Abstract
Purpose
This study aimed to evaluate the clinical course and prognostic factors of acquired third, fourth, and sixth cranial nerve (CN) palsy grouped according to etiology.
Methods
This study involved a retrospective review of the medical records of 153 patients who were diagnosed with acquired paralytic strabismus from January 2004 to July 2015. Outcomes, recovery rates, and time to recovery were investigated according to the affected CN: CN3, CN4, and CN6 palsies. The patients were classified into four groups based on etiology: idiopathic, traumatic, neoplastic, and vascular.
Results
The mean age of the patients was 59.8 ± 14.5 years and the mean follow-up period was 10.8 months. Out of the 153 patients, 63 (41.2%) had CN3 palsy, 35 (22.9%) had CN4 palsy, and 55 (35.9%) had CN6 palsy. The most common causes were vascular related (54.9%), followed by idiopathic (28.1%), trauma (8.5%), and neoplasm (5.88%). About 50% of the patients recovered within six months. Among the four etiologic groups, the idiopathic group showed the best prognosis because about 50% of the patients in this group recovered within three months. This was followed by the vascular, traumatic, and neoplastic groups. Cox proportional hazard analysis revealed a significant association between the baseline prism diopter and recovery rate.
Conclusions
The prognosis and natural history of paralytic strabismus vary depending on its cause. The vascular group had the best recovery rate and shortest recovery time, whereas the neoplastic group required the longest time to recover.
Keywords: Cranial nerve diseases, Paralytic strabismus
Strabismus is a misalignment of ocular movement characterized by a horizontal, vertical, or torsional deviation of one eye. Palsies of the third, fourth, and sixth cranial nerves (CNs; CN3, CN4, and CN6), which innervate six extraocular muscles, are identified as major causes of adult-onset secondary paralytic strabismus. Diplopia and head tilting are common symptoms present in patients with strabismus. CN palsies may result from vascular ischemia, hemorrhage, trauma, intracranial neoplasm, systemic neurologic disease, or idiopathic causes. The relative frequency of the different causes may vary slightly according to the studies reviewed [1,2,3]. Diabetes mellitus and hypertension are among the most frequently reported systemic causes of acquired paralytic strabismus, as they cause ischemic changes in the cerebral nerve [4,5,6,7]. The prognosis in most cases of ocular nerve paresis is generally good, with spontaneous regression of symptoms occurring within several weeks; the time to and degree of recovery may differ depending on the cause of the condition [8,9,10,11,12,13]. In this study, we investigated the clinical characteristics and natural history of acquired paralytic strabismus and tried to identify prognostic factors for recovery in Korean patients.
Materials and Methods
This retrospective study was approved by the institutional review board of Kyung Hee University Hospital and the requirement for informed consent was waived. Medical records of patients diagnosed with CN3, CN4, and CN6 palsy from January 2004 to July 2015 at Kyung Hee University Hospital were retrospectively reviewed to confirm the diagnosis and determine the etiology. Patients were excluded if they (1) were younger than 19 years, (2) had a history of strabismus diagnosed prior to the study, (3) had a history of orbital surgery, (4) had been lost to follow-up within one year of the first visit without being fully recovered, or (5) had an onset of symptoms two weeks or more prior to the first diagnosis. Age of onset, sex, presence of comorbidities such as hypertension and diabetes mellitus, and findings from brain magnetic resonance imaging (MRI), if performed, were all reviewed to identify the cause of each case. At first visit, the angle of deviation was measured using the alternate prism cover test in a primary position at close range and at a distance, and the larger value of the two was recorded as the baseline prism diopter (PD). If both horizontal and vertical deviations existed, the larger PD was recorded as the baseline value. All patients were asked about their subjective diplopia at every visit.
The patients were classified into four etiologic groups: idiopathic, vascular, traumatic, and neoplastic. The four groups were compared in terms of total recovery rate and mean time to recovery. Complete recovery of nerve palsies was defined as the condition where the angle of deviation was less than 8 PD, and the patient feeling free of diplopia without surgical intervention. The vascular group consisted of those who were diagnosed with vascular comorbidities such as diabetic mellitus, hypertension, or hypercholesterolemia with no history of trauma. Patients who had microangiopathy or infarction evident in brain MRI were also included in the vascular group. Patients with obvious recent head trauma or evidence of intracranial neoplasm on brain imaging studies were assigned to the traumatic or neoplastic groups, respectively. Patients who did not meet any of the etiologic criteria above were classified into the idiopathic group.
Statistical analyses were done using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Differences in clinical characteristics and prognosis among the four etiologic groups were identified using one-way analysis of variance and the chi square test. Kaplan-Meier analysis with the log-rank test was used to plot and compare the survival curve by etiology, and the mean time taken to achieve recovery for each group was calculated. To assess the factors that were significantly associated with recovery, Cox proportional hazards regression analysis was performed with adjustment. For all statistical tests, the threshold of significance was set at a p-value of 0.05.
Results
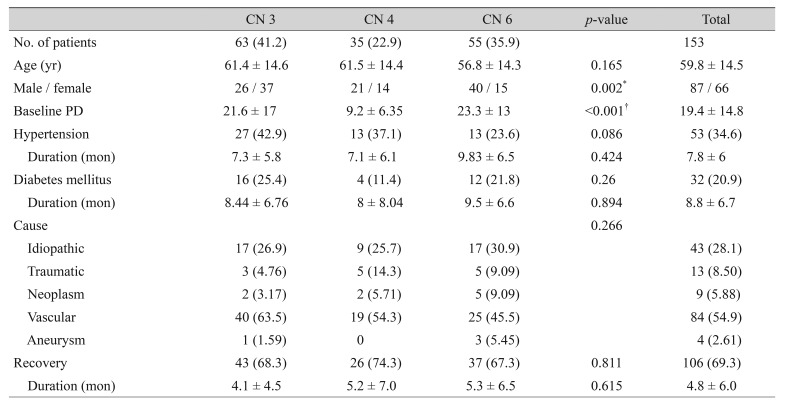
A total of 153 patients with new onset paralytic strabismus were assessed in this study. There were 87 (56.86%) men and 66 (44.14%) women. The mean age was 59.8 ± 14.5 years old, and the mean follow-up duration was 10.8 months. One hundred six patients (69.3%) achieved recovery, and the mean time to recovery was 4.8 ± 6.0 months. CN3, CN4, and CN6 palsies were observed in 63 (41.2%), 35 (22.9%), and 55 (35.9%) patients, respectively. The baseline characteristics, frequency of etiology, and time to recovery for each CN palsy type are shown in Table 1.
Table 1. Demographic data by CN affected.
Values are presented as number (%), mean ± standard deviation, or number.
CN = cranial nerve; PD = prism diopter.
*Chi-square test; †One-way analysis of variance.
The baseline angle of deviation was significantly smaller in the CN4 palsy group (9.2 ± 6.35 PD) than in the CN3 and CN6 palsy groups (21.6 ± 17 and 23.3 ± 13 PD, respectively, p < 0.001). Vascular was the most common etiology, followed by idiopathic, trauma, and neoplasm for all CN palsies. The recovery rate was 68.3% in CN3, 74.3% in CN4, and 67.3% in CN6, but the difference was not statistically significant (p = 0.811). Mean time to recovery was not significantly different among the three CN palsy groups (p = 0.615).
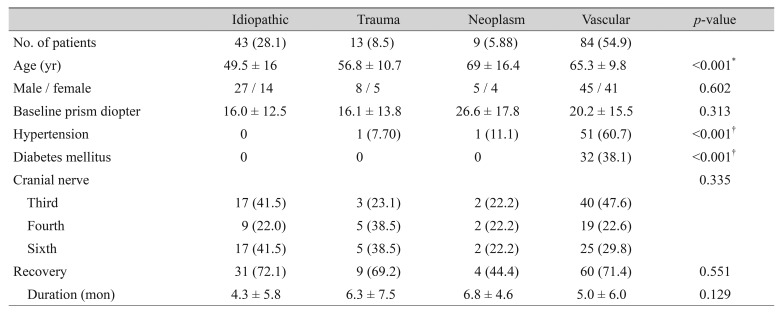
The most common causes of paralytic strabismus were vascular diseases (54.9%), followed by idiopathic causes (28.1%), trauma (8.5%), and neoplasm (5.88%) (Table 2). The mean age at onset was significantly lower (p < 0.001) in the idiopathic group (49.5 years) than in the other groups (vasculopathic, 65.3 years; traumatic, 56.8 years; neoplastic, 69 years). The baseline angle of deviation was largest in the neoplastic group (26.6 ± 17.8 PD), followed by vascular (20.2 ± 15.5 PD), idiopathic (16.4 ± 12.5 PD), and traumatic (16.1 ± 12.8 PD) groups, with no significant difference among them. Brain MRI was performed on 130 (85.1%) of the study patients following the diagnosis of paralytic strabismus. Brain MRI was performed on 82 of 84 patients in the vascular group, and ischemic small vessel lesions or microinfarctions were found in 61 of these patients (74.4%). In the vascular group, the odds ratio (OR) or association between risk factors and recovery was calculated (Table 3). Diabetes mellitus was more frequently reported in the group of patients who failed to recover from strabismus (non-recovery group) at 45.8% than in the recovery group (35.0%), but the difference was not significant (p = 0.384), with an OR of 1.22 (95% confidence interval [CI], 0.692 to 3.908). Systemic hypertension was slightly more common in the non-recovery group (62.5%) than in the recovery group (60.0%), but the difference was not significant (p = 0.816), with an OR of 1.02 (95% CI, 0.352 to 2.774). Ischemic findings on brain MRI were more commonly found in the non-recovery group (87.5%) than in the recovery group (66.7%, p = 0.012), with an OR of 3.23 (95% CI, 1.252 to 8.039).
Table 2. Demographic data by etiology.
Values are presented as number (%), mean ± standard deviation, or number.
*One-way analysis of variance; †Chi-square test.
Table 3. Odds ratio for disease recovery across three vascular risk factors by chi-square analysis.
Values are presented as number (%)
CI = confidential interval; MRI = magnetic resonance imaging.
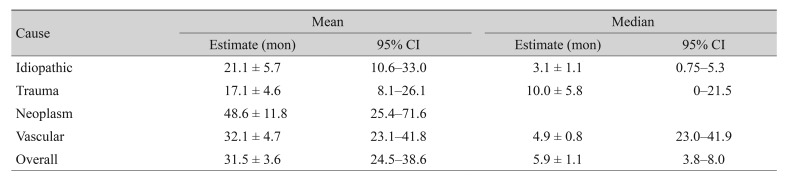
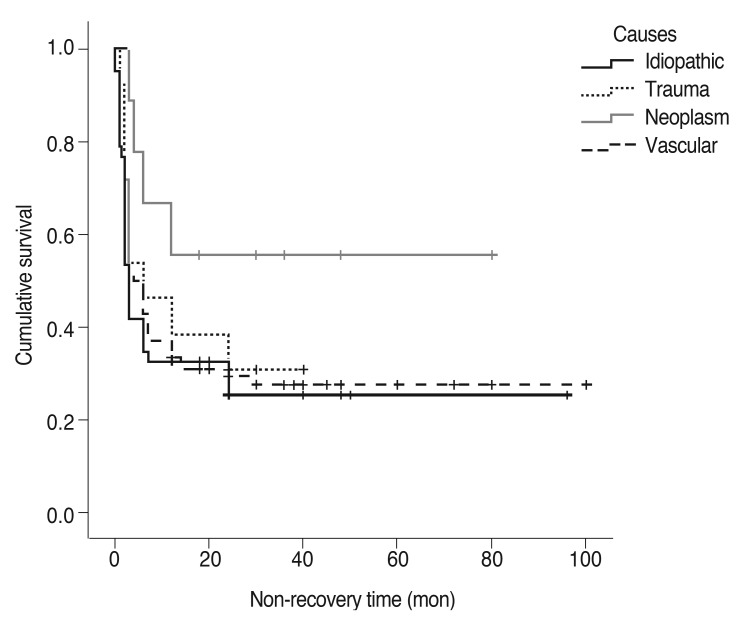
At the end of the follow-up period, the recovery rate was highest in the idiopathic group (72.1%) followed by the vascular (71.4%), traumatic (69.2%), and neoplastic (44.4%) groups. The results of a log-rank test showed that the distribution of recovery rate across different causes were significantly different (p = 0.031). Mean time to recovery was 4.3 ± 5.8 months in the idiopathic group, 5.0 ± 6.0 months in the vascular group, 6.3 ± 7.5 months in the traumatic group, and 6.8 ± 4.6 months in the neoplastic group, but the difference was not significant (p = 0.129). In addition, Kaplan-Meier analysis was performed for the different etiologic groups. Half of the patients in the idiopathic group took more than three months to recover, half of the patients in the vascular group took more than five months to recover, and half of the trauma patients took more than 10 months to recover. Overall, about half of the patients needed more than six months to recover (Table 4). Survival curves for different disease etiologies were plotted against the recovery rate of each group (Fig. 1). This further supported the same results already found in Table 4: patients with idiopathic paralytic strabismus recovered most quickly while patients with tumors required the longest time to recover.
Table 4. Median time to recovery from disease by different causes.
Values are presented as mean ± standard deviation; Kaplan-Meier survival analysis.
CI = confidential interval.
Fig. 1. The recovery curve by etiologic group (for cranial nerve palsy).
Cox proportional hazard regression analysis was performed to find factors associated with the recovery of paralytic strabismus. Univariate analysis revealed that the baseline angle of deviation was significantly associated with patient recovery (p = 0.006). After adjustment was made for age, sex, CN, and disease etiology, multivariate analysis was performed. The baseline angle of deviation was found to be inversely related to patient recovery (p = 0.01).
Discussion
The incidence and prevalence of paralytic strabismus have been reported in several population-based studies. The current study revealed that the prevalence of paralytic strabismus involving oculomotor nerve paresis ranged from 25% to 40%, trochlear nerve paresis from 17% to 19%, and abducens nerve paresis from 30% to 58% [8,9,10,11,12,13]. The distribution pattern of palsy in the patients reviewed in our study was consistent with the general trend: 41.2% involved the oculomotor nerve, 22.9% involved the trochlear nerve, and 35.9% involved the abducens nerve. These results showed a relatively higher portion of CN3 than CN6 palsy. This can be explained by the different referral patterns depending on the distribution of the population, in particular, the low incidence of head trauma and frequent referral of patients with vasculopathic causes. CN3 palsy most commonly stems from systemic vascular origin, specifically, brain MRI suggested a focal mesemcephalic infarct as a possible cause for the majority of diabetic mononeuropathies of CN3 [14]. Moreover, due to a long intradural course of the abducens nerve, CN6 is known to be particularly vulnerable to head trauma [15]. Previous studies also reported that CN6 palsy had a higher proportion of traumatic causes of paralytic strabismus compared to CN3 and CN4 palsies [10,11,12,13,16,17].
The distribution of paralytic strabismus by etiology varied depending on the study. Ho et al. [10] reported that vasculopathy (35.2%) was the most common cause, while Tiffin et al. [11] reported that idiopathic cases (35%) were the most common. In studies involving Korean populations, trauma (48.9%) was the most common cause in Lee et al.'s study [12], while Park et al. [13] and Park and Chang [17] reported that vasculopathy (30.0% and 42.9%, respectively) was the most common etiology. Our study found that patients with palsies caused by vasculopathy comprised the largest group (54.9%), followed by idiopathic (28.1%), trauma (8.5%), and neoplasm (5.88%). Such differences in distribution seemed attributable to the differences in patient characteristics, diagnostic equipment, and classification criteria used in the study. In the current study, 85.1% of patients received brain MRIs to identify the cause of the palsy. Those who were found to have ischemic small vessel lesions or microinfarction on brain MRI were classified into the group with vasculopathy. Unlike the current study, previous studies included only patients with systemic vascular risk factors such as diabetes or hypertension in the vasculopathy group. Having a wider scope of vasculopathy in the current study seemed to have resulted in a higher number of patients being included in the vasculopathy group. In particular, 87.5% of patients in this study's vascular group were found to have ischemic small vessel lesions or microinfarction, with a hazard ratio of OR 3.23 (Table 3).
Existing papers and studies identified diabetes and hypertension as major risk factors for acquired paralytic strabismus [4,5,6,7]. It was reported that left ventricular hypertrophy resulting from hypertension and HbA1c indicating the glucose level in the blood were associated with CN palsy [6,7]. Patel et al. [9] reported that patients with diabetes were six times more likely to have CN6 palsy while there was no significant association between hypertension and CN6 palsy. Patients with both diabetes and hypertension were found to be eight times more likely to have CN6 palsy. When it comes to prognosis, Sanders et al. [5] and Ho et al. [10] reported that the presence of hypertension and diabetes had no significant association with recovery from paralytic strabismus. Our study found no significant association between the presence of hypertension and diabetes and recovery from paralytic strabismus (p = 0.923, p = 0.223). Co-existing systemic morbidities such as diabetes and hypertension may affect systemic circulation and cause ischemic changes in small vessels. Also, ischemic lesions evident on brain MRI can directly indicate blood vessel changes around CNs, which consequently cause demyelination in a portion of CNs. Therefore, our results assumed that ischemic findings on brain MRI may be a more significant related factor in predicting prognosis of vasculopathic paralytic strabismus (p = 0.011).
Rush and Younge [8] analyzed the recovery of 1,000 patients with paralytic strabismus to find that those in the vascular group had the highest recovery at 71%, followed by the idiopathic group at 50%, traumatic group at 40%, and neoplastic group at 14.3%. Ho et al. [10] reported that of 196 subjects, the time to recovery was shortest in patients in the vascular (median, 3 months), followed by the idiopathic (median, 4 months), and traumatic (median, 6 months) groups. Patients in the neoplastic group had the lowest recovery rate and the longest time to recovery. Half of the patients in this study recovered within six months. The patients in the idiopathic group recovered much more quickly than others (median, 3 months), followed by the vascular (median, 5 months) and traumatic (median, 10 months) groups; patients in the neoplastic group had the longest time to recovery. The recovery rate was highest in the idiopathic group (72.1%), followed by the vascular (71.4%), traumatic (69.2%), and neoplastic (44.4%). CN palsy due to vascular reasons causes the blood vessel walls around the CN to become thicker and hyalinized, which in turn may cause demyelination in some of the nerves. Over time, it seems that demyelinated lesions remyelinate, resulting in spontaneous recovery [18]. Most previous studies reported that both vasculopathic and idiopathic causes demonstrated a consistently high recovery rate when compared to other causes [10,11,12,13]. Because differential diagnosis between vascular and idiopathic causes may be challenging, they may overlap, depending on the inclusion criteria for risk factors. In addition, younger age in the idiopathic group could be an acceleration factor for spontaneous remyelination of affected nerves.
Cox regression analysis revealed that the larger the deviation angle at the time of first diagnosis, the lower the recovery rate (OR, 0.9; 95% CI, 0.885 to 0.942; p = 0.01), which was consistent with the results of previous studies [16,17,19]. In the idiopathic group whose recovery rate was highest, the baseline deviation angle was smallest but the difference among the various etiological groups was not statistically significant. This means that there should be factors other than a small deviation angle that led the idiopathic group to have a higher recovery rate than others.
This study has some limitations. Existing studies on the natural history of acquired paralytic strabismus adopted somewhat different criteria for recovery. Ho et al. [10] defined recovery as the total loss of the subjective symptoms of diplopia. Park et al. [13] defined the total loss of the strabismus and diplopia as complete recovery, and decline in the angle of deviation by more than 10 PD from the onset of the disease as partial recovery. Shin and Park [16] defined recovery as no complaint of diplopia by the patient with the angle of deviation ranging from 8 to 10 PD as recovery. The authors did not have access to the final angle of deviation for all patients, so recovery was defined as the condition where the angle of deviation was less than 8 PD or the patient feeling free of diplopia. Since all patients in the current study were enrolled in the same hospital, there could also have been selection bias. A prospective, multi-center study that adopts an objective and unified definition of recovery would be able to supplement the limitations of this study.
In conclusion, this study was meaningful in that the long-term natural history of acquired paralytic strabismus and its predictive factors for prognosis were analyzed with a relatively large number of Korean patients. Over the follow-up period, 69.3% of all the patients recovered. According to etiology, patients in the idiopathic group showed the best prognosis followed by the vascular, traumatic, and neoplastic groups, with those in the neoplastic group having the lowest recovery rate and longest time to recovery. In addition, the study found that the smaller the angle of deviation at baseline, the higher the recovery rate. This kind of population-based data may provide ophthalmologists and neurologists with more comprehensive insight into the diagnosis and evaluation of paralytic strabismus with CN palsy.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.de Camargo GB, Hida WT, Goldchmit M, et al. Paralytic strabismus: review of 24 years at “Santa Casa de São Paulo”. Arq Bras Oftalmol. 2007;70:585–587. doi: 10.1590/s0004-27492007000400005. [DOI] [PubMed] [Google Scholar]
- 2.Mwanza JC, Ngweme GB, Kayembe DL. Ocular motor nerve palsy: a clinical and etiological study. Indian J Ophthalmol. 2006;54:173–175. doi: 10.4103/0301-4738.27068. [DOI] [PubMed] [Google Scholar]
- 3.Richards BW, Jones FR, Jr, Younge BR. Causes and prognosis in 4,278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992;113:489–496. doi: 10.1016/s0002-9394(14)74718-x. [DOI] [PubMed] [Google Scholar]
- 4.Patel SV, Holmes JM, Hodge DO, Burke JP. Diabetes and hypertension in isolated sixth nerve palsy: a population-based study. Ophthalmology. 2005;112:760–763. doi: 10.1016/j.ophtha.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Sanders SK, Kawasaki A, Purvin VA. Long-term prognosis in patients with vasculopathic sixth nerve palsy. Am J Ophthalmol. 2002;134:81–84. doi: 10.1016/s0002-9394(02)01439-3. [DOI] [PubMed] [Google Scholar]
- 6.Choung HK, Chang BL. Clinical features of ischemic ophthalmoplegia caused by diabetes mellitus or hypertension. J Korean Ophthalmol Soc. 2002;43:131–135. [Google Scholar]
- 7.Jacobson DM, McCanna TD, Layde PM. Risk factors for ischemic ocular motor nerve palsies. Arch Ophthalmol. 1994;112:961–966. doi: 10.1001/archopht.1994.01090190109029. [DOI] [PubMed] [Google Scholar]
- 8.Rush JA, Younge BR. Paralysis of cranial nerves III, IV, and VI: cause and prognosis in 1,000 cases. Arch Ophthalmol. 1981;99:76–79. doi: 10.1001/archopht.1981.03930010078006. [DOI] [PubMed] [Google Scholar]
- 9.Patel SV, Mutyala S, Leske DA, et al. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004;111:369–375. doi: 10.1016/j.ophtha.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Ho TH, Lin HS, Lin MC, Sheu SJ. Acquired paralytic strabismus in Southern Taiwan. J Chin Med Assoc. 2013;76:340–343. doi: 10.1016/j.jcma.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Tiffin PA, MacEwen CJ, Craig EA, Clayton G. Acquired palsy of the oculomotor, trochlear and abducens nerves. Eye (Lond) 1996;10(Pt 3):377–384. doi: 10.1038/eye.1996.77. [DOI] [PubMed] [Google Scholar]
- 12.Lee WY, Kim JH, Shin H. A clinical study of paralytic strabismus. J Korean Ophthalmol Soc. 1993;34:549–554. [Google Scholar]
- 13.Park UC, Kim SJ, Yu YS. Clinical features and natural history of acquired third, fourth, and sixth cranial nerve palsy. J Korean Ophthalmol Soc. 2005;46:1555–1562. doi: 10.1038/sj.eye.6702720. [DOI] [PubMed] [Google Scholar]
- 14.Hopf HC, Gutmann L. Diabetic 3rd nerve palsy: evidence for a mesencephalic lesion. Neurology. 1990;40:1041–1045. doi: 10.1212/wnl.40.7.1041. [DOI] [PubMed] [Google Scholar]
- 15.Coello AF, Canals AG, Gonzalez JM, Martin JJ. Cranial nerve injury after minor head trauma. J Neurosurg. 2010;113:547–555. doi: 10.3171/2010.6.JNS091620. [DOI] [PubMed] [Google Scholar]
- 16.Shin H, Park SE. A clinical study of acquired paralytic strabismus in a secondary hospital. J Korean Ophthalmol Soc. 2007;48:311–314. [Google Scholar]
- 17.Park KH, Chang BL. The etiology and clinical feature of the third, fourth, and sixth cranial nerve palsy. J Korean Ophthalmol Soc. 1997;38:1432–1436. [Google Scholar]
- 18.Asbury AK, Aldredge H, Hershberg R, Fisher CM. Oculomotor palsy in diabetes mellitus: a clinico-pathological study. Brain. 1970;93:555–566. doi: 10.1093/brain/93.3.555. [DOI] [PubMed] [Google Scholar]
- 19.Hyun J, Kim SY. Clinical features and natural course of superior oblique palsy. J Korean Ophthalmol Soc. 2013;54:627–631. [Google Scholar]