CDC. [cited 2017 May 25];Arboviral Diseases, Neuroinvasive and Non-neuroinvasive 2015 Case Definition. Available from: https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/
- Deibel R, Srihongse S, Woodall JP. Arboviruses in New York State: an attempt to determine the role of arboviruses in patients with viral encephalitis and meningitis. Am J Trop Med Hyg. 1979;28:577–82. [PubMed] [Google Scholar]
- Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg. 2004;71:268–71. [PubMed] [Google Scholar]
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol. 2010;55:95–110. doi: 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- El Khoury MY, Camargo JF, White JL, Backenson BP, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerging Infect Dis. 2013;19:1926–1933. doi: 10.3201/eid1912.130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embil JA, Camfield P, Artsob H, Chase DP. Powassan virus encephalitis resembling herpes simplex encephalitis. Arch Intern Med. 1983;143:341–343. [PubMed] [Google Scholar]
- Goldfield M, Austin SM, Black HC, Taylor BF, et al. A non-fatal human case of Powassan virus encephalitis. Am J Trop Med Hyg. 1973;22:78–81. doi: 10.4269/ajtmh.1973.22.78. [DOI] [PubMed] [Google Scholar]
- Hermance ME, Thangamani S. Powassan virus: An emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis. 2017;17:453–462. doi: 10.1089/vbz.2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinten SR, Beckett GA, Gensheimer KF, Pritchard E, et al. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733–740. doi: 10.1089/vbz.2008.0022. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Staples JE, Sotir MJ, Warshauer DM, et al. Tickborne Powassan virus infections among Wisconsin residents. WMJ. 2010;109:91–97. [PubMed] [Google Scholar]
- Kari K, Liu W, Gautama K, Mammen MP, et al. A hospital-based surveillance for Japanese encephalitis in Bali, Indonesia. BMC Med. 2006;4:8. doi: 10.1186/1741-7015-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey NP, Staples JE, Lehman JA, Fischer M. Surveillance for human West Nile virus disease — United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- Main AJ, Carey AB, Downs WG. Powassan virus in Ixodes cookei and mustelidae in New England. J Wildl Dis. 1979;15:585–591. doi: 10.7589/0090-3558-15.4.585. [DOI] [PubMed] [Google Scholar]
- McLean DM, Macpherson LW, Walker SJ, Funk G. Powassan virus: surveys of human and animal sera. Am J Public Health Nations Health. 1960;50:1539–1544. doi: 10.2105/ajph.50.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitzel DF, Lynfield R, Smith K. Powassan virus encephalitis, Minnesota, USA. Emerg Infect Dis. 2013;19:686. doi: 10.3201/eid1904.121651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A, Rubin DB, McQuillen DP, Hsu L, et al. Emerging cases of Powassan virus encephalitis in New England: Clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707–713. doi: 10.1093/cid/civ1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann CA, Hayes EB, DiGuiseppi C, Hoffman R, et al. Epidemiology of neuroinvasive arboviral disease in the United States, 1999–2007. Am J Trop Med Hyg. 2008;79:974–979. [PubMed] [Google Scholar]
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, et al. Surveillance for Lyme disease - United States, 2008–2015. MMWR Surveill Summ. 2017;66:1–12. doi: 10.15585/mmw.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. [cited 2017 May 25];American fact finder: Community facts. Available from: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml#.