Abstract
Purpose
To compare the effect of posterior corneal astigmatism on the estimation of total corneal astigmatism using anterior corneal measurements (simulated keratometry [K]) between eyes with keratoconus and healthy eyes.
Methods
Thirty-three eyes of 33 patients with keratoconus of grade I or II and 33 eyes of 33 age- and sex-matched healthy control subjects were enrolled. Anterior, posterior, and total corneal cylinder powers and flat meridians measured by a single Scheimpflug camera were analyzed. The difference in corneal astigmatism between the simulated K and total cornea was evaluated.
Results
The mean anterior, posterior, and total corneal cylinder powers of the keratoconus group (4.37 ± 1.73, 0.95 ± 0.39, and 4.36 ± 1.74 cylinder diopters [CD], respectively) were significantly greater than those of the control group (1.10 ± 0.68, 0.39 ± 0.18, and 0.97 ± 0.63 CD, respectively). The cylinder power difference between the simulated K and total cornea was positively correlated with the posterior corneal cylinder power and negatively correlated with the absolute flat meridian difference between the simulated K and total cornea in both groups. The mean magnitude of the vector difference between the astigmatism of the simulated K and total cornea of the keratoconus group (0.67 ± 0.67 CD) was significantly larger than that of the control group (0.28 ± 0.12 CD).
Conclusions
Eyes with keratoconus had greater estimation errors of total corneal astigmatism based on anterior corneal measurement than did healthy eyes. Posterior corneal surface measurement should be more emphasized to determine the total corneal astigmatism in eyes with keratoconus.
Keywords: Astigmatism, Cornea, Keratoconus, Posterior corneal astigmatism, Total corneal astigmatism
Keratoconus is a chronic, progressive, noninflammatory, ectatic corneal disorder that deteriorates vision because of myopia and irregular astigmatism [1]. The condition usually arrests in the third to fourth decades of life, although it can commence later and progress at any age [1]. Currently, a rigid gas-permeable contact lens, intrastromal corneal ring segment implantation, corneal collagen cross-linking, photorefractive keratectomy, and a phakic intraocular lens (IOL) are the treatment options for keratoconus.
Collagen cross-linking affects the progression of and can suppress keratoconus [2]. A previous study demonstrated that combined collagen cross-linking and toric phakic IOL implantation was associated with good clinical outcomes for correcting myopic astigmatism for mild to moderate progressive keratoconus [3]. The cylinder power of a toric phakic IOL is determined by ocular astigmatism, not by corneal astigmatism. In comparison, when cataract surgery with toric IOL implantation is considered, the cylinder power of toric IOL is determined by corneal astigmatism, because the lenticular astigmatism disappears [4].
Recently, the importance of posterior corneal astigmatism has been recognized when toric IOL is considered, because selecting toric IOL based on anterior corneal measurements and neglecting posterior corneal astigmatism could lead to an incorrect estimation of total corneal astigmatism [5,6]. Unlike the anterior corneal surface, most eyes had against-the-rule (ATR) astigmatism on the posterior corneal surface. Thus, estimating the total corneal astigmatism using anterior corneal measurements (simulated keratometry [K]) could lead to overcorrection in eyes with with-the-rule (WTR) astigmatism and undercorrection in eyes with ATR astigmatism [5,7,8]. This phenomenon might be more pronounced in patients with keratoconus, because keratoconus involves a high degree of corneal astigmatism [9,10,11].
Eyes with keratoconus cannot avoid cataract development, and cataract surgery with toric IOL implantation can be considered for progression of cataracts. Alio et al. [12] reported that cataract surgery with toric IOL implantation is a safe and effective procedure in eyes with cataracts and stable keratoconus. Thus, the aim of this study was to compare the anterior, posterior, and total corneal powers and astigmatisms of keratoconus with those of healthy eyes and to evaluate the effect of posterior corneal astigmatism on the estimation of total corneal astigmatism using anterior corneal measurements in eyes with keratoconus using a single Scheimpflug camera.
Materials and Methods
Study population
This retrospective cross-sectional study was conducted at the department of ophthalmology in the Korea University College of Medicine. The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Korea University Ansan Hospital (AS14068). According to the institutional review board standard operating procedures on retrospective single center clinical study, ethics committee of the Korea University Ansan Hospital ruled that subject consent was not required for this study. Retrospective reviews were performed on all patients diagnosed with keratoconus at our institution between May 8, 2009 and May 31, 2017. We included patients who underwent a single Scheimpflug camera examination (Oculus, Wetzler, Germany) at our institution [7]. All patients also underwent measurement of refractive error using an autorefractometer (KR-8100; Topcon, Tokyo, Japan).
Keratoconus was defined as exhibiting at least one typical keratoconus sign (i.e., anterior bulging of the cornea, stromal thinning, Fleischer ring, Vogt striae, or Descemet's breaks) on slit-lamp examination and topographic findings (i.e., asymmetric bow-tie pattern with or without skewed axes and central or paracentral steepening of the cornea) [13]. Eyes with grade I or II keratoconus according to the Amsler-Krumeich classification (keratometric astigmatism <8.00 diopters [D], mean central K reading <53.00 D, absence of corneal scarring, or minimum corneal thickness > 400 µm) and no history of treatment for keratoconus were included [14,15]. Because corneal opacity precludes accurate corneal topography measurement, patients with corneal opacity such as subepithelial fibrosis or anterior stromal scarring were excluded.
The patients were matched for age (±3 years), sex, and laterality at a ratio of 1 : 1 to a normal control group who underwent a single Scheimpflug camera examination at our institution during the same study period. The normal control group was selected by reviewing charts and the single Scheimpflug examination results. We excluded controls with abnormal findings on both the slit-lamp examination and the single Scheimpflug examination.
Main outcome measures
For each subject, we measured anterior, posterior, and total mean corneal power; cylinder power; flat meridian; and central corneal thickness using a single Scheimpflug camera. The refractive indices used in the Scheimpflug camera were 1 for air, 1.376 for cornea, and 1.336 for aqueous humor. Anterior corneal power, or simulated K, was calculated using a single value for the keratometric index (nk = 1.3375). The total corneal power, or true net power, was calculated using the Gaussian total corneal power with the Gullstrand eye model without regard for corneal thickness in the 4.0-mm zone [16]. Anterior, posterior, and total corneal powers were calculated in a single Scheimpflug camera based on the following equations:
where rant is the radius of the anterior corneal surface, and rpost is the radius of the posterior corneal surface.
Correlations among the anterior, posterior, and total corneal measurements were evaluated. The cylinder power difference between the simulated K and total cornea was defined as the difference in cylinder power between the simulated K and total cornea (cylinder power difference between the simulated K and total cornea = anterior corneal cylinder power – total corneal cylinder power) [6]. The mean absolute corneal power difference between the simulated K and total cornea was defined as the mean absolute value of the difference between the corneal power of the simulated K and total cornea. To compare the astigmatism of the simulated K and total cornea, the vector difference between the astigmatism of the simulated K and total cornea was calculated using vector analysis, and double-angle plots were drawn [5,6,17]. The mean absolute flat meridian difference between the simulated K and the total cornea was defined as the mean absolute value of the difference between the anterior and total corneal flat meridian.
WTR astigmatism was defined as a flat meridian of the anterior or posterior corneal surface of 180 ± 30 degrees, ATR astigmatism as 90 ± 30 degrees, and the rest were defined as oblique astigmatism.
Statistical analysis
Descriptive statistics for all patient data were calculated using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). Student's t-tests were performed to compare anterior, posterior, and total mean corneal powers; cylinder powers; and flat meridians. Student's t-tests were also performed to compare the mean magnitudes of the vector differences of the astigmatism and the mean absolute corneal power and the flat meridian differences between the simulated K and total cornea between keratoconus and healthy eyes. Linear regression and Pearson's correlation analyses were performed to evaluate correlations between corneal power, cylinder power, and flat meridian among anterior and posterior corneal surfaces and the total cornea. Chi-square tests were performed to compare the proportions of anterior and posterior corneal astigmatism according to the flat meridian between eyes with keratoconus and healthy eyes. Results were considered statistically significant at a p-value <0.05.
Results
Sixty-six eyes of 66 subjects were enrolled in this study (33 eyes of 33 patients with keratoconus and 33 eyes of 33 controls). The mean age (±standard deviation, SD) of all subjects was 28.4 ± 9.2 years. There were 22 females (66.7%) and 18 left eyes (54.5%) in each group. The mean anterior, posterior, and total corneal powers (±SD) of the keratoconus group (46.99 ± 2.83, −6.92 ± 0.54, and 45.92 ± 2.94 D, respectively) were greater than those of the control group (42.98 ± 1.42, −6.30 ± 0.24, and 42.00 ± 1.38 D, respectively). The mean total corneal power was significantly smaller than the mean anterior corneal power in both groups (p < 0.001 and p < 0.001, respectively). The mean anterior, posterior, and total corneal cylinder powers (±SD) of the keratoconus group (4.37 ± 1.73, 0.95 ± 0.39, and 4.36 ± 1.74 cylinder diopters [CD], respectively) were also greater than those of the control group (1.10 ± 0.68, 0.39 ± 0.18, and 0.97 ± 0.63 CD, respectively). The mean central corneal thickness (±SD) of the keratoconus group (509.8 ± 37.5 µm) was significantly smaller than that of the control group (567.8 ± 30.2 µm) (Table 1).
Table 1. Baseline clinical characteristics of participants and their eyes.
Values are presented as mean ± standard deviation or number (%).
D = diopters; CD = cylinder diopters.
*Student's t-test; †Fisher's exact test; ‡True net power in the 4.0-mm zone, which was measured by a single Scheimpflug camera.
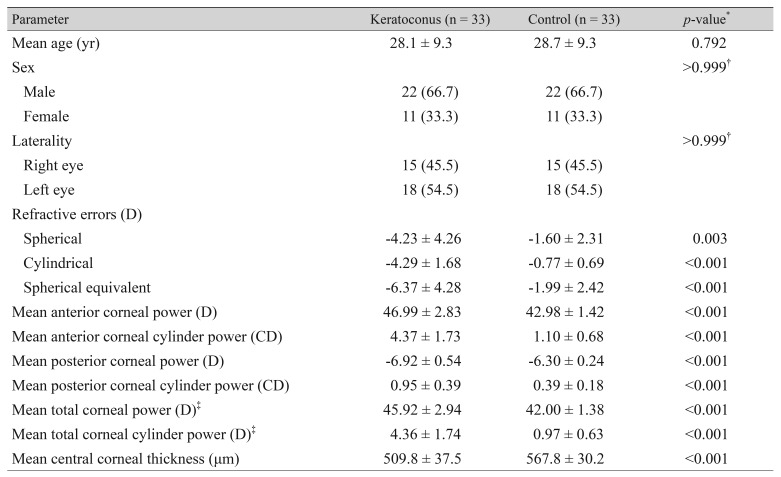
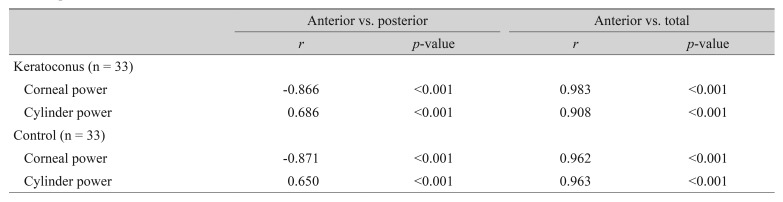
All correlation coefficients of corneal power and cylinder power showed significant correlations among anterior and posterior corneal surfaces and with the total cornea in the keratoconus and control groups (Table 2). According to linear regression analysis, the correlation between the anterior and total corneal cylinder powers was more prominent in both groups than between the anterior and posterior corneal cylinder powers (Fig. 1, 2).
Table 2. Pearson correlation coefficient (r) and p-values for correlation of mean corneal power and cylinder power among the anterior and posterior corneal surfaces and the total cornea.
Fig. 1. Linear regression analysis of the relationship between anterior and posterior corneal cylinder power. The solid line represents a linear regression line (Y = 0.156X + 0.270, R2 = 0.470, p < 0.001) for the keratoconus group (filled triangles), and the dashed line represents a linear regression line (Y = 0.174X + 0.194, R2 = 0.422, p < 0.001) for the normal controls (open circles). CD = cylinder diopters.
Fig. 2. Linear regression analysis of the relationship between anterior and total corneal cylinder power. The solid line represents a linear regression line (Y = 0.914X + 0.361, R2 = 0.824, p < 0.001) for the keratoconus group (filled triangles), and the dashed line represents a linear regression line (Y = 0.894X − 0.011, R2 = 0.928, p < 0.001) for the normal controls (open circles). CD = cylinder diopters.
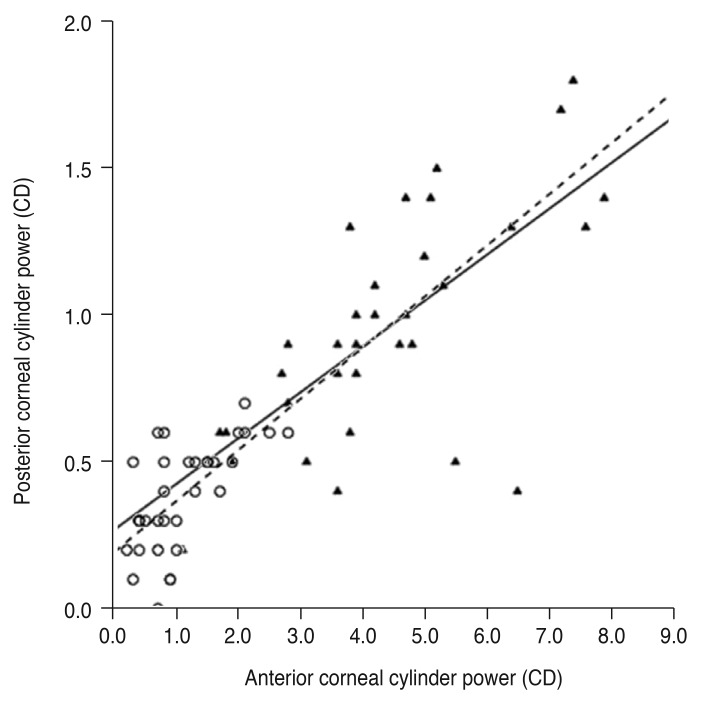
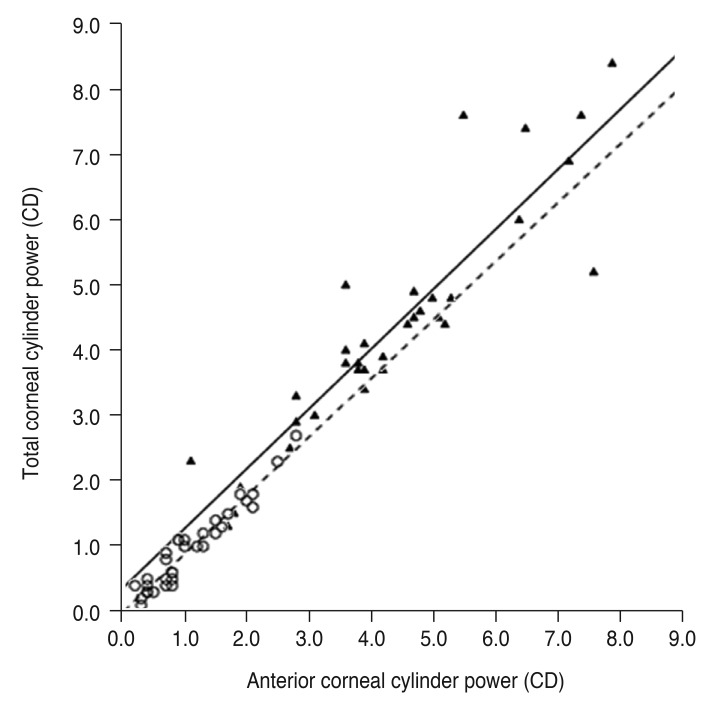
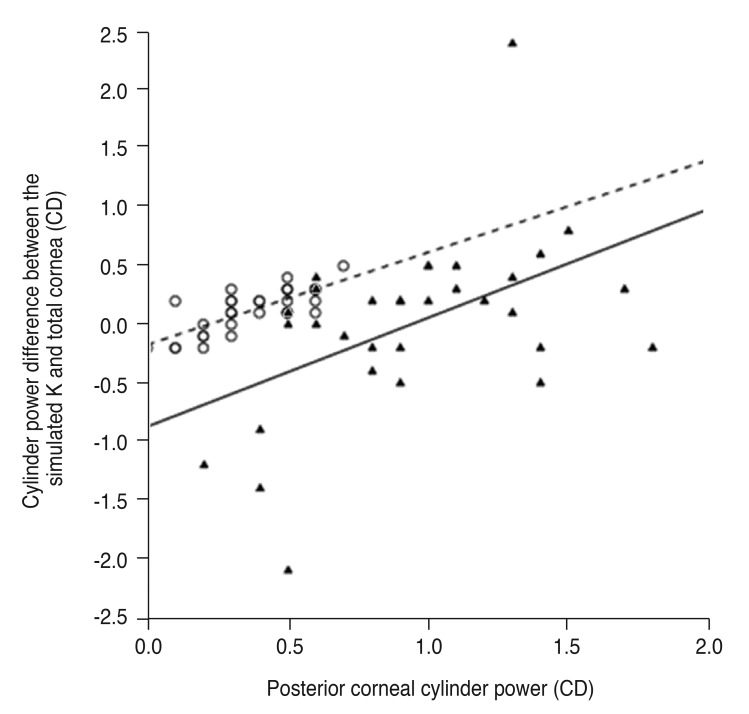
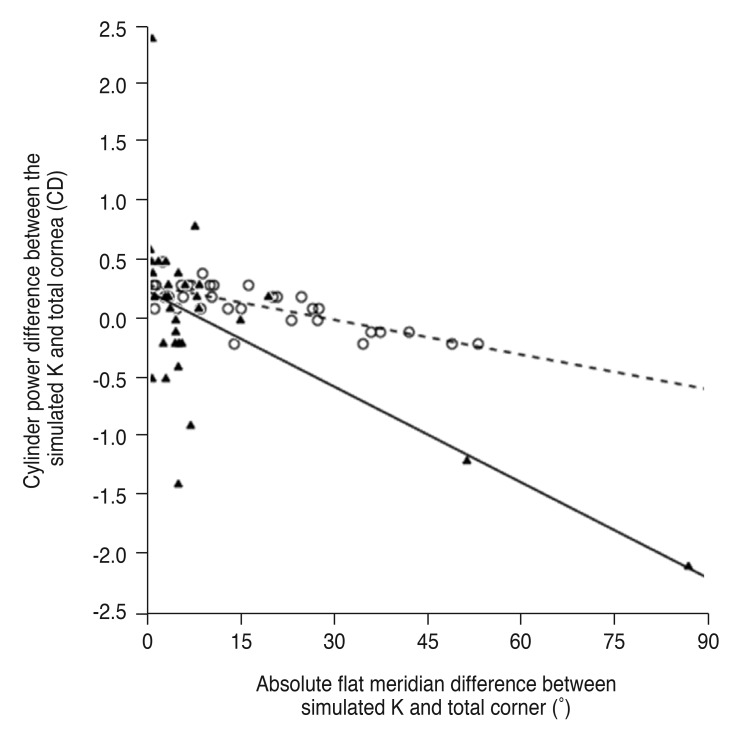
The cylinder power difference between the simulated K and total cornea was positively correlated with posterior corneal cylinder power (R2 = 0.240 and p = 0.004 in the keratoconus group, R2 = 0.592 and p < 0.001 in the control group) (Fig. 3) and negatively correlated with the absolute flat meridian difference between the simulated K and total cornea in both groups (R2 = 0.370 and p < 0.001 in the keratoconus group, R2 = 0.592 and p < 0.001 in the control group) (Fig. 4).
Fig. 3. Linear regression analysis of the relationship between posterior corneal cylinder power and cylinder power difference between the simulated keratometry (K) and total cornea. The solid line represents a linear regression line (Y = 0.928X − 0.868, R2 = 0.240, p = 0.004) for the keratoconus group (filled triangles), and the dashed line represents a linear regression line (Y = 0.786X − 0.172, R2 = 0.592, p < 0.001) for the normal controls (open circles). CD = cylinder diopters.
Fig. 4. Linear regression analysis of the relationship between absolute flat meridian difference and cylinder power difference between the simulated keratometry (K) and total cornea. The solid line represents a linear regression line (Y = −0.027X + 0.249, R2 = 0.370, p < 0.001) for the keratoconus group (filled triangles), and the dashed line represents a linear regression line (Y = −0.010X + 0.300, R2 = 0.592, p < 0.001) for the normal controls (open circles). CD = cylinder diopters.
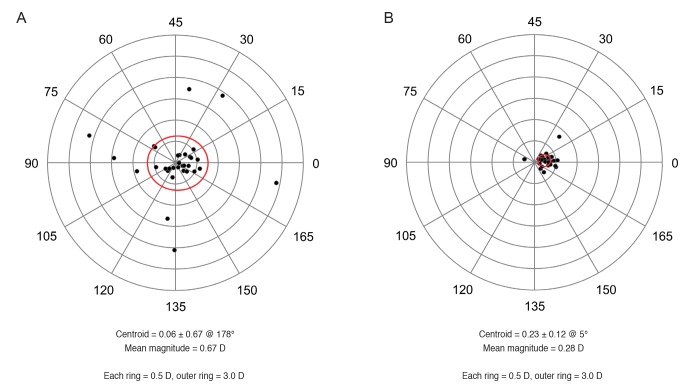
The mean magnitude of the vector difference between the astigmatism of the simulated K and the total cornea of the keratoconus group, 0.67 ± 0.67 CD, was significantly greater than that of the control group, 0.28 ± 0.12 CD (p = 0.002) (Table 3 and Fig. 5A, 5B), although the mean absolute corneal power difference between the simulated K and total cornea was not significantly different between the two groups. The magnitude of the vector difference between the astigmatism of the simulated K and total cornea was a maximum of 2.42 CD in the keratoconus group and 0.77 CD in the control group. In contrast, the mean absolute flat meridian difference between the simulated K and total cornea of the keratoconus group, 2.3 ± 3.8 degrees, was significantly smaller than that of the control group, 9.2 ± 12.6 degrees (p = 0.005) (Table 3). The percentage of eyes with a flat meridian difference between the simulated K and total cornea >10 degrees in the keratoconus group, 9.1%, was significantly smaller than that in the control group, 30.3% (p = 0.030).
Table 3. Comparison of corneal power difference, magnitude of the vector difference of astigmatism, and flat meridian difference between simulated K and the total cornea (Student's t-test).
Values are presented as mean ± standard deviation.
K = keratometry; MAKDSimK-Tot = mean absolute corneal power (K) difference between the simulated K and total cornea; D = diopters; MMVDSimK-Tot = mean magnitude of vector difference between the astigmatism of the simulated K and total cornea; CD = cylinder diopters; MAMDSimK-Tot = mean absolute flat meridian difference between the simulated K and total cornea.
Fig. 5. Double-angle plots of the vector difference between the astigmatism of the anterior corneal surface and total cornea. The red ellipse indicates one standard deviation. (A) Keratoconus group. (B) Normal controls. D = diopters.
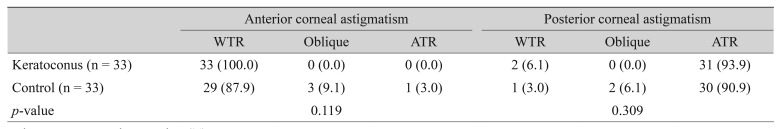
Our analysis indicated that 100.0% of the keratoconus group and 87.9% of the control group had WTR astigmatism on the anterior corneal surface, and 93.9% of the keratoconus group and 90.9% of the control group had ATR astigmatism on the posterior corneal surface (Table 4). There were no significant differences between the two groups in the proportions of anterior and posterior corneal astigmatism according to the flat meridian.
Table 4. Anterior and posterior corneal astigmatism proportions according to the flat meridian in each group (chi-square test).
Values are presented as number (%).
WTR = with-the-rule astigmatism; ATR = against-the-rule astigmatism.
Discussion
In this study, we compared the anterior, posterior, and total corneal measurements of healthy eyes and eyes with grade I or II keratoconus. The results of this study indicated that both eyes with keratoconus and healthy eyes showed a significant correlation among anterior, posterior, and total corneal astigmatisms. These results are similar to previous studies that showed that the correlation between anterior and posterior corneal astigmatism was good in eyes with keratoconus [9,10,11]. In addition, the analysis of this study determined that eyes with keratoconus had a large vector difference but a small flat meridian difference between the astigmatism of the simulated K and total cornea compared to healthy eyes. The results indicated that the amount of posterior corneal astigmatism rather than the posterior corneal flat meridian had a greater effect on the total corneal astigmatism in eyes with grade I or II keratoconus.
The magnitude of vector difference between the astigmatism of the simulated K and total cornea was up to 2.42 CD in the keratoconus group and 0.77 CD in the control group. In addition, the range of cylinder power difference between the simulated K and total cornea was wider in the keratoconus group than the control group. The cause of this wide range of cylinder power difference between the simulated K and total cornea is mainly due to the diversity of the posterior corneal curvature in eyes with keratoconus [9,10,11]. The mean posterior corneal cylinder power of the keratoconus group (0.95 CD) was significantly greater than that of the control group (0.39 CD) in this study. Thus, posterior corneal astigmatism has more influence on the total corneal astigmatism in eyes with keratoconus, and there could be a large vector difference in eyes with keratoconus compared to healthy eyes.
The reason for the small flat meridian difference between the astigmatism of the simulated K and total cornea in eyes with keratoconus is that the flat meridians of the anterior and posterior corneal surfaces tended to be the same as those of healthy eyes. In the keratoconus group, the flat anterior corneal meridian was horizontally aligned in 100.0% of patients, and the flat posterior corneal meridian was horizontally aligned in 93.9%. As a result, in 90.9% of eyes with keratoconus, the flat meridian difference between the anterior corneal surface and the total cornea was less than or equal to 10 degrees. In line with this study, Savini et al. [18] demonstrated that eyes with keratoconus showed a tendency for the steep meridians of the anterior and posterior corneal surfaces to be aligned regardless of the type of astigmatism (WTR, ATR, or oblique astigmatism). In that study, the steep meridian difference between the anterior corneal surface and the total cornea was greater than 10 degrees in only 8.4% of eyes with keratoconus [18].
In this study, the cylinder power difference between the simulated K and total cornea was positively correlated with posterior corneal cylinder power and negatively correlated with the absolute flat meridian difference between the simulated K and total cornea in both groups. Similar to the results of this study, a previous study showed the same correlations among the posterior corneal cylinder power, the absolute flat meridian difference, and the cylinder power difference between the simulated K and total cornea in healthy eyes [6]. These results mean that the cylinder power of simulated K tends to overestimate total corneal cylinder power as the posterior corneal cylinder power increases or as the absolute flat meridian difference decreases in both healthy and keratoconus eyes. To avoid incorrect estimation of the total corneal astigmatism in eyes with keratoconus, it is necessary to measure the cylinder power and axis of the posterior corneal surface, because the estimation error for total corneal cylinder power from anterior corneal measurements is larger in eyes with keratoconus. The importance of posterior corneal surface measurement when considering toric IOL should be emphasized for patients with keratoconus.
Pinero et al. [16] demonstrated that the central corneal power, which was estimated with a single value of the keratometric index (nk = 1.3375), is imprecise in eyes with keratoconus, and that overestimation was observed in most cases compared to the true net power determined with the Gaussian equation. There was also a significant difference in corneal power between the conventional keratometric approach and the Gaussian equation in normal healthy eyes [19,20,21]. The results of this study were similar to the results of previous studies [16,19,20,21]. In this study, the anterior corneal power, which was estimated with a keratometric index of 1.3375, was significantly larger than the total corneal power, which is the true net power, in both groups. Park et al. [22] reported that the IOL power calculation that used the conventional keratometric approach was inaccurate and showed a hyperopic shift in patients with posterior keratoconus. This hyperopic shift occurs because the conventional keratometric approach ignores the posterior corneal surface and overestimates the corneal power.
There were some limitations to this study. First, the sample size was relatively small, and medical records were reviewed retrospectively. Second, for eyes with grade I or II keratoconus, this study did not verify whether the condition had progressed or remained stable, although progression of keratoconus induces severe irregular myopic astigmatism [23], and corneal astigmatism correction using toric IOLs is more suitable for eyes with stable keratoconus. Third, the mean age of the patients in this study was 28 years, which is too young for typical cataract surgery. Therefore, a prospective study to assess posterior and total corneal astigmatism in a large number of patients with keratoconus is necessary.
In conclusion, eyes with keratoconus had larger posterior corneal astigmatism and greater estimation errors of total corneal astigmatism based on anterior corneal measurements than healthy eyes. Thus, posterior corneal surface measurement should be emphasized for patients that are considering toric IOL in eyes with keratoconus.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1A02937003), by a Korea University Grant (K1625491), and by the Alumni of the Department of Ophthalmology, Korea University College of Medicine in 2017.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Wittig-Silva C, Chan E, Islam FM, et al. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121:812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Guell JL, Morral M, Malecaze F, et al. Collagen crosslinking and toric iris-claw phakic intraocular lens for myopic astigmatism in progressive mild to moderate keratoconus. J Cataract Refract Surg. 2012;38:475–484. doi: 10.1016/j.jcrs.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Eom Y, Nam KT, Kang SY, et al. Axis difference between corneal and internal astigmatism to consider for toric intraocular lenses. Am J Ophthalmol. 2013;156:1112–1119. doi: 10.1016/j.ajo.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 5.Koch DD, Ali SF, Weikert MP, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38:2080–2087. doi: 10.1016/j.jcrs.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Eom Y, Kang SY, Kim HM, Song JS. The effect of posterior corneal flat meridian and astigmatism amount on the total corneal astigmatism estimated f rom anterior corneal measurements. Graefes Arch Clin Exp Ophthalmol. 2014;252:1769–1777. doi: 10.1007/s00417-014-2737-9. [DOI] [PubMed] [Google Scholar]
- 7.Eom Y, Rhim JW, Kang SY, et al. Toric intraocular lens calculations using ratio of anterior to posterior corneal cylinder power. Am J Ophthalmol. 2015;160:717–724. doi: 10.1016/j.ajo.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Eom Y, Ryu D, Kim DW, et al. Development of a program for toric intraocular lens calculation considering posterior corneal astigmatism, incision-induced posterior corneal astigmatism, and effective lens position. Graefes Arch Clin Exp Ophthalmol. 2016;254:1977–1986. doi: 10.1007/s00417-016-3446-3. [DOI] [PubMed] [Google Scholar]
- 9.Tomidokoro A, Oshika T, Amano S, et al. Changes in anterior and posterior corneal curvatures in keratoconus. Ophthalmology. 2000;107:1328–1332. doi: 10.1016/s0161-6420(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 10.Pinero DP, Alio JL, Aleson A, et al. Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in subclinical and different stages of clinical keratoconus. J Cataract Refract Surg. 2010;36:814–825. doi: 10.1016/j.jcrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Montalban R, Alio JL, Javaloy J, Pinero DP. Correlation of anterior and posterior corneal shape in keratoconus. Cornea. 2013;32:916–921. doi: 10.1097/ICO.0b013e3182904950. [DOI] [PubMed] [Google Scholar]
- 12.Alio JL, Pena-Garcia P, Abdulla Guliyeva F, et al. MICS with toric intraocular lenses in keratoconus: outcomes and predictability analysis of postoperative refraction. Br J Ophthalmol. 2014;98:365–370. doi: 10.1136/bjophthalmol-2013-303765. [DOI] [PubMed] [Google Scholar]
- 13.Muftuoglu O, Ayar O, Ozulken K, et al. Posterior c orneal elevation and back difference corneal elevation in diagnosing forme fruste keratoconus in the fellow eyes of unilateral keratoconus patients. J Cataract Refract Surg. 2013;39:1348–1357. doi: 10.1016/j.jcrs.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Krumeich JH, Daniel J, Knulle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998;24:456–463. doi: 10.1016/s0886-3350(98)80284-8. [DOI] [PubMed] [Google Scholar]
- 15.Choi JA, Kim MS. Progression of keratoconus by longitudinal assessment with corneal topography. Invest Ophthalmol Vis Sci. 2012;53:927–935. doi: 10.1167/iovs.11-8118. [DOI] [PubMed] [Google Scholar]
- 16.Pinero DP, Camps VJ, Caravaca-Arens E, et al. Estimation of the central corneal power in keratoconus: theoretical and clinical assessment of the error of the keratometric approach. Cornea. 2014;33:274–279. doi: 10.1097/ICO.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 17.Holladay JT, Moran JR, Kezirian GM. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J Cataract Refract Surg. 2001;27:61–79. doi: 10.1016/s0886-3350(00)00796-3. [DOI] [PubMed] [Google Scholar]
- 18.Savini G, Naeser K, Schiano-Lomoriello D, Mularoni A. Influence of posterior corneal astigmatism on total corneal astigmatism in eyes with keratoconus. Cornea. 2016;35:1427–1433. doi: 10.1097/ICO.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 19.Fam HB, Lim KL. Validity of the keratometric index: large population-based study. J Cataract Refract Surg. 2007;33:686–691. doi: 10.1016/j.jcrs.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Olsen T. On the calculation of power from curvature of the cornea. Br J Ophthalmol. 1986;70:152–154. doi: 10.1136/bjo.70.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho JD, Tsai CY, Tsai RJ, et al. Validity of the keratometric index: evaluation by the Pentacam rotating Scheimpflug camera. J Cataract Refract Surg. 2008;34:137–145. doi: 10.1016/j.jcrs.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Park DY, Lim DH, Chung TY, Chung ES. Intraocular lens power calculations in a patient with posterior keratoconus. Cornea. 2013;32:708–711. doi: 10.1097/ICO.0b013e3182797900. [DOI] [PubMed] [Google Scholar]
- 23.Visser N, Gast ST, Bauer NJ, Nuijts RM. Cataract surgery with toric intraocular lens implantation in keratoconus: a case report. Cornea. 2011;30:720–723. doi: 10.1097/ICO.0b013e31820009d4. [DOI] [PubMed] [Google Scholar]