Abstract
It has been hypothesized that the increased risk of obesity among African Americans may be partially explained by low energy expenditure (EE) and impaired fat oxidation. Twelve White adults without obesity were pair-matched by age, sex and body mass index (BMI) to a sample of 12 African Americans. Resting EE (REE), 24-h EE, 24-h RQ, Sleep EE, Sleep RQ and spontaneous physical activity were measured in a respiratory chamber; and free-living total daily EE (TDEE) and physical activity EE were measured using doubly labeled water. There were no race differences in age, body composition, 24-h RQ, sleep RQ, or spontaneous or free-living physical activity; however, Whites had significantly higher REE (p=0.02), 24-h EE (p=0.02), Sleep EE (p=0.005); but not TDEE (p=0.30) compared to African Americans after adjustment for FFM. African Americans may have a higher risk for obesity because of lower energy expenditure.
African Americans experience higher obesity rates than Whites, especially among women. Low 24-h or resting energy expenditure (REE) and low fat oxidation rates [high respiratory quotient (RQ)] have been associated with weight gain [1–4]. It has been hypothesized that the increased risk of obesity among African Americans may be explained, in part, by lower levels of energy expenditure and fat oxidation. There is evidence that African Americans have lower REE compared to Whites, after adjustment for body composition [5]. Evidence for race differences in RQ is less robust. There was no difference in fasting RQ between African Americans and Whites in the CARDIA study [6]; however, other studies have reported higher RQ in African Americans compared to Whites [7].
The objective of this study was to compare measures of energy expenditure, substrate oxidation and physical activity among African American and White adults without obesity.
Subjects and Methods
Healthy men and women without obesity (BMI 18.5–27.5 kg/m2) aged 20–35 years participated in the InSight study. The total sample includes 12 African Americans, 72 Whites, and 6 individuals of “other” races. The present sample consists of 12 African Americans (4 men) and a pair-matched [sex, age, and BMI] sub-sample of 12 Whites. All procedures were approved by Pennington Biomedical Research Center’s Institutional Review Board, and participants provided informed consent.
Height and weight were measured using standard procedures. Body fat percentage was measured using dual-energy x-ray absorptiometry (DXA; QDR 4500A, Hologic, Inc., Marlborough, MA), and multiplied by weight to determine fat mass (FM; kg). Fat-free mass (FFM; kg) was computed by subtracting FM from weight. Further, we estimated soft-tissue lean body mass (LBM) by subtracting bone mineral content (kg) from FFM.
Measurements of 24-h EE, 24-h RQ, Sleep EE, and Sleep RQ were performed in a respiratory chamber as previously described [8]. Energy expenditure and substrate oxidation parameters, including 24-h RQ and 24-h oxidation rates of fat, carbohydrate, and protein were calculated from O2 consumption, CO2 production and nitrogen excretion [9]. Sleep EE and Sleep RQ were computed for a 3-hour period (2:00 am to 5:00 am), and Sleep EE was extrapolated to 24 hours. Non-fasting REE was estimated from measurements of EE and a microwave activity detection using data from 30 minutes after entry to 11 pm. Activity counts and EE are averaged in 15-minute intervals, and then plotted as average EE vs average activity. The Y-intercept was used as an estimate of REE. Spontaneous physical activity in the chamber was estimated using three methods. First, spontaneous physical activity EE (SPAEE; kcal/day) was estimated by the following formula: (slope of the regression line between EE and activity as described above X ((sum of the 15-min averages of activity)/96) X 100). Second, the physical activity level (PALCH) was computed as 24-h EE/Sleep EE. Third, physical activity EE (PAEECH) was calculated as 0.9 X 24-h EE – Sleep EE.
Free-living total daily EE (TDEE) was measured over two weeks using doubly labeled water as previously described [10]. Free-living physical activity was estimated using three methods. First, the physical activity level (PALFL) was computed as TDEE/Sleep EE. Second, physical activity EE (PAEEFL) was calculated as 0.9 X TDEE – Sleep EE, which assumes a 10% thermic effect of feeding (TEF). Third, we expressed physical activity as the residual value of the regression between measured TDEE and Sleep EE, which we termed activity-related energy expenditure (AREE).
Independent-samples t-tests were used to determine differences between Whites and African Americans. Race differences in energy expenditure were also determined using general linear models, adjusting for FFM.
Results
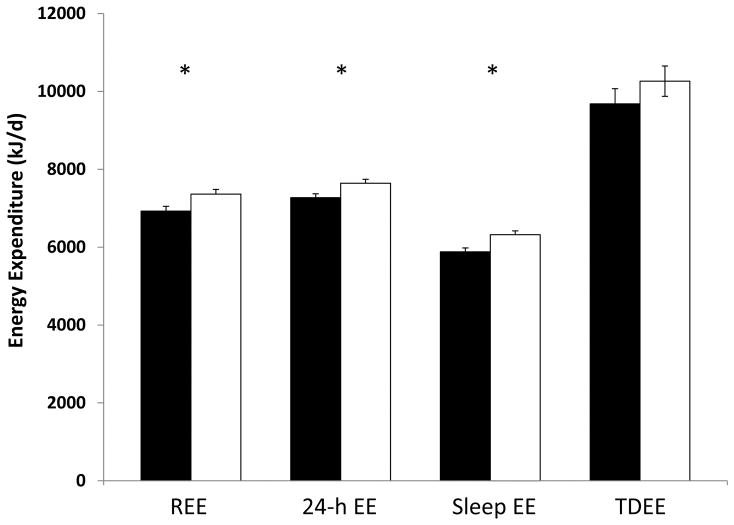
The mean values for age and BMI were 27.0 y (SD 4.3 y) and 22.9 kg/m2 (SD 2.9 kg/m2), respectively. There were no significant differences in age, BMI, FM or FFM between Whites and African Americans. Without adjustment for FFM, there were no significant race differences in REE, 24-h EE, sleep EE or TDEE, 24-h RQ or Sleep RQ. There were also no race differences in spontaneous physical activity in the chamber (PALCH, AEECH, and SPAEECH) or in free-living physical activity (PALFL, AEEFL and AREE). After adjustment for FFM, African Americans had significantly lower REE (6928 vs 7363 kJ/d; p=0.02), 24-h EE (7271 vs 7644 kJ/d; p=0.02), and sleep EE (5881 vs 6321 kJ/d; p=0.005); but not TDEE (9682 vs 10 264 kJ/d; p=0.30) compared to Whites (Figure 1).
Figure 1.
Measurements of energy expenditure in African Americans (black bars) and Whites (white bars). Means are presented as least squares means from general linear models, adjusted for differences in fat-free mass. Error bars represent standard errors.*p<0.02 between groups.
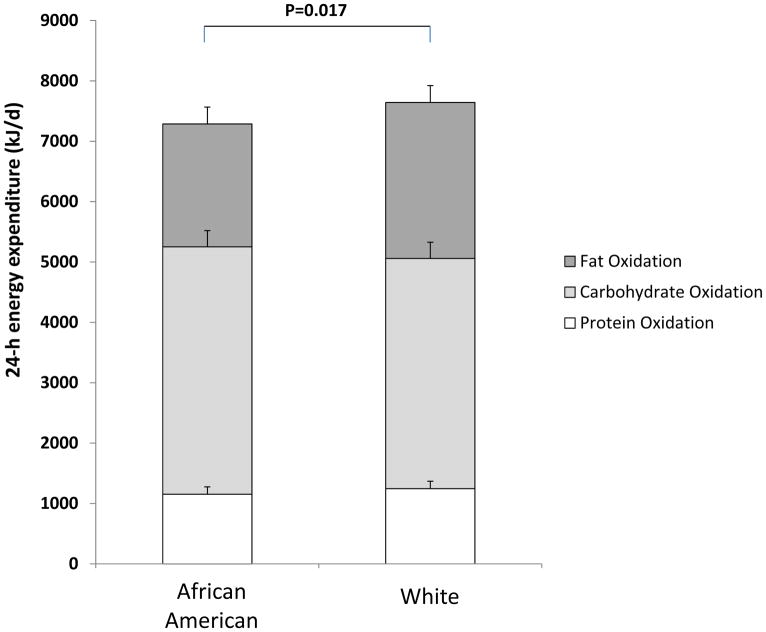
Figure 2 presents proportions of 24-h EE derived from the oxidation of protein, carbohydrate and fat. Although overall 24 h EE was significantly lower in African Americans, there were no differences the energy expended through oxidation of the major macro-nutrients (all p>0.05).
Figure 2.
Twenty-four-hour energy expenditure (total height of the columns) and proportions of 24-h oxidation of protein, carbohydrate, and fat between African American and White subjects. Means are presented as least squares means from general linear models, adjusted for differences in fat-free mass. Error bars represent standard errors for each macronutrient.
In sensitivity analyses where we replaced FFM with LBM, or additionally included FM in the models, the results were unchanged. Further, when adjusting for extremity lean mass (arms + legs), the results remained significant; however, when we adjusted for trunk lean mass, the results were no longer significant. When we restricted the analysis to women, the results remained unchanged with the exception that the difference in REE was of similar magnitude but no longer statistically significant (p=0.09).
Discussion
Our results demonstrate that in adults without obesity carefully matched on age, sex and BMI, African Americans have lower levels of some measures of energy expenditure but similar substrate oxidation rates compared to Whites. Further, there were no race differences in free-living energy expenditure or physical activity levels. All participants in this study were non-obese; however, it is unknown whether they will develop obesity in the future. Given the equivocal evidence about the role of RQ in weight gain, combined with the lack of evidence for differences in RQ between Whites and African Americans, it is unlikely that differences in RQ explain any significant fraction of the observed race differences in obesity.
In conclusion, we observed that African Americans had lower levels of REE, 24-h EE and Sleep EE, which could put them at higher risk for obesity. Longitudinal studies of bi-racial cohorts are required to better understand the role of racial differences in associations among energy expenditure, substrate oxidation and the development of obesity.
Acknowledgments
This study was supported by the USDA and NORC Grant #1P30 DK072476. This work was created in the performance of a Specific Co-operative Agreement with the U.S. Department of Agriculture. The Government of the United States has a royalty-free government purpose license to use, duplicate, or disclose the work, in whole or in part and in any manner, and to have or permit others to do so, for government purposes. Peter Katzmarzyk is supported, in part, by 1U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. Peter Katzmarzyk is partially supported by the Marie Edana Corcoran Endowed Chair in Pediatric Obesity and Diabetes and Eric Ravussin is partially supported by the Douglas L. Gordon Chair in Diabetes and Metabolism.
Conflict of Interest: Drs. Katzmarzyk, Ravussin, Redman and Rood report grants from United States Department of Agriculture and from National Institutes of Health during the conduct of the study. Dr. Katzmarzyk reports speaker fees from Baptist Health South Florida and Renown Health. Dr. Ravussin reports personal fees from ICAN, Amway (Nutrilite) and Janssen, grants from Amway, Nestle, NuSI, Novartis, Sanofi-Aventis, Weight Watchers and Ethicon, speaker fees from Open Academy – Venice and Center for Medical Weight Loss, and a patent pending for Nightly Moderated Hypoxia to Treat Insulin Resistance and Cardiometabolic Syndrome, outside the submitted work. Dr. Most reports nothing to disclose.
Authors’ Contributions: PTK, ER, JR designed and conducted research; PTK analyzed data and wrote paper; ER, JR, JM, ad LR provided critical input; PTK had primary responsibility for final content. All authors read and approved the final manuscript.
Abbreviations
- AREE
activity related energy expenditure
- BMI
body mass index
- DXA
dual energy x-ray absorptiometry
- EE
energy expenditure
- FFM
fat free mass
- FM
fat mass
- PAEE
physical activity energy expenditure
- PAL
physical activity level
- REE
resting energy expenditure
- RQ
respiratory quotient
- SPAEE
spontaneous physical activity energy expenditure
- TEF
thermic effect of feeding
- TDEE
total daily energy expenditure
Footnotes
Clinical Trial Registry Number: NCT00945633, ClinicalTrials.gov
References
- 1.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WGH, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 2.Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int J Obes. 2005;29(3):287–91. doi: 10.1038/sj.ijo.0802888. [DOI] [PubMed] [Google Scholar]
- 3.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: The Baltimore Longitudinal Study on Aging. Int J Obes. 1992;16:667–74. [PubMed] [Google Scholar]
- 4.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. The American journal of physiology. 1990;259:E650–E7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 5.Luke A, Dugas L, Kramer H. Ethnicity, energy expenditure and obesity: are the observed black/white differences meaningful? Curr Opin Endocrinol Diabetes Obes. 2007;14(5):370–3. doi: 10.1097/MED.0b013e3282c48a7c. [DOI] [PubMed] [Google Scholar]
- 6.Sharp TA, Bell ML, Grunwald GK, Schmitz KH, Sidney S, Lewis CE, et al. Differences in resting metabolic rate between white and African-American young adults. Obes Res. 2002;10(8):726–32. doi: 10.1038/oby.2002.99. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. Am J Clin Nutr. 1999;70(1):13–20. doi: 10.1093/ajcn/70.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Lam YY, Redman LM, Smith SR, Bray GA, Greenway FL, Johannsen D, et al. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. Am J Clin Nutr. 2014;99(4):834–42. doi: 10.3945/ajcn.113.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jequier E, Acheson KJ, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Ann Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 10.Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4(2):e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]