Abstract
Historically, Monotherium had been one of the few genera of extinct Phocidae (true seals) that served as a wastebin taxon. Consequently, it did neither aid in understanding phylogenetic relationships of extinct Phocidae, nor in understanding seal diversity in deep time. This urged the reassessment of the genus. Before our review, Monotherium included five different species: Monotherium aberratum, Monotherium affine, and Monotherium delognii from Belgium; Monotherium gaudini from Italy; and Monotherium? wymani from the east coast USA. In this work we redescribe the fossil record of the genus, retaining the type species M. delognii. Monotherium aberratum and M. affine are reassigned to the new phocine genus Frisiphoca. Monotherium gaudini is renamed and considered a stem-monachine (Noriphoca gaudini). The holotype of the monachine M.? wymani requires further study pending the discovery of new fossil material that could be attributed to the same taxon. Reinvestigating the stratigraphic context reveals that N. gaudini most likely represents one of the two oldest named phocid seals, or even the oldest, dated to the late Oligocene–earliest Miocene. Our results allow questioning the widespread idea that Phocidae originated in the western Atlantic and better appreciate their palaeobiogeography during the late Oligocene–Miocene interval in the North Atlantic realm.
Keywords: Phocidae, Monotherium, Neogene, North Atlantic, North Sea Basin
1. Introduction
The extinct genus Monotherium Van Beneden, 1876 (Monatherium in Van Beneden, 1877) is a particular taxon among Phocidae (true seals). Of all currently known extant and extinct phocid seal taxa, most are considered monospecific or include only two species [1], and only two genera include three species: the extant Pusa and the extinct middle to late Miocene Praepusa [2,3]. However, the Miocene monachine genus Monotherium surpasses these and currently includes five species: Monotherium aberratum Van Beneden, 1876; Monotherium affine Van Beneden, 1876; Monotherium delognii Van Beneden, 1876; Monotherium gaudini (Guiscardi, 1870); and Monotherium? wymani (Leidy, 1853), from Belgium (first three species), Italy and the east coast of the United States, respectively. Furthermore, historically other species have been assigned to this genus, e.g. Monotherium maeotica [Cryptophoca maeotica] (Nordmann, 1860), and Monotherium rugosidens (Owen, in Adams, 1879) [holotype is odontocete tooth] [4,5]. On the other side, M. aberratum, M. affine and M. delognii have been recently considered nomina dubia [6], but without any evidence provided. A major issue for the taxonomy of Monotherium is that the fossil record of the different species within the genus varies strongly in terms of preserved skeletal elements: most Monotherium species cannot be directly compared to other species within the genus. For example, M. gaudini is known from a partial skull [7], while no cranial bones have been attributed to the other species of the genus.
In the light of recently discovered specimens showing affinities with part of the species of this genus, a reinvestigation is essential, with a description of the new material and reassessment of the previously described species.
2. Material and methods
2.1. Terminology and comparative material
This study follows the anatomical nomenclature of the recent publications of Amson & Muizon [8], Berta et al. [6] and Dewaele et al. [9,10]. Whenever terms have not been used in any of the aforementioned publications, anatomical nomenclature follows Evans & Lahunta's description of the dog [11]. Length measurements were taken to the nearest 0.1 mm, using analogue calipers and are presented as tables 2, 4 and 5 and electronic supplementary material (Supplementary Information 1: tables S1–S3). For reasons of consistency, these measurements were taken following the same scheme as Koretsky [2], which has more recently been applied to other extinct phocids [6,9,10]. Comparative specimens of extant and extinct taxa are listed as electronic supplementary material (Supplementary Information 1: lists 1 (extant taxa) and 2 (extinct taxa)).
Table 2.
Measurements of the humerus of Frisiphoca aberratum and Frisiphoca affine (in mm). Measurements based on the scheme presented by Koretsky [2].
| Frisiphoca aberratum | Frisiphoca affine | |
|---|---|---|
| IRSNB 1191-M266 (lectotype) | IRSNB 1118-M260 (lectotype) | |
| total length | 142.5 | 177.1 |
| length deltopectoral crest | 77.0 | n.a. |
| height head | 33.9 | 36.8 |
| height trochlea | n.a. | 2.28 |
| width head | 35.9 | 41.5 |
| width proximal epiphysis | 48.3 | 60.2 |
| width distal epiphysis | 53.4 | 61.8 |
| distal width trochlea | 32.8 | n.a. |
| transverse width mid-diaphysis | 22.0 | 24.8 |
Table 4.
Measurements of the astragalus IRSNB 1126-M262, identified as Phocidae aff. Frisiphoca affine (in mm). ‘+’ indicates that the measured length is smaller than the real length, due to post-mortem wear of the specimen.
| Phocidae aff. Frisiphoca affine | |
|---|---|
| IRSNB 1126-M262 | |
| absolute length | +75.5 |
| maximum dorsoplantar height | +47.7 |
| mediolateral width across tibial facet | +31.5 |
| dorsoplantar height astragalar head | +28.0 |
| mediolateral width astragalar head | n.a. |
| dorsoplantar height caudal process | +30.0 |
| mediolateral width caudal process | +16.6 |
| maximal length ectal facet | n.a. |
| maximal length sustentacular facet | +22.7 |
Table 5.
Measurements of the calcaneum IRSNB 1125-M263 identified as Phocidae aff. Frisiphoca affine (in mm).
| Phocidae aff. Frisiphoca affine | |
|---|---|
| IRSNB 1125-M263 | |
| absolute proximodistal length | 78.2 |
| maximal mediolateral width | n.a. |
| least mediolateral width of calcaneal tuber | n.a. |
| mediolateral width across the medial calcaneal tuberosity | n.a. |
| maximal dorsoplantar height | 38.8 |
| maximal length of ectal facet | 20.8 |
| height of ectal facet | 8.6 |
| maximal length of sustentacular facet | 33.6 |
| mediolateral width of facet for navicular | n.a. |
| dorsoplantar height of facet for navicular | 24.9 |
2.2. Dinoflagellate cyst biostratigraphy
The palynological preparation of the sediments followed standard techniques described by Louwye et al. [12]. Acid treatments with HCl and HF were applied for the removal of carbonates and silicates, respectively. Sieving of the organic residue was carried out on a nylon screen with a 10 µm mesh size. The residue was placed on glass slides with glycerol gelatin jelly. The microscopic analysis was carried out with a transmitted light microscope Zeiss AxioImager A1 under 400× magnification. The entire slide was scanned in non-overlapping traverses. The taxonomy of the dinocysts and acritarchs follows Fensome et al. [13]. A table showing all the observed dinocyst and acritarch taxa is presented as electronic supplementary material (Supplementary Information 1: table S4).
2.3. Phylogenetic analysis
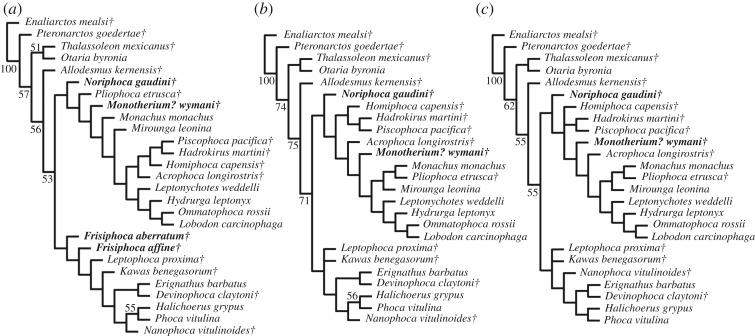
The phylogenetic analysis largely follows the methodology of Dewaele et al. [9] for the assessment of the phylogenetic position of Nanophoca vitulinoides Dewaele, Amson, Lambert & Louwye, 2017 among Phocidae. The analysis was performed using PAUP version 4.0b10 for Macintosh [14] with a heuristic search option with simple sequence addition, using the tree-bisection-reconnection (TBR) algorithm. Bootstrap values were obtained after a full heuristic search with 10 000 replications with random number seed zero and the best tree saved for each replication. Character states were optimized with accelerated transformation criterion (ACCTRAN). The phylogenetic analysis has been performed both without down-weighting homoplastic characters and with the k-value of the Goloboff criterion set at three, for down-weighting homoplastic characters. The phylogenetic matrix includes 80 morphological characters (Supplementary Information 1: list 3, table S5; Supplementary Information 2) and 27 operational taxonomic units (OTUs), including the extinct Pinnipedimorpha Enaliarctos mealsi Mitchell & Tedford, 1973 and Pteronarctos goedertae Barnes, 1989, the Otariidae Otaria byronia Blainville, 1820 (extant) and Thalassoleon mexicanus Repenning & Tedford, 1977 (extinct), and the desmatophocid Allodesmus kernensis Kellogg, 1922 as outgroup taxa; the extinct Monachinae Acrophoca longirostris Muizon, 1981, Hadrokirus martini Amson & Muizon, 2013, Homiphoca capensis (Hendey & Repenning, 1971), Piscophoca pacifica Muizon, 1981, and Pliophoca etrusca Tavani, 1941; the extant Monachinae Hydrurga leptonyx (Blainville, 1820), Leptonychotes weddellii (Lesson, 1826), Lobodon carcinophaga (Hombron & Jacquinot, 1842), Mirounga leonina (Linnaeus, 1758), Monachus monachus Hermann, 1779, and Ommatophoca rossii (Gray, 1844); the extinct Phocinae Devinophoca claytoni Koretsky & Holec, 2002, Kawas benegasorum Cozzuol, 2001, Leptophoca proxima (Van Beneden, 1876), Nanophoca vitulinoides (Van Beneden, 1871); and the extant Phocinae Erignathus barbatus Erxleben, 1777, Halichoerus grypus (Fabricius, 1791), and Phoca vitulina Linnaeus, 1758. Four (former) Monotherium species are included for the first time in a phylogenetic analysis: Monotherium aberratum (Van Beneden, 1876; as Frisiphoca aberratum), Monotherium affine (Van Beneden, 1876; as Frisiphoca affine), Monotherium gaudini (Guiscardi, 1870; as Noriphoca gaudini) and Monotherium? wymani (Leidy, 1853). Two characters are parsimony-uninformative (23, 35) and three (31, 35, 76) are ordered. The choice of outgroups is such that early stem Pinnipedimorpha are represented (E. mealsi and Pt. goedertae), as well as two of the three non-phocid pinniped families with Desmatophocidae (A. kernensis) and Otariidae (O. byronia and T. mexicanus). Odobenidae are excluded in order to keep the outgroup appreciably small.
2.4. Institutional abbreviations
ELNRP, East Libya Neogene Research Project collection, housed at Garyounis University, Benghazi, Libya; IRSNB, Institut Royal des Sciences Naturelles de Belgique (‘M’ representing type and figured specimens from the fossil mammal collection), Brussels, Belgium; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA; MSNUN, Museo di Storia Naturale del'Università di Napoli, Naples, Italy; USNM, Department of Paleobiology, National Museum of Natural History, Washington, DC, USA.
3. Historical background
Prior to the current study, five species have been considered within the genus Monotherium: Monotherium aberratum Van Beneden, 1876; Monotherium affine Van Beneden, 1876; Monotherium delognii Van Beneden, 1876; Monotherium gaudini (Guiscardi, 1870); and Monotherium? wymani (Leidy, 1853). Unfortunately, as with many historically longstanding extinct taxa, its history has been turbulent. Monotherium? wymani and M. gaudini have been described prior to the erection of the genus Monotherium, but had been named Phoca wymani Leidy, 1853, on the basis of a few isolated cranial and postcranial specimens from the Miocene (presumably the Calvert formation) of Richmond, Virginia [15], and Phoca gaudini Guiscardi, 1870, on the basis of one partial skull and a mandible from 3 km east of Roccamorice, Abruzzo Region, Italy [7], respectively. Later, the genus Monotherium was erected by Van Beneden [16], including M. delognii, M. affine and M. aberratum based on isolated postcranial material from Antwerp (Belgium, southern margin of the North Sea Basin). Although somewhat vague and very concise, Van Beneden [16] provided descriptive elements for M. delognii (‘similarities with Phoca barbata’) and M. aberratum (‘size greater than Monachus’), but not for M. affine. However, it is argued that M. affine comprises all material from the original collection that could not be assigned to either of the two other species. For neither of the three species, Van Beneden [16] provided illustrations or collection numbers.
Having met the requirements of naming a species under the International Code on Zoological Nomenclature (ICZN) Article 11, and being published before 1931, ICZN Article 12 applies to Monotherium and ‘indications’ suffice for naming taxa. Therefore, Monotherium is a valid name, irrespective of Van Beneden [17] using the name Monatherium one year later and implicitly providing the etymology of Monatherium, and rendering the original name of Monotherium a typographical error. Van Beneden corrected the spelling, providing the etymology, after observing affinities between the genus ‘Monatherium’ and the extant Pelagius monachus, which is a junior synonym of Monachus monachus. Therefore, it can safely be implied that the name Monatherium etymologically refers to Monachus monachus and that the former name of Monotherium by Van Beneden [16] is a typographical error; yet, Monotherium is the valid name for the taxon and Monatherium should be considered a junior synonym of it. In the 1877 publication, Van Beneden [17] described Monotherium delognii, Monotherium affine and Monotherium aberratum in much more detail and provided the collection numbers of specific specimens.
Van Beneden did not assign a type species to the genus Monotherium. In 1922, Kellogg retained the name Monotherium for all three taxa and assigned Monotherium delognii as the type species of the genus on the basis of page priority in Van Beneden [17] [18, p. 72]. He also regarded Monotherium affine as a junior synonym to M. delognii, stating ‘Monotherium delognii is based upon too fragmentary material to distinguish it from Monotherium affine. Therefore, since Monotherium delognii has page priority, it is here interpreted to include Van Beneden's second species, Monotherium affine, as well.’ Kellogg [18] also retained Monotherium affine as a separate taxon and renamed the Italian species Phoca gaudini to Monotherium gaudini.
More recently, Ray [15] studied the North American Phoca wymani in detail, removing it from the genus Phoca and tentatively placing it among Monotherium: Monotherium? wymani. However, M.? wymani is based on very fragmentary isolated specimens that bear little diagnostic value for comparison with other extinct Phocidae. Following Kellogg [18], Ray [15] did not use the generic name Monatherium, but instead used the first, and correct, name Monotherium for the three taxa described by Van Beneden [16,17].
4. Dinoflagellate cyst biostratigraphy of Monotherium from Belgium
Historically, the stratigraphic context of Monotherium aberratum, Monotherium affine and Monotherium delognii had been poorly defined. Van Beneden [17] assigned a ‘Diestian’ age to the entire record of Monotherium from Belgium. However, the ‘Diestian’ is a currently abandoned term and it had been shown that the term should not be used any more [19]. Moreover, shortly after the description of Monotherum by Van Beneden [16,17], the ‘Anversien’ (=‘Antwerpian’) was erected and considerably restricted the extent of the ‘Diestian’ [19,20]. Nowadays, the same toponym is used for the upper Miocene Diest Formation, which is roughly the lithostratigraphical equivalent of the ‘Diestian’ stage. The Diest Formation is a diachronous formation deposited in a marginal marine setting [19]. Near the city of Antwerp, the deposits of the Diest Formation (Deurne Member) are of late Tortonian age [19–23].
Only two sediment samples could be recovered from bone cavities of specimens formerly attributed to the genus Monotherium: sample 1108LDW-1100Lab from the thoracic or lumbar vertebrae of Monotherium delognii (either from specimen IRSNB 1108 or from specimen IRSNB 1108-M255; Phocidae indet. in this study, see Supplementary Information 3) and sample 1132LDW-1102Lab from the cervical or thoracic vertebrae originally assigned to Monotherium aberratum (IRSNB 1132-M269; Phocidae indet. in this study, see Supplementary Information 3). The samples were palynologically analysed for organic-walled dinoflagellate cysts (dinocysts) and acritarchs (see Supplementary Information 1, table S4).
The preservation and diversity of the dinocysts in sample LDW1108-1100Lab is poor. A total of 10 dinocyst species and one reworked acritarch were recorded (Supplementary Information 1: table S4). Dybkjaer & Piasecki [24] defined the Achomosphaera andalousiensis zone as the interval from the lowest common occurrence of the eponymous species to the lowest occurrence of Gramocysta verricula, and suggest an age of 13.2 Ma for the lower boundary of the zone. The dinoflagelatte cysts thus indicate a maximum age of 13.2 Ma (early Serravallian, late middle Miocene) for the sediment sample. The other recorded dinoflagellate cysts are stratigraphically long ranging species with no biostratigraphical value.
The preservation and diversity of the dinocysts in sample LDW1132-1102Lab is similarly poor. Only eleven dinocyst species and two acritarch were recorded. A maximum age for the sample is provided by the key species Habibacysta tectata. This species has a lowest occurrence in high latitudes dated at 14.2 Ma by Schreck et al. [25], and this datum was later confirmed by Quaijtaal et al. [26] in lower latitudes (Porcupine Basin, off southwest Ireland). A lowest occurrence of Operculodinium? eirikianum at ca 14 Ma (upper Langhian) is provided by Louwye et al. [27] in a low-resolution palynological study of the Miocene of the Porcupine Basin. Operculodinium? eirikianum has a persistent highest occurrence at the lower–upper Pliocene boundary at ca 2.617 Ma [28]. The lowest occurrence of Operculidinium tegillatum is located at the Tortonian–Messinian boundary (7.25 Ma). The persistent highest occurrence is noted in the Zanclean at 3.7 Ma [28]. Quinquecuspis concreta has a poorly specified lowest occurrence in the upper Tortonian of Germany [29]. The dinoflagellate cysts in this second sample indicate an age situated between ca 7.25 Ma (or somewhat older in the late Tortonian), and 3.7 Ma (late Zanclean).
5. Systematic palaeontology
Family Phocidae Gray, 1821
Subfamily Monachinae Gray, 1869
Genus Noriphoca gen. nov.
LSID. urn:lsid:zoobank.org:act:CF6ABB16-8EEA-4490-8D6D-918B48910613
Type and only included species. Noriphoca gaudini (Guiscardi, 1870).
Diagnosis. As for the only included species
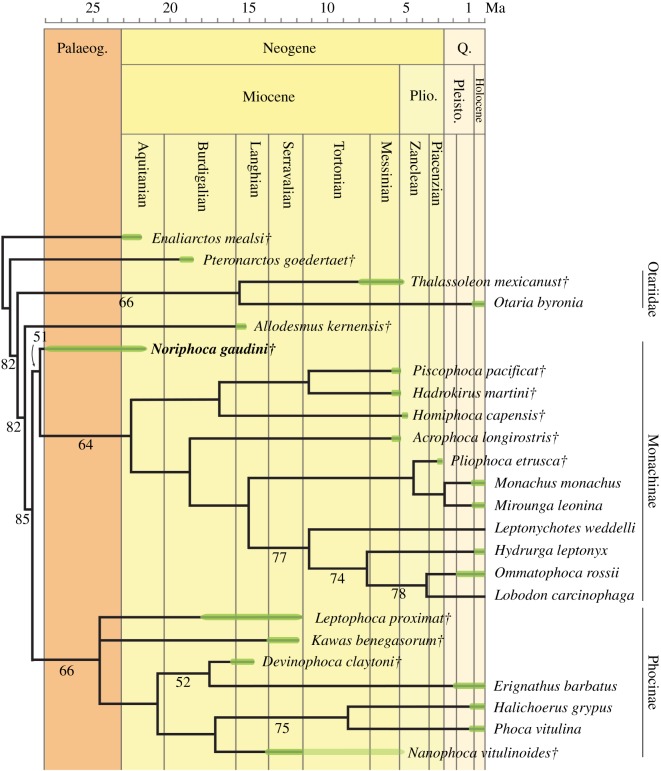
Etymology. From the Greek adjective ‘noris’ and the Greek noun ‘phoke’. Meaning ‘early’ and ‘seal’, respectively, referring to the geologically old age of the species. An age interval of late Oligocene to early Miocene is presented here (see below). Hence, this taxon may possibly represent the first unquestionable phocid from the Palaeogene ([10] versus [30,31]).
Noriphoca gaudini (Guiscardi, 1870)
Figure 1.
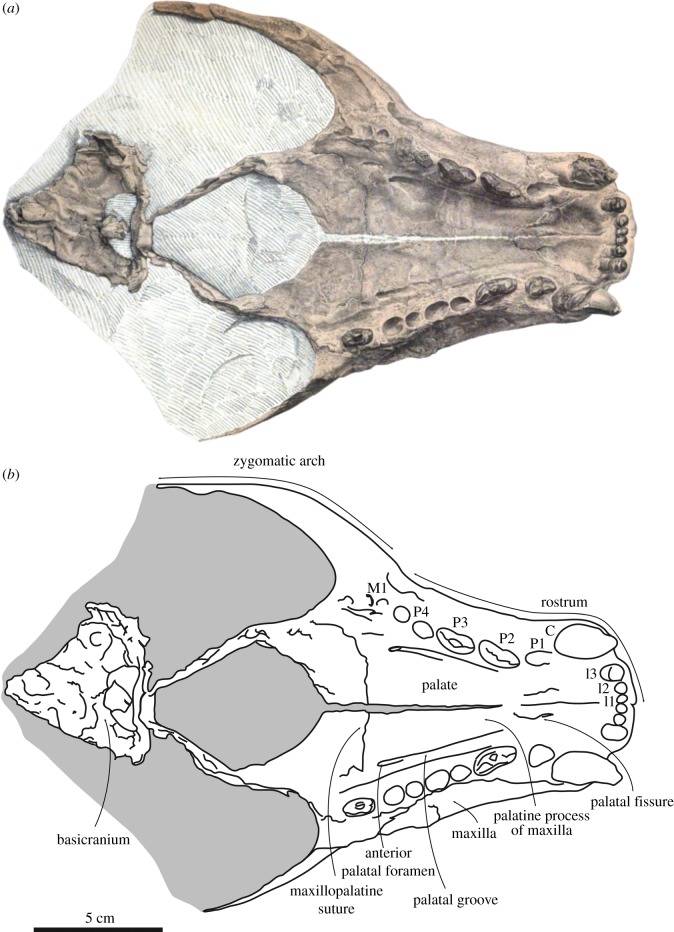
Holotype skull of the stem monachine Noriphoca gaudini, MSNUN123, presumably from the late Oligocene–early Miocene Lepidocyclina Limestone of the Bolognano Formation near Roccamorice, Italy, and originally described as Phoca gaudini by Guiscardi ([7]: plate 1). Original drawing from Guiscardi [7] (a), and line drawing (b). Skull in ventral view. Sediment and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Figure 2.
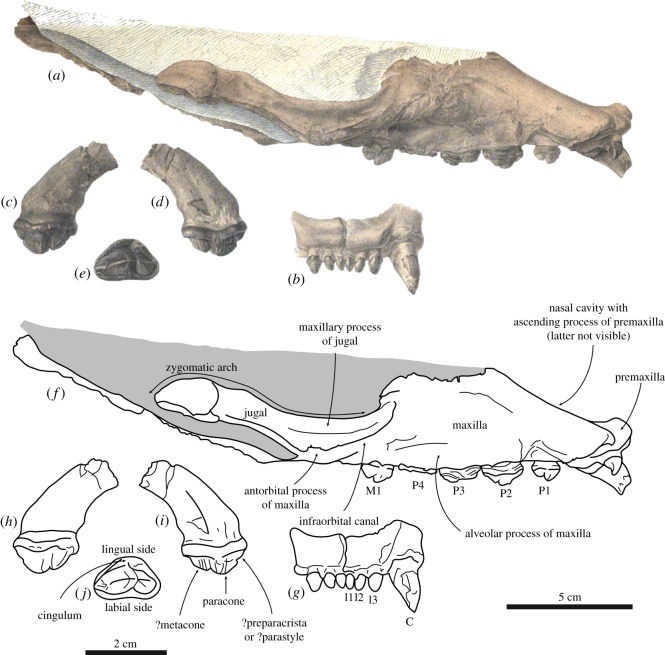
Holotype skull of the stem monachine Noriphoca gaudini, MSNUN123, presumably from the late Oligocene–early Miocene Lepidocyclina Limestone of the Bolognano Formation near Roccamorice, Italy, and originally described as Phoca gaudini by Guiscardi ([7]: plate 2), also including isolated teeth originally assigned to P. gaudini. Original drawing from Guiscardi [7] (a–e), and line drawing (f–j). Skull in right lateral view (a,f), and snout in anterior view (b,g). Corresponding scale bar equals 5 cm. Isolated right postcanine tooth in lingual (c,h), labial (d,i) and occlusal (e,j) view. Corresponding scale bar equals 2 cm. Sediment and obliterated parts are indicated in grey.
LSID. urn:lsid:zoobank.org:act:0A15AA71-8B86-495B-BF9B-5FE3D11FB2CF
Diagnosis. Large phocid, comparable in size to the leopard seal, Hydrurga leptonyx. Stem phocid, but still yielding typically monachine characters in having: an ascending process of the premaxilla that is (at least partially) within the nasal cavity and not visible laterally; roots of incisors not (or only weakly) laterally compressed. The anterior termination of the maxillary process of the jugal is located lateral to the infraorbital foramen, which is shared with extinct Monachinae (Acrophoca longirostris, Hadrokirus martini, Homiphoca capensis and Piscophoca pacifica) and the extant Monachinae Mirounga. In the phylogenetic analysis, the identification of Noriphoca gaudini as a separate taxon is supported by one unequivocal autapomorphy: the ventral edge of the zygomatic arch is level with the alveolar plane. Furthermore, the skull of N. gaudini differs from all other Monachinae by the presence of three upper incisors, and a paracone on the postcanine teeth that is low.
Holotype. MSNUN123, partial skull. Only the ventral and anterior portion of the skull are visible. The dorsal portion of the skull is missing and the preserved part is embedded in the matrix, inhibiting description of the specimen in dorsal view.
Type locality. Approximately 3 km east of the village of Roccamorice (Abruzzo Region, Italy) [7].
Stratigraphy and age. Guiscardi [7] noted that the specimen comes from calcareous deposits, rich in bitumen. Based on more recent literature, it is evident that the outcropping formations, 3 km east of Roccamorice are the Santo Spirito and Bolognano formations [32,33]. Because it had been hypothesized that the earliest Phocidae lived around 23 Ma (divergence date of Phocidae from other Pinnipedia taken from Higdon et al. [34]), around the Oligocene–Miocene boundary, the late Oligocene to Miocene Bolognano Formation is the most likely candidate as the origin of the Monotherium gaudini holotype. It is indeed very unlikely that the holotype of M. gaudini comes from the underlying Eocene Santo Spirito Formation, which also outcrops in the area. Furthermore, the bituminous layers of the Bolognano Formation are found in its lower part, mainly restricted to the Lepidocyclina Limestone dated to the Oligocene or earliest Miocene (Aquitanian) ([33,35]; and references therein). Consequently, the exact age of the holotype specimen of M. gaudini is still unknown; but nevertheless, this holotype is most likely as old as, or even older than the Aquitanian. Hence, N. gaudini is most likely older than Afrophoca libyca, known from the Burdigalian of Libya [36].
Remarks. Other specimens from the same locality and from the same level, i.e. a partial mandible and isolated teeth, had been presented by Guiscardi [7], but these specimens are currently lost (Giovanni Bianucci 2017, personal communication). Additionally, the little informative illustration of the mandible ([7]: fig. 6) precludes its description. Therefore, we do not deem it appropriate to redescribe these specimens in depth. However, the isolated teeth are comparable in shape to the teeth of the holotype skull and are unspecialized (contrasting to extant Monachinae, except Monachus). In their review of the palaeobiogeography of Pinnipedia, Deméré et al. [4] disregarded Monotherium gaudini, but referred to cf. Monotherium sp. indet. isolated teeth from the Bismantova Formation in the Stirone River, Italy, described by Cigala-Fulgosi & Pilleri [37] and Pilleri & Cigala-Fulgosi [38]. As noted by Dewaele et al. [10], the geological age of the Bismantova Formation is strongly debated, with proposed ages ranging from the Burdigalian–early Langhian [39] (adopted by Berta et al. [6]) to the late Langhian–Serravalian–early Tortonian [40,41] (adopted by Deméré et al. [4]). Although these teeth are clearly monachine, we observe only few similarities between those and the teeth of the holotype of Noriphoca gaudini. Although both have strongly pronounced cusps and a crenulated enamel layer, the cusps of the teeth of N. gaudini are much less raised than in the specimens from the Stirone River. Therefore, we deem it impossible to identify these isolated teeth more precisely than Monachinae indet. and they will no further be considered in this study. The redescription of M. gaudini (as N. gaudini) is entirely based on observations made on the descriptions, images and drawings presented by Guiscardi [7]. We, the authors, have not studied the type specimen in person.
Description and comparison
The type and only known specimen of Noriphoca gaudini comprises an incomplete skull (figures 1 and 2). The known parts of the skull include the ventral part of the rostrum and parts of the basicranium and the right zygomatic arch. A number of maxillary teeth are known as well [7]. The dorsal portion of the skull is missing and only the anterior portion of the snout is visible in dorsal view. The remainder of the skull is embedded in the matrix.
Only the anterior portion of the premaxilla is preserved and it is restricted to the interior of the nasal cavity, which means that it is not visible in lateral view. This is a typically monachine characteristic [42,43]. The anterior alveolar plane faces anteroventrally and the canines have a strong anterior aspect in their orientation. Amson & Muizon [8] observed a similar condition in the monachine Homiphoca capensis, but we conclude that this is considerably more pronounced in N. gaudini than it is in H. capensis. In lateral outline, the nasal cavity is weakly curved and almost rectilinear, and has a strong dorsal aspect to its orientation. This corresponds, notably, with Acrophoca longirostris from the late Miocene of Peru, and Allodesmus spp. [42,44–46]. Other Monachinae and pinnipedimorphs usually have snouts that face more anteriorly and that are either more strongly concave in lateral view (Phocidae), or convex (early pinnipedimorphs), or vary between concave or convex in lateral view within a single clade [8,42,47–57].
The palate of N. gaudini is slightly constricted at the level of the first premolar, after which the combined tooth rows diverge posteriorly. This is a typically phocid characteristic [58] and it is far less expressed in stem pinnipedimorphs and other non-phocid pinnipeds, where this constriction is minimal or absent [8,44–55,58]. The posterior divergence of the tooth row is minimal in early pinnipedimorphs, Odobenidae and Otariidae [47–55], but is present to varying degrees in Desmatophocidae and Phocidae [8,42,44–46]. On the palate, a small, slit-like and narrow palatine fissure is located at the suture between the premaxilla and maxilla, at the level of P1. The shape of the palatine fissure varies among extinct Phocidae and is, for instance, small in Homiphoca capensis [56,57], but large in Hadrokirus martini and Piscophoca pacifica [8,42], and this palatine fissure is generally large in other extinct Pinnipedia and early pinnipedimorphs [47–55], although a strongly reduced palatine fissure has also been observed in some desmatophocids [44]. A significant portion of the maxilla is preserved. The alveolar process, bearing the teeth, is slightly raised over the palatine process of the maxilla. In lateral view, the alveolar process faces ventrally, as in other Pinnipedimorpha, except the Monachinae Hadrokirus martini, Ommatophoca rossii and Piscophoca pacifica [8]. The palate is slightly arching dorsally in N. gaudini. This condition varies within different clades of Pinnipedimorpha. Among stem pinnipedimorphs, for instance, it is arching in Enaliarctos spp., but nearly flat in Pinnarctidion bishopi [47]. Even among Monachinae, Muizon [42] noted variation in the degree of arching of the palate. The palatal groove on the palatine process of the maxilla is narrow and becomes gradually more pronounced towards the anterior palatal foramen, which is located at the level of the posterior foramen of P4. Among Phocidae, the location of the anterior palatal foramen varies from the level of P3 (Erignathus barbatus, Monachus spp.) to posterior to the level of M1 (Homiphoca capensis, Mirounga spp., Ommatophoca rossii and Pusa spp.) [56,57,59]. Among extant and other Pinnipedimorpha, the position of this foramen varies, but it is generally well anterior to the level of the last postcanine tooth, notwithstanding exceptions such as Desmatophoca brachycephala [46–51]. The maxillopalatine suture is transversely straight between the M1. Consequently, for N. gaudini, the maxillopalatine suture is located posterior to the anterior palatine foramina. Muizon [42] noted that in some Phocinae, the anterior palatine foramina are located on the maxillopalatine suture, while they are anterior to that suture in Monachinae. Differences in the terminology used combined with inadequate illustrations inhibit studying this trait in detail for other pinnipedimorphs, based on literature alone. The posterior margin of the joined palatines is rounded, forming a half circle in N. gaudini. At the posterior extremity of the palatine, where right and left palatines meet, there is no true apex. This condition varies among Phocidae, ranging from a strongly-developed anterior invagination between the left and right palatines, to a caudal nasal spine of the palatines. Among other Pinnipedimorpha, the posterior margin of the palatines is smoothly rounded [44–54]. Compared to all other pinnipedimorphs, including Phocidae (except the Monachinae Acrophoca longirostris and Hadrokirus martini), the (rounded) posterior margin of the palatines is located relatively anterior, with the anteriormost tip located little posterior to the last postcanine tooth and the anterior extremity of the orbit in ventral view. In all other pinnipedimorphs, the palatine extends much more posteriorly, reaching the anteroposterior level of the jugal-squamosal contact and much more posterior to the last postcanine tooth.
The anterior margin of the infraorbital canal on the antorbital process of the maxilla is located at the level of M1. In Monachinae, this compares to the extant Lobodon carcinophaga and Monachus spp., and the extinct Hadrokirus martini and Pi. pacifica, whereas this canal is located either anterior (e.g. Leptonychotes weddellii) or posterior (e.g. to M1) in other Monachinae. In Phocinae, the anterior margin of the infraorbital canal is located posterior to M1. In stem pinnipedimorphs, the anterior margin of the infraorbital canal is usually located at the level of M1 [46–49]. Only rarely is the anterior margin of the infraorbital canal located anterior to M1 (e.g. Enaliarctos spp.) [52,53]. Related to that, the last postcanine is as the level of the root of the jugal process of the maxilla in Noriphoca gaudini, as in many Monachinae. In Phocinae, the last postcanine tooth is located anterior to the jugal process. In other early pinnipedimorphs and early pinnipeds, the last postcanine tooth reaches the level of the posterior portion of the root of the jugal process of the maxilla or posterior. The maxillary process of the jugal contacts the maxilla, terminating dorsal to the infraorbital canal, as in extinct Monachinae and Mirounga. In extant Monachinae (except Mirounga) and Phocinae, this process terminates lateral to the infraorbital canal. The jugal is incomplete, but comparison with more complete monachine skulls show that the anterior portion of the arch of the jugal is flat to slightly oriented downwards in N. gaudini. This condition varies among Monachinae: flat in Acrophoca longirostris, Hadrokirus martini, and Mirounga, upward in Homiphoca capensis, and Piscophoca pacifica, and downward in other Monachinae. In Phocinae, the anterior portion of the arch of the jugal is directed flat to upward.
In the upper tooth row, Noriphoca gaudini is characterized by having three incisors. Among Phocidae, the presence of three upper incisors is generally considered a characteristic of Phocinae (except Cystophora cristata having two upper incisors), while Monachinae are characterized by having two upper incisors. Three upper incisors are also present in early pinnipedimorphs and desmatophocids [18,44–49,53,54]. The lateral incisor I3 is larger than I1 and I2, which are similar in size, but still much smaller than the canine, and all incisors form a transversely straight row. In most Phocinae (except Halichoerus grypus) and early pinnipedimorphs, the lateral incisor is comparable in size to or only slightly larger than the medial incisor(s). A number of extinct Monachinae retain relatively small lateral incisors (Hadrokirus martini, Homiphoca capensis and Piscophoca pacifica), while the lateral incisor is clearly intermediate in size between medial incisors and canines in other extinct (Acrophoca longirostris) and extant Monachinae. Though, overall the incisors of N. gaudini are relatively smaller than the incisors in Monachinae (except Acrophoca longirostris and Ommatophoca rossii), while the incisors are almost always comparatively small in Phocinae (except H. grypus). The roots of the incisors of N. gaudini are not transversely compressed. In Monachinae and stem pinnipedimorphs, roots are not or only faintly compressed transversely, while in Phocinae, incisor roots are strongly compressed. Morphologically, there is little variation between the mesial incisors and the lateral incisors, apart from the size. The mesial incisors are more slender transversely than the lateral incisors. The incisors are single cusped and bear no cingulum. They are conical, but slightly recurved lingually and bear two occlusal facets on their lingual surfaces: one posterolateral and one posteromedial. Both canines are only partially preserved, but they are conical and appear labially curved. The canine alveolus is oval, i.e. slightly mediolaterally compressed.
The maxillary postcanine teeth include four premolars and one—noticeably smaller—molar. This is typically phocid, as other early stem pinnipedimorphs, desmatophocids, and many extinct odobenids and otariids have at least two upper molars [44–55,58]. Apart from the single-rooted P1, the postcanine teeth of Noriphoca gaudini are all double-rooted. This is common among Phocidae, in which only few taxa (Halichoerus grypus and Mirounga spp.) show a tendency towards single-rooted postcanine teeth. Whereas Devinophoca claytoni is the only known phocid to have a triple-rooted upper first molar [60], early stem pinnipedimorphs have triple-rooted upper first molars [46–49,52–54]. Desmatophocids, odobenids and otariids all show a trend towards reduction of the roots to single-rooted postcanine teeth during their evolution [44–46,50,58]. All postcanine teeth of N. gaudini are labiolingually broad and the lingual cingulum is well developed, yielding a semicircular or subtriangular shape in occlusal view. This is clearly a plesiomorphic character observed in early stem pinnipedimorphs, Hadrokirus martini, Monachus spp., and Piscophoca pacifica, while other (extant) Phocidae all display a specialized dentition with the cingulum being strongly reduced or absent [8,46–49,53,54].
The first premolar (P1) is subtriangular in occlusal view and is implanted parallel to the tooth row. The labial margin is slightly concave, while the lingual margin strongly projects lingually, yielding a subtriangular outline in occlusal view. The paracone (central cusp) and the ?metacone distal to the paracone are lowly raised and only little defined. The ?metacone is closely appressed against the paracone, further reducing the prominence of both. The term ‘metacone’ is based on usage in publications on other pinnipedimorphs [47], but contrasts with dedicated literature on dentition in carnivorans. For example, Solé et al. [61] stated that there is no metacone present on the maxillary premolars of Dormaalocyon latouri, the oldest known carnivoran. Anterior to the paracone, there is a strongly reduced and rounded cusp. Similar to the ?metacone, this cusp may be considered the ?preparacrista or the ?parastyle, following Solé et al. [61], and pending further studies on the evolution of the dentition in (early) Pinnipedimorpha.
P2–M1 are severely damaged, precluding detailed description. They are more elongate than P1, the lingual convexity is less pronounced, and the labial concavity is slightly more pronounced than in P1. P2–P4 are implanted slightly obliquely to the tooth row axis, with the distal extremity of P2 located labial to the mesial extremity of P3 and the distal extremity of P3 located labial to the mexial extremity of P4. P2–P4 are morphologically similar to P1, having a lowly-raised paracone and a ?metacone distal to it. The height of the cusp mesial to the paracone is strongly reduced but mesiodistally long and may be considered the ?preparacrista or the ?parastyle. Posterior to the ?metacone, there is a small protuberance that can be considered the ?postmetacrista or the ?metastyle.
The first upper molar (M1) is separated from and slightly smaller than the premolars. M1 is morphologically strongly similar to P1, and is clearly premolariform, having lost the trigonid morphology and the protocone that are still present in early stem pinnipedimorphs [46–49,52–54]. The prominence of the paracone and the ?metacone is greater than in the premolars.
The enamel of the postcanine teeth is ‘wrinkled’, as had been observed for H. martini by Amson & Muizon [8]. The protocone is not as prominent as in other Monachinae, but broad: contributing to the robust appearance of the teeth. The metacone is large, about half the size of the protocone. The paracone anterior to the protocone is strongly reduced. The robust upper dentition of N. gaudini is most similar to that of the extant Monachus and a number of extinct Monachinae, such as H. martini and Pl. etrusca, while extant Monachinae (except Monachus) have highly specialized teeth. The mandibles have been described for both Afrophoca libyca and N. gaudini, and both are geographically and possibly geochronologically close [7,36]. However, the state of preservation of the holotype, and only specimen, of A. libyca (ELNRP 2Z131) is poor, and the illustrated mandible of N. gaudini ([7]: fig. 6) is missing (Giovanni Bianucci 2017, personal communication). Therefore, formal comparison between both taxa is for now precluded.
Monotherium? wymani (Leidy, 1853)
(Figure 3)
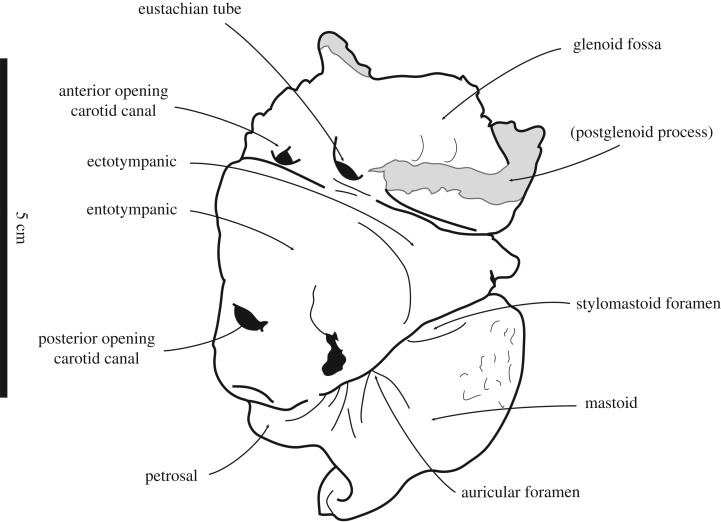
Figure 3.
Line drawings of the holotype tympanic bulla MCZ 8741 of Monotherium? wymani (?Calvert Formation at Richmond, Virginia) in ventral view. After figures from Ray [15]. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Holotype. MCZ 8741, left and right temporal bones, originally assigned to Phoca wymani by Leidy [62], ‘Tertiary’, Richmond, Virginia, USA.
Type locality. ‘Shockoe creek ravine near the base of Church Hill’ [63, p. 229], which is located in Richmond, Virginia, USA. Ray [15] provided evidence supporting Wyman's statement.
Type horizon. Ray [15] elaborated on the probable type horizon of MCZ 8741, concluding on the Calvert Formation. However, the Calvert Formation spans across the entire early Miocene and into the late middle Miocene (ca 23.03–13.8 Ma). At the Calvert Cliffs in Maryland, the oldest published record phocid fossils come from zone 10 of the Calvert Formation [64] which is dated to the early middle Miocene ([65]; and references therein). This renders a pre-middle Miocene age for MCZ 8741 less likely.
Comments. Originally presented as Phoca wymani, Ray [15] tentatively placed the original material of the species in Monotherium? wymani, as well as newly described specimens. The fossil record of M.? wymani is difficult to assess: The holotype MCZ 8741, a cranial fragment including the ear region and malleus, is valuable for the differentiation between different taxa of Phocidae [8,59], but the fossil record of Phocidae from the North Atlantic and Paratethys contains only a few ear regions [2]. Hence, the diagnostic value of fossil phocid ear regions is undermined. Additionally, a study of tympanic bullae of elephant seals, Mirounga, has shown that intraspecific variation is prominent [52]. Because other specimens attributed to M.? wymani only include disassociated and/or postcranial bones, Ray [15] could only tentatively assign them to M.? wymani. Moreover, the fossil record of referred specimens of M.? wymani does not include either humeri or femora, despite these bones being the most valuable postcranial bones for the identification of Phocidae [2,15]. The lack of humeri in the fossil record of M.? wymani precludes any comparison of the taxon with the original Monotherium humeri from the Miocene of Belgium (lectotype humerus of Frisiphoca aberratum IRSNB 1191-M266, and lectotype humerus of Frisophoca affine IRSNB 1118-M260 in this study). The holotype ear region of M.? wymani is moderately well inflated, yielding a tympanic bulla that is roughly triangular in ventral view, and the posterior carotid foramen is clearly visible in ventral view (figure 3). These characteristics clearly support the identification of MCZ 8741 as a monachine (e.g. [15,43]). However, because the genus Monotherium is restricted to its type species, M. delognii, and because this type species is restricted to its lectotype pelvis IRSNB 1153-M257a, b, the holotype specimen of M.? wymani is reidentified as a monachine of uncertain affinities. The other specimens tentatively referred to M.? wymani are USNM 187410 (partial right mandible, left ulna, and right tibia and fibula) and USNM 214625 (partial fibula). The trochlear notch of the ulna of USNM 187410 is very similar to that of the ulna IRSNB 1121-M261a, and both are considered Phocidae cf. Frisiphoca affine. Muizon [42] already noted the value of the shape of the trochlear notch of the ulna as a means to distinguish Phocidae to the generic level, also indicating similarities between the ulnae of Phocidae cf. Frisiphoca [Monotherium] affine (IRSNB 1121-M261a) and Monotherium? wymani with the ulnae of Homiphoca capensis and Piscophoca pacifica of South Africa and Peru, respectively. Ulna USNM 187410 can be considered as Phocidae cf. Frisiphoca affine. Contrastingly, we deem it inappropriate to identify the mandible and the partial tibia and fibula of USNM 187410 beyond the subfamily level. Ray [15] stated that the specimens came from one single block and are probably of one individual, but he implicitly expressed his doubt. Indeed, their association can be considered questionable in the absence of other preserved parts of the skeleton.
Pending the discovery of more complete fossil specimens of Monotherium? wymani, we propose to restrict the species M.? wymani to the holotype tympanic bulla (MCZ 8741), discarding the remainder of the fossil record proposed by Ray [15], because it cannot be compared to the holotype. The holotype of ‘Phoca’ wymani cannot be compared to the original Monotherium material from Belgium and Italy, i.e. the holotype skull of Noriphoca gaudini, the lectotype humeri of Frisiphoca aberratum and Frisiphoca affine, and the lectotype pelvis of Monotherium delognii. Apart from M.? wymani, tympanic bullae have been presented for three other Neogene seals from the North Atlantic: Leptophoca proxima [2], Terranectes magnus and Terranectes parvus [66]. However, Dewaele et al. [10] questioned the former designation of the skull of L. proxima to the species. And similarly, new research by Dewaele et al. (in preparation) questions whether the tympanic bullae referred to T. magnus and T. parvus can be securely attributed to these taxa. Thus, the holotype of M.? wymani cannot be compared to other contemporaneous Phocidae from the North Atlantic. Therefore, we consider M.? wymani to be a monachine of unknown affinities, pending the discovery of more complete specimens that can be attributed to the taxon. Despite being uncomparable to Monotherium delognii, we provisionally retain the genus name Monotherium with a question mark. It is unknown whether M.? wymani indeed belongs to the genus Monotherium. It is likely that the holotype will eventually be designated into another genus, but this cannot be ascertained based on the current fossil record. Contrastingly, Monotherium gaudini is given a new genus name in this study, based on the argumentation that the systematic comparison and phylogenetic analysis (see above and below) of the more complete type material places it as a stem monachine, while the phylogenetic affinities of the holotype of M.? gaudini with other Monachinae cannot be ascertained (see below). The current genus name Monotherium is preferred as placeholder over the genus name Phoca, which was used prior to Ray's redescription [15]. The name Phoca is currently restricted to Phoca largha and Phoca vitulina: two phocine seals that show no affinities with M.? wymani (see below).
Subfamily Phocinae Gray, 1821
Genus Frisiphoca nov. gen.
LSID. urn:lsid:zoobank.org:act:C4514A72-F792-4414-8130-2D595E413954
Type species. Frisiphoca aberratum (Van Beneden, 1876).
Other included species. Frisiphoca affine (Van Beneden, 1876).
Diagnosis. Identification as a phocid seal supported by the large development of the deltopectoral crest on the humerus. Identified as a phocine based on the presence of an entepicondylar foramen (also in Homiphoca capensis) and the overall slenderness. Differs from most Phocinae by having a very strongly reduced humeral neck (also in Histriophoca fasciata, Leptophoca proxima and Pagophilus groenlandicus). Differs from all Phocidae in the following unique combination of characteristics: lesser tubercle slightly below the level of the humeral head (also in Devinophoca emryi, Le. proxima, Monachopsis pontica, Nanophoca vitulinoides, Pachyphoca chapskii, Pachyphoca ukrainica, Praepusa vindobonensis, Properiptychus argentinus and Sarmatonectes sintsovi), transverse bar in bicipital groove (also in Lobodon carcinophaga, Monachus monachus, Ommatophoca rossii, Piscophoca pacifica and Pliophoca etrusca), deep fossa for m. triceps brachii distal to the humeral head (also in Pi. pacifica), and deltopectoral crest tapering smoothly distally (also in Australophoca changorum, Acrophoca longirostris, Cryptophoca maeotica, De. emryi, Kawas benegasorum, Messiphoca mauretanica, Mo. pontica, Pachyphoca chapskii, Pachyphoca ukrainica, Pi. pacifica, Pl. etrusca, Pra. vindobonensis, Prophoca rousseaui, Properiptychus argentinus and S. sintsovi).
Etymology. From the Latin pronoun ‘Frisicum’ and the Greek noun ‘phoke’. ‘Frisicum’ refers to the historical region of Frisia and the much smaller modern Dutch province with the same name. Here, the term is used in reference to the Mare Frisicum, the Latin name for the North Sea, alluding to the geographical origin of the two species of the genus listed here. ‘Phoké’ means ‘seal’.
Comments. Although Frisiphoca shares characteristics with both Monachinae and Phocinae (see diagnosis), the presence of an entepicondylar foramen is regarded as a characteristic uniting Frisiphoca with Phocinae. Unfortunately, this is not reflected in the phylogenetic analysis due to the poor scoring of the fragmentary fossil record of the genus.
Frisiphoca aberratum (Van Beneden, 1876)
(Figure 4a–d, i–l)
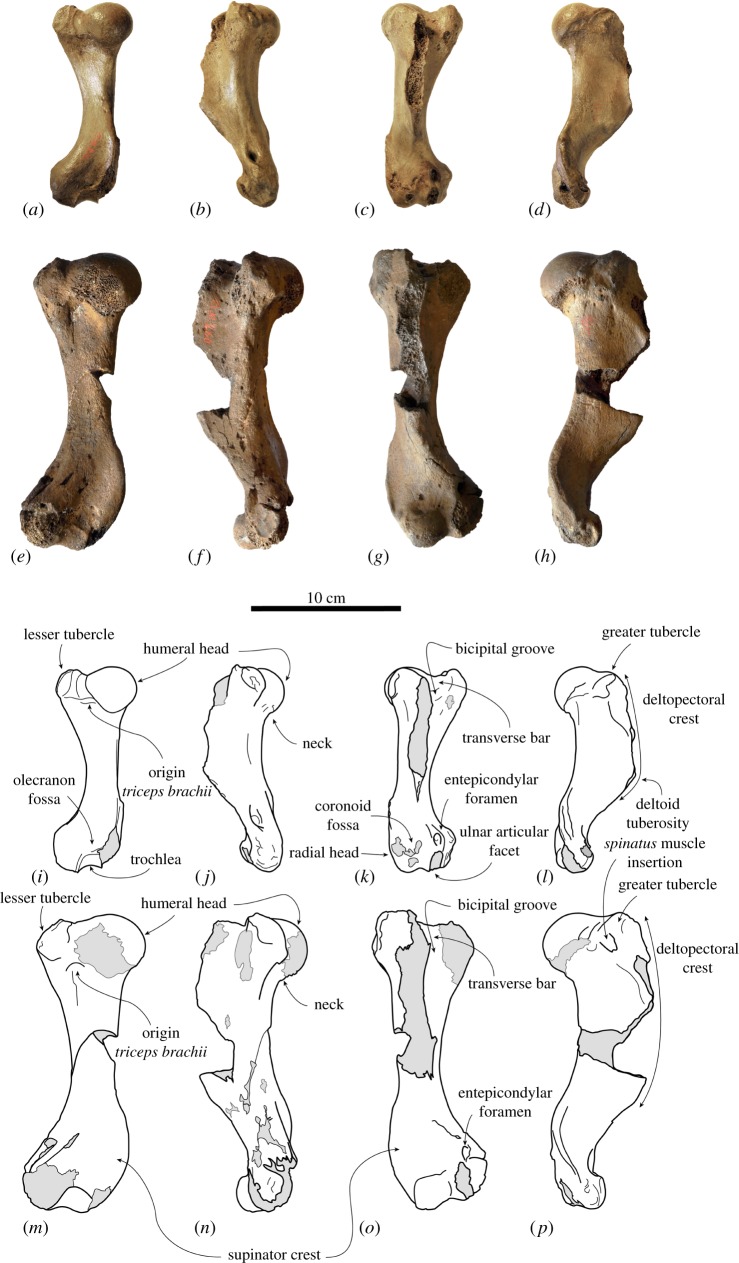
Figure 4.
Lectotype right humerus IRSNB 1191-M266 of the stem phocine Frisiphoca aberratum from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in posterior (a), medial (b), anterior (c) and lateral (d) view. Lectotype right humerus IRSNB 1118-M260 of the stem phocine Frisiphoca affine from the ‘Diestian’ of the ‘third section’ at Deurne, Antwerp, in posterior (e), medial (f), anterior (g) and lateral (h) view. Corresponding labelled drawings of right humerus IRSNB 1191-M266 of Frisiphoca aberratum in posterior (i), medial (j), anterior (k) and lateral (l) view; and lectotype right humerus IRSNB 1118-M260 of Frisiphoca affine in posterior (m), medial (n), anterior (o) and lateral (p) view. Broken and obliterated parts are indicated in grey. Scale bar equals 10 cm.
LSID. urn:lsid:zoobank.org:act:E8382F26-74B1-4D16-AA5E-21F85821FF4D
Lectotype. IRSNB 1191-M266, partial right humerus. Van Beneden nor any subsequent author assigned a type specimen to Frisiphoca [Monotherium] aberratum. In the absence of more completely preserved material, we consider the humerus figured by Van Beneden ([17]; pl. 17, figs 1–4) the most diagnostic specimen to identify F. aberratum.
Type locality. Third section at Borgerhout (see original label with specimen), Antwerp, Belgium. The ‘third section’ follows Van Beneden's discretization of the nineteenth-century fortification constructions around the city of Antwerp, with the third section at Borgerhout being located northeast to the Borgerhout district of Antwerp ([9]: fig. 1; [10]: fig. 2) [9,10,17,67]. However, it should be noted that this type locality is derived from the original labels associated to the specimen. In his original publications Van Beneden [16,17] did not discuss the geographical provenance of individual specimens of the original fossil record of Frisiphoca aberratum.
Type horizon. Van Beneden (unpublished handwritten notes in the IRSNB archives) assigned the specimen IRSNB 1191-M266 to the ‘Diestien’ (Diestian). However, as mentioned above, the Diestian is currently considered an obsolete term and should not be used any more [20]. Different authors assign different ages and stratigraphic intervals to the Diestian (see [19]: table 1), but in general it is considered that the Diestian is roughly equivalent to the Deurne Sands Member of the Diest Formation. Louwye et al. [21] assigned a Messinian to Tortonian (late Miocene) age to the Diest Formation north of Antwerp and in the Campine area. In a more detailed description of the Neogene stratigraphy of the Antwerp area, Mourlon [68] mentions the occurrence of Frisiphoca aberratum (and possibly also Frisiphoca [Monotherium] affine and Monotherium delognii) in one of the strata he described. This unnamed stratum, composed of greenish glauconiferous sands overlays—also unnamed—darker green and black sands [68]. Extrapolating this to the current knowledge on the stratigraphy of the Antwerp area, these greenish glauconiferous sands most likely represent the Deurne Sands Member of the Diest Formation, while the underlying darker green sands and black sands represent the Antwerpen Sands Member of the Berchem Formation [69]. Therefore, it can be assumed that F. aberratum comes from the Deurne Sands Member of the Diest Formation (table 1).
Table 1.
Selection of cranial measurements of Noriphoca gaudini redrawn from Guiscardi [7] (originally for Phoca gaudini).
| length (mm) | ||
|---|---|---|
| bizygomatic width skull | 184.0 | |
| sagittal length of the palate | 122.0 | |
| transverse width across pterygoid processes | 63.0 | |
| width across the canines (excluding canines) | 36.0 | |
| width across the canines (including canines) | 61.0 | |
| width across the incisor tooth row | 29.0 | |
| length of postcanine tooth row | 83.0 | |
| anteroposterior length (mm) | labiolingual length (mm) | |
| diameter upper premolars | ||
| first premolar | 11.2 | 7.6 |
| second premolar | 17.4 | 9.0 |
| third premolar | 16.8 | 9.5 |
| diameter upper first molar (isolated specimen) | 12.0 | 7.0 |
Diagnosis. Medium-sized phocine, comparable in size to the harbour seal, Phoca vitulina. Differs from all Phocidae, including Frisiphoca affine, by the strong posteroproximal orientation of the humeral head, and differs from Phocinae, including F. affine, by the weak development of the supinator crest. Differs further from F. affine by the little medial curvature of the distal portion of the humerus, the shallow olecranon fossa (deeper in F. affine), and the smaller size (80.5% of length of humerus in F. affine; see table 2).
Comments. Although Van Beneden [17] assigned partially articulated specimens to Frisiphoca [Monotherium] aberratum, these specimens, including two partial pes associated with a baculum and three caudal vertebrae (IRNSB 1187-M273a-o); a phalanx and a fifth metatarsal (IRSNB 1188-M270a, b); a partial hind limb including a second and third metatarsal, a partial fibula, and an ectocuneiform (IRSNB 1189-M271a, b); and a thoriac and cervical vertebra (IRSNB 1132-M269a, b), bear little diagnostic value. Given the overall rarity of such bones in the fossil record, they cannot be compared with other extinct phocid taxa from the southern North Sea basin, and the lectotype humerus has been selected as the type specimen of F. aberratum, degrading the other specimens to Monachinae indet., Phocidae indet., or Phocinae indet. (see Supplementary Information 3). A sediment sample associated with the vertebrae IRSNB 1132-M269 was analysed biostratigraphically with dinoflagellate cysts for our study. The identification of this specimen as F. aberratum is questioned (this study; considered Phocidae indet.), but it should be mentioned that this sediment sample returned an age range from 7.25 Ma (latest Tortonian) to 3.7 Ma (late Zanclean), thus providing a minimum age interval for IRSNB 1132-M269 that does not contradict the stratigraphic assignment of the lectotype of F. aberratum.
Description and comparison
Humerus (figure 4a–d, i–l). In the absence of more complete, e.g. cranial, material, the humerus is the most diagnostic bone in Phocidae [2]. The humerus IRSNB 1191-M266 is overall well preserved, only missing part of the deltopectoral crest and portions of the distal epiphysis. The bone is straight and moderately slender. Ray [70] already noted that the humerus is relatively straight in some early Phocidae, such as Leptophoca proxima, as well as early stem pinnipedimorphs and terrestrial carnivorans, while most extinct and recent Phocidae have a more strongly curved humeral diaphysis. A measure for the ‘slenderness’ or ‘robustness’ of the humerus of Monachinae has been provided by Muizon & Bond [71]. This has been expanded to include Phocinae (table 3) and shows that the humerus in Phocinae is generally more slender than in Monachinae, although there is noticeable overlap. The humerus of F. aberratum is moderately slender and falls within the range observed in extinct and extant Phocinae (table 3).
Table 3.
Measurement of the robustness of phocid humeri, adapted from Muizon & Bond [71]. The robustness R is calculated as the ratio of l/L or (l1 + l2 + l3 + l4)/L, with l1 = maximum transverse width of the proximal epiphysis, l2 = minimum transverse width of the diaphysis, l3 = maximum transverse width of the distal epiphysis, l4 = maximum anteroposterior width of the diaphysis at the level of the deltoid tuberosity; L = maximum length of the humerus. Measurements with an asterisk are estimations. Measurements in mm. Sources provided for measurements retrieved from the literature. Measurements by Koretsky [2] represent averages of multiple specimens.
| taxon | l1 | l2 | l3 | l4 | l | L | R = l/L |
|---|---|---|---|---|---|---|---|
| Acrophoca longirostris [71] | 65.0 | 26.2 | 53.0 | 57.5 | 201.7 | 154 | 1.309 |
| 64.6 | 26.4 | 54.8 | 51.0 | 201.5 | 155 | 1.300 | |
| 62.0 | 27.6 | 55.5 | 51.2 | 196.3 | 153 | 1.283 | |
| 63.0 | 28.0 | 53.2 | 51.0 | 195.2 | 144.6 | 1.349 | |
| 62.0 | 26.2 | 51.7 | 52.5 | 192.4 | 145.7 | 1.320 | |
| 61.0 | 30.0 | 51.0 | 54.0 | 196.0 | 146.0 | 1.340 | |
| Cryptophoca maeotica [2] | 34.2 | 14.5 | 37.0 | 33.5 | 119.2 | 107.1 | 1.113 |
| Halichoerus grypus | 50.9 | 25.3 | 52.7 | 40.9 | 169.8 | 124.4 | 1.365 |
| 56.8 | 24.2 | 58.4 | 41.2 | 180.6 | 134.5 | 1.343 | |
| Homiphoca capensis [71] | 62.8 | 25.0 | 51.5 | 53.5 | 192.8 | 136.4 | 1.413 |
| 55.1 | 20.7 | 45.7 | 46.5 | 168.0 | 119.4 | 1.407 | |
| Hydrurga leptonyx [71] | 75.3 | 36.6 | 64.3 | 76.5 | 252.7 | 166.0 | 1.522 |
| 78.0 | 34.0 | 67.0 | 90.0 | 269.0 | 168.0 | 1.601 | |
| Leptonychotes weddellii [71] | 59.7 | 26.0 | 58.3 | 58.7 | 202.7 | 139.7 | 1.450 |
| 66.0 | 26.0 | 59.0 | 65.0 | 216.0 | 154.0 | 1.402 | |
| Leptophoca proxima | 38.0 | 14.4 | 38.0 | 37.3 | 127.7 | 131.7 | 0.970 |
| Lobodon carcinophaga [71] | 67.4 | 27.0 | 57.0 | 63.5 | 214.9 | 132.3 | 1.624 |
| 65.0 | 32.7 | 62.0 | 69.7 | 227.5 | 124.0 | 1.826 | |
| 59.0 | 29.0 | 55.0 | 59.0 | 202.0 | 119.0 | 1.697 | |
| Mirounga leonina [71] | 130.0 | 60.0 | 130.0 | 133.0 | 453.0 | 290.0 | 1.562 |
| Monachus monachus [71] | 61.0 | 26.7 | 58.4 | 56.0 | 202.1 | 144.0 | 1.403 |
| Nanophoca vitulinoides [9] | 27.5 | 9.8 | 24.0 | 20.0 | 81.3 | 72.4 | 1.123 |
| 28.1 | 9.5 | 26.6 | 20.8 | 85.0 | 78.2 | 1.087 | |
| Phoca vitulina | 48.5 | 18.0 | 41.5 | 36.2 | 144.2 | 110.4 | 1.306 |
| 49.9 | 18.8 | 44.2 | 36.4 | 149.3 | 122.9 | 1.215 | |
| Phocanella pumila | 45.7 | 15.8 | 47.1 | 39.9 | 148.5 | 127.8 | 1.162 |
| Piscophoca pacifica [71] | 64.1 | 28.0 | 55.5 | 63.2 | 211.2 | 148.9 | 1.418 |
| 64.5 | 30.3 | 62.0 | 63.6 | 220.4 | 160.8 | 1.370 | |
| Praepusa vindobonensis [2] | 27.6 | 10.6 | 25.6 | 24.2 | 88.0 | 86.3 | 1.020 |
| Properiptychus argentinus [71] | 58.8 | 18.4 | 40.7 | 40.0* | 157.9 | 125.9 | 1.254 |
| 46.0 | 18.0 | 42.0 | 40.0 | 146 | 120.9 | 1.207 | |
| Pusa sibirica | 36.6 | 12.6 | 36.1 | 24.8 | 110.1 | 91.4 | 1.205 |
| 43.1 | 15.4 | 42.4 | 32.7 | 133.6 | 103.1 | 1.296 | |
| Frisiphoca aberratum | 48.3 | 22.0 | 53.4 | 51.0* | 174.7 | 142.5 | 1.226 |
The humeral head is small and strongly hemispherical. As in Acrophoca longirostris, Hydrurga leptonyx and Otariidae, the head faces relatively proximally (contra [42]), relatively more posteroproximal than in other Phocidae, including F. aberratum. Unlike other Phocinae, except the extinct Leptophoca proxima [10], and the extant Histriophoca fasciata and Pagophilus groenlandicus, the neck is poorly developed in the species of Frisiphoca.
The deltopectoral crest is slender in anterior view. Although incompletely preserved, it appears that the deltoid tuberosity must have been located approximately halfway the length of the bone. The bicipital groove bears a small but noticeable transverse bar, which is also observed in the Monachinae Lobodon carcinophoca, Monachus spp., Ommatophoca rossii, Piscophoca pacifica and Pliophoca etrusca, and in the phocine Frisiphoca affine [42]. The lesser and greater tubercles reach slightly below the level as the head (contra [42]; for the greater tubercle). Among Phocidae, most extant taxa (except Monachus) have a strongly developed lesser tubercle and a small greater tubercle. While this condition is variable among extant taxa, many early phocid taxa bear a relatively little-developed lesser tubercle (e.g. Leptophoca proxima, Nanophoca vitulinoides). Geologically younger extinct Phocidae (e.g. Homiphoca capensis, Pliophoca etrusca) tend to have a lesser tubercle that shows a degree of development intermediate between early extinct Phocidae and extant Phocidae [9]. On the lateral surface of the greater tubercle, the insertion area for the infraspinatus and supraspinatus muscles is well outlined. The deltopectoral crest is overall slender and tapers smoothly towards the coronoid fossa, distally, as is typical for many extinct monachines and phocines (see genus diagnosis) [10,42]. Just distal to the humeral head and lesser tubercle, on the posterior surface of the diaphysis, there is a prominent fossa for the origin of the triceps brachii muscles.
An entepicondylar foramen is present, which is a characteristic shared with other Phocinae; among Monachinae, only Homiphoca capensis has an entepicondylar foramen [42,58]. The supinator crest is reduced, as in Monachinae, but contrasting to other Phocinae, including F. affine. The medial epicondyle is broad and flaring, as in F. affine. The lateral margin of this supinator crest is rugose, providing an origin area for powerful manual extensor muscles. The medial epicondyle is broad at the distal extremity of the supinator crest and medial to the medial condyle. The olecranon fossa and coronoid fossa are strongly reduced, these regions being almost completely flat. When compared to other Phocidae (except Homiphoca), at the distal epiphysis the ulnar articular facet of the trochlea is prominent in relation to the radial capitulum. This contrasts with F. affine, in which the coronoid fossa is less reduced. Overall, the humerus of F. aberratum combines characters which are otherwise considered typically monachine, or typically phocine. The poorly developed supinator crest is considered a plesiomorphic character retained in Monachinae, while the presence of an entepicondylar foramen is regarded as a plesiomorphic character retained in Phocinae [8,58].
Phocinae cf. Frisiphoca aberratum
(Figure 5a)
Figure 5.
Line drawings of right humerus USNM 214625 (a) (Phocinae cf. Frisiphoca aberratum) (St Marys Formation of the Gay Head Greensands at Martha's Vineyard, Massachusetts) in lateral (a) view; and left ulna USNM 187410 (b,c) (Phocinae cf. Frisiphoca affine) (?Calvert Formation at Richmond, Virginia) in medial (b) and anterior (c) view. After figures from Ray [15]. USNM 214625 was considered ?Monatherium aberratum, and USNM 187410 was considered Monotherium? wymani. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Referred specimen. USNM 214625, right humerus, Gay Head Greensand, Martha's Vineyard, Massachusetts, USA.
Comments. In his redescription of Monotherium? wymani, Ray [15] identified specimen USNM 214625, a partial humerus, as ?Monotherium aberratum ([15]: figs 8–11, subset 1). Despite the poor state of preservation, its general shape and size, and more specifically the proximal projection of the humeral head, the morphology of the greater tubercle, the shape of the insertion pit for the spinatus muscles, and the weak development of the supinator crest indicate similarities with the lectotype of Frisiphoca aberratum (figure 5a). However, given the poor state of preservation of the specimen, we deem it best to consider the specimen Phocidae cf. Frisiphoca aberratum. Dall [72] and Ray [15] considered the Gay Head Greensand to be part of the St Marys Formation, which is nowadays considered to be Tortonian (upper Miocene) in age (dinocyst zones DN 8 and 9 in Kidwell et al. [65]; 11.2–7.6 Ma from Köthe [73]).
Frisiphoca affine (Van Beneden, 1876)
(Figure 4e–h, m–p)
LSID. urn:lsid:zoobank.org:act:75D28CBF-B02F-4727-ACD6-BFF2A7EB77EC
Lectotype. IRSNB 1118-M260, partial right humerus. Van Beneden nor any subsequent author assigned a type specimen to Frisiphoca affine. In the absence of cranial material, we consider the humerus the most diagnostic specimen to identify F. affine.
Type locality. ‘Third section at Deurne’ (see original label with specimen), Antwerp, Belgium. The third section at Deurne is located southwest along the Deurne district of Antwerp ([9]: fig. 2; [10]: fig. 1) [9,10,17,67]. This type locality is derived from the original labels associated to the specimen, while Van Beneden [17,18] did not discuss the geographical provenance of all specimens, restricting the description to ‘third section’.
Type horizon. Van Beneden (unpublished handwritten notes in the IRSNB archives) assigned IRSNB 1118-M260 to the ‘Diestien’ (Diestian). However, as mentioned above, the Diestian is currently considered an obsolete term and should not be used any more [19]; in general, it is considered roughly equivalent to the Deurne Sands Member of the Diest Formation, dated to Messinian to Tortonian (late Miocene; [21]). In addition to Monotherium aberratum, Mourlon [68] mentions the possible occurrence of Monotherium affine and Monotherium delognii in an unnamed stratum, composed of greenish glauconiferous sands overlays—also unnamed—darker green and black sands that most likely represents the Deurne Sands Member of the Diest Formation [69].
Diagnosis. Large phocine, comparable in size to the leopard seal, Hydrurga leptonyx, and larger than all other extant and extinct Phocinae, except Erignathus barbatus. The humerus of Frisiphoca affine differs from Frisiphoca aberratum by the strong medial curvature of the distal portion of the humerus, the moderately deep olecranon fossa (shallow in F. aberratum), and the larger size (humerus of F. aberratum is 80.5% the size of the humerus of F. affine, see table 2). The presence of a well-developed supinator crest on the humerus is another difference between F. affine (present) and F. aberratum (absent), commonly observed among Phocinae.
Comments. When Kellogg [18] elected Monotherium delognii as the type species for the genus Monotherium, he considered the differences between M. delognii and Monotherium affine minimal and regarded M. affine as a junior synonym to M. delognii. Based on the current fossil record, it is clear that, apart from the partial pelvis IRSNB 1153-M257a, b, none of the bones originally assigned to M. delognii bears enough diagnostic characteristics to identify it beyond the family level. This partial pelvis is selected as the lectotype of M. delognii, showing similarities to the pelvis of Prophoca rousseaui. However, it cannot be compared with F. affine due to the lack of a preserved pelvis in the known fossil record of F. affine. Therefore, we do not follow Kellogg [18] and do not regard M. affine as a junior synonym to M. delognii.
Description and comparison
Humerus (figure 4e–h, m–p). The lectotype humerus of Frisiphoca affine IRSNB 1118-M260 is the only known humerus for the species and it is moderately well preserved. The humerus of F. affine differs relatively little from the humerus of Frisiphoca aberratum. Overall, the humerus is also slender and straight, but it is noticeably longer than the humerus of F. aberratum (table 2). The incompleteness of the humerus precludes quantification of the ‘slenderness’ or ‘robustness’ as has been done for F. aberratum (table 3). However, the similar shape allows assuming similar robustness values for both Frisiphoca species. The humeral head of F. affine is strongly hemispherical. The bicipital groove is moderately wide and relatively open, i.e. the margins of the bicipital groove almost form a straight angle and are not U-shaped in section. The bicipital groove bears a little-developed but noticeable transverse bar. This transverse bar was also observed in a large number of Monachinae and F. aberratum [42]. The lesser tubercle does not reach the level of the head and the greater tubercle reaches the same level as the head. Among other Phocidae, comparable conditions have been observed in extinct taxa, such as the middle Miocene phocines Leptophoca proxima and Pachyphoca spp. In extant phocids only Monachus has a somewhat reduced lesser tubercle (see above). As in F. aberratum, the insertion area for the infraspinatus and supraspinatus muscles is well outlined on the lateral surface of the greater tubercle. Distally, the deltopectoral crest tapers smoothly towards the coronoid fossa. As already pointed out by Muizon [42], the posterior surface of the diaphysis bears a prominent fossa just distal to the humeral head and lesser tubercle, for the origin of the triceps brachii muscles. This was also observed in the South American stem lobodontins Acrophoca and Piscophoca, as well as in F. aberratum [42]. An entepicondylar foramen is present. The supinator crest is well developed, as in other Phocinae except F. aberratum.
Phocidae cf. Frisiphoca affine
(Figure 5b,c)
Referred specimens. IRSNB 1121-M261a, right ulna, ‘Diestian’, third section at Borgerhout, Antwerp, Belgium. IRSNB 1126-M262, left astragalus, ‘Diestian’, third section at Borgerhout. IRSNB 1125-M263, right calcaneum, ‘Diestian’, third section at Borgerhout. USNM 187410, left ulna, Calvert Formation?, Richmond, Virginia, USA.
Comments. All Belgian specimens considered as Phocidae cf. Frisiphoca affine in this study had originally been identified and illustrated as Monotherium affine [17]. However, these specimens have been found isolated and are generally considered of very little diagnostic value. Another radius (IRSNB 1138-M267), astragalus (IRSNB 1144-M272) and calcaneum (IRSNB 1187-M273d) have originally been assigned to Monotherium aberratum, which are much smaller than the referred specimens IRSNB 1121-M261b, IRSNB 1126-M262, IRSNB 1125-M263. Therefore, the fossil record of phocids from the ‘Diestian’ includes the larger bones referred to in this section, as well as comparatively smaller radius, astragalus and calcaneum. The larger set can be assigned to Phocidae cf. Frisiphoca affine, based on the larger size of the specimens better matching F. affine, presumably from the same lithological unit. The American specimen of Phocidae cf. Frisiphoca affine, specimen USNM 187410, had previously been considered as Monotherium? wymani [15].
Description and comparison of the Belgian material
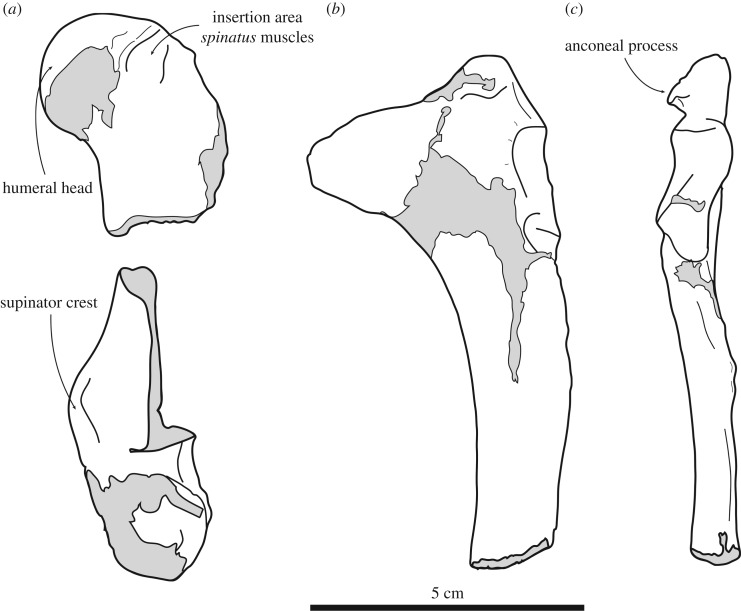
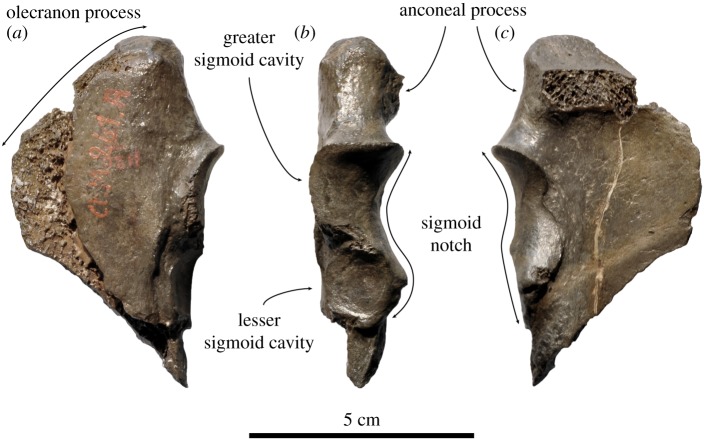
Ulna (figures 5b,c and 6). The anteroproximal portion of a right ulna is preserved (IRSNB 1121-M261a) (figure 6). The anconeal process is located relatively more distal on the proximomedial surface of the olecranon process than it is in any other phocid, except specimen USNM 187410 (figure 5b,c), formerly assigned to Monotherium? wymani [15]. This yields a strongly sloping appearance for the anconeal process. The prominence of this anconeal process is similar to that in other Phocinae, while it is generally much reduced in Monachinae (except Homiphoca) [57]. The greater sigmoid cavity for articulation with the humerus is mushroom-shaped, with the upper, greater facet facing anteriorly. Gradually curving around the sigmoid notch, this facet transits distally in a smaller facet facing medially. The lesser sigmoid cavity (=radial notch) is located anterodistally of the greater sigmoid cavity. This cavity is circular, flat, and faces anterolaterally. Again, this matches well M.? wymani, but it should be highlighted that little attention has historically been given to the description of the shape of the sigmoid notch of fossil Phocidae. Published drawings and descriptions have shown appreciable differences in the shape of the sigmoid notch among extant Phocinae [59], but no detailed description has been provided for Monachinae [42,74]. Overall, the position of the anconeal process suggests that the ulna is phocine. However, being only one characteristic combined with the poor state of preservation of the specimens raises doubt on the validity of this assumption. Therefore, it is safer to consider the specimens Phocidae cf. Frisiphoca affine.
Figure 6.
Right ulna IRSNB 1121-M261a assigned here to Phocidae cf. Frisiphoca affine (originally Monatherium affine by Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in lateral (a), anterior (b) and medial (c) view. Scale bar equals 5 cm.
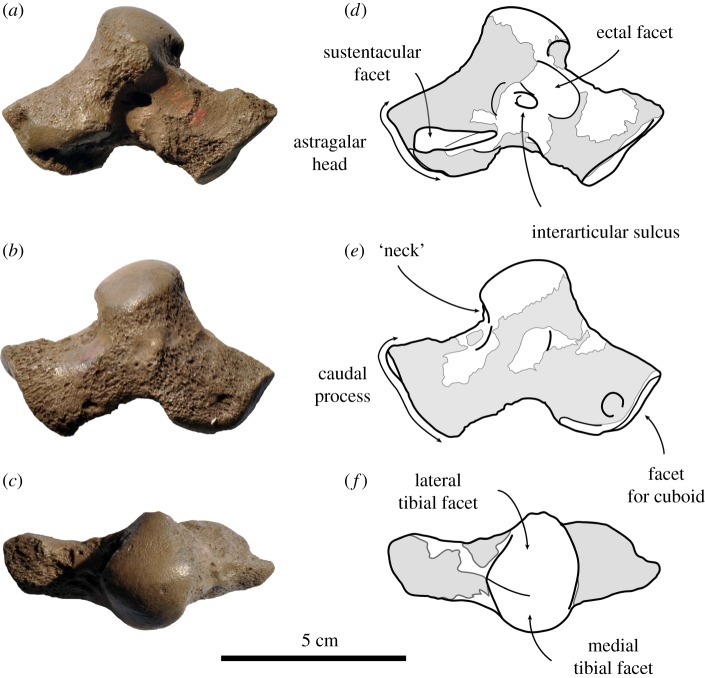
Astragalus (figure 7). One isolated left astragalus (IRSNB 1126-M262) can very tentatively be assigned to Phocidae cf. Frisiphoca affine. Although it had been found isolated, at an absolute length of 75.5 mm (table 4), it is noticeably larger than the isolated specimen originally assigned to Monotherium aberratum, IRSNB 1144-M272 (considered Phocidae indet. in this study, see Supplementary Information 3), which has an estimated absolute length of 5.62 mm, and much larger than the adult male pes originally assigned to M. aberratum (IRSNB 1187-M273; also Phocidae indet. in this study, see Supplementary Information 3). In addition, a number of differences can be observed, separating this astragalus from the indeterminate phocid astragalus IRSNB 1144-M272.
Figure 7.
Left astragalus IRSNB 1126-M262 assigned here to Phocidae cf. Frisiphoca affine (originally Monatherium affine by Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in lateral (a), medial (b) and dorsal (c) view. Corresponding labelled drawings of IRSNB 1126-M262 in lateral (d), medial (e) and dorsal (f) view. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
The tibial facet is small, and proportionally smaller than in IRSNB 1144-M272 and other phocid astragali. The lateral and medial tibial facets form a right angle in proximal view, as in Monachus sp., Phocinae and Piscophoca pacifica; but this angle is slightly larger than in IRSNB 1144-M272. In lateral view, the tibial facet is convex. The tibial facet is separated from the caudal process by a ‘neck’, which is more pronounced than in other phocids, except Acrophoca longirostris. The caudal process is long, which is a typically phocid characteristic [58], and plantardorsally elongate, but mediolaterally not as thick as in astragalus IRSNB 1144-M272. The dorsoplantar height of the caudal process of IRSNB 1126-M262 is 30.0 mm, and the mediolateral width of the process is 16.6 mm. The dorsoplantar height of the caudal process of IRSNB 1144-M272 cannot be measured due to the poor state of preservation, but the mediolateral width is 18.5 mm, which is somewhat wider than in IRSNB 1126-M262. Although the proximodistally elongate ectal facet is not completely preserved, it appears proportionally thicker and less elongate than in IRSNB 1144-M272. However, the elongation of the ectal and sustentacular facets is marked, uniting both specimens with Australophoca changorum, Monachus sp., Phocinae and Piscophoca pacifica. The elongate sustentacular facet is well developed and highly raised over the astragalar head. This facet is convex and facing ventrolaterally; it is separated from the ectal facet by an interarticular sulcus. This sulcus forms a distinct oval fossa just ventroproximally of the ectal facet. Although this sulcus is present in other Phocidae as well [57], it only forms a deep and narrow fossa in IRSNB 1126-M262. The sustentacular facet is separated from the facet for the navicular. The facet for the navicular is plantardorsally elongate and runs along the entire distal margin and the distal portion of the plantar margin of the astragalus. The long axes of the caudal process and of the head form an obtuse angle of approximately 120°, making the astragalus arched in lateral view, as in extant Lobodontini. Given the strong similarities between the astragalus in Phocinae and some Monachinae, it is difficult to elucidate whether IRSNB 1126-M262 is monachine or phocine.
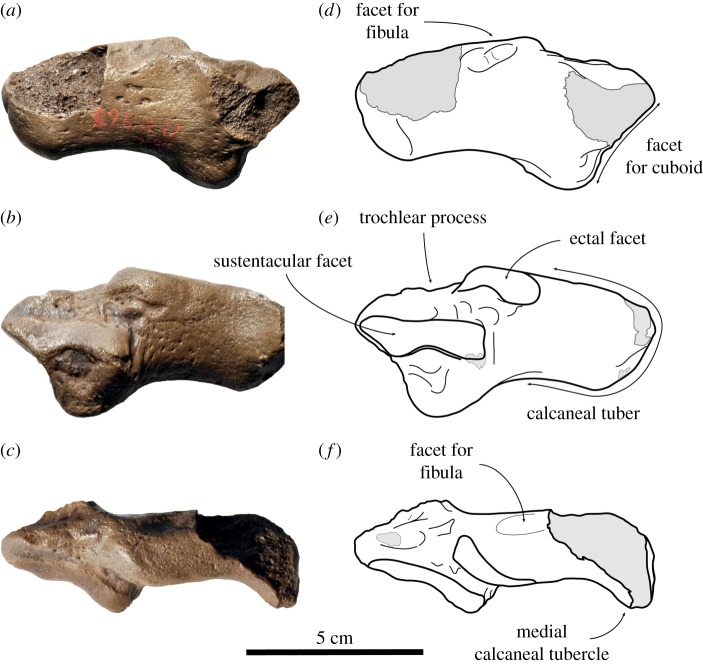
Calcaneum (figure 8). Similar to the astragalus, one isolated right calcaneum (IRSNB 1125-M263) can very tentatively be assigned to Phocidae cf. Frisiphoca affine, largely based on its relatively large size in comparison to another calcaneum from the ‘Diestian’ of Antwerp that was originally assigned to Monotherium aberratum (IRSNB 1187-M273d; Phocidae indet. this study, see Supplementary Information 3). Although it has been found isolated, the specimen is much larger than the adult male calcaneum IRSNB 1187-M273d (see Supplementary Information 3: figure S2a–c), precluding the possibility of sexual dimorphism as an argument to group both specimens in the same species. The total length of calcaneum IRSNB 1125-M263 is 78.8 mm, while the total length of calcaneum IRSNB 1187-M273d is only 51.2 mm (table 5; Supplementary Information 1: table S3). Furthermore, both calcanea differ morphologically. In lateral view, the calcaneum IRSNB 1125-M263 is distally much wider than it is proximally. However, the calcaneal tuber is plantardorsally relatively thicker in IRSNB 1144-M272 than in IRSNB 1125-M263. Muizon ([42]: table 7) employed the ratio of the plantardorsal height versus the total length of the calcaneum as a means to separate extant Lobodontini (high ratio; greater than 0.55) from other Monachinae (intermediate ratio) and Phocinae (low ratio; less than 0.50). For IRSNB 1125-M263, this ratio equals 0.503 (39.3 mm : 78.2 mm), at the boundary between Monachinae and Phocinae. The variability in dimensions is in correspondence to the differing length of the calcaneal tuber among Phocidae: the calcaneal tuber of IRSNB 1125-M263 is very long, as in Phocinae, Monachini and extinct Phocidae; but contrasts with extant Lobodontini, where the calcaneal tuber is short. Muizon [42] considered a long calcaneal tuber to be a plesiomorphic characteristic. IRSNB 1125-M263 bears a prominent medial process at its proximal end that is well developed, as in IRSNB 1187-M273d. Halfway on the dorsolateral margin of the calcaneum, there is an oval, concave facet for the articulation with the fibula. Such a facet has also been observed in IRSNB 1187-M273d, and is generally considered more prominent in Monachinae than in Phocinae [42]. The trochlear process extends across the dorsal surface of the calcaneum, anterior to the ectal facet (=proximal astragalar facet). The astragalar articular facets are relatively long, as in Phocinae. The sustentacular facet (=distal articular facet) is slightly less slender than in IRSNB 1187-M273d and the curvature of the facet in medial view is less pronounced. The ectal facet (=proximal articular facet) is oriented anterodorsally--posteroventrally, but nearly horizontal, and is shorter than in IRSNB 1187-M273d and slightly thicker. In IRSNB 1125-M263, the length of the ectal facet is 26.6% of the total length (20.8 mm : 78.2 mm), and in IRSNB 1187-M273d, the ectal facet is 28.3% of the total length (14.5 mm : 51.2 mm). The height-to-length ratio of the ectal facet is 41.3% in IRSNB 1125-M263 (8.6 mm : 20.8 mm) and 37.9% in IRSNB 1187-M273d (5.5 mm : 14.5 mm). Anteriorly, the sustentacular facet transits into the cuboid facet. The concave and lozenge-shaped cuboid facet is higher than wide and, contrasting to IRSNB 1187-M273d, not restricted to the dorsal two-thirds of the distal margin of the calcaneum. Although sharing a number of characteristics with extant Phocinae and not with extant Monachinae, because extinct Monachinae and Phocinae tend to exhibit an overall intermediate morphology [42], it is impossible to confidentially assign this specimen to either of both subfamilies and its comparison with F. affine is largely based on its size.
Figure 8.
Right calcaneum IRSNB 1125-M263 assigned here to Phocidae cf. Frisiphoca affine (originally Monatherium affine by Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in lateral (a), medial (b) and dorsal (c) view. Corresponding labelled drawings of IRSNB 1125-M263 in lateral (d), medial (e) and dorsal (f) view. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Phocidae indet.
Monotherium Van Beneden, 1876
Type species and only included species. Monotherium delognii Van Beneden, 1876.
Diagnosis. As for the species
Monotherium delognii Van Beneden, 1876
(figure 9)
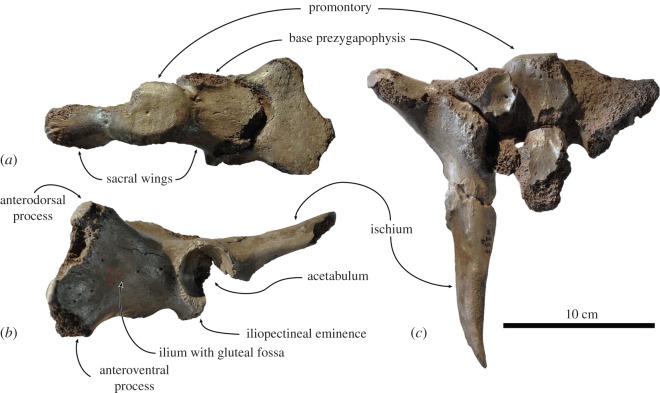
Figure 9.
Partial pelvis IRSNB 1153-M257a, b including a sacrum (a) and a left innominate (b), assigned to Monatherium delognii (Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Deurne, Antwerp in anterior (a), left lateral (b) and dorsal (c) view. Scale bar equals 10 cm.
Lectotype. IRSNB 1153-M257a, b, partial sacrum including the sacral wings and the bodies of the first and second sacral vertebrae, and the associated left innominate represented by the ilium and the acetabular branch of the ischium, originally assigned to Monatherium delognii by Van Beneden ([17]: pl. 16, figs 5, 6), ‘Diestian’, third section at Deurne, Antwerp, Belgium.
Type locality. Third section at Deurne, Antwerp, Belgium. The ‘third section’ follows Van Beneden's discretization of the nineteenth-century fortification constructions around the city of Antwerp, with the third section at Deurne being located southwest to the Deurne district of Antwerp ([9]: fig. 2; [10]: fig. 1) [9,11,17,67]. It should be noted that this type locality is derived from the original labels associated to the specimen. In his original publications Van Beneden [16,17] did not discuss the geographical provenance of individual specimens of the original fossil record of Monotherium delognii.
Type horizon. Van Beneden (unpublished handwritten notes in the IRSNB archives) assigned the specimen IRSNB 1153-M257a, b to the ‘Diestien’ (Diestian). However, as mentioned above, the Diestian is currently abandoned, with different authors providing different ages and stratigraphic intervals for the Diestian ([19]: table 1). Generally, it is considered that the Diestian is equivalent to the Deurne Sands Member of the Diest Formation. Louwye et al. [21] assigned a Tortonian (late Miocene; 8.8–11.4 Ma) age to the Diest Formation north of Antwerp and in the Campine area.
Diagnosis. Large phocid, comparable in size to the monachine Leptonychotes weddelli, larger than the extinct phocine Prophoca rousseaui. Differences from other Phocidae (except Prophoca rousseaui) in: straight horizontal ventral margin of the sacral wings, wide base for the prezygapophysis of S1, the anterior offset of the promontory to the sacral wings, an elongate ilium, weak lateral eversion of the ilium (also in Monachinae, Erignathus barbatus and Kawas benegasorum), and the weak development of a gluteal fossa on the ilium (also in Monachinae, E. barbatus and K. benegasorum). Differs from P. rousseaui by a dorsoventral compression of the promontory of the sacrum and more rounded lateral margins of the sacral wings.
Comments. Neither Van Beneden [16,17] nor subsequent researchers (e.g. [18]) assigned a type specimen to Monotherium delognii. Of all specimens assigned to M. delognii, the specimen IRSNB 1153-M257a, b is the least unsatisfactory in terms of diagnostic value. As mentioned above, Van Beneden [16,17] did not assign a type species to the genus Monotherium; later, Kellogg [18] considered Monotherium delognii as the type species, giving it page priority over Monotherium affine and Monotherium aberratum. Originally, Van Beneden [17] assigned a much more extensive number of specimens to M. delognii, also including vertebrae (IRSNB 1108, IRSNB 1108-M255a, b, IRSNB 1216, IRSNB 1217, IRSNB 1217-M256a), a radius (IRSNB 1139), a fibula (IRSNB 1149), phalanges (IRSNB 1217-M256b, IRSNB 1227) and indeterminate remains (IRSNB 1115). The state of preservation of these specimens, composed of moderately to poorly preserved disassociated bones, does not allow identification to a specific phocid taxon; they are, hence, considered indeterminate monachine, phocid or phocine specimens (Supplementary Information 3). From the current study it is evident that the lectotype of M. delognii shows similarities with the pelvis of the phocine Prophoca rousseaui [10]. However, the size of the pelvis matches well the size of the lectotype humerus IRSNB 1118-M260 of Frisiphoca affine (see below). Because no pelvis is currently known for the latter species, no further comparison can be done. Due to its similarities to P. rousseaui, this pelvis of M. delognii is tentatively considered a phocine pelvis, pending the discovery of more complete specimens and a detailed phylogenetic analysis.
Description and comparison
Sacrum (figure 9). The sacrum IRSNB 1153-M257a is only very partially preserved: only the sacral wings, and the bodies of the first and second sacral vertebrae are incompletely preserved and severely abraded, inhibiting a detailed description. The promontory is dorsoventrally slightly compressed (43.9 mm : 60.9 mm). The sacral wings (alae) are strongly laterally enlarged, but not as pronounced as in Prophoca rousseaui or some other phocids [10]: the transverse width across the wings is 2.70 times the lateral width across the promontory (164.4 mm : 60.9 mm) (3.31× in P. rousseaui, [10]). Although past studies indicated that this ratio is higher in Monachinae than in Phocinae [42], Dewaele et al. [10] showed that there is considerable overlap between the ratio ranges of Monachinae and Phocinae, preventing clear distinction between both subfamilies. The lateral margins of the sacral wings are badly preserved, but they appear to have been originally rounded. The ventral margins of the sacral wings are remarkably straight horizontally, as in P. roussseaui, but unlike other Phocidae. Other similarities with P. rousseaui are the dorsal and posterior offset of the wings in relation to the promontory, and the wide base of the prezygapophysis.
Innominate (figure 9). The partial left innominate IRSNB 1153-M257b associated with the partial sacrum IRSNB 1153-M273a is noticeably larger than the innominates IRSNB 1192-M276 and IRSNB M2234 (both assigned to Prophoca rousseaui [10]). Similar to what we observe for the sacrum, the innominate IRSNB 1153-M273 strongly resembles that of P. rousseaui (most noticeably IRSNB 1192-M276). The ilium is strongly elongate in comparison with other Phocidae, except the monachines Monachus monachus and Piscophoca pacifica, and the phocine P. rousseaui. Following the procedure of Dewaele et al. [9] to quantify the lateral eversion of the ilium of Phocidae, the angle of the ilium to the postacetabular region of innominate IRSNB 1153-M257b is 63.4°. This falls within the range observed for Monachinae (average 65.6°), but at the lower end of the range of extant Phocinae (average 74.6°) [9]. The lateral eversion of the ilium and the degree of development of the gluteal fossa are similar to that in Monachinae, the extinct phocines Kawas benegasorum and P. rousseaui, and the extant phocine Erignathus barbatus. As in P. rousseaui the shape of the iliac crest is slender, with the anteroventral process located anterior to the level of the anterodorsal process. The posterodorsal and posteroventral processes are well developed as well. The iliopectineal eminence is very incompletely preserved, but appears well developed. Lastly, a deep acetabulum is shared with Monachus spp. and Phocinae. Contrasting to P. rousseaui, the ilium is not as markedly triangular in outline in IRSNB 1153-M273b. It should be noted that Dewaele et al. [10] suggested some degree of sexual dimorphism in P. rousseaui, considering the larger IRSNB 1192-M276 as a hypothetical male and IRSNB M2234 as a female. Given the observed number of differences and the incompleteness of the fossil record of M. delognii, it is not replaced into Prophoca, pending the discovery of more complete specimens.
Phylogenetic analyses
First analysis (figure 10a). A first phylogenetic analysis (k-value of the Goloboff criterion set at three) includes all three redescribed and reassigned taxa Frisiphoca aberratum, Frisiphoca affine and Noriphoca gaudini, as well as Monotherium? wymani. The pinnipedimorphs Enaliarctos mealsi and Pteronarctos goedertae, the otariids Otaria byronia and Thalassoleon mexicanus, and the desmatophocid Allodesmus kernensis are selected as outgroups. This analysis resulted in 29 most parsimonious phylogenetic trees (50% majority consensus tree figure 10a) with score −60.10 (tree length 220) after 133 718 tried rearrangements. Consistency index (CI) is 0.45, homoplasy index (HI) is 0.55, retention index (RI) is 0.69, and rescaled consistency index (RC) is 0.31. Higher level phylogenetic relationships correspond better with previously published phylogenetic analysis, returning Enaliarctos mealsi and Pteronarctos goedertae as stem Pinnipedimorpha, Allodesmus kernensis (Desmatophoca), Otariidae and Phocidae as Pinnipedia, and A. kernensis and Phocidae as Phocoidea [58,75]. Kawas benegasorum and Leptophoca proxima are returned as stem Phocinae, as has been shown before [9,10]. However, Devinophoca claytoni and Nanophoca vitulinoides are not returned as stem Phocinae, contrasting with a recent analysis by Dewaele et al. [9]. Historically, there has been little consensus on the phylogenetic relationships of extant and extinct Monachinae [6,8,76]. This first phylogenetic analysis returns F. aberratum and F. affine as stem Phocinae (figure 10a), branching off prior to K. benegasorum and L. proxima. Monotherium? wymani and Noriphoca gaudini are returned as stem Monachinae (figure 10a). Both Frisiphoca aberratum and Frisiphoca affine are very incompletely scored (7/80 characters) and share typical features with both Monachinae and Phocinae (see description), which makes it difficult to clarify phylogenetic affinities. Indeed, a test to elucidate the bootstrap support for this analysis resulted in poorly supported phylogenetic relationships among all Phocidae included in the analysis.
Figure 10.
Phylogenetic tree resulting from the (a) first, (b) second and (c) third analysis. (a) The first analysis includes Frisiphoca aberratum, Frisiphoca affine, Monotherium? wymani and Noriphoca gaudini. 50% majority consensus tree of 29 most parsimonious trees without equal weighting of homoplastic characters. (b) The second analysis includes M.? wymani and N. gaudini. 50% majority consensus tree of 75 most parsimonious trees without equal weighting of homoplastic characters. (c) The third analysis includes M.? wymani and N. gaudini (a). 50% majority consensus tree of 174 most parsimonious trees with equal weighting of homoplastic characters. Frisiphoca aberratum, F. affine, M.? wymani and N. gaudini highlighted in bold. Extinct taxa are indicated by a dagger. All bootstrap values equal to or higher than 50 (after 10 000 replicates) are shown.
Second analysis (figure 10b). A second phylogenetic analysis (with down-weighting homoplastic characters and k-value of the Goloboff criterion set at three) excludes both Frisiphoca species, but includes Monotherium? wymani and Noriphoca gaudini. This analysis resulted in 75 most parsimonious phylogenetic trees (50% majority consensus tree, figure 10b) with score −60.21 (tree length 217) after 331 705 tried rearrangements. CI is 0.46, HI is 0.55, RI is 0.69 and RC is 0.32. The second analysis returns extinct Monachinae from South America (Acrophoca longirostris, Hadrokirus martini and Piscophoca pacifica) and South Africa (Homiphoca capensis) as stem Monachinae, while the first analysis returned them as a clade nested within crown Monachine. As in the first analysis (figure 10a), M.? wymani and N. gaudini are returned as stem monachines, with N. gaudini being the earliest branching stem monachine included in the analysis.
Third analysis (figure 10c). The same analysis as the second analysis (see above), but without down-weighting homoplastic characters, resulted in 174 most parsimonious phylogenetic trees (50% majority consensus tree, figure 10c) with score 216 after 727 267 tried rearrangements. CI is 0.46, HI is 0.54, RI is 0.69 and RC is 0.32. The topology of this analysis is largely similar to the topology of the second analysis (figure 10b), except for the phylogenetic position of Nanophoca vitulinoides among Phocinae and the phylogenetic position of Homiphoca capensis among Monachinae. The phylogenetic relationships of M.? wymani and N. gaudini are identical to the relationships observed in the second analysis. Noriphoca gaudini is again returned as the earliest branching stem monachine.
Fourth analysis (figure 11). The fourth phylogenetic analysis excludes Frisiphoca and Monotherium? wymani and focuses on elucidating the phylogenetic relationships of Noriphoca gaudini. The analysis with down-weighting homoplastic characters and k-value of the Goloboff criterion set at three resulted in three most parsimonious phylogenetic trees (50% majority consensus tree, figure 11) with score −57.23 (tree length 246) after 15 494 tried rearrangements. CI is 0.40, HI is 0.60, RI is 0.62 and RC is 0.25. The analysis returns the same topology as for the second analysis (figure 10b), but with the exclusion of M.? wymani. Noriphoca gaudini is the first stem monachine to branch off the Monachinae clade. A bootstrap value of 51 supports the inclusion of N. gaudini among Monachinae. A complete list of the apomorphies that resulted from the phylogenetic analysis is provided as electronic supplementary material (Supplementary Information 1: figure S1 and table S5). In the phylogenetic analysis, the identification of N. gaudini is supported by one unequivocal autapomorphy: the ventral edge of the zygomatic arch is level with the alveolar plane (character 13, state ‘0’ to ‘1’).
Figure 11.
Phylogenetic tree resulting from the fourth analysis, excluding Frisiphoca aberratum, Frisiphoca affine and Monotherium? wymani. 50% majority consensus tree of the six most parsimonious trees. The age ranges for extinct OTUs are expressed as a green bar over each relevant terminal branch. Bootstrap values exceeding 50% are indicated on the relevant branches. Geochronological ages for the included species, whenever fossil or subfossil specimens have been documented: Acrophoca longirostris [42], Allodesmus kernensis [76] Enaliarctos mealsi [52], Devinophoca claytoni [30], Erignathus barbatus [77,78], Hadrokirus martini [8], Halichoerus grypus [79], Homiphoca capensis [80], Hydrurga leptonyx [18,81], Kawas benegasorum [82], Leptophoca proxima [10], Mirounga leonina [83], Monachus monachus [84], Nanophoca vitulinoides [9], Noriphoca gaudini (this study), Ommatophoca rossii [81], Otaria byronia [85], Phoca vitulina [79], Piscophoca pacifica [8], Pliophoca etrusca [6], Pteronarctos goedertae [54] and Thalassoleon mexicanus [55]. Extinct taxa are indicated by a dagger. Noriphoca gaudini indicated in bold.
Fifth analysis. Rerunning the fourth analysis without down-weighting homoplastic characters resulted in six most parsimonious phylogenetic trees with tree length 216 after 24 773 tried rearrangements. CI is 0.46, HI is 0.54, RI is 0.69 and RC is 0.32. The topology of this analysis differs from the fourth analysis for the Phocinae, with the clade composed of Kawas benegasorum + Leptophoca proxima returned as a sister clade to all other included Phocinae, and with Nanophoca vitulinoides returned as the last stem phocine before crown Phocinae. However, the phylogenetic position of Noriphoca gaudini as the earliest-branching stem monachine is unchanged.
6. Discussion
6.1. Systematic palaeontology
Prior to our reassessment of the extinct phocid Monotherium, this genus included five species: Monotherium aberratum, Monotherium affine, Monotherium delognii, Monotherium gaudini and Monotherium? wymani. Our study strongly impacts this content, questioning the validity of some species and reassigning others to different genera in different subfamilies. Following the current study, Monotherium is split in three different genera, including four species. The monachine M.? wymani cannot be compared to Monotherium delognii, the type species of Monotherium with unknown subfamilial attributions. However, the holotype of M.? wymani is two associated tympanic bullae, which are considered diagnostic in Phocidae. Hence, the redescription of M.? wymani is left in limbo, pending the discovery of more complete specimens that will allow comparison with other extinct Phocidae. Monotherium gaudini is identified as the earliest branching monachine seal. This is supported by multiple phylogenetic analyses. Monotherium gaudini is renamed to Noriphoca gaudini. The fossil records of M. aberratum and M. affine are limited to their respective holotype humeri. Despite the incompleteness of their fossil records, a detailed redescription and one preliminary phylogenetic analysis return both as stem Phocinae. Both species are regrouped into the new genus Frisiphoca: Frisiphoca aberratum and Frisiphoca affine.
6.2. Stratigraphy
Prior to the current study, the stratigraphic position and geological age of Noriphoca gaudini was poorly known and no formal reinvestigation of the original stratigraphic data have been published since the original publication by Guiscardi in 1870 [7]. To date, it remains impossible to retrace the exact geographical and stratigraphic position of the specimen. However, the Eocene Santo Spirito and late Oligocene to Miocene Bolognano formations are the only geological formations outcropping at the approximate type locality of N. gaudini, 3 km east of Roccamorice, Italy. Guiscardi [7] stated that the specimen came from a bituminous layer, which corresponds to the Lepidocyclina Limestone of the Bolognano Formation, dated to the Oligocene or earliest Miocene (Aquitanian) ([33,35]; and references therein). Consequently, N. gaudini can be considered the oldest known phocid seal, older than the Burdigalian Afrophoca libyca from Libya ([36]; contra [30,31]).
N. gaudini is clearly a phocid (e.g. the tooth row is diverging posteriorly (character 31, state ‘0’ to ‘1’) and M2 is absent (character 42, state ‘0’ to ‘1’)) (Supplementary Information 1, table S5), N. gaudini is arguably one of the oldest known crown pinnipeds, being equally old as or older than Desmatophoca brachycephala from the Aquitanian of the northeast Pacific [46]. Formerly, Koretsky & Sanders [30] and Diedrich [31] presented allegedly older phocid seal specimens from the late Oligocene of South Carolina and the Lutetian (middle Eocene) of Germany, respectively. Although these specimens are clearly phocid as well, Dewaele et al. [10] formally questioned the validity of stratigraphy of both findings. Noriphoca gaudini is also of the same age as Enaliarctos spp. from the Oligocene and early Miocene of the northeast Pacific [53]. This ascertains a late Oligocene diversification of Pinnipedimorpha and that different families of Pinnipedia already existed during the late Oligocene--early Miocene.
Formerly, a Diestian age had been assigned to specimens of Monotherium from Belgium. However, the Diestian age has been abandoned more recently. A detailed reinvestigation of the literature allows assuming that Frisiphoca aberratum, Frisiphoca affine and Monotherium delognii all come from the Deurne Sands Member of the Diest Formation [17,68]. Louwye et al. [21], Louwye [22] and Louwye & De Schepper [23] concluded that the deposits of the Diest Formation (Deurne Sands Member) near the city of Antwerp are of late Tortonian age. This age is moderately well supported by dinoflagellate cyst biostratigraphy of a sediment sample (1132LDW-1102Lab) associated with specimen IRSNB 1132-M269 (formerly M. aberratum, currently Phocidae indet.), which yielded an age range of 7.25–3.7 Ma (latest Tortonian to late Zanclean).
Overall, the stratigraphy of North American specimens that have been related to Monotherium [15] remains poorly resolved. One partial humerus sharing affinities with Frisiphoca aberratum comes from the Gay Head Greensand, which is considered to be part of the St Marys Formation (Tortonian, upper Miocene) (Dinocyst zones DN 8 and 9 in Kidwell et al. [65]; dates from Köthe [73]; see also Dall [72] and Ray [15]). This roughly matches the late Tortonian age proposed for the Deurne Sands Member of the Diest Formation, from where F. aberratum comes. Contrastingly, the stratigraphic origin of an ulna showing affinities with Frisiphoca affine and of the holotype ear region of Monotherium? wymani is poorly resolved.
6.3. Phylogenetic analysis and palaeobiogeographical considerations
The phylogenetic relationships among the Phocinae in the cladistic analyses correspond with the previously published trees by Dewaele et al. [9,10], with Leptophoca proxima and Kawas benegasorum as stem Phocinae. The phylogenetic relationships of extant and extinct Monachinae are less well established and different previously published analyses show different topologies [6,8,76]: the specific relationships among the four lobodontin species remain poorly resolved [34,43,47,86], and there is no agreement whether extinct Monachinae from the Southern Hemisphere form a distinct clade [8] or not [6,87], or whether they are stem Monachinae or not.
The phylogenetic trees obtained here clearly show that the extremely fragmentary fossil record of Frisiphoca aberratum and Frisiphoca affine precludes any detailed phylogenetic analysis and that it is for now impossible to resolve the subfamilial affinities of these taxa based on the current phylogenetic analysis. However, the presence of an entepicondylar in the lectotype humeri of F. aberratum and F. affine supports assigning the genus Frisiphoca to the Phocinae subfamily. Monotherium? wymani, on the other hand, is returned as a stem monachine, but the holotype is very incompletely coded (7/80 characters), and the exact phylogenetic relationships vary between different analyses (figure 10b,c) and remain unclear. Therefore, M.? wymani is considered a monachine of unknown phylogenetic affinities.
Noriphoca gaudini is a stem monachine, a result that agrees well with its geological age. Assuming that the type specimen of N. gaudini had indeed been collected from the bituminous layers of the Lepidocyclina Limestone of the Bolognano Formation, this species can be dated to the Oligocene or earliest Miocene (Aquitanian) ([33,35]; and references therein), which is older than the Burdigalian (ca 19 Ma) Afrophoca libyca from Libya [36]. Consequently, our phylogenetic analysis, together with the presence of A. libyca in the early Miocene Mediterranean, may suggest a Mediterranean origin for the subfamily Monachinae or even the entire family Phocidae. Ancestral non-phocid pinnipedimorphs were all restricted to the North Pacific realm [4,44–55]. The oldest pinnipedimorphs are Enaliarctos spp. from the late Oligocene, and the previously oldest known crown pinniped is Desmatophoca brachycephala from the Aquitanian of the northeast Pacific. It is broadly accepted that the direct ancestors of Phocidae crossed the Central American Seaway, with an origin of Phocidae along the east coast of North America ([4]; contra [88]). However, no fossils of these stem Phocidae have been found or recognized, either near the Central American Seaway, or on the east coast of North America, or in Europe. Hence, it can be hypothesized that direct ancestors of Phocidae or the earliest Phocidae crossed the North Atlantic Ocean from the Central American Seaway to the Mediterranean Sea during the late Oligocene or earliest Miocene, shortly after diversification of early Pinnipedimorpha and Pinnipedia in the northeast Pacific. Direct migration from the Central American Seaway to the Mediterranean may indeed explain (1) the record of a monachine mandible in the Burdigalian to Langhian of Libya (Afrophoca libyca) [36], (2) the early diversification of Phocidae taking place in Europe [9,10], and (3) the lower diversity of the Phocidae during the early and middle Miocene along the east coast of North America, as compared to Europe [10].
7. Conclusion
Formerly, the extinct phocid genus Monotherium included five different species: Monotherium aberratum Van Beneden, 1876; Monotherium affine Van Beneden, 1876; Monotherium delognii Van Beneden, 1876; Monotherium gaudini (Guiscardi, 1870); and Monotherium? wymani (Leidy, 1853). After a careful revision, only the type species M. delognii is retained, and considered Phocidae indet., sharing affinities with the late middle to late Miocene North Sea stem phocine species Prophoca rousseaui. Monotherium aberratum and M. affine are replaced into the new, most likely phocine, genus Frisiphoca. Most of the original material from the late Miocene of the North Sea is considered non-diagnostic, with only a lectotype humerus retained for F. aberratum, one lectotype humerus for F. affine, and one lectotype pelvis for M. delognii. Frisiphoca aberratum, F. affine and M. delognii are dated to the late Miocene, based on stratigraphic provenance in the literature.
First identified as Phoca wymani and being restricted here to its holotype ear region, the species Monotherium? wymani from the east coast of North America cannot be compared to M. delognii, but the type tympanic bullae could be considered diagnostic; the taxon is not treated in more detail, pending the discovery of more complete fossils. However, some specimens previously referred to Monotherium from the east coast of North America share affinities with F. aberratum and F. affine from the North Sea Basin, indicating a certain degree of faunal communication across the North Atlantic during the late Miocene.
Finally, the Italian species Monotherium gaudini is replaced into a new genus within the subfamily Monachinae (as confirmed with the phylogenetic analysis): Noriphoca gaudini. A careful literature study of N. gaudini reveals the taxon as one of the two oldest, or even the oldest known phocid, dated to the Chattian (late Oligocene) or Aquitanian (early Miocene). This is also the oldest known record of a crown pinniped, replacing the desmatophocid Desmatophoca brachycephala from the Aquitanian of the northeast Pacific as the oldest known crown pinniped. Previously, Dewaele et al. [10] questioned the validity of published phocid fossil records from the Oligocene of South Carolina, USA [30] and the Eocene of Germany [31]. The revised stratigraphic origin of N. gaudini pushes the origination and the migration of the direct ancestors of Phocidae or the earliest Phocidae through the Central American Seaway and eastwards across the Atlantic back to the late Oligocene and indicates a very early diversification of Pinnipedimorpha into the different families of Pinnipedia.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The research presented in this publication is part of the PhD research of L.D. at Ghent University, with O.L. and S.L. as advisors. We would like to thank G. Bianucci from the Università di Pisa and M. del Re from the Università di Napoli for information on Noriphoca gaudini; C. de Muizon (Muséum national d'Histoire naturelle, Paris, France), D.J. Bohaska and N.D. Pyenson (USNM), and S. Bruaux, C. Cousin and A. Folie (IRSNB) for access to museum collections, and S. Van Cauwenberghe (Ghent University) for the preparation of the palynological samples; and Paul and Pierre Gigase from Antwerp for access to their private collection of fossil pinnipeds from the Antwerp area. We would also like to thank F.G. Marx (IRSNB), C.M. Peredo and M.D. Uhen (George Mason University) for discussing aspects of the research with us. Special thanks to R. Sansom (Associate Editor), J. Blundy (Subject Editor), R.W. Boessenecker (Reviewer) and one anonymous reviewer for helpful and constructive comments on earlier drafts of this paper.
Data accessibility
All data for this paper are available within this paper or as electronic supplementary material of this paper. Length measurements of specimens, dinoflagellate cyst biostratigraphy results, and the phylogenetic matrix are provided as electronic supplementary material. This published work and the nomenclatural acts it contains have been registered in ZooBank. The LSID for this publication is urn:lsid:zoobank.org:pub:17B81201-199E-4577-B465-79F65A108A8.
Authors' contributions
L.D. designed the study, carried out the description and phylogenetic analysis, and contributed to the discussion and writing of the paper. O.L. carried out the description and phylogenetic analysis and contributed to the discussion and writing of the paper. S.L. carried out the dinoflagellate cyst biostratigraphy and contributed to the discussion and writing the paper. All authors gave their approval for the publication of this version of the manuscript.
Competing interests
The authors declare that there are no competing interests.
Funding
Funding is provided as an FWO PhD Fellowship for L.D. (grant no. 11V9115N). Additional funding for a research visit to the National Museum of Natural History, Washington, DC, USA, by L.D., was provided as an FWO long stay travel grant (grant no. V411116N). There was no additional funding received for this study. Funders played no role in study design, data collection, analysis, publishing decisions or preparation of the manuscript.
References
- 1.Berta A, Churchill M. 2012. Pinniped taxonomy: review of currently recognized species and subspecies, and evidence used for their description. Mammal Rev. 42, 207–234. (doi:10.1111/j.1365-2907.2011.00193.x) [Google Scholar]
- 2.Koretsky IA. 2001. Morphology and systematics of the Miocene Phocinae (Mammalia: Carnivora) from Paratethys and the North Atlantic Region. Geol. Hungarica Series Palaeontol. 54, 1–109. [Google Scholar]
- 3.Koretsky IA. 2003. New finds of Sarmatian seals (Mammalia, Carnivora, Phocinae) from southern Hungary. In Advances in Vertebrate Paleontology ‘Hen to Panta’: a tribute to Constantin Rădulescu and Petre Mihai Samson (eds Petculescu A, Ştiucă E), pp. 63–70. Bucharest, Romania: Emil Racoviţă Institute of Speleology. [Google Scholar]
- 4.Deméré TA, Berta A, Adam PJ. 2003. Pinnipedimorph evolutionary biogeography. Bull. Am. Museum Nat. His. 279, 32–76. (doi:10.1206/0003-0090(2003)279<0032:C>2.0.CO;2) [Google Scholar]
- 5.Bianucci G, Gatt M, Catanzariti R, Sorbi S, Bonavia CG, Curmi R, Varola A. 2011. Systematics, biostratigraphy and evolutionary pattern of the Oligo-Miocene marine mammals from the Maltese Islands. Geobios 44, 549–585. (doi:10.1016/j.geobios.2011.02.009) [Google Scholar]
- 6.Berta A, Kienle S, Bianucci G, Sorbi S. 2015. A Reevaluation of Pliophoca etrusca (Pinnipedia, Phocidae) from the Pliocene of Italy: phylogenetic and biogeographic implications. J. Vertebr. Paleontol. 35, e889144 (doi:10.1080/02724634.2014.889144) [Google Scholar]
- 7.Guiscardi G. 1870. Sopra un teschio fossile di foca. Rendiconti dell'Accademia delle Science Fisiche e Matematiche 5, 1–8. [Google Scholar]
- 8.Amson E, de Muizon C. 2014. A new durophagous phocid (Mammalia: Carnivora) from the late Neogene of Peru and considerations on monachine seal phylogeny. J. Syst. Paleontol. 12, 523–548. (doi:10.1080/14772019.2013.799610) [Google Scholar]
- 9.Dewaele L, Amson E, Lambert O, Louwye S. 2017. Reappraisal of the extinct seal ‘Phoca’ vitulinoides from the Neogene of the North Sea Basin, with bearings on its geological age, phylogenetic affinities, and locomotion. PeerJ 5, e3316 (doi:10.7717/peerj.3316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewaele L, Lambert O, Louwye S. 2017. On Prophoca and Leptophoca (Pinnipedia, Phocidae) from the Miocene of the North Atlantic realm: redescription, phylogenetic affinities and paleobiogeographic implications. PeerJ 5, e3024 (doi:10.7717/peerj.3024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans HE, de Lahunta A. 2013. Miller's anatomy of the dog, 4th edn, 850 p St Louis, MO: Elsevier Saunders. [Google Scholar]
- 12.Louwye S, Head MJ, De Schepper S. 2004. Dinoflagellate cyst stratigraphy and palaeoecology of the Pliocene in northern Belgium, southern North Sea Basin. Geol. Mag. 141, 353–378. (doi:10.1017/S0016756804009136) [Google Scholar]
- 13.Fensome RA, MacRae RA, Williams GL. 2008. DINOFLAG2, Version 1. American Association of Stratigraphic Palynologists, Data Series No. 1.
- 14.Swofford DL. 2001. PAUP*: phylogenetic analysis using parsimony (*and other methods). version 4b10. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 15.Ray CE. 1976. Phoca wymani and other tertiary seals (Mammalia: Phocidae) described from the eastern seaboard of North America. Smithsonian Contrib. Paleobiol. 28, 1–36. (doi:10.5479/si.00810266.28.1) [Google Scholar]
- 16.Van Beneden P-J. 1876. Les phoques fossiles du basin d'Anvers. Bulletin de l'Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique 41, 783–802. [Google Scholar]
- 17.Van Beneden P-J. 1877. Description des ossements fossiles des environs d'Anvers, première partie. Pinnipèdes ou amphithériens. Annales du Musée Royal d'Histoire Naturelle de Belgique 1, 1–88. [Google Scholar]
- 18.Kellogg R. 1922. Pinnipeds from Miocene and Pleistocene deposits of California. University of California Publications, Bulletin of the Department of Geological Sciences 13, 23–132. [Google Scholar]
- 19.Laga P, Louwye S. 2006. Disused Neogene and Quaternary regional stages from Belgium: Bolderian, Houthalenian, Antwerpian, Diestian, Deurnian, Kasterlian, Kattendijkian, Scaldisian, Poederlian, Merksemian and Flandrian. Geol. Belgica 9, 215–224. [Google Scholar]
- 20.Van Ertborn O. 1880. Lettre de O. Van Ertborn concernant la position du Diestien et l’âge du sable d'Hérenthals. Annales de la Societé géologique du Nord 7, 191–192. (letter of Van Ertborn, read by Gosselet at the session of 5 May 1880). [Google Scholar]
- 21.Louwye S, De Coninck J, Verniers J. 1999. Dinoflagellate cyst stratigraphy and depositional history of Miocene and Lower Pliocene formations in northern Belgium (southern North Sea Basin). Geol. Mijnbouw 78, 31–46. (doi:10.1023/A:1003793300214) [Google Scholar]
- 22.Louwye S. 2002. Dinoflagellate cyst biostratigraphy of the Upper Miocene Deurne Sands (Diest Formation) of northern Belgium, southern North Sea Basin. Geol. J. 37, 55–67. (doi:10.1002/gj.900) [Google Scholar]
- 23.Louwye S, De Schepper S. 2010. The Miocene-Pliocene hiatus in the southern North Sea Basin (northern Belgium) revealed by dinoflagellate cysts. Geol. Mag. 147, 760–776. (doi:10.1017/S0016756810000191) [Google Scholar]
- 24.Dybkjaer K, Piasecki S. 2010. Neogene dinocyst zonation of the eastern North Sea Basin, Denmark. Rev. Palaeobot. Palynol. 161, 1–29. (doi:10.1016/j.revpalbo.2010.02.005) [Google Scholar]
- 25.Schreck M, Matthiesen J, Head MJ. 2012. A magnetostratigraphic calibration of Middle Miocene through Pliocene dinoflagellate cyst and acritarch events in the Iceland Sea (Ocean Drilling Program Hole 907A). Rev. Palaeobot. Palynol. 187, 66–94. (doi:10.1016/revpalbo.2012.08.006) [Google Scholar]
- 26.Quaijtaal W, Donders T, Persico D, Louwye S. 2014. Characterizing the middle Miocene Mi-events in the eastern North Atlantic realm: a first high-resolution marine palynological record from the Porcupine Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 399, 140–159. (doi:10.1016/j.palaeo.2014.02.017) [Google Scholar]
- 27.Louwye S, De Schepper S, Laga P, Vandenberghe N. 2007. The Upper Miocene of the southern North Sea Basin (northern Belgium): a palaeoenvironmental and stratigraphical reconstruction using dinoflagellate cysts. Geol. Mag. 144, 33–52. (doi:10.1017/S0016756806002627) [Google Scholar]
- 28.De Schepper S, Head MJ. 2008. Age calibration of dinoflagellate cyst and acritarch events in the Pliocene-Pleistocene of the eastern North Atlantic (DSDP Hole 610A). Stratigraphy 5, 137–161. [Google Scholar]
- 29.Köthe A, Stäger S.. 2015. Marine late Miocene to early Pleistocene near shore environments at the southern rim of the North Sea Basin (Kranenvurg/Kleve, Germany). Dinoysts, calcareous nannoplankton and Pediastrum biostratigraphy, taxonomy and paleoecology. Bundesanstalt für Geowissenschaften und Rohstoffe, 257 p.
- 30.Koretsky IA, Sanders A. 2002. Paleontology of the late oligocene Ashley and Chandler Bridge Formations of South Carolina, 1: Paleogene pinniped remains; the oldest known seal (Carnivora: Phocidae). C Smithsonian Contributions to Paleobiology 93, 179–184. [Google Scholar]
- 31.Diedrich CG. 2011. The world's oldest fossil seal record. Nat. Sci. 3, 914–920. (doi:10.4236/ns.2011.311117) [Google Scholar]
- 32.Vezzani L, Ghisetti F. 1998. Carta geologica dell'Abruzzo, scala 1:100.000. Florence, Italy: SELCA. [Google Scholar]
- 33.Brandano M, Scrocca D, Lipparini L, Petracchini L, Tomassetti L, Campagnoni V, Meloni D, Mascaro G. 2013. Physical stratigraphy and tectonic setting of Bolognano Formation (Majella): a potential carbonate reservoir. J. Mediterranean Earth Sci. Special Issue 2013, 151–176. [Google Scholar]
- 34.Higdon JW, Bininda-Emonds ORP, Beck RMD, Ferguson SH. 2007. Phylogeny and divergence of the pinnipeds (Carnivora: Mammalia) assessed using a multigene dataset. BMC Evol. Biol. 7, 216 (doi:10.1186/1471-2148-2-216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuter M, Piller WE, Brandano M, Harzhauser M. 2012. Oligo-Miocene stratigraphy in neritic platform carbonates of the central Mediterranean region (Majella, Abruzzi, Italy) In 29th IAS Meeting of Sedimentology, Schladming, Austria, 10–13 September 2012, meeting programme and abstract book, p. 578.
- 36.Koretsky IA, Domning DP. 2014. One of the oldest seals (Carnivora, Phocidae) from the Old World. J. Vertebr. Paleontol. 34, 224–229. (doi:10.1080/02724634.2013.787428) [Google Scholar]
- 37.Cigala-Fulgosi F, Pilleri G. 1985. The lower Serravallian cetacean fauna of Visano (northern Appenines, Parma, Italy). Invest. Cetacea 17, 55–73. [Google Scholar]
- 38.Pilleri G, Cigala-Fulgosi F. 1989. Additional observations on the Lower Serravallian marine mammals fauna of Visano and the Stirone River (northern Apennines). In Contributions to the paleontology of some Tethyan Cetacea and Sirenia (mammalia) II: Ostermundigen (Switzerland), (ed. Pilleri G.), pp. 63–85. Berne, Switzerland: Brain Anatomy Institute. [Google Scholar]
- 39.Odin GS, Amorosi A, Tateo F, Coccioni R, Cosca M, Negri A, Pini GA, Hunziker JC. 1997. Chapter 2: Integrated stratigraphy (biostratigraphy and geochronology) of the early Miocene sequence from the Emilian Apennines (Italy). In Developments in palaeontology and stratigraphy, 15, Miocene stratigraphy: An integrated approach (eds Montanari A, Odin GS, Coccioni R). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 40.Pini GA. 1999. Tectonosomes and Olistostromes in the Argille Scagliose of the Northern Apennines, Italy. U.S. Geol. Sur. Professional Paper 335, 1–71. [Google Scholar]
- 41.Carena S, Borgia A, Pasquarè G, Battaglia A, Ferraris M, Martelli L, De Nardo MT. 2000. Gravity Synclines. J. Geophys. Res. 105, 21 819–21 833. (doi:10.1029/2000JB900206) [Google Scholar]
- 42.de Muizon C. 1981. Les vertébrés fossils de la Formation Pisco (Pérou) Première partie: deux nouveaux Monachinae (Phocidae: Mammalia) du Pliocène de Sud Sacaco. Institut Français d'Etudes Andines, Mémoire 6, 20–161. [Google Scholar]
- 43.Bininda-Emonds ORP, Russell AP. 1996. A morphological perspective on the phylogenetic relationships of the extant phocid seals (Mammalia: Carnivora: Phocidae). Bonner Zoologische Monographien 41, 1–256. [Google Scholar]
- 44.Mitchell ED. 1966. The Miocene pinniped Allodesmus. University of California Publications in Geological Sciences 61, 1–105. [Google Scholar]
- 45.Barnes LG. 1972. Miocene Desmatophocinae (Mammalia: Carnivora) from California. University of California Publications in Geological Sciences 89, 1–76. [Google Scholar]
- 46.Barnes LG. 1987. An early Miocene pinniped of the genus Desmatophoca (Mammalia: Otariidae) from Washington. Contrib. Sci. Nat. His. Museum of Los Angeles County 382, 1–20. [Google Scholar]
- 47.Barnes LG. 1979. Fossil enaliarctine pinnipeds (Mammalia: Otariidae) from Pyramid Hill, Kern County, California. Contrib. Sci. Nat. His. Museum Los Angeles County 318, 1–41. [Google Scholar]
- 48.Barnes LG. 1989. A new enaliarctine pinniped from the Astoria Formation, Oregon, and a classification of the Otariidae (Mammalia: Carnivora). Contrib. Sci. Nat. His. Museum Los Angeles County 403, 1–28. [Google Scholar]
- 49.Barnes LG. 1990. A new Miocene enaliarctine pinniped of the genus Pteronarctos (Mammalia: Otariidae) from the Astoria Formation, Oregon. Contrib. Sci. Nat. His. Museum Los Angeles County 422, 1–20. [Google Scholar]
- 50.Repenning CA, Tedford RH. 1977. Otarioid seals of the Neogene. US Geol. Sur. Professional Paper 992, 1–87. [Google Scholar]
- 51.Kohno N. 2006. A new Miocene odobenid (Mammalia: Carnivora) from Hokkaido, Japan, and its implications for odobenid phylogeny. J. Vertebr. Paleontol. 26, 411–421. (doi:10.1671/0272-4634(2006)26[411:ANMOMC]2.0.CO;2) [Google Scholar]
- 52.Mitchell ED, Tedford RH. 1973. The Enaliarctinae: a new group of extinct aquatic carnivore and a consideration of the origin of Otariidae. Bull. Am. Museum Nat. His. 151, 203–284. [Google Scholar]
- 53.Berta A. 1991. New Enaliarctos* (Pinnipedimorpha) from the Oligocene and Miocene of Oregon and the role of ‘enaliarctids’ in pinniped phylogeny. Smithsonian Contrib. Paleobiol. 69, 1–33. (doi:10.5479/si.00810266.69.1) [Google Scholar]
- 54.Berta A. 1994. New specimens of the pinnipediform Pteronarctos from the Miocene of Oregon. Smithsonian Contrib. Paleobiol. 78, 1–30. (doi:10.5479/si.00810266.78.1) [Google Scholar]
- 55.Deméré TA, Berta A. 2005. New skeletal material of Thalassoleon (Otariidae: Pinnipedia) from the late Miocene–early Pliocene (Hemphillian) of California). Bull. Florida Museum Nat. His. 45, 379–411. [Google Scholar]
- 56.Hendey QB, Repenning CA. 1972. A Pliocene phocid from South Africa. Ann. South Af. Museum 59, 71–98. [Google Scholar]
- 57.de Muizon C, Hendey QB. 1980. Late Tertiary seals of the South Atlantic Ocean. Ann. South Af. Museum 82, 91–128. [Google Scholar]
- 58.Berta A, Wyss AR. 1994. Pinniped phylogeny. Proc. San Diego Soc. Nat. His. 29, 33–56. [Google Scholar]
- 59.Hodgetts LM. 1999. Animal bones and human society in the late Younger Stone Age of arctic Norway; volume 2 of 2: figures and appendices. PhD thesis, University of Durham. [Google Scholar]
- 60.Koretsky IA, Holec P. 2002. A primitive seal (Mammalia: Phocidae) from the early middle Miocene of Central Paratethys. Smithsonian Contrib. Paleobiol. 93, 163–178. [Google Scholar]
- 61.Solé F, Smith R, Coillot T, de Bast E, Smith T. 2014. Dental and tarsal anatomy of ‘Miacis’ latouri and a phylogenetic analysis of the earliest carnivoraforms (Mammalia, Carnivoramorpha). J. Vertebr. Paleontol. 34, 1–21. (doi:10.1080/02724634.2013.793195) [Google Scholar]
- 62.Leidy J. 1853. The ancient fauna of Nebraska. Smithsonian Contrib. Knowledge 6, 1–126. [Google Scholar]
- 63.Wyman J. 1850. Notice of remains of vertebrated animals found at Richmond, Virginia. Am. J. Sci. Arts, second series 10, 228–235. [Google Scholar]
- 64.True FW. 1906. Description of a new genus and species of fossil seal from the Miocene of Maryland. Proc. United States Natl Museum 30, 835–840. (doi:10.5479/si.00963801.30-1475.835) [Google Scholar]
- 65.Kidwell SM, Powars DS, Edwards LE, Vogt PR. 2015. Miocene stratigraphy and paleoenvironments of the Calvert Cliffs, Maryland. Field Guides 40, 231–279. (doi:10.1130/2015.0040(08)) [Google Scholar]
- 66.Rahmat SJ, Koretsky IA, Osborne JE, Alford AA. 2017. New Miocene Monachinae from the western shore of the Chesapeake Bay (Maryland, USA). Vestnik zoologii 51, 221–242. (doi:10.1515/vzoo-2017-0029) [Google Scholar]
- 67.Vanden Broeck E. 1878. Esquisse géologique et paléontologique des dépôts pliocènes des environs d'Anvers, fascicule II: les sables moyens et les sables supérieurs d'Anvers. Brussels, Belgium: Mayolez. [Google Scholar]
- 68.Mourlon M. 1876. Sur les depots qui, aux environs d'Anvers, séparent les sables noirs miocènes des couches pliocènes scaldiennes. Bulletin de l'Académie royale de Belgique, deuxième série 42, 760–789. [Google Scholar]
- 69.Laga P, Louwye S, Geets S. 2001. Paleogene lithostratigraphic units (Belgium). In Guide to a revised lithostratigraphic scale of Belgium. Volume 4. (eds Bultynck P, Dejonghe L), pp. 135–152. Brussels, Belgium: Geologica Belgica. [Google Scholar]
- 70.Ray CE. 1976. Geography of phocid evolution. Syst. Zool. 25, 391–406. (doi:10.2307/2412513) [Google Scholar]
- 71.de Muizon C, Bond M. 1982. Le Phocidae (Mammalia) miocène de la formation Paraná (Entre Ríos, Argentine). Bulletin du Muséum national d'Histoire naturelle 4, 165–207. [Google Scholar]
- 72.Dall WH. 1894. Notes on the Miocene and Pliocene of Gay Head, Martha's Vineyard, Mass., and on the ‘Land Phosphate’ of the Ashley River District, South Carolina. Am. J. Sci. series 3 48, 296–301. [Google Scholar]
- 73.Köthe A. 2003. Dinozysten-Zonierung im Tertiär Norddeutschlands. Revue de Paléobiologie 22, 895–923. [Google Scholar]
- 74.Valenzuela-Toro AM, Pyenson ND, Gutstein CS, Suárez ME. 2016. A new dwarf seal from the late Neogene of South America and the evolution of pinnipeds in the southern hemisphere. Papers Palaeontol. 2, 101–115. (doi:10.1002/spp2.1033) [Google Scholar]
- 75.Boessenecker RW, Churchill M. 2015. The oldest known fur seal. Biol. Lett. 11, 20140835 (doi:10.1098/rsbl.2014.0835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnes LG. 1988. A new fossil pinniped (Mammalia: Otariidae) from the middle Miocene Sharktooth Hill bonebed, California. Contrib. Sci. Nat. His. Museum Los Angeles County 396, 1–11. [Google Scholar]
- 77.West RG. 1980. The Pre-glacial Pleistocene of the Norfolk and Suffolk coasts. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 78.Harington CR. 2008. The evolution of arctic marine mammals. Ecol. Appl. 18, S23–S40. (doi:10.1890/06-0624.1) [DOI] [PubMed] [Google Scholar]
- 79.Repenning CA. 1983. New evidence for the age of the Gubik Formation, Alaska North Slope. Quat. Res. 19, 356–372. (doi:10.1016/0033-5894(83)90041-8) [Google Scholar]
- 80.Roberts DL, et al. 2011. Regional and global context of the Late Cenozoic Langebaanweg (LBW) palaeontological site: west coast of South Africa. Earth Sci. Rev. 106, 191–214. (doi:10.1016/j.earscirev.2011.02.002) [Google Scholar]
- 81.Fleming CA. 1968. New Zealand fossil seals. N. Z. J. Geol. Geophys. 11, 1184–1187. (doi:10.1080/00288306.1968.10420246) [Google Scholar]
- 82.Cozzuol MA. 2001. A ‘northern’ seal from the Miocene of Argentina: implications for phocid phylogeny and biogeography. J. Vertebr. Paleontol. 21, 415–421. (doi:10.1671/0272-4634(2001)021[0415:ANSFTM]2.0.CO;2) [Google Scholar]
- 83.Avery G, Klein RG. 2011. Review of fossil phocid and otariid seals from the southern and western coasts of South Africa. Trans. R. Soc. South Africa 66, 14–24. (doi:10.1080/0035919x.2011.564490) [Google Scholar]
- 84.Stringer CB, et al. 2008. Neanderthal exploitation of marine mammals in Gibraltar. Proc. Natl Acad. Sci. USA 105, 14 319–14 323. (doi:10.1073/pnas.0805474105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drehmer CJ, Ribeiro AM. 1998. A temporal bone of an Otariidae (Mammalia, Pinnipedia), from the Pleistocene of Rio Grande de Sul State, Brazil. Revista Universidade Guarulhos Geociencias 3, 39–44. [Google Scholar]
- 86.Loza CM, Scarano AC, Soibelzon LH, Negrete J, Carlini AA. 2015. Morphology of the tympanic-basicranial region in Mirounga leonina (Phocidae, Carnivora), postnatal ontogeny and sexual dimorphism. J. Anat. 226, 354–372. (doi:10.1111/joa.12286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Govender R. 2015. Preliminary phylogenetics and biogeographic history of the Pliocene seal, Homiphoca capensis from Langebaanweg, South Africa. Trans. R. Soc. South Africa 70, 25–39. (doi:10.1080/0035919X.2014.984258) [Google Scholar]
- 88.Koretsky IA, Barnes LG, Rahmat SJ. 2016. Re-evaluation of morphological characters question current views of pinniped origins. Vestnik zoologii 50, 327–354. (doi:10.1515/vzoo-2016-0040) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for this paper are available within this paper or as electronic supplementary material of this paper. Length measurements of specimens, dinoflagellate cyst biostratigraphy results, and the phylogenetic matrix are provided as electronic supplementary material. This published work and the nomenclatural acts it contains have been registered in ZooBank. The LSID for this publication is urn:lsid:zoobank.org:pub:17B81201-199E-4577-B465-79F65A108A8.