Abstract
Purpose
Androgen deprivation therapy (ADT) is used as a salvage treatment for men with biochemical recurrence (BCR) of prostate cancer (PCa) following initial radical prostatectomy (RP). The optimal time at which to begin salvage ADT (sADT) remains controversial. In this retrospective study, we evaluated the efficacy of initiating sADT in patients before prostate-specific antigen (PSA) values met the clinical definition of BCR.
Materials and Methods
We identified 484 PCa patients who received sADT for BCR after RP. Median follow-up was 82 months. Propensity score matching was performed based on preoperative PSA level, pathologic T stage, and Gleason score. Patients were assigned to two groups of 169 patients each, based on PSA levels at the time of sADT: Group A (without meeting of the definition of BCR) and Group B (after BCR). Kaplan-Meier survival analyses and Cox regression analyses were performed.
Results
The median PSA level at sADT initiation was 0.12 ng/mL in group A and 0.42 ng/mL in group B. Kaplan-Meier analyses showed that group A had favorable disease progression-free survival (DPFS) and distant metastasis-free survival (DMFS), but did not have better cancer-specific survival (CSS) than group B. In subgroup analyses, group A showed better CSS rates in the non-organ confined PCa group. In Cox regression analyses, early sADT was associated significantly with DPFS and DMFS rates, however, did not correlate with CSS (p=0.107).
Conclusion
Early sADT after RP improved DPFS and DMFS. Furthermore, early sADT patients demonstrated better CSS in non-organ confined PCa.
Keywords: Radical prostatectomy, androgen deprivation therapy, prostate specific antigen, salvage therapy
INTRODUCTION
Prostate cancer (PCa) is the most common, newly diagnosed cancer in men.1 Choice of therapy is based on risk stratification of the disease or the patient's morbidity. Androgen deprivation therapy (ADT) represents a cornerstone of treatment for PCa.2 The association between androgen suppression and tumor regression was first described by Huggins3 in 1942, and the efficacy and usage of ADT for various stages of PCa have since been extensively studied.
National Comprehensive Cancer Network (NCCN®) guidelines currently recommend ADT as the primary treatment for metastatic PCa.4 In addition, external irradiation combined with ADT in patients with locally advanced PCa improves both clinical disease-free and overall survival (OS), as compared to radiotherapy alone.5 Radical prostatectomy (RP) followed by immediate adjuvant ADT for lymph node-positive metastatic PCa also improves cancer-specific survival (CSS) and OS.6 The efficacy of ADT for the treatment of clinical localized PCa remains controversial. Primary ADT does not improve survival in cases of localized PCa7; however, adjuvant ADT improves CSS and systematic progression-free survival (PFS) after RP in node-positive PCa.8 ADT benefits patients with biochemical recurrence (BCR) or non-metastatic PCa recurrence after local treatment, especially in high-risk PCa patients.9 While early ADT delays biochemical and clinical disease progression,10 however, the optimal prostate-specific antigen (PSA) levels for initiation of ADT as a salvage treatment for BCR are not well defined.
In this retrospective study, we evaluated the impact of early salvage ADT (sADT) initiated in the presence of increasing PSA levels that did not yet meet the clinically-defined criteria for BCR. Our goal was to determine the optimal timing of ADT as a salvage treatment for BCR after RP to improve outcomes for these patients.
MATERIALS AND METHODS
Study population
After Institutional Review Board approval (4-2017-1206), we retrospectively reviewed medical records of 484 node-negative PCa (T1–T4, N0, and M0) patients who received sADT after RP, performed at Yonsei University Health System, from 1995 to 2014. Patients with clinical distant metastasis or lymph node metastasis observed by lymph node dissection, and those who received neo-adjuvant therapy were excluded from the study. Salvage treatment was defined as ADT or radiotherapy initiated following rising PSA levels after RP. We considered ADT or radiotherapy administered in the absence of rising PSA levels after RP as adjuvant therapy, and excluded these patients from our analysis. The type and timing of sADT administered following observation of rising PSA levels were determined by discretion of physicians. Clinical and pathologic variables assessed for this study included age, body mass index (BMI), PSA level, risk classification, type of sADT, PSA doubling time, and pathologic outcomes.
D'Amico risk classification for PCa was performed.11 PSA doubling time was calculated, based on PSA levels at the commencement of sADT, compared to the last PSA level recorded prior to sADT. Data regarding mortality and cause of death were obtained from the Yonsei Cancer Registry Center database at Severance Hospital. TNM stage was determined according to the American Joint Committee on Cancer, 8th edition.12
Statistical analyses
We divided patients into two groups based on PSA level at the initiation of sADT. Group A (early sADT) included patients administered sADT when PSA levels rose consecutively two or more times without meeting the clinical definition of BCR. BCR was defined as two consecutive increases in PSA levels >0.2 ng/mL after RP.13 Group B (delayed sADT) included patients administered sADT after standard BCR criteria were reached. Propensity score matching was performed to adjust for confounders (preoperative PSA level, pathologic T stage (pT), and pathologic Gleason score after RP). We compared clinical and pathologic parameters of each group before and after propensity score matching using Student-T tests and chi-square tests. Age, surgical margin status, type of ADT, and other parameters were compared in each group.
Kaplan-Meier survival analyses were performed to compare disease progression-free survival (DPFS), distant metastasis-free survival (DMFS), and CSS between treatment groups. Subgroups included patients divided by risk classification (low/intermediate vs. high-risk), Gleason score (<8 vs. ≥8), and T stage [organ confined (pT2) vs. non-organ confined (pT3 or 4)]. Disease progression was defined as any progression of disease confirmed by radiologic evaluations, and distant metastasis was defined as metastasis to bone, non-regional lymph nodes or other sites. Cancer-specific mortality (CSM) was defined as death caused by PCa or PCa-related complications. Univariable and multivariable Cox regression analyses were performed to identify associations between clinical parameters and survival outcomes. All statistical analyses were performed using SPSS Statistics software version 23.0 (IBM Corp., Armonk, NY, USA).
RESULTS
A total of 484 node-negative PCa patients who received sADT were evaluated for this study, and their characteristics are summarized in Table 1. Of these patients, 190 received early sADT, while 294 received delayed sADT. Median follow-up duration was 82 months. Significantly more patients with delayed sADT had high-risk PCa (45.3% vs. 64.3%, p<0.001) and higher PSA levels preoperatively (8.3 ng/mL vs. 11.8 ng/mL, p<0.001) compared to those with early sADT. Among delayed sADT patients, a pathologic Gleason Score ≥8 was observed more frequently than in patients with early sADT (18.9% vs. 32.7%, p=0.001).
Table 1. Baseline Patient Characteristics (n=484).
| Variable | Early sADT (n=190) | Delayed sADT (n=294) | p value |
|---|---|---|---|
| Age (yr) | 66 (60–71) | 67 (63–70) | 0.217 |
| BMI (kg/m2) | 23.9 (22.2–26.1) | 24.3 (22.6–25.8) | 0.387 |
| Prostate volume measure by TRUS (mL) | 28.5 (23.0–38.0) | 29.0 (24.0–39.0) | 0.461 |
| PSA (ng/mL) | 8.3 (5.9–13.1) | 11.8 (8.0–17.8) | <0.001 |
| PSA (categorical) | <0.001 | ||
| <20 | 174 (91.6) | 230 (78.2) | |
| ≥20 | 16 (8.4) | 64 (21.8) | |
| D'Amico risk classification | <0.001 | ||
| Low | 32 (16.8) | 22 (7.5) | |
| Intermediate | 72 (37.9) | 83 (28.2) | |
| High | 86 (45.3) | 189 (64.3) | |
| Pathologic T stage | 0.323 | ||
| Organ confined (T2) | 63 (33.2) | 85 (28.9) | |
| Non-organ confined (>T2) | 127 (66.8) | 209 (71.1) | |
| Pathologic Gleason score | 0.001 | ||
| <8 | 154 (81.1) | 198 (67.3) | |
| ≥8 | 36 (18.9) | 96 (32.7) | |
| Positive surgical margin | 125 (65.8) | 200 (68.0) | 0.609 |
| PSA at sADT (ng/mL) | 0.12 (0.11–0.15) | 0.50 (0.29–1.26) | 0.021 |
| Type of ADT | 0.073 | ||
| LHRH agonist or antagonist with anti-androgens | 104 (54.7) | 177 (60.2) | |
| LHRH agonist or antagonist only | 64 (33.7) | 72 (24.5) | |
| Anti-androgens only | 22 (11.6) | 45 (15.3) | |
| PSA doubling time at ADT (month) | 3.7 (1.9–7.5) | 3.5 (1.7–6.9) | 0.283 |
| Salvage radiotherapy | 29 (15.3) | 103 (35.0) | <0.001 |
| PSA >0.2 ng/mL after sADT | 47 (24.9) | 175 (59.9) | <0.001 |
BMI, body mass index; LHRH, luteinizing hormone-releasing hormone; PSA, prostate-specific antigen; sADT, salvage androgen deprivation therapy; TRUS, transrectal ultrasonography.
Data are expressed as median (interquartile range) or n (%).
Propensity score matching was used to adjust for confounding variables, resulting in 169 patients being assigned to two treatment groups (Groups A and B) based on PSA levels at the time of sADT. Basic characteristics of these two groups are displayed in Table 2. No statistical differences were observed in age, BMI, risk classification, and surgical margin status between groups. The median PSA level at the initiation of sADT was 0.12 ng/mL in group A and 0.42 ng/mL in group B (p=0.010). The majority of patients in both groups underwent similar treatment regimens which comprised of sADT with luteinizing hormone-releasing hormone (LHRH) agonist with or without anti-androgens (Group A 87.0%, Group B 83.4%). Salvage radiotherapy was performed more frequently in group B than in group A (15.4% vs. 35.5%, p<0.001). After sADT, group A demonstrated a significantly slower rate of PSA increase to levels above 0.2 ng/mL than did group B (26.8% vs. 57.1%, p<0.001). During the follow-up period, there were 17 cases of disease progression in group A and 49 cases in group B. Distant metastasis was reported in four cases in group A and 24 cases in group B. A total of 11 deaths from PCa were recorded. There was one death from PCa in group A and 10 deaths in group B.
Table 2. Baseline Patient Characteristics after Propensity Score Matching (n=338).
| Variable | Early sADT (n=169) | Delayed sADT (n=169) | p value |
|---|---|---|---|
| Age (yr) | 66 (60–71) | 66 (62–70) | 0.208 |
| BMI (kg/m2) | 23.9 (22.1–26.1) | 24.1 (22.4–25.8) | 0.262 |
| Prostate volume measure by TRUS (mL) | 28.0 (23.0–38.0) | 28.0 (23.0–35.0) | 0.558 |
| PSA (ng/mL) | 9.5 (6.2–14.0) | 9.3 (6.4–13.0) | 0.882 |
| PSA (categorical) (ng/mL) | 0.841 | ||
| <20 | 155 (91.7) | 156 (92.3) | |
| ≥20 | 14 (8.3) | 13 (7.7) | |
| D'Amico risk classification | 0.530 | ||
| Low | 23 (13.6) | 22 (13.0) | |
| Intermediate | 66 (39.1) | 57 (33.7) | |
| High | 80 (47.3) | 90 (53.3) | |
| Pathologic T stage | 0.817 | ||
| Organ confined (T2) | 55 (32.5) | 57 (33.7) | |
| Non-organ confined (>T2) | 114 (67.5) | 112 (66.3) | |
| Pathologic Gleason score | 0.790 | ||
| <8 | 134 (79.3) | 132 (78.1) | |
| ≥8 | 35 (20.7) | 37 (21.9) | |
| Positive surgical margin | 117 (69.2) | 111 (65.7) | 0.486 |
| PSA at sADT (ng/mL) | 0.12 (0.11–0.16) | 0.42 (0.27–1.10) | 0.010 |
| Type of ADT | 0.115 | ||
| LHRH agonist or antagonist with anti-androgens | 90 (53.3) | 101 (59.8) | |
| LHRH agonist or antagonist only | 57 (33.7) | 40 (23.7) | |
| Anti-androgens only | 22 (13.0) | 28 (16.6) | |
| PSA doubling time (month) | 3.4 (1.9–6.1) | 3.6 (1.7–7.9) | 0.572 |
| Salvage radiotherapy | 26 (15.4) | 60 (35.5) | <0.001 |
| PSA >0.2 ng/mL after sADT | 45 (26.8) | 96 (57.1) | <0.001 |
BMI, body mass index; LHRH, luteinizing hormone-releasing hormone; PSA, prostate-specific antigen; sADT, salvage androgen deprivation therapy; TRUS, transrectal ultrasonography.
Data are expressed as median (interquartile range) or n (%).
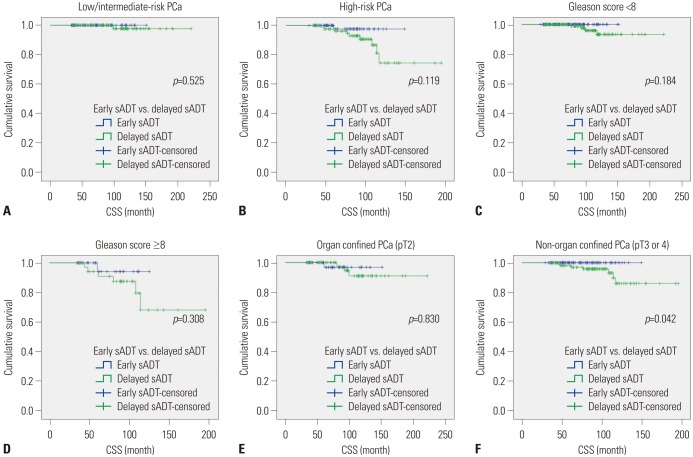
Kaplan-Meier analyses revealed that group A had a significantly more favorable DPFS than group B, with 7-year DPFS rates of 87.3% and 73.3% (p=0.001), respectively, as shown in Fig. 1. Group A also demonstrated a significantly higher 7-year DMFS than group B (96.1% vs. 88.7%, p=0.001) as displayed in Fig. 2. The 7-year CSS rates of groups A and B were not significantly different (98.9% vs. 96.3%, p=0.071) (Fig. 3); however, group A experienced fewer mortalities. In a subgroup analysis of patients with non-organ confined PCa, the early sADT group showed statistically favorable CSS compared to those receiving delayed sADT (p=0.042), as shown in Fig. 4. This was not observed in other subgroups.
Fig. 1. Kaplan-Meier curves of DPFS in early sADT group (Group A) and delayed sADT group (Group B). sADT, salvage androgen deprivation therapy. DPFS, disease progression-free survival.
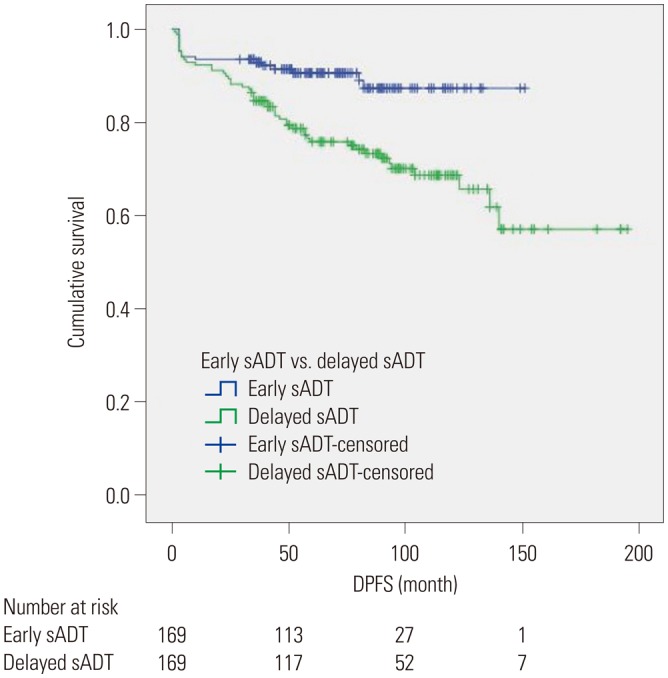
Fig. 2. Kaplan-Meier curves of DMFS in early sADT group (Group A) and delayed sADT group (Group B). sADT, salvage androgen deprivation therapy. DMFS, distant metastasis-free survival.
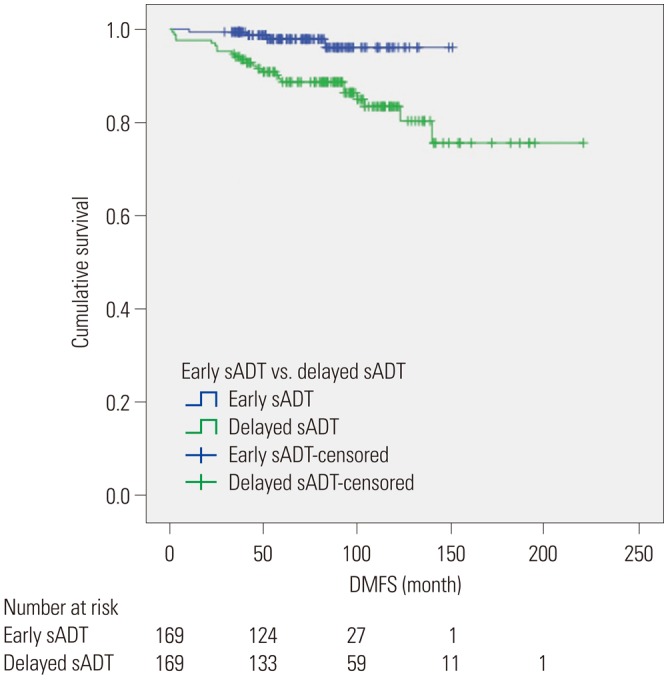
Fig. 3. Kaplan-Meier curves of CSS in early sADT group (Group A) and delayed sADT group (Group B). sADT, salvage androgen deprivation therapy. CSS, cancer-specific survival.
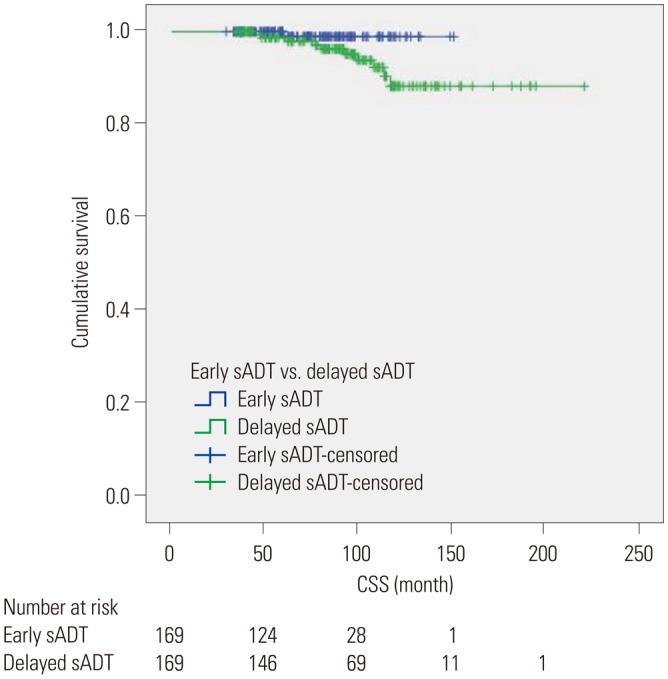
Fig. 4. Kaplan-Meier curves of CSS in subgroups of early sADT group (Group A) and delayed sADT group (Group B). (A) CSS in low/intermediate-risk PCa, (B) CSS in high-risk PCa, (C) CSS in Gleason score <8 PCa, (D) CSS in Gleason score ≥8 PCa, (E) CSS in organ confined PCa, and (F) CSS in non-organ confined PCa. CSS, cancer-specific survival; PCa, prostate cancer; sADT, salvage androgen deprivation therapy.
Univariable and multivariable Cox regression analyses revealed several factors that were significantly associated with DPFS (Table 3). These included high-risk PCa [hazard ratio (HR) 2.272, 95% confidential interval (CI) 1.359–3.799], salvage radiotherapy (HR 0.447, 95% CI 0.274–0.730), and early sADT (HR 0.457, 95% 0.260–0.801). The results of univariable and multivariable Cox regression analyses for predictive factors of DMFS are presented in Table 4. These predictive factors included pathologic Gleason score (HR 2.463, 95% CI 1.073–5.654), PSA doubling time (HR 0.875, 95% CI 0.773–0.990), and salvage radiotherapy (HR 0.389, 95% CI 0.183–0.826). Early sADT (HR 0.244, 95% CI 0.084–0.714) was positively associated with DMFS. High-risk PCa (HR 9.550, 95% CI 1.049–86.947) and a pathologic Gleason score ≥8 (HR 3.688, 95% CI 1.009–13.484) correlated significantly with CSS, while early sADT was not overall predictive of CSS (Supplementary Table 1, only online). As shown in Supplementary Table 2 (only online), early sADT in non-organ confined PCa was not associated with CSS in Cox regression analysis, while only pathologic Gleason score was associated with CSS in non-organ confined PCa (HR 10.092, 95% CI 1.930–52.785).
Table 3. Univariate and Multivariate Analyses of Factors Associated with Disease Progression-Free Survival.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 0.993 (0.958–1.029) | 0.705 | ||
| BMI | 0.949 (0.856–1.053) | 0.327 | ||
| Prostate volume measure by TRUS | 1.000 (0.981–1.019) | 0.983 | ||
| Risk classification | 0.002 | 0.002 | ||
| Low & intermediate | 1 (ref) | 1 (ref) | ||
| High | 2.225 (1.332–3.716) | 2.272 (1.359–3.799) | ||
| PSA | 0.413 | |||
| <20 | 1 (ref) | |||
| ≥20 | 1.387 (0.633–3.040) | |||
| Pathologic T stage | 0.895 | |||
| Organ confined (T2) | 1 (ref) | |||
| Non-organ confined (>T2) | 0.966 (0.579–1.612) | |||
| Pathologic Gleason score | 0.454 | |||
| <8 | 1 (ref) | |||
| ≥8 | 1.240 (0.706–2.178) | |||
| Positive surgical margin | 0.830 (0.502–1.370) | 0.466 | ||
| PSA doubling time | 0.972 (0.928–1.019) | 0.242 | ||
| Salvage radiotherapy | 0.396 (0.244–0.643) | <0.001 | 0.447 (0.274–0.730) | 0.001 |
| Early salvage ADT vs. delayed salvage ADT | <0.001 | 0.002 | ||
| Delayed salvage | 1 (ref) | 1 (ref) | ||
| Early salvage | 0.389 (0.223–0.677) | 0.457 (0.260–0.801) | ||
ADT, androgen deprivation therapy; BMI, body mass index; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography.
Table 4. Univariate and Multivariate Analysis of Factors Associated with Distant Metastasis-Free Survival.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 0.994 (0.940–1.050) | 0.822 | ||
| BMI | 1.000 (0.857–1.166) | 0.999 | ||
| Prostate volume measure by TRUS | 1.000 (0.971–1.029) | 0.991 | ||
| Risk classification | 0.005 | 0.073 | ||
| Low & intermediate | 1 (ref) | 1 (ref) | ||
| High | 3.458 (1.464–8.170) | 2.391 (0.922–6.203) | ||
| PSA | 0.395 | |||
| <20 | 1 (ref) | |||
| ≥20 | 0.421 (0.057–3.097) | |||
| Pathologic T stage | 0.867 | |||
| Organ confined (T2) | 1 (ref) | |||
| Non-organ confined (>T2) | 1.070 (0.483–2.370) | |||
| Pathologic Gleason score | 0.001 | 0.033 | ||
| <8 | 1 (ref) | 1 (ref) | ||
| ≥8 | 3.653 (1.737–7.686) | 2.463 (1.073–5.654) | ||
| Positive surgical margin | 0.556 (0.264–1.170) | 0.122 | ||
| PSA doubling time | 0.871 (0.779–0.975) | 0.016 | 0.875 (0.773–0.990) | 0.034 |
| Salvage radiotherapy | 0.324 (0.154–0.682) | 0.003 | 0.389 (0.183–0.826) | 0.014 |
| Early salvage ADT vs. delayed salvage ADT | 0.004 | 0.010 | ||
| Delayed salvage | 1(ref) | 1 (ref) | ||
| Early salvage | 0.205 (0.071–0.593) | 0.244 (0.084–0.714) | ||
ADT, androgen deprivation therapy; BMI, body mass index; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography.
DISCUSSION
Current NCCN® guidelines recommend ADT for the treatment of advanced PCa.4 Immediate ADT has demonstrated benefits for patients with metastatic PCa in OS or CSS,14 as well as those with node-positive PCa following RP with pelvic lymph node dissection.6,15 In contrast, however, a population-based cohort study demonstrated that primary ADT is not associated with improved survival among men with localized PCa.7 Current guidelines do not recommend ADT for PCa without local definitive treatment.4
The utility of ADT after local definitive treatment of clinically localized PCa remains unresolved in the current literature. A study performed by Siddiqui, et al.8 suggests that adjuvant ADT improves CSS and systemic PFS after RP in node-negative PCa. The benefits of ADT, however, are lost when treatment is initiated after BCR or systemic progression. In a study involving 2096 PCa patients, Garcia-Albeniz, et al.16 suggest that ADT initiated within three months after PSA-only relapse results in similar survival to that observed with deferred ADT (initiated within three months after clinical progression). Furthermore, Duchesne, et al.17 report that immediate ADT improves OS and time to clinical progression for PCa patients after BCR following curative therapy in a randomized trial (TOAD trial). In yet another study, sADT for BCR following treatment for localized PCa was not associated with all-cause mortality or CSM; however, it was observed in a subgroup analysis that men with rapidly progressing disease have decreased all-cause mortality and CSM with sADT.18 Finally, in a recently published systematic review, van den Bergh, et al.9 suggest that early systemic hormonal therapy for non-metastatic recurrence should be reserved for those patients with the highest risk of disease progression, defined by short PSA doubling time or high Gleason score. Clearly, these incongruous findings confound opportunities to optimize PCa therapy for patients with recurrent disease.
Similarly, previously published studies, that seek to identify the ideal time to initiate sADT for BCR following RP, remain controversial. Based on a study of 1352 PCa patients who underwent RP, Moul, et al.19 concluded that initiation of ADT in patients with PSA levels <5 ng/mL for treatment of “PSA-only recurrence” can delay clinical metastasis, and suggested that early ADT is associated with delayed clinical metastases in patients with high-risk cases, including those with a Gleason score of greater than 7 or a PSA doubling time shorter than 12 months. The benefits of early ADT on clinical metastasis, however, were lost in the overall cohort and they did not further evaluate the impact of early ADT on OS or CSS. The benefits of early ADT, administered before disease progression, including BCR, have previously been addressed by Siddiqui, et al.8 who showed improved 10-year systemic PFS and 10-year CSS, while they did not suggest an optimal PSA value at which to initiate ADT in a salvage treatment setting. Taguchi, et al.20 suggested that early sADT before reaching a PSA level of 0.2 ng/mL might reduce BCR in pT2-4N0 PCa. However, their studies were limited due to a relatively small patient cohort and a lack of survival outcome analysis.
In the present study, we reported that early sADT could have benefits for DPFS and DMFS. In a similar concept, very early salvage radiotherapy improved DMFS.21 These findings suggest that sADT might be particularly effective when tumor burden was minimal.6
We did not exclude patients who received salvage radiotherapy. Salvage radiotherapy was associated with DPFS or DMFS in our analyses; however, proportions of patients with salvage radiotherapy were significantly different in groups A and B. This difference should be considered for comparison of survivals between the two groups. We will examine in the future further details through a more precisely designed study.
We recognize that our present study has several limitations. This study is retrospective and evaluated outcomes for a relatively small number of patients. Additional randomized, prospective multi-center studies are needed to confirm our data. Furthermore, because there were few mortalities and a small number of cases of indolent localized or regional PCa, it was difficult to draw a definitive association between early sADT and CSS. Finally, side effects associated with ADT and patient anxiety were not considered in the study. We recognize that these should be considered prior to the initiation of sADT after RP and should be included in future studies.
Our current study aimed to identify the optimal timing for sADT following RP. Our findings suggest that early sADT, administered prior to the current definition of BCR, is associated with improved disease progression and DMFS. In non-organ confined PCa, early sADT had better CSS than did delayed sADT. Furthermore, we demonstrated that early sADT has a positive impact on DPFS and DMFS. Although early sADT was not associated with CSS in this study, early sADT showed favorable CSS in non-organ confined PCa. Physicians may wish to consider early ADT as a salvage treatment after RP, especially in patients with non-organ confined PCa.
Footnotes
The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIALS
Univariable and Multivariable analysis of Factors Associated with Cancer-Specific Survival
Cox Regression Analysis of Factors Associated with Cancer Specific Survival in Non-Organ Confined Prostate Cancer
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Resnick MI. Hormonal therapy in prostatic carcinoma. Urology. 1984;24(5 Suppl):18–23. [PubMed] [Google Scholar]
- 3.Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann Surg. 1942;115:1192–1200. doi: 10.1097/00000658-194206000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN®): prostate cancer. ver. 2. Fort Washington (PA): National Comprehensive Cancer Network Inc; 2017. [Google Scholar]
- 5.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 6.Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 7.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui SA, Boorjian SA, Inman B, Bagniewski S, Bergstralh EJ, Blute ML. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008;179:1830–1837. doi: 10.1016/j.juro.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 9.van den Bergh RC, van Casteren NJ, van den Broeck T, Fordyce ER, Gietzmann WK, Stewart F, et al. Role of hormonal treatment in prostate cancer patients with nonmetastatic disease recurrence after local curative treatment: a systematic review. Eur Urol. 2016;69:802–820. doi: 10.1016/j.eururo.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Ryan CJ, Small EJ. Early versus delayed androgen deprivation for prostate cancer: new fuel for an old debate. J Clin Oncol. 2005;23:8225–8231. doi: 10.1200/JCO.2005.03.5311. [DOI] [PubMed] [Google Scholar]
- 11.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 12.Buyyounouski MK, Choyke PL, McKenney JK, Sartor O, Sandler HM, Amin MB, et al. Prostate cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:245–253. doi: 10.3322/caac.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Kirk D. Timing and choice of androgen ablation. Prostate Cancer Prostatic Dis. 2004;7:217–222. doi: 10.1038/sj.pcan.4500733. [DOI] [PubMed] [Google Scholar]
- 15.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Albeniz X, Chan JM, Paciorek A, Logan RW, Kenfield SA, Cooperberg MR, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. Eur J Cancer. 2015;51:817–824. doi: 10.1016/j.ejca.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D'Este C, Frydenberg M, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17:727–737. doi: 10.1016/S1470-2045(16)00107-8. [DOI] [PubMed] [Google Scholar]
- 18.Fu AZ, Tsai HT, Haque R, Yood MU, Cassidy-Bushrow AE, Van Den Eeden SK, et al. Mortality and androgen deprivation therapy as salvage treatment for biochemical recurrence after primary therapy for clinically localized prostate cancer. J Urol. 2017;197:1448–1454. doi: 10.1016/j.juro.2016.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moul JW, Wu H, Sun L, McLeod DG, Amling C, Donahue T, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–1147. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi S, Fukuhara H, Azuma T, Suzuki M, Fujimura T, Nakagawa T, et al. Ultra-early versus early salvage androgen deprivation therapy for post-prostatectomy biochemical recurrence in pT2-4N0M0 prostate cancer. BMC Urol. 2014;14:81. doi: 10.1186/1471-2490-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abugharib A, Jackson WC, Tumati V, Dess RT, Lee JY, Zhao SG, et al. Very early salvage radiotherapy improves distant metastasis-free survival. J Urol. 2017;197(3 Pt 1):662–668. doi: 10.1016/j.juro.2016.08.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariable and Multivariable analysis of Factors Associated with Cancer-Specific Survival
Cox Regression Analysis of Factors Associated with Cancer Specific Survival in Non-Organ Confined Prostate Cancer