Summary
A model to predict response to chemoradiotherapy in patients with rectal cancer was developed by integrating volumetric and DWI MRI parameters of 85 patients and was validated in an external cohort of 55 patients. Based on this model, patients can be selected for organ preservation.
Keywords: Rectal cancer, chemoradiotherapy, response prediction, MRI
Introduction
Multimodality treatment combining preoperative chemoradiotherapy and total mesorectal excision (TME) improved local control of locally advanced rectal cancer [1–3]. Notwithstanding this reduction in local recurrences, significant morbidity (e.g. postoperative complications, long-term bowel dysfunction, decreased bladder and sexual functioning, permanent stoma care) is associated with such multimodality treatment [4–7]. For patients achieving a pathologic complete response [8], individualised organ-preserving treatment strategies such as local excision [9] or a watch-and-wait policy [10] could spare these patients the morbidity and mortality associated with extensive surgery.
Well-considered selection of patients with favourable response is the key element to safely implement less invasive treatment strategies. However, the gold standard of conventional histopathological analysis [11] cannot be used for preoperative selection of these patients [12]. Moreover, computed tomography (CT), endorectal ultrasound (EUS) and conventional magnetic resonance imaging (MRI) all lack accuracy for restaging after chemoradiotherapy [13–15].
In contrast to the limited accuracy of imaging techniques using morphological tumour changes, functional imaging techniques are appealing as accurate tools to predict response to chemoradiotherapy. 18F-Fluorodeoxyglucose positron emission photography (18F-FDG PET) assesses tumour metabolism and a decrease in standardised uptake value (SUV) has been shown to be associated with pathologic complete response [16,17]. In addition, tumour microstructure and cellularity can be measured by the amount of water diffusion, quantified as the apparent diffusion coefficient (ADC) on diffusion-weighted imaging (DWI) MRI. Decreasing cellularity as expressed by an increase in ADC during and after chemoradiotherapy has been associated with tumour response [18]. Furthermore, visual assessment of DWI-MRI and DWI-MRI volumetry provide high diagnostic performance in predicting tumour response [19,20]. Nevertheless, the major strength of DWI-MRI and 18F-FDG PET lies in the identification of non-responders who are not candidates for organ preservation [21].
Recently, a promising model allowing for prediction of pathological (near-)complete response to chemoradiotherapy by fusion of 18F-FDG-PET parameters, DWI-MRI parameters and volumetric MRI parameters has been developed [22]. To allow for patient selection for an organ-preserving treatment, the model remains to be validated. Validation in an external patient cohort however is cumbersome, as in most institutions patients do not receive 18F-FDG-PET in addition to MRI in the preoperative setting. A clinical prediction model with MRI features only would therefore be favourable.
In this study we have developed an MRI based model to predict response following chemoradiation. We validated this model in an external cohort of locally advanced rectal cancer patients.
Methods
Development cohort (Leuven)
Development of the MRI-based prediction model was performed on the same study population as the recently published prediction model including data from 85 patients with locally advanced rectal cancer, included in a prospective trial (NCT01171300) between January 2012 and February 2015, under approval of the institutional ethical committee of the University Hospitals Leuven [22]. Locally advanced rectal cancer was defined as primary histologically proven adenocarcinoma of the rectum, clinical stage T3-4N0 or T1-4N1-2. Patients with distant metastases, prior chemotherapy or radiotherapy for rectal cancer, previous or concurrent malignancies at other sites, and known allergies to intravenous contrast agents or other contraindications for MRI acquisition were excluded.
Patients received chemoradiotherapy up to 45 Gy delivered in 1.8 Gy fractions, with a continuous infusion of 5-fluorouracil (225mg/m2/d). Six patients received capecitabine (825 mg/m2 twice daily). TME was performed after an interval of eight weeks from completion of chemoradiotherapy. MRI studies were acquired prior to chemoradiotherapy and prior to surgery (6 weeks after chemoradiotherapy) at a 3 Tesla MRI scanner (Ingenia, Philips Healthcare, Best, The Netherlands).
Validation cohort (Utrecht)
Model validation was performed in a cohort of 55 patients with histologically proven locally advanced rectal cancer treated at the Radiation Oncology Department of the University Medical Center (UMC) Utrecht (the Netherlands) between November 2008 and December 2011. Locally advanced rectal cancer was defined as primary histologically proven adenocarcinoma of the rectum, clinical stage T3-4N0-2. Exclusion criteria were nonresectable and/or metastatic disease and insufficient MR image quality. All patients gave written informed consent prior to study entry.
Patients received 50 Gy delivered in 25 fractions of 2 Gy, 5 times a week in supine position, with concurrent capecitabine 825 mg/m2 bid. TME was performed seven to ten weeks after chemoradiation. MRIs were obtained before start of chemoradiation and within one or two weeks before surgery at a 3 Tesla MRI scanner (AchievaTX, Philips Medical System, Best, the Netherlands).
Outcome definition
The primary outcome measure for our study was (near-)complete pathological tumour response defined as ypT0-1N0. Histologic evaluation of the resection specimen was performed by experienced pathologists according to the method described by Quirke et al. [11]. In the development cohort, 2 patients had strong clinical evidence of a clinical complete response (repeated digital rectal examination, endoscopic evaluation, and DWI) with prolonged disease-free survival of at least 1 year when entered in a watch-and wait protocol, which was considered a surrogate endpoint for pCR.
MRI parameters
Details on MRI acquisition are described in the supplementary material. Tumour volumetry was assessed by manually delineating tumor boundaries on the axial T2-weighted images. Besides the tumor volume in cm3, a diameter of the equivalent sphere was calculated. DWI images were acquired using different b-values. Additionally, absolute and relative changes in T2-volumetry and relative changes in ADC between different time points were calculated. The 17 candidate MRI parameters extracted for each MRI are summarised in supplementary Table 1.
Statistical Analysis
Patients who had more than 30 % missing variables were excluded from the analysis. Remaining missing data were estimated using a 15-Nearest Neighbour algorithm using the data of the respective cohort [23]. Baseline characteristics and pathological response outcomes of both cohorts, were summarised using descriptive statistics.
The predictive model was developed using only the Leuven cohort. First, models were built using T2-volumetry and DWI data separately to identify the baseline performance of each data mode. Secondly, we built models using different combinations of T2-volumetry and DWI data applying linear regression with lasso regularization and the logistic transfer function. We used a ten-fold cross validation strategy on this cohort to assess the models’ performance on unseen data. We repeated this process ten times to randomise the process to split the data in ten folds. The performance of this model was estimated using the area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and positive predictive value (PPV). After acceptable performance of this step, we developed a model on the complete development cohort (i.e. Leuven cohort), and tested it on the validation cohort (i.e. Utrecht cohort). The performance in the external validation set was again evaluated using AUC, sensitivity, specificity, and PPV. All statistical analyses were done in the statistical language R [24].
Results
Patient characteristics of both cohorts are presented in Table 1. In both cohorts, median age at diagnosis was 64 years. All patients completed their planned chemoradiotherapy schedule. Chemoradiotherapy was followed by surgery after a median interval of 54 days in the development cohort and 55 days in the validation cohort. Most patients received sphincter-saving surgery (91.8 % in the development cohort and 65.5 % in the validation cohort). At histopathology, 11 patients of the development cohort had a pathologic complete response. Furthermore, 2 patients presented with a clinical complete response persisting 30 and 26 months after the end of chemoradiotherapy. Since we consider this a surrogate endpoint for pathologic complete response, this adds up to 13 patients (15.3 %) with pCR in the development cohort. Additionally, 9 patients (10.6 %) had a near-complete response (ypT1-0N0) at histopathology. In the validation cohort, 8 patients (14.5 %) achieved a pathologic complete response and 5 patients (9.1 %) presented with a near-complete response. In summary, a total of 22 patients (25.9 %) were considered to have a ypT0-1N0 response in the development cohort whereas 13 patients (23.6 %) achieved ypT0-1N0 in the validation cohort.
Table 1.
Patient characteristics, Surgery and Response Outcome.
| Leuven (n = 85) (development) | Utrecht (n = 55) (validation) | |
|---|---|---|
| Age (y) | 64 (38.3 – 85.7) | 64 (40.9 – 79.7) |
| Gender | ||
| F | 25 (29.4 %) | 13 (23.6 %) |
| M | 60 (70.6 %) | 42 (76.4 %) |
| cT | ||
| 2 | 13 (15.3 %) | 0 (0 %) |
| 3 | 68 (80 %) | 46 (83.6 %) |
| 4 | 4 (4.7 %) | 9 (16.4 %) |
| cN | ||
| 0 | 3 (3.5 %) | 5 (9.1 %) |
| 1 | 26 (30.6 %) | 15 (27.3 %) |
| 2 | 56 (65.9 %) | 35 (63.6 %) |
| Interval to Surgery (d) | 54 (25 – 83) | 55 (37 – 96) |
| Type of surgery | ||
| Sphincter-sparing | 78 (91.8 %) | 36 (65.5 %) |
| APR | 5 (5.9 %) | 18 (32.7 %) |
| W&W | 2 (2.4 %) | 0 (0 %) |
| TEM | 0 (0%) | 1 (1.8 %) |
| pCR | ||
| 0 | 72 (84.7 %) | 47 (85.5 %) |
| 1 | 13 (15.3 %) | 8 (14.5 %) |
| ypT0-1N0 | ||
| 0 | 63 (74.1 %) | 42 (76.4 %) |
| 1 | 22 (25.9 %) | 13 (23.6 %) |
Data are presented as n (%) or median (range). Abbreviations: APR = Abdominoperineal Resection; W&W = Watch and Wait; TEM = Transanal Endoscopic Microsurgery; pCR = pathologic Complete Response.
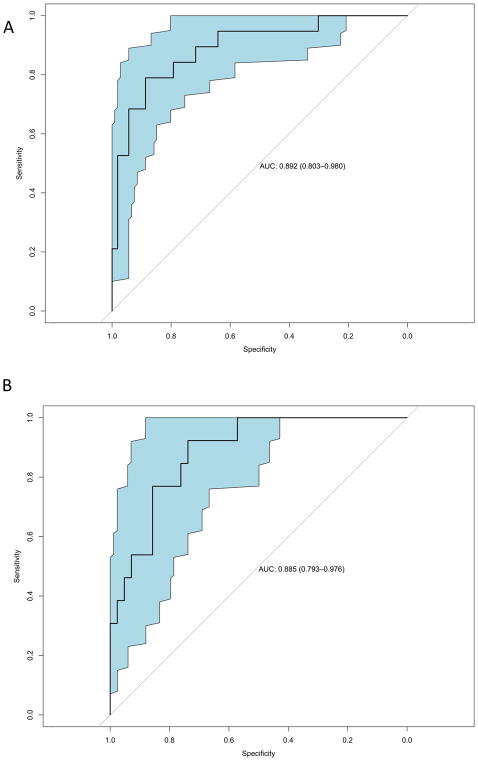
For the model building, 13 out of 85 patients were excluded from the development cohort because of > 30 % missing data. Previously, using these data, Joye et al. reported on the performance of individual features extracted from DWI-MRI and volumetric MRI parameters for response prediction in the development cohort [22]. After variable selection of these candidate MRI parameters, two T2-volumetric parameters (ΔVolume% and Sphere_post) and two DWI parameters (ADC_avg_post and ADCratio_avg) were retained (Figure 1A) in a model predicting (near-)complete response (ypT0-1N0) to chemoradiotherapy for rectal cancer patients with a mean AUC of 0.81 ± 0.03 (Supplementary Figure 1). At a positive predictive value of at least 80%, this model showed a sensitivity of 68 % and specificity of 94 %. The final model was applied to the validation set which showed a similar AUC of 0.80 (95 % CI 0.68–0.93) (Figure 1B).
Figure 1. Predictive model and its performance on the development and validation cohort.
(A) Receiver Operating Characteristic curve for the development cohort of the MRI- based prediction model on pathological (near-)complete tumour response in rectal cancer; AUC = 0.892 with 95% confidence interval shown. (B) Receiver Operating Characteristic curve for the validation cohort of the MRI-based prediction model on pathological (near-)complete tumour response in rectal cancer; AUC = 0.885 with 95% confidence interval shown. Abbreviations: MRI = Magnetic Resonance Imaging; AUC = Area Under the Curve.
Discussion
In the present study, we developed a model with conventional volumetric parameters and DWI features of MRI scans before and 6 weeks after treatment to predict (near-)complete response to chemoradiotherapy for patients with locally advanced rectal cancer. The model was validated in an external validation cohort resulting in a good performance (AUC 0.80).
The results presented in this study confirm earlier reports that T2-MRI and DWI measurements before and 6 weeks after chemoradiotherapy can be used for response assessment. The two T2-MRI parameters retained in the model are volume change over treatment (ΔVolume%) and equivalent sphere diameter after chemoradiotherapy (Sphere_post), which is concordant with previous findings of Lambregts et al. illustrating the predictive power of tumour volumetry [25]. Furthermore, retention of the two DWI-MRI parameters, average ADC after chemoradiotherapy (ADC_avg_post) and the ratio of average ADC before and after chemoradiotherapy (ADCratio_avg), is concordant with previous reports demonstrating changes in ADC value over treatment being useful for prediction of pathological complete response [18,26]. Additionally to ADC measurements, other studies have reported on the application of visual assessment and volumetry using DWI-MRI to predict response after chemoradiotherapy. Visual assessment of DWI-MRI combined with standard MRI led to a good predictive performance (AUC = 0.80) [19]. DWI-MRI volumetry provided higher diagnostic performance in assessing complete response (AUC = 0.92) to chemoradiation and was more accurate than T2-volumetry [20].
Combining MRI and DWI data with clinical assessment including digital rectal examination (DRE) and endoscopy proved to be the most accurate strategy to select patients who may experience a complete remission with a high predictive value of 98% [30]. Unfortunately, DRE and endoscopy are subjective measures that cannot be incorporated into a mathematical prediction model. Furthermore, also high seated tumours not evaluable by DRE were included in the current study. For all the above reasons clinical assessment with DRE and endoscopy was not taken into account in the development of the prediction model.
Our previous report showed that integrating DWI-MRI, T2-volumetry parameters and 18F-FDG-PET parameters in a prediction model looks promising (AUC 0.81 ± 0.03) but lacks external validation as in most institutions patients do not receive 18F-FDG-PET in addition to MRI in preoperative setting [22]. The currently reported prediction model in which 18F-FDG-PET data were excluded had a performance (AUC = 0.83 ± 0.03) slightly worse than the previously discussed DWI-MRI studies, but similar to the previously described model integrating MRI and 18F-FDG-PET data.
The strength of this prediction model however lies in its validation, which was possible even though MRI machines, image acquisition and ADC calculations differed between the development and the validation cohort. This illustrates the robustness and applicability of the presented model and resembles clinical practice in which different scanners and protocols are used in different hospitals. Notwithstanding the use of an external patient cohort for validation as a major strength, this external cohort is also a limitation for this study. Patients from the development cohort and validation cohort slightly differed in patient and tumour characteristics. However, these differences, hardly evitable in an external patient cohort, are minimal and do not hamper the development and validation of the prediction model in this study.
In our goal to offer patients an organ preserving treatment, we chose the outcome measure to be (near-)complete response (ypT0-1N0) and not pCR. We believe that these patients are eligible for organ preserving strategies such as watch & wait or local excision, given the low rate of positive lymph node involvement for ypT0 and ypT1 tumours [27,28]. In this study, reassessment was performed at six weeks after the end of CRT, with surgery eight weeks after the end of CRT, whereas increasing evidence suggests that a prolonged interval after chemoradiation increases pCR rate [29]. Furthermore, the higher ypT0-1N0 rate as compared to pCR rate (25.9 vs 15.3 %) allows for a more robust prediction model development.
The use of MRI parameters that are clinically relevant and easily interpretable, makes this prediction model a valuable tool for an organ preservation approach from a clinician’s point of view. This differs from predictive modelling with novel techniques such as radiomics, for which to our knowledge no externally-validated models yet exist for the prediction of response outcome in patients with rectal cancer [31–33]. Extraction and analysis of the required 4 parameters by an experienced radiologist or radiation oncologist require some extra effort. However, MRI staging and restaging are standard practice for patients with rectal cancer. Furthemore, future automated segmentation tools could be used for this purpose. Therefore, for experienced readers, the presented prediction model could be a fairly simple tool applicable in the clinical routine to select patients with a favourable response that are good candidates for organ-preserving strategies.
Conclusion
An MRI-based prediction model on (near-)complete pathological response following chemoradiotherapy in locally advanced rectal cancer patients, integrating volumetric and diffusion-weighted MRI features, shows a high predictive performance in an external validation cohort. The clinically relevant features in the model make it an interesting tool for implementation of organ preserving strategies in rectal cancer.
Supplementary Material
Acknowledgments
This work was funded by the Belgian Government Agency for Innovation by Science and Technology (IWT). Philippe Bulens is an aspirant investigator at the Research Foundation Flanders (FWO). Karin Haustermans is a senior clinical investigator at the Research Foundation Flanders (FWO). The funding source had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript and in the decision to submit the manuscript for publication. We thank all participating patients and data managers who were involved in this project. Research reported in this publication was further supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R01 EB020527.
References
- 1.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–60. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative Radiotherapy combined with Total Mesorectal Excision for Resectable Rectal Cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 4.Marijnen CAM, Kapiteijn E, Van de Velde CJH, Martijn H, Steup WH, Wiggers T, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: Report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–25. doi: 10.1200/JCO.20.3.817. [DOI] [PubMed] [Google Scholar]
- 5.Peeters KCMJ, van de Velde CJH, Leer JWH, Martijn H, Junggeburt JMC, Klein Kranenbarg E, et al. Late Side Effects of Short-Course Preoperative Radiotherapy Combined With Total Mesorectal Excision for Rectal Cancer: Increased Bowel Dysfunction in Irradiated Patients--A Dutch Colorectal Cancer Group Study. J Clin Oncol. 2005;23:6199–206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 6.Pietrzak L, Bujko K, Nowacki MP, Kepka L, Oledzki J, Rutkowski A, et al. Quality of life, anorectal and sexual functions after preoperative radiotherapy for rectal cancer: Report of a randomised trial. Radiother Oncol. 2007;84:217–25. doi: 10.1016/j.radonc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Braendengen M, Tveit KM, Bruheim K, Cvancarova M, Berglund K, Glimelius B. Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: Results from a randomized phase III study. Int J Radiat Oncol Biol Phys. 2011;81:1017–24. doi: 10.1016/j.ijrobp.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo L-J, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 9.Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99:1211–8. doi: 10.1002/bjs.8821. [DOI] [PubMed] [Google Scholar]
- 10.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, et al. Operative Versus Nonoperative Treatment for Stage 0 Distal Rectal Cancer Following Chemoradiation Therapy. Ann Surg. 2004;240:711–8. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quirke P, Dixon MF, Durdey P, Williams NS. Local Recurrence of Rectal Adenocarcinoma Due To Inadequate Surgical Resection. Lancet. 1986;328:996–9. doi: 10.1016/S0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 12.Perez RO, Habr-Gama A, Pereira GV, Lynn PB, Alves PA, Proscurshim I, et al. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: Can they rule out persisting cancer? Color Dis. 2012;14:714–20. doi: 10.1111/j.1463-1318.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhao R-S, Wang H, Zhou Z-Y, Zhou Q, Mulholland MW. Restaging of Locally Advanced Rectal Cancer With Magnetic Resonance Imaging and Endoluminal Ultrasound After Preoperative Chemoradiotherapy. Dis Colon Rectum. 2014;57:388–95. doi: 10.1097/DCR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 14.Hanly AM, Ryan EM, Rogers AC, McNamara DA, Madoff RD, Winter DC. Multicenter Evaluation of Rectal cancer ReImaging pOst Neoadjuvant (MERRION) Therapy. Ann Surg. 2014;259:723–7. doi: 10.1097/SLA.0b013e31828f6c91. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Jang HS, Kim J-G, Lee MA, Kim DY, Kim TH, et al. Prediction of pathologic staging with magnetic resonance imaging after preoperative chemoradiotherapy in rectal cancer: Pooled analysis of KROG 10-01 and 11-02. Radiother Oncol. 2014;113:18–23. doi: 10.1016/j.radonc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Lambrecht M, Deroose C, Roels S, Vandecaveye V, Penninckx F, Sagaert X, et al. The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol. 2010;49:956–63. doi: 10.3109/0284186X.2010.498439. [DOI] [PubMed] [Google Scholar]
- 17.Perez RO, Habr-Gama A, São Julião GP, Lynn PB, Sabbagh C, Proscurshim I, et al. Predicting complete response to neoadjuvant CRT for distal rectal cancer using sequential PET/CT imaging. Tech Coloproctol. 2014;18:699–708. doi: 10.1007/s10151-013-1113-9. [DOI] [PubMed] [Google Scholar]
- 18.Lambrecht M, Vandecaveye V, De Keyzer F, Roels S, Penninckx F, Van Cutsem E, et al. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: Preliminary results. Int J Radiat Oncol Biol Phys. 2012;82:863–70. doi: 10.1016/j.ijrobp.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 19.Lambregts DMJ, Vandecaveye V, Barbaro B, Bakers FCH, Lambrecht M, Maas M, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18:2224–31. doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curvo-Semedo L, Lambregts DMJ, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal Cancer: Assessment of Complete Response to Preoperative Combined Radiation Therapy with Chemotherapy—Conventional MR Volumetry versus Diffusion-weighted MR Imaging. Radiology. 2011;260:734–43. doi: 10.1148/radiol.11102467. [DOI] [PubMed] [Google Scholar]
- 21.Joye I, Deroose CM, Vandecaveye V, Haustermans K. The role of diffusion-weighted MRI and 18F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: A systematic review. Radiother Oncol. 2014;113:158–65. doi: 10.1016/j.radonc.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Joye I, Debucquoy A, Deroose CM, Vandecaveye V, Cutsem E, Van Wolthuis A, et al. Quantitative imaging outperforms molecular markers when predicting response to chemoradiotherapy for rectal cancer. Radiother Oncol. 2017;124:104–9. doi: 10.1016/j.radonc.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17:520–5. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. 2016. [Google Scholar]
- 25.Lambregts DMJ, Rao S-X, Sassen S, Martens MH, Heijnen La, Buijsen J, et al. MRI and Diffusion-Weighted MRI Volumetry for Identification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Ann Surg. 2014:1–6. doi: 10.1097/SLA.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 26.Intven M, Reerink O, Philippens MEP. Diffusion-weighted MRI in locally advanced rectal cancer: Pathological response prediction after neo-adjuvant radiochemotherapy. Strahlentherapie Und Onkol. 2013;189:117–22. doi: 10.1007/s00066-012-0270-5. [DOI] [PubMed] [Google Scholar]
- 27.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Kępka L, Winkler-Spytkowska B, Suwiński R, et al. Prediction of mesorectal nodal metastases after chemoradiation for rectal cancer: results of a randomised trial. Implication for subsequent local excision. Radiother Oncol. 2005;76:234–40. doi: 10.1016/j.radonc.2005.04.004. doi: https://doi.org/10.1016/j.radonc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Rullier E, Rouanet P, Tuech J-J, Valverde A, Lelong B, Rivoire M, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390:469–79. doi: 10.1016/S0140-6736(17)31056-5. [DOI] [PubMed] [Google Scholar]
- 29.Sloothaak DAM, Geijsen DE, van Leersum NJ, Punt CJA, Buskens CJ, Bemelman WA, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer (Br J Surg 2013; 100: 933–939) Br J Surg. 2013;100:933–9. doi: 10.1002/bjs.9130. [DOI] [PubMed] [Google Scholar]
- 30.Maas M, Lambregts DMJ, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JWA, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI. Ann Surg Oncol. 2015;22:3769–71. doi: 10.1093/annonc/mdv223. [DOI] [PubMed] [Google Scholar]
- 31.Lovinfosse P, Polus M, Van Daele D, Martinive P, Daenen F, Hatt M, et al. FDG PET/CT radiomics for predicting the outcome of locally advanced rectal cancer. Eur J Nucl Med Mol Imaging. 2017 doi: 10.1007/s00259-017-3855-5. [DOI] [PubMed] [Google Scholar]
- 32.Nie K, Shi L, Chen Q, Hu X, Jabbour S, Yue N, et al. Rectal Cancer: Assessment of Neoadjuvant Chemo-Radiation Outcome Based on Radiomics of Multi-Parametric MRI. Am Assoc Cancer Res. 2016;22 doi: 10.1158/1078-0432.ccr-15-2997. clincanres.2997.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Zhang X-Y, Shi Y-J, Wang L, Zhu H-T, Tang Z, et al. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.