Abstract
Purpose
To investigate the efficacy and safety of umbilical cord blood (UCB) infusion (UCBI) plus immunosuppressive therapy (IST) treatment in comparison to IST treatment, as well as predictive factors for clinical responses, in severe aplastic anemia (SAA) patients.
Materials and Methods
Totally, 93 patients with SAA were enrolled in this cohort study. In the IST group, rabbit antithymocyte globulin (r-ATG) combined with cyclosporine A (CsA) was administered, while in the IST+UBCI group, r-ATG, CsA, and UCB were used.
Results
After 6 months of treatment, UCBI+IST achieved a higher complete response (CR) rate (p=0.002) and an elevated overall response rate (ORR) (p=0.004), compared to IST. Regarding hematopoietic recovery at month 6, platelet responses in the UCBI+IST group were better than those in the IST group (p=0.002), and UCBI+IST treatment facilitated increasing trends in absolute neutrophil count (ANC) response (p=0.056). Kaplan-Meier curves illuminated UCBI+IST achieved faster ANC response (p<0.001) and platelet response (p<0.001), compared with IST therapy. There was no difference in overall survival (OS) between the two groups (p=0.620). Furthermore, logistic regression analysis demonstrated that UCBI+IST was an independent predicting factor for both CR (p=0.001) and ORR (p<0.001), compared to IST; meanwhile, very severe aplastic anemia (VSAA) and ANC could predict clinical responses as well. However, Cox proportional hazard regression indicated that VSAA (p=0.003), but not UCBI+IST, affected OS. Safety profiles showed that UCBI+IST therapy did not elevate adverse events, compared with IST treatment.
Conclusion
UCBI+IST achieved better clinical responses and hematopoietic recovery than IST, and was well tolerated in SAA patients.
Keywords: Effect, predictive factors, immunosuppressive therapy (IST), umbilical cord blood infusion (UCBI), severe aplastic anemia (SAA)
INTRODUCTION
Aplastic anemia (AA), characterized by hemorrhages, anemia, and infections, is a prototype of bone marrow failure caused by destruction of hematopoietic progenitor or stem cells.1,2 According to the severity of marrow failure and clinical course, AA is categorized into non-severe AA and severe aplastic anemia (SAA). Despite much improvement in the diagnosis and treatment of AA, which has increased treatment response and prolonged long-term survival, SAA is still a life-threatening disease due to its sudden onset, rapid disease progression, and lack of efficacy for conventional treatments.3,4,5
Accumulating evidence suggests that bone marrow transplantation (BMT) is the first option of treatment for SAA patients at most medical institutions, although only a small quantity of patients has human leukocyte antigen (HLA)-matched sibling donors, limiting its application.5,6 Nowadays, immunosuppressive therapy (IST), usually referred to as antithymocyte globulin (ATG) combined with cyclosporine A (CsA), is primarily used for pharmacotherapy of SAA, facilitating a hematologic recovery in 60–80% of untreated SAA patients.6,7 Nevertheless, 20–40% patients still present with a sufficient response to initial courses of IST therapy; almost 40% patients relapse.1,8,9 As a result, more novel and effective treatment options are needed.
Umbilical cord blood (UCB), as first adopted in the treatment of Fanconi anemia in 1989, has been widely used in various hematological diseases. UCB contains abundant hematopoietic stem cells (HSCs), providing durable engraftment and offering the patients without matched sibling donor an alternative stem cell source.10,11,12 As early as 1994, Auerbach13 improved the application of UCB on Fanconi anemia disease. In other research, transplantation derived from unrelated UCB has achieved favorable effects in acute leukemia, and is likely to decrease the risk of graft-versus-host disease (GVHD), compared to adult bone marrow or peripheral blood stem cells.14 Hence, we presumed that UCB would also offer benefits in SAA patients. Indeed, a recent study with 16 treatment-naïve SAA patients indicated that treatment combining UCB infusion (UCBI) and IST is effective and well tolerated.2 However, the sample size was small and only treatment-naïve patients were recruited, thereby limiting the drawing of conclusion on the clinical efficacy and safety of UCBI treatment in SAA patients. Thus, this study enrolled 93 SAA patients, and aimed to investigate the efficacy and safety of UCBI plus IST treatment in comparison to IST treatment, as well as predictive factors for clinical response, in SAA patients.
MATERIALS AND METHODS
Participants
A total of 93 patients with SAA on admission at the Department of Hematology in Taihe Hospital Affiliated to Hubei University of Medicine between August 2008 and January 2012, were consecutively enrolled in this cohort study. The inclusion criteria were 1) diagnosis with SAA according to Camitta criteria,15 2) starting or about to receive IST with or without UCBI, 3) age above 2 years, and 4) weight more than 12 kg. All 93 enrolled patients lacked HLA-matched bone marrow donors, and they were treated by UCBI+IST or IST therapy according to their actual conditions. Patients who had matched donors and were about to undergo BMT were not included in this study. Patients complicated with Fanconi anemia, heart failure, serious cardiac arrhythmias, coronary artery disease, or human immunodeficiency virus positive were excluded from this study. Pregnant or breast feeding women and cognitive impairment patients were excluded as well.
This study was approved by Ethics Committee of Taihe Hospital Affiliated to Hubei University of Medicine, and all patients signed informed consents. This study was conducted in line with the Declaration of Helsinki.
Treatments
Overall, 52 patients chose to be treated by IST, while 41 patients chose to be treated by UCBI+IST. This study was a cohort study without any interventions, and in total, 93 SAA patients received one of the two options according to their actual conditions or willingness. Thus, we divided them into UCBI+IST and IST groups accordingly. In the UCBI+IST group, patients were treated by 2.5 mg/kg/day of rabbit ATG (r-ATG) from −5 to −1 day and 3–5 mg/kg/day of CsA from −1 day. The latter was continued to 2 years. At day 0 UCB was transfused. In the IST group, patients were treated with 2.5 mg/kg/day of r-ATG from day 1 to day 5 combined with 3–5 mg/kg/day of CsA from day 5 after enrollment. Patients in the IST group also received CsA for 2 years, and concentrations of CsA were monitored regularly in order to readjust the dose to maintain the trough level. Most patients were unavailable for BMT from matched donors due to high cost or lack of a matched donor, and patients who had matched donors and were about to undergo BMT were not included in this study. UCB used in this study was obtained from Hubei Cord Blood Bank.
The following complicated treatments were performed in the meantime: 1) All patients were set in laminar flow beds before and during the initiation of IST to prevent infection. 2) 0.5 mg–1.0 mg/kg of promethazine was injected intramuscularly in patients pre-ATG treatment and 1–2 mg/kg of glucocorticoids was given to prevent allergic reaction (with the dose tapering after 5 days of treatment). 3) Condensed erythrocyte infusion was performed when hemoglobin B (Hb) <60 g/L, and platelet infusion was performed when platelet <10×109/L or platelet >10×109/L combined with the occurrence of bleeding or symptoms of high risk of intracranial hemorrhage. 4) Patients were treated by leukocytes elevating therapy on the condition of absolute neutrophil count (ANC) <1.5×109/L.
Definitions, assessments and follow ups
SAA was diagnosed by Camitta criteria.15 This comprised a bone marrow cellularity less than 30 percent (excluding lymphocytes) and at least two of the following: ANC less than 0.5×109 cell/L, platelet count less than 20×109 cell/L, and absolute reticulocyte count less than 20×109 cell/L. While very severe aplastic anemia (VSAA) was defined as patients diagnosed as SAA with a ANC less than 0.2×109/L.16
The efficacy of treatment in our study was evaluated according to international Camitta criteria:17 1) complete response (CR) (ANC>1.5×109/L, Hb>110 g/L, and platelet count ≥150×109/L), 2) partial response (PR) (no longer meeting the criteria for SAA with no transfusion dependence for platelets or red blood cells), and 3) no remission (NR) (CR or PR not achieved). Overall response rate (ORR) was defined as the rate of CR+PR. CR, PR, ORR, and NR were assessed at 1, 3, and 6 months post treatment initiation.
The time to hematopoietic recovery was also evaluated, including ANC response (defined as ANC >0.5×109/L at least three consecutive days), platelet response (platelet count >20×109/L), and Hb response (Hb >80 g/L at least three consecutive days). Chimerism was not included in this study. Overall survival (OS) of patients was calculated from the day starting treatment to the time of death by any cause or last-follow up. All patients were followed up for 5 years post initiation of treatment in this study by telephone or clinic visits; the last follow-up date was January 2017. Patients were regularly followed up every month (3–5 weeks) for the first six months, while most patients lived in other areas and returned to residences after 6 months. Thus, telephone call reviews were adopted to continue the follow up in the remaining 4.5 years. Safety profiles during the study duration were evaluated at 6 months after treatment.
Statistics
SPSS software (ver. 22.0, IBM Corp., Armonk, NY, USA) and Microsoft Office software 2010 (IBM Corp.) were used for statistical analysis. Data are presented as means±standard deviations, medians (interquartile range), or counts (percentages). Differences between groups was detected by t test, Wilcoxon rank sum test, or chi-square test. Kaplan-Meier (K-M) curve and log-rank test were performed to evaluate the time achieving hematopoietic recovery and OS of patients between groups. Univariate logistic regression was performed to investigate the factors affecting CR or ORR achievement, and all factors with a p value below 0.1 were further analyzed by multivariate logistic regression model. Univariate Cox proportional hazard regression was conducted to assess the factors affecting OS, while all factors with a p value below 0.1 were further analyzed by multivariate analysis. p<0.05 was considered significant.
RESULTS
Baseline characteristics
Baseline characteristics of the patients are presented in Table 1. No differences were found in age (p=0.712) and gender (p=0.883) between the two groups. There were 14 males and 27 females in UCBI+IST group, with a mean age of 29.95±13.11 years (range: 9–51 years), and 17 males and 35 females in the IST group, with mean age of 28.77±16.79 years (range: 5–60 years). Interval from first diagnosis to IST treatment was markedly longer in the UCBI+IST group than in the IST group (p<0.001). Body mass index and platelet counts in the UCBI+IST group were both numerically lower than those in IST group, although differences were not statistically significant (p=0.095 and p=0.068). No differences were found in other baseline characteristics between the two groups.
Table 1. Baseline Characteristics.
| Parameters | IST group (n=52) | UCBI+IST group (n=41) | p value |
|---|---|---|---|
| Age (yr) | 28.77±16.79 (range: 5–60) | 29.95±13.11 (range: 9–51) | 0.712 |
| Gender (male/female) | 17/35 | 14/27 | 0.883 |
| BMI (kg/m2) | 22.28±2.04 | 21.59±1.86 | 0.095 |
| Interval from first diagnosis to IST (month) | 38.0 (20.0–50.8) | 60.0 (38.0–151.0) | <0.001 |
| Etiology | 0.850 | ||
| Idiopathic | 3 (6) | 2 (5) | |
| Other | 49 (94) | 39 (95) | |
| Severity of disease | 0.667 | ||
| VSAA | 12 (23) | 11 (27) | |
| SAA | 40 (77) | 30 (73) | |
| ANC (×109 cell/L) | 0.309 (0.202–0.431) | 0.293 (0.194–0.426) | 0.792 |
| ANC ≤0.5×109 cell/L | 48 (92) | 33 (80) | |
| ANC >0.5×109 cell/L | 4 (8) | 8 (20) | |
| Platelet (×109 cell/L) | 14.92 (10.29–23.40) | 12.66 (5.81–18.76) | 0.068 |
| Platelet ≤20×109 cell/L | 35 (67) | 35 (85) | |
| Platelet >20×109 cell/L | 17 (33) | 6 (15) | |
| Reticulocyte (×109 cell/L) | 19.29 (11.60–26.06) | 17.57 (10.97–26.73) | 0.775 |
| Reticulocyte ≤20×109 cell/L | 30 (58) | 27 (66) | |
| Reticulocyte >20×109 cell/L | 22 (42) | 14 (34) | |
| Hemoglobin (g/dL) | 59.98 (48.88–77.99) | 66.65 (50.35–83.83) | 0.279 |
| Hemoglobin ≤80 g/dL | 41 (79) | 30 (73) | |
| Hemoglobin >80 g/dL | 11 (21) | 11 (27) | |
| HLA match | - | ||
| 4/6 | - | 12 (29) | |
| 5/6 | - | 15 (37) | |
| 6/6 | - | 14 (34) | |
| Cell dose | - | ||
| TNC (107/kg) | - | 2.394±0.710 | |
| CD34+ (105/kg) | - | 0.570±0.308 |
IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count; HLA, human leukocyte antigen; TNC, total nuclear cell.
Data are presented as means±standard deviations, medians (interquartile range), or counts (percentages). Comparison between two groups were conducted by t test, Wilcoxon rank sum test, or chi-square test. p<0.05 was considered significant.
Clinical efficacy by UCBI+IST and IST treatment
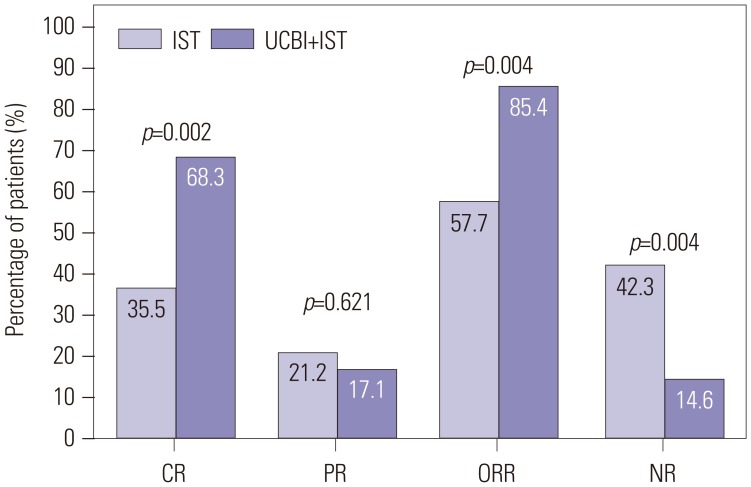
As disclosed in Fig. 1, after 6 months of treatment, the UCBI+IST group achieved a higher CR rate (68.3%) than that in the IST group (36.5%, p=0.002), as well as an elevated ORR (85.4% vs. 57.7%, p=0.004), indicating that UCBI+IST treatment was more effective than IST treatment. Clinical responses to UCBI+IST and IST at 1 month (CR: 4.9% vs. 0.0%, p=0.107; ORR: 34.1% vs. 7.7%, p=0.001) and 3 months (CR: 34.1% vs. 11.5%, p=0.008; ORR: 68.3% vs. 34.6%, p=0.001) after treatment are shown in Supplementary Fig. 1 (only online).
Fig. 1. Comparison of treatment responses between UCBI+IST and IST treatments at 6 months. Both CR and ORR were higher in the UCBI+IST group than the IST group. Differences between groups were detected by chi-square test. p<0.05 was considered significant. IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; ORR, overall response rate; CR, complete response; PR, partial response; NR, no remission.
Hematopoietic recovery rate in UCBI+IST and IST groups
There were 33, 35, and 30 patients in the UCBI+IST group with ANC ≤0.5×109 cell/L, platelet ≤20×109 cell/L, and hemoglobin ≤80 g/dL at baseline, respectively, while the numbers were 48, 35, and 41 in the IST group. As shown in Table 2, after 6 months of treatment, platelet response rate in the UCBI+IST group (86%) was higher than in the IST group (51%, p=0.002). Meanwhile, UCBI+IST treatment achieved an increased trend in ANC responses, compared to IST therapy, although without significance (100% vs. 90%, p=0.056). No significant difference was observed in hemoglobin responses between the UCBI+IST and IST groups (83% vs. 68%, p=0.150).
Table 2. Hematopoietic Recovery Rate in IST and UCBI+IST Groups.
| Parameters | IST group | UCBI+IST group | p value |
|---|---|---|---|
| ANC response | 43/48 (90) | 33/33 (100) | 0.056 |
| Platelet response | 18/35 (51) | 30/35 (86) | 0.002 |
| Hemoglobin response | 28/41 (68) | 25/30 (83) | 0.150 |
IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; ANC, absolute neutrophil count.
Data are presented as counts (percentages). Comparison between two groups were conducted by the chi-square test. p<0.05 was considered significant.
Time to hematopoietic recovery in UCBI+IST and IST groups
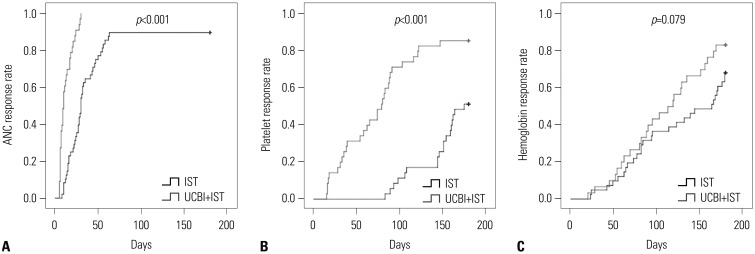
In order to evaluate the differences in time to hematopoietic recovery between the UCBI+IST and IST groups, K-M curves and log-rank test were performed, which indicated that UCBI+IST achieved faster ANC responses (p<0.001) (Fig. 2A) and platelet responses (p<0.001) (Fig. 2B), compared with IST treatment. While hemoglobin response was only observed to be numerically achieved more rapidly in the UCBI+IST than in the IST group, the difference lacked significance (p=0.079) (Fig. 2C).
Fig. 2. K-M curves analysis for days to ANC, platelet, and hemoglobin responses in the UCBI+IST and IST groups. K-M curves revealed that patients in the UCBI+IST group achieved faster ANC (A) and platelet (B) responses, compared to the IST group. While no difference was discovered in hemoglobin response (C). Differences between groups were detected by K-M curves and log-rank test. p<0.05 was considered significant. K-M, Kaplan-Meier; ANC, absolute neutrophil count; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion.
OS analysis between UCBI+IST and IST treatment
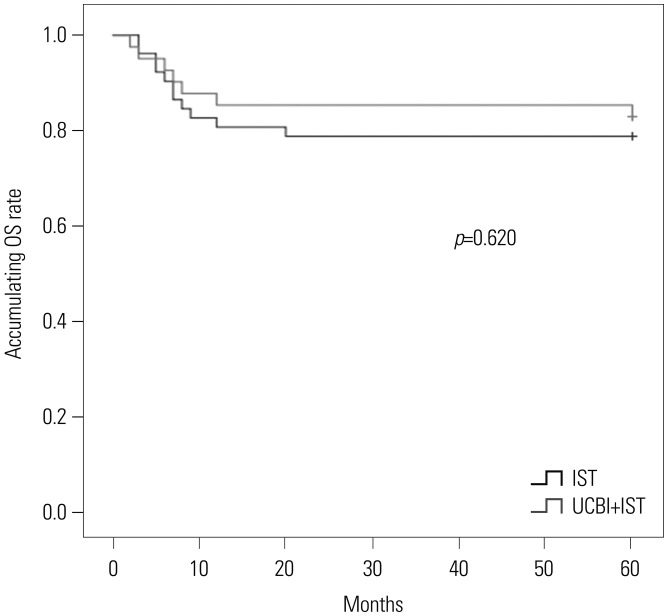
UCBI+IST realized a 5-year survival rate of 82.9%, compared to 78.8% in the IST group. K-M curves were drawn to analyze differences in OS for UCBI+IST treatment and IST treatment, although no difference was discovered, as presented in Fig. 3 (p=0.620).
Fig. 3. Accumulating OS rates of patients in UCBI+IST and IST groups. No difference in accumulating OS rates between the two groups was found. Differences between groups were detected by Kaplan-Meier curve and log-rank test. p<0.05 was considered significant. OS, overall survival; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion.
Analysis of factors affecting CR
Factors affecting CR were assessed by univariate logistic regression (Table 3), which indicated that UCBI+IST treatment achieved a higher CR than IST treatment (p=0.003). VSAA and ANC were also predictive factors predicting CR (p=0.001 and p=0.010). All factors with a p value <0.1 were subsequently analyzed by a multivariate model, which indicated that UCBI+IST treatment is an independent factor for predicting better CR, compared with IST treatment (p=0.001). Moreover, VSAA was found to predict worse CR independently (p=0.004).
Table 3. Analysis of Factors Affecting CR.
| Parameters | Univariate logistic regression | Multivariate logistic regression | ||||||
|---|---|---|---|---|---|---|---|---|
| p value | OR | 95% CI | p value | OR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| UCBI+IST (vs. IST) | 0.003 | 3.741 | 1.572 | 8.900 | 0.001 | 5.626 | 1.982 | 15.969 |
| Age (yr) | 0.715 | 0.995 | 0.969 | 1.022 | - | - | - | - |
| Gender (female) | 0.769 | 1.138 | 0.480 | 2.696 | - | - | - | - |
| BMI (kg/m2) | 0.264 | 0.887 | 0.720 | 1.094 | - | - | - | - |
| Interval from first diagnosis to IST (month) | 0.250 | 0.995 | 0.987 | 1.003 | - | - | - | - |
| Etiology (Idiopathic) | 0.665 | 1.500 | 0.239 | 9.420 | - | - | - | - |
| VSAA (vs. SAA) | 0.001 | 0.132 | 0.041 | 0.431 | 0.004 | 0.118 | 0.027 | 0.512 |
| ANC (×109 cell/L) | 0.010 | 16.974 | 1.972 | 146.097 | 0.345 | 2.954 | 0.312 | 27.976 |
| Platelet (×109 cell/L) | 0.234 | 1.023 | 0.986 | 1.062 | - | - | - | - |
| Reticulocyte (×109 cell/L) | 0.522 | 0.991 | 0.963 | 1.020 | - | - | - | - |
| Hemoglobin (g/L) | 0.940 | 1.001 | 0.980 | 1.023 | - | - | - | - |
CR, complete response; CI, confidence interval; OR, odds ratio; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count.
Univariate logistic regression was performed to analyze the factors affecting CR achievement, while factors with p value <0.1 was subsequently analyzed by multivariate model. p value <0.05 was considered significant.
Analysis of factors affecting ORR
As for the factors affecting ORR, univariate logistic regression results in Table 4 showed that UCBI+IST treatment is a predictive factor for achieving ORR (p=0.005), while VSAA was a predictive factor for absence of ORR (p=0.002). All factors with a p value <0.1 was subsequently analyzed by multivariate model. UCBI+IST was demonstrated to be an independent factor, which endowed the patients with more of a possibility to obtain ORR (p<0.001), as did platelet counts (p=0.012). Meanwhile VSAA was identified as an independent factor related to a lower possibility of ORR (p=0.001).
Table 4. Analysis of Factors Affecting ORR.
| Parameters | Univariate logistic regression | Multivariate logistic regression | ||||||
|---|---|---|---|---|---|---|---|---|
| p value | OR | 95% CI | p value | OR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| UCBI+IST (vs. IST) | 0.005 | 4.278 | 1.533 | 11.934 | <0.001 | 10.634 | 2.870 | 39.405 |
| Age (yr) | 0.254 | 0.983 | 0.955 | 1.012 | - | - | - | - |
| Gender (female) | 0.749 | 1.164 | 0.459 | 2.955 | - | - | - | - |
| BMI (kg/m2) | 0.237 | 0.871 | 0.693 | 1.095 | - | - | - | - |
| Interval from first diagnosis to IST (month) | 0.755 | 0.999 | 0.990 | 1.007 | - | - | - | - |
| Etiology (Idiopathic) | 0.617 | 1.770 | 0.189 | 16.592 | - | - | - | - |
| VSAA (vs. SAA) | 0.002 | 0.210 | 0.077 | 0.572 | 0.001 | 0.108 | 0.029 | 0.405 |
| ANC (×109 cell/L) | 0.194 | 4.006 | 0.494 | 32.510 | - | - | - | - |
| Platelet (×109 cell/L) | 0.053 | 1.055 | 0.999 | 1.114 | 0.012 | 1.114 | 1.024 | 1.211 |
| Reticulocyte (×109 cell/L) | 0.550 | 0.991 | 0.963 | 1.021 | - | - | - | - |
| Hemoglobin (g/L) | 0.112 | 1.018 | 0.996 | 1.040 | - | - | - | - |
ORR, overall response rate; CI, confidence interval; OR, odds ratio; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count.
Univariate logistic regression was performed to analyze the factors affecting ORR achievement, while factors with p value <0.1 was subsequently analyzed by multivariate model. in vivo value <0.05 was considered significant.
Analysis of factors affecting OS
In the univariate Cox proportional hazards regression analysis, as shown in Table 5, UCBI+IST treatment did not affect OS, compared with IST treatment (p=0.623). Patients with VSAA were associated with worse OS (p=0.005). Factors with a p value <0.1 was subsequently analyzed by multivariate model, and VSAA was proven to be an independent predicting factor for worse OS (p=0.003).
Table 5. Analysis of Factors Affecting OS.
| Parameters | Univariate Cox regression | Multivariate logistic regression | ||||||
|---|---|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| UCBI+IST (vs. IST) | 0.623 | 0.789 | 0.306 | 2.034 | - | - | - | - |
| Age (yr) | 0.699 | 1.006 | 0.977 | 1.036 | - | - | - | - |
| Gender (female) | 0.630 | 0.792 | 0.307 | 2.044 | - | - | - | - |
| BMI (kg/m2) | 0.127 | 1.203 | 0.949 | 1.526 | - | - | - | - |
| Interval from first diagnosis to intense-IST (month) | 0.316 | 1.004 | 0.996 | 1.012 | - | - | - | - |
| Etiology (Idiopathic) | 0.485 | 0.045 | 0.000 | 267.668 | - | - | - | - |
| VSAA (vs. SAA) | 0.005 | 3.804 | 1.507 | 9.601 | 0.003 | 4.118 | 1.604 | 10.573 |
| ANC (×109 cell/L) | 0.489 | 0.468 | 0.054 | 4.021 | - | - | - | - |
| Platelet (×109 cell/L) | 0.086 | 0.947 | 0.890 | 1.008 | 0.135 | 0.953 | 0.895 | 1.015 |
| Reticulocyte (×109 cell/L) | 0.800 | 1.004 | 0.975 | 1.033 | - | - | - | - |
| Hemoglobin (g/L) | 0.672 | 0.995 | 0.971 | 1.019 | - | - | - | - |
OS, overall survival; CI, confidence interval; HR, hazard ratio; IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; BMI, body mass index; VSAA, very severe aplastic anemia; SAA, severe aplastic anemia; ANC, absolute neutrophil count.
Univariate Cox proportional hazards regression was performed to analyze the factors affecting OS, while factors with p value <0.1 were subsequently analyzed by a multivariate model. p value <0.05 was considered significant.
Safety profiles of IST and UCBI+IST treatment
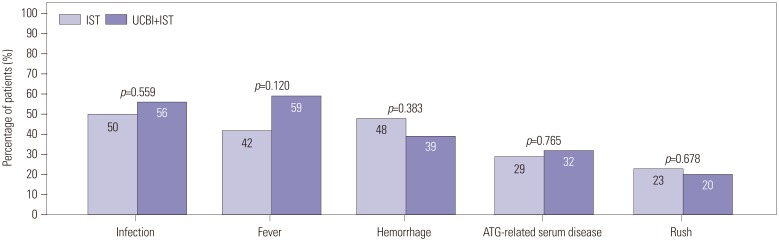
Safety profiles over the study duration were evaluated at 6 months after treatment, and adverse events, including infection, fever, hemorrhage, ATG-related serum disease, and rush, were all recorded and presented in Fig. 4. We found no difference in infection (50% vs. 56%, p=0.559), fever (42% vs. 59%, p=0.120), hemorrhage (48% vs. 39%, p=0.383), ATG-related serum disease (29% vs. 32%, p=0.765), or rush (23% vs. 20%, p=0.678) between IST and UCBI+IST groups, indicating that the UCBI+IST therapy did not elevate adverse events, compared with IST treatment.
Fig. 4. Comparison of adverse events between IST and UCBI+IST treatments. No differences were observed in infection, fever, hemorrhage, ATG-related serum diseases, and rush between IST and UCBI+IST groups. Differences between groups were evaluated by chi-square test. p<0.05 was considered significant. IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; ATG, antithymocyte globulin.
DISCUSSION
In our study, UCBI+IST treatment achieved a higher clinical response rate and realized a better hematopoietic recovery than IST treatment without elevated adverse effects. Meanwhile, logistic regression indicated that UCBI+IST (vs. IST) was an independent predictive factor for both higher CR and ORR.
IST, widely applied in various diseases, including kidney disease, inflammatory disease, etc., is commonly used in SAA treatment, especially for patients without suitable HLA donors. Although IST has been demonstrated to achieve a moderate clinical response rate, ranging from 60% to 80%, the treatment is still far from satisfactory due to its delayed acting, adverse effects, and high relapse rate.1,8,9 Hence, novel treating options are greatly needed to improve prognosis in SAA patients, especially for patients lacking efficacy or intolerance to IST.
UCB, originating from residual blood in the umbilical cord of newborns, has been extensively used in hematologic diseases, such as acute leukemia, Fanconi anemia, and thalassemia profiting from its following features:18,19,20 1) UCB consists of abundant HSCs that could generate healthy blood cells, contributing to reconstructing new hematopoietic systems.6 2) UCB brings plentiful hematopoietic factors, such as Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF), that could stimulate proliferation and differentiation of hematopoietic progenitors, consequently promoting hematopoiesis.21,22,23,24 3) Colony forming unit-fibroblastic (CFU-F) in UCB, which plays a critical role in hematopoietic microenvironment, improves the recovery of hematopoiesis.25,26,27 4) HSCs from UCB are characterized by weak antigenicity that decreases the risk of GVHD.28 These findings confirm the utility of UCB in various hematologic diseases, and considering the pancytopenia syndrome as the key property of SAA, we hypothesized that UCB could realize a good efficacy in treating SAA patients.
Recently, some pilot studies have explored the effect of UCBI in SAA treatment. A small-sample pilot study including 16 SAA patients receiving UCBI+IST treatment revealed that 65% patients achieve CR after 12-month treatment.2 Another cohort study enrolled 36 pediatric SAA patients treated by UCBI+IST, while a historic cohort of 20 SAA patients treated by IST served as a control. The study disclosed that UCBI+IST increases ORR at 3, 6, 9, and 12 months, compared to IST (61.1, 76.7, 80.5, and 86.2% in UCBI+IST group, and 20, 25, 45, and 55% in IST group, respectively).3 Another cohort study recruited relative larger samples, with 62 SAA patients divided into UCBI+IST and non-subsampled contourlet transform (NSCT) groups, and showed UCBI+IST reached an equal ORR (69%), compared to NSCT (80%), after 6 months of therapy.1 These indicated that UCBI+IST is effective in SAA treatment. As to our study, we observed UCBI+IST achieves elevated CR and ORR rates, compared to IST treatment, which is consistent with a previous study.3 These results might have resulted from: 1) Although IST decreases abnormal immunity, the quantity of normal autologous HSCs in SAA patients was too small to recover the hematopoietic function effectively, and the UCBI provides abundant HSCs that improved this situation, thus leading to better treatment response.29,30 2) Immunology is not the only cause for SAA, thus some patients would lack efficacy with immunosuppressants.31,32 3) UCBI offers a large amount of G-CSF, GM-CSF, and CFU-F to increase the recovery of SAA.21,22,23,24,25,26,27 It was noteworthy that intervals from first diagnosis to IST at baseline was longer in the UCBI+IST group than the IST group. The detailed reasons were as follows. Firstly, the interval from diagnosis to IST referred to the first diagnosis of AA. Most patients in our study had been diagnosed as AA in local hospitals with less experience with AA, while they developed SAA and were recruited in our study. Thus, the interval from the first diagnosis to IST might be much different among total patients. Secondly, this was a cohort study without any interventions, and patients received UCBI+IST or IST according to their actual conditions. We inferred that patients who experienced longer disease course might have severe disease conditions, and tended to receive UCBI+IST therapy. Thus, patients in the UCBI+IST group showed a longer interval from diagnosis to IST treatment at baseline. Although there was a difference in the interval from diagnosis to IST at baseline between the two groups, logistic regression was performed to assess the influence of this difference, which verified that the interval from first diagnosis to IST did not affect CR or ORR achievement, as well as OS. Moreover, regarding GVHD, it is a complication that happens in patients with graft, thus, GVHD could be observed only in the UCBI+IST group, whose comparison between two groups is not available. Meanwhile, the purpose of our study was to compare efficacy of UCBI+IST and IST in SAA patients; therefore, we did not perform analysis of GVHD. Cyclophosphamide, which is used in the conditioning followed by UCBI in similar previous studies, is not recommended by Chinese Guidelines for the Diagnosis and Management of Aplastic Anemia due to its high fatality rate and severe adverse events.33 According to a previous study, which used a combination of ATG, CsA, danazol, and G-CSF as the conditioning for subsequent UCB transplantation in Japanese SAA patients, cyclophosphamide is also excluded due to its cardiotoxicity.34 Hence, cyclophosphamide was not used in our study.
As to hematopoietic recovery time of UCBI+IST treatment, a median day of 50 days and 57 days were found in platelet and hemoglobin recovery in the study by Yu, et al.,1 while Li, et al.2 showed a median platelet recovery time for UCBI+IST of 37 days and a ANC recovery time of 23 days. In our study, a better and faster recovery time was observed for ANC and platelet counts in the UCBI+IST group, compared with the IST group. These might be on account of the following aspects: 1) UCB contains HSCs and hematopoietic factors, such as G-CSF and GM-CSF, the latter activate the production of blood cells and thus contribute to the recovery of hematopoiesis. 2) UCB provides CFU-F, which does not stimulate cells directly, but offers a suitable hematopoietic microenvironment for hematopoietic system reconstruction. 3) Better responses for ANC and platelet were observed in the UCBI+IST group than the IST group, while no superior response in Hb was observed. The reason for no superior response of Hb in the UCBI+IST group might be that IST contributes to the rapid resumption of erythropoietin, which promotes erythropoiesis, and thus leads to a remarkable increase of Hb.35 Therefore, IST therapy plays a critical role in improving Hb level, and no difference in Hb recovery between the UCBI+IST group and IST group was found. Additionally, we found no differences in infection, fever, hemorrhage, ATG-related serum disease, and rush between UCBI+IST treatment and IST treatment, which indicated UCBI+IST is well tolerated in SAA patients.
Our study had some limitations. 1) Interval from first diagnosis to IST treatment showed a marked difference between groups; however, we conducted multivariate logistic regression to minimize its influence. 2) Sample size was relatively small even though it was much larger than that in previous studies. 3) This study was conducted as a cohort study without randomization, in which some compounding factors might exist. 4) Detailed information on relapse, response, safety, or treatment-related immunologic complications after 6 months was not provided in this study. The reasons for this were as follows: Firstly, considering the uncontrollability of follow up in the prospective study, we set 6 months after treatment as the primary endpoint, thus follow up in this study was regularly performed every month in each patient during the first 6 months. Meanwhile, follow up in the remaining 4.5 years was not standardized. Secondly, it was difficult to request all patients to return to our hospital after 6 months because most patients were from different areas and they came back to their residences after 6 months. Although we continued to perform follow up by telephone in the remaining 4.5 years, detailed information after 6 months was still not available to collect due to missed calls or calls that were not answered by patients themselves. Moreover, events of relapse or treatment-related complications were not evaluated in local. Furthermore, we have tried again to communicate with patients to collect more follow-up information, while limited information on response rate or hematopoietic recovery were obtained from the patients' answers. Therefore, the long-term information about relapse, response, safety, or treatment-related immunologic complications could not be provided. According to a previous study, which evaluated the efficacy of UCBI+IST and IST therapies at 3, 6, 9, and 12 months in SAA patients with small sample size (n=36), UCBI+IST therapy showed superior efficacy at 3 months after treatment, and this superiority for UCBI+IST was still observed in the remaining observation period, including 6, 9, and 12 months.3 Based on these results, although our study lacked comparison of efficacy and safety between two groups after 6 months, we inferred that the superiority of UCBI+IST in 6-month efficacy in our study would continue in prolonged observation. Hence, evaluations in our study were able to differentiate efficacy and safety between the two therapies. 5) This was a cohort study without any interventions, and all treatments, as well as examinations, were performed according to patients' actual conditions or their willingness. As to chimerism examination, it is not a routine examination in our hospital, and only nine of 93 patients finished chimerism examination. Thus, the data of chimerism was unavailable in our study. Moreover, according to previous studies, although SAA patients finish chimerism exanimation, failure to produce abundant blood cells still occurs, suggesting that response of blood cells might be more sufficient evidence of successful hematopoietic reconstruction.36,37 Therefore, despite analysis of chimerism data was not performed in our study, we finished the examination of blood cells responses, which also could assess the hematopoietic reconstruction.
In summary, this study indicated that UCBI+IST achieves better clinical responses and hematopoietic recovery, compared to IST, and is well tolerated in SAA patients.
Footnotes
The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIAL
Comparison of treatment responses between UCBI+IST and IST treatments at 1 month and 3 months. No difference in CR was observed between the two groups at 1 month; meanwhile, ORR was higher in the UCBI+IST group than the IST group (A). Both CR and ORR were increased in the UCBI+IST group, compared with the IST group, at 3 months (B). Differences between groups were detected by chi-square test. p<0.05 was considered significant. IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; ORR, overall response rate; CR, complete response; PR, partial response; NR, no remission.
References
- 1.Yu Z, Zhou F, Ge LF, Liu XM, Fang Y, Xie L, et al. High-dose immunosuppressive therapy combined with cord blood infusion and non-myeloablative peripheral blood stem cell transplantation for patients with severe aplastic anemia. Eur Rev Med Pharmacol Sci. 2013;17:2613–2618. [PubMed] [Google Scholar]
- 2.Li Y, Sheng Z, Niu S, Ge L, Ren C, Zou Y. Rapid and complete reconstitution of autologous haemopoiesis after cord blood infusion in treatment-naive patients with severe aplastic anemia receiving high-dose cyclophosphamide/ATG therapy. Eur J Haematol. 2013;90:45–50. doi: 10.1111/ejh.12033. [DOI] [PubMed] [Google Scholar]
- 3.Xie LN, Fang Y, Yu Z, Song NX, Kong FS, Liu XM, et al. Increased immunosuppressive treatment combined with unrelated umbilical cord blood infusion in children with severe aplastic anemia. Cell Immunol. 2014;289:150–154. doi: 10.1016/j.cellimm.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Kurre P, Johnson FL, Deeg HJ. Diagnosis and treatment of children with aplastic anemia. Pediatr Blood Cancer. 2005;45:770–780. doi: 10.1002/pbc.20322. [DOI] [PubMed] [Google Scholar]
- 5.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185–1196. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Füreder W, Valent P. Treatment of refractory or relapsed acquired aplastic anemia: review of established and experimental approaches. Leuk Lymphoma. 2011;52:1435–1445. doi: 10.3109/10428194.2011.568646. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky RA, Chen AR, Dorr D, Fuchs EJ, Huff CA, Luznik L, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136–2141. doi: 10.1182/blood-2009-06-225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori M, Terasawa T, Tsushita K, Utsumi M, Kawano F, Saito H, et al. The status of antithymocyte globulin therapy for adult patients in Japan: retrospective analysis of a nationwide survey. Int J Hematol. 2008;87:48–55. doi: 10.1007/s12185-007-0016-9. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 11.Mao P, Wang S, Wang S, Zhu Z, Liv Q, Xuv Y, et al. Umbilical cord blood transplant for adult patients with severe aplastic anemia using anti-lymphocyte globulin and cyclophosphamide as conditioning therapy. Bone Marrow Transplant. 2004;33:33–38. doi: 10.1038/sj.bmt.1704295. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal J, Woolfrey AE, Pawlowska A, Thomas SH, Appelbaum F, Forman S. Hematopoietic cell transplantation with autologous cord blood in patients with severe aplastic anemia: an opportunity to revisit the controversy regarding cord blood banking for private use. Pediatr Blood Cancer. 2011;56:1009–1012. doi: 10.1002/pbc.22970. [DOI] [PubMed] [Google Scholar]
- 13.Auerbach AD. Umbilical cord blood transplants for genetic disease: diagnostic and ethical issues in fetal studies. Blood Cells. 1994;20:303–309. [PubMed] [Google Scholar]
- 14.Eapen M, Rubinstein P, Zhang MJ, Camitta BM, Stevens C, Cairo MS, et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006;24:145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- 15.Camitta BM, Storb R, Thomas ED. Aplastic anemia (first of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645–652. doi: 10.1056/NEJM198203183061105. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177–182. doi: 10.1111/j.1365-2141.1988.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 17.Camitta BM. What is the definition of cure for aplastic anemia? Acta Haematol. 2000;103:16–18. doi: 10.1159/000040999. [DOI] [PubMed] [Google Scholar]
- 18.Ustun C, Giannotti F, Zhang MJ, Wang HL, Brunstein C, Labopin M, et al. Outcomes of UCB transplantation are comparable in FLT3+ AML: results of CIBMTR, EUROCORD and EBMT collaborative analysis. Leukemia. 2017;31:1408–1414. doi: 10.1038/leu.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raut S, Shah S, Shah K, Patel K, Talati S, Parikh S, et al. Improving outcome of thalassemia with hematopoetic stem cell transplantation: an experience of Gujarat Cancer Research Institute. Indian J Hematol Blood Transfus. 2016;32:284–291. doi: 10.1007/s12288-015-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacMillan ML, DeFor TE, Young JA, Dusenbery KE, Blazar BR, Slungaard A, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125:3798–3804. doi: 10.1182/blood-2015-02-626002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu LX, Cao YB, Liu ZY, Wu YM, Wang ZH, Yan B, et al. [Transplantation of haploidentical-hematopoietic stem cells combined with two kind of third part cells for chronic aplastic anemia: one case report] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:1522–1525. doi: 10.7534/j.issn.1009-2137.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Liu D. Related HLA-mismatched/haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: observations of a single Chinese center. Clin Transpl. 2011:237–245. [PubMed] [Google Scholar]
- 23.Baron F, Nagler A. Novel strategies for improving hematopoietic reconstruction after allogeneic hematopoietic stem cell transplantation or intensive chemotherapy. Expert Opin Biol Ther. 2017;17:163–174. doi: 10.1080/14712598.2017.1269167. [DOI] [PubMed] [Google Scholar]
- 24.Azuma H, Watanabe E, Otsuka Y, Negishi Y, Ohkura S, Shinya E, et al. Induction of langerin+ Langerhans cell-like cells expressing reduced TLR3 from CD34+ cord blood cells stimulated with GM-CSF, TGF-β1, and TNF-α. Biomed Res. 2016;37:271–281. doi: 10.2220/biomedres.37.271. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Chen XH, Si YJ, Li ZJ, Gao L, Gao L, et al. Reconstruction of hematopoietic inductive microenvironment after transplantation of VCAM-1-modified human umbilical cord blood stromal cells. PLoS One. 2012;7:e31741. doi: 10.1371/journal.pone.0031741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Yi L, Wang L, Chen L, Chen X, Wang Y. Ginsenoside Rg1 protects human umbilical cord blood-derived stromal cells against tert-Butyl hydroperoxide-induced apoptosis through Akt-FoxO3a-Bim signaling pathway. Mol Cell Biochem. 2016;421:75–87. doi: 10.1007/s11010-016-2786-y. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Wong WH, Chan S, Chim JC, Cheung KM, Lee TL, et al. Factors affecting mesenchymal stromal cells yield from bone marrow aspiration. Chin J Cancer Res. 2011;23:43–48. doi: 10.1007/s11670-011-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanz J, Arango M, Carpio N, Montesinos P, Moscardó F, Martín G, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single-center experience. Bone Marrow Transplant. 2014;49:1084–1088. doi: 10.1038/bmt.2014.107. [DOI] [PubMed] [Google Scholar]
- 29.Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015;101:527–535. doi: 10.1007/s12185-015-1787-z. [DOI] [PubMed] [Google Scholar]
- 30.Peffault de Latour R, Rocha V, Socié G. Cord blood transplantation in aplastic anemia. Bone Marrow Transplant. 2013;48:201–202. doi: 10.1038/bmt.2012.252. [DOI] [PubMed] [Google Scholar]
- 31.Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15:162–168. doi: 10.1097/MOH.0b013e3282fa7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA. 2010;304:1358–1364. doi: 10.1001/jama.2010.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Red Blood Cell Disease (Anemia) Group; Chinese Society of Hematology; Chinese Medical Association. Chinese expert consensus on the diagnosis and treatment of aplastic anemia (2017) Zhonghua Xue Ye Xue Za Zhi. 2017;38:1–5. doi: 10.3760/cma.j.issn.0253-2727.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohga S, Ichino K, Goto K, Hattori S, Nomura A, Takada H, et al. Unrelated donor cord blood transplantation for childhood severe aplastic anemia after a modified conditioning. Pediatr Transplant. 2006;10:497–500. doi: 10.1111/j.1399-3046.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- 35.Ruangkanchanasetr P, Srichaikul T, Supaporn T. Triple immunosuppressive therapy can accelerate the recovery of antibody-mediated pure red cell aplasia and allow successful concurrent resumption of erythropoietin. J Med Assoc Thai. 2012;95(Suppl 5):S92–S95. [PubMed] [Google Scholar]
- 36.Sun YQ, He GL, Chang YJ, Xu LP, Zhang XH, Han W, et al. The incidence, risk factors, and outcomes of primary poor graft function after unmanipulated haploidentical stem cell transplantation. Ann Hematol. 2015;94:1699–1705. doi: 10.1007/s00277-015-2440-x. [DOI] [PubMed] [Google Scholar]
- 37.Kong Y, Chang YJ, Wang YZ, Chen YH, Han W, Wang Y, et al. Association of an impaired bone marrow microenvironment with secondary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1465–1473. doi: 10.1016/j.bbmt.2013.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of treatment responses between UCBI+IST and IST treatments at 1 month and 3 months. No difference in CR was observed between the two groups at 1 month; meanwhile, ORR was higher in the UCBI+IST group than the IST group (A). Both CR and ORR were increased in the UCBI+IST group, compared with the IST group, at 3 months (B). Differences between groups were detected by chi-square test. p<0.05 was considered significant. IST, immunosuppressive therapy; UCBI, umbilical cord blood infusion; ORR, overall response rate; CR, complete response; PR, partial response; NR, no remission.