Abstract
In comparative studies of evolution of communication, the function and use of animal quiet calls have typically been understudied, despite that these signals are presumably under selection like other vocalizations, such as alarm calls. Here, we examine vocalization diversification of chimpanzee quiet ‘hoos’ produced in three contexts—travel, rest and alert—and potential pressures promoting diversification. Previous playback and observational studies have suggested that the overarching function of chimpanzee hoos is to stay in contact with others, particularly bond partners. We conducted an acoustic analysis of hoos using audio recordings from wild chimpanzees (Pan troglodytes schweinfurthii) of Budongo Forest, Uganda. We identified three acoustically distinguishable, context-specific hoo variants. Each call variant requires specific responses from receivers to avoid breaking up the social unit. We propose that callers may achieve coordination by using acoustically distinguishable calls, advertising their own behavioural intentions. We conclude that natural selection has acted towards acoustically diversifying an inconspicuous, quiet vocalization, the chimpanzee hoo. This evolutionary process may have been favoured by the fact that signallers and recipients share the same goal, to maintain social cohesion, particularly among those who regularly cooperate, suggesting that call diversification has been favoured by the demands of cooperative activities.
Keywords: animal communication, chimpanzee, call diversification, cooperation
1. Introduction
Within the framework of the evolution of communication, how and why some species have greater call diversity than others—and more context specificity of vocalizations—remain much discussed. One extensive debate has centred on the variety of context-specific vocalizations within a species' repertoire, such as how precisely primate calls refer to objects and events external to themselves [1–7]. Context specificity has been particularly well documented in alarm calls, for example the predator-specific vocalizations of vervet monkeys ([8,9] but see [5]). Various evolutionary scenarios have been proposed to explain the origins of this type of signalling behaviour [9–11]. For instance, kin selection predicts that callers will gain a fitness benefit, provided a costly behaviour, like producing conspicuous vocal behaviour in the presence of a predator, favours close genetic relatives [12]. However, alarm calls can also be directly beneficial for the caller, for example if calling fosters group-level defence or if it has aversive effects on the predator [9]. Alarm calls have also been well studied when examining the evolution of vocal diversity. One relevant line of research has demonstrated that if a prey species regularly encounters various predators that differ in their hunting behaviour, then this can lead to the evolution of acoustically distinct, predator-specific alarm calls, a process well documented in social carnivores [11,13] and non-human primates [9]. Predation pressure, in other words, can be one of the main causes for the evolution of call diversity.
However, the evolution of call diversity also seems to be favoured by social factors. Species living in complex social units are permanently caught between cooperation and competition, and communication plays a key role in navigating between these two forces. Illustrating this, chacma baboons produce grunts during a range of social interactions. Dominant females, for example, grunt during approaches to handle a subordinate female's infant [14,15]. When grunts are emitted, grunters are more likely to be permitted access to infants, suggesting that grunts aid predictability of benign intent [14,15]. This and other observations have led to the hypothesis that social complexity is one of the key drivers for the evolution of vocal behaviour [16,17]. The main argument is that if individuals are regularly challenged by competing with others over resources, then selection is likely to favour the evolution of signals to minimize costs.
Selection for call diversity may also emerge when coordination with other group members becomes essential [11], provided such social interactions confer fitness gains [18–22]. Furrer & Manser [23] suggest that species that require coordinated escape responses from predators may evolve more context-specific alarm calls than species that do not. Species that coordinate hunting are also expected to evolve specific signals to increase hunting success: chimpanzees produce acoustically distinct hunt barks only when they hunt monkeys [24], which observations suggest function to recruit group members [24,25]. A last example regards mutual grooming, a highly coordinated and cooperative activity that, in some species, can involve exposure to vulnerable body parts. Chimpanzees use a specific signal, lip smacking, which appears to facilitate this type of cooperative interaction [26].
A particularly common coordination problem in social animals is group travel, and many primates living in visually dense habitats have evolved specific vocal signals, contact calls, to help maintain proximity [27]. Positive selection may particularly shape contact call evolution in species when reproductive benefits are accrued through coalition formation, and vocalizations enhance cohesion between coalition partners, such as in chimpanzees. Contact calls can be loud, reaching across hundreds of metres, or quiet, reaching only 50–150 m. Examples of quiet calls used to maintain contact within social groups are the coo calls of Japanese macaques [28], the peeps of bonobos [29] or the ‘move’ grunts of chacma baboons [30] and vervet monkeys [31].
Chimpanzees (Pan troglodytes) are an interesting species to investigate coordination problems, given that they gain benefits from coalition formation when engaged in both within and between group competition [20,32,33]. Chimpanzees also live in low visibility forest such that vocalizations become key in predicting the behaviour of others. Additionally, they have a fission–fusion social system, which makes group travel a more difficult problem due to the continuously changing social units, which are mediated by differentiated association preferences [34–36]. Thus, chimpanzee travel units can range from large groups to small parties to solitary travel, which requires negotiation with adequate signals and flexibility [37,38]. To this end, long-distance calls (pant hoots) are likely to play an important role in promoting fusion and coordination, especially during feeding and travelling [37,39]. More recently, quiet ‘hoo’ calls have also been noted to aid coordination in relation to travel [40].
Chimpanzees emit hoos in at least three distinguishable contexts, when initiating or during travel (travel hoo), when stationary, particularly when resting or feeding (rest hoo) or when seeing hidden threats, such as snakes (alert hoo). Chimpanzees are more likely to emit travel and alert hoos when cooperation partners are present than absent [40,41]. Field experiments show that different responses are elicited from receivers after hearing either a rest or an alert hoo broadcast from a hidden speaker. Specifically, receivers engage in more search behaviour after hearing alert than rest hoos [42] and appear to take the hoo variant as an indicator of the caller's awareness of a threat [43]. In the current study, we focus on the acoustic features of chimpanzee hoo calls that can serve as potential carriers of contextual information.
2. Material and methods
2.1. Study site and subjects
Subjects were wild-living, habituated chimpanzees of the Sonso community in Budongo Forest, Uganda [44]. Vocalizations were recorded between February 2008 and September 2010 by two observers, T.G. and C.C., from adult and subadult chimpanzees of both sexes. T.G. mainly recorded calls in rest and travel contexts, whereas C.C. mainly recorded calls in alert and rest contexts. Out of a total of 77 chimpanzees, we obtained good quality hoo recordings from 29 individuals: 14 males (nine adults greater than 14 years, five subadults 10–14 years) and 15 females (11 adults greater than 13 years and four subadults 10–13 years) (table 1). Data are available in the electronic supplementary material, data file.
Table 1.
Distribution of calls across subjects.
| subject | sex | age | alert | rest | travel |
|---|---|---|---|---|---|
| BB | M | A | 2 | ||
| FD | M | A | 5 | 5 | |
| FK | M | S | 4 | 5 | |
| HT | F | A | 1 | 1 | |
| HW | M | A | 7 | 5 | |
| JN | F | A | 6 | 4 | |
| KA | F | S | 2 | ||
| KLa | F | A | 3 | 4 | 5 |
| KTa | M | A | 5 | 9 | 5 |
| KU | F | A | 6 | 5 | |
| KWa | F | A | 2 | 8 | 5 |
| KY | F | A | 2 | 2 | |
| KZa | M | S | 2 | 12 | 5 |
| MK | F | A | 2 | ||
| ML | F | A | 5 | 5 | |
| MSa | M | A | 1 | 7 | 5 |
| NBa | F | A | 1 | 7 | 5 |
| NKa | M | A | 2 | 11 | 5 |
| NR | F | S | 1 | 1 | |
| OK | F | A | 3 | ||
| PSa | M | S | 2 | 6 | 5 |
| REa | F | S | 2 | 4 | 5 |
| SQa | M | A | 4 | 7 | 5 |
| TK | M | A | 2 | 1 | |
| VR | F | S | 2 | ||
| ZF | M | A | 7 | 5 | |
| ZG | M | S | 1 | 2 | |
| ZLa | M | S | 2 | 6 | 5 |
| ZM | F | A | 5 | 5 |
aSubjects with calls in each context used to make the discriminant functions. Remaining individuals' calls were permuted into the analyses. N = 271 calls; 29 chimpanzees.
2.2. Recording
We recorded hoos opportunistically using either a Sennheiser directional microphone MKH416 or MKH418 microphone with a Marantz PMD-660 solid-state recorder, an external Sennheiser directional MKE-400 microphone attached to a Panasonic NV-GS 330 DV camera or a Panasonic NV-GS 330 DV camera with an internal microphone. In all cases, calls were digitized at a 44.1 kHz sampling rate and 16-bit sampling depth. Each time hoos were recorded, the signaller, date and context of calling were noted. Although, when listening to recordings, we could detect no obvious acoustic differences from hoos recorded on different devices, given that recording devices may cause slight acoustic variation, we controlled for the recording device used in statistical models (see below).
2.3. Behavioural context
We classified hoos according to their context of production. We classified calls as ‘alert hoos’, if emitted in response to a hidden threat, such as a sedentary viper, viper model or a wire snare. We classified calls as ‘rest hoos’ if emitted when resting. By contrast, we classified hoos as ‘travel hoos’ if emitted immediately before (on average 0.8 s prior departure; N = 15, range: 0–3.0 s) or during travel (N = 72). N = 7 cases where individuals failed to recruit other individuals for travel while producing hoos were also included [40]. (Audio recordings are included in the electronic supplementary material.)
2.4. Acoustic analyses
Any call of sufficient quality and produced in the three contexts was subjected to acoustic analysis. Selection criteria were that at least the lowest frequency band had to be clearly visible throughout the call with no overlap from the calls of other individuals, that the signaller could be clearly identified, that the context of production was unambiguous and that all acoustic variables (table 2) could be measured. A maximum of two calls per bout were measured, although these were never sequential neighbours. Calls from digital audio files or digital videos were analysed using the PRAAT software [45]. Sound files were lifted from digital video using a VLC player (VideoLan Project 2001).
Table 2.
Acoustic differences of hoos across contexts: discriminant function scores for analyses 1 and 2. F0: fundamental frequency; analysis 1: contexts rest, travel and alarm; analysis 2: contexts: travel and alarm, enabling bout information to be included (inter-call interval). Italics: greater than 1 or less than −1 (highly influential).
| acoustic variable | analysis 1 (three contexts) | analysis 2 (two contexts) | |
|---|---|---|---|
| discriminant function 1 | discriminant function 2 | discriminant function 1 | |
| call duration (log) | −1.41 | −0.40 | −0.91 |
| maximum F0 (log) | −0.15 | 4.22 | −2.17 |
| drop in F0 (sqrt) | −0.17 | −0.05 | 0.00 |
| peak frequency positiona (sqrt) | 0.08 | −0.01 | 0.03 |
| maximum F0 positiona (log) | 0.01 | 0.13 | −0.05 |
| inter-call interval (sqrt) | — | — | −3.81 |
aAs a proportion of call duration.
Hoos are relatively quiet calls, thus even when recording using a directional microphone in a range of 5–20 m from the signaller, the signal-to-noise ratio is too low to allow for extracting reliable measures using automated software programs. Thus, 10 acoustic variables describing temporal and frequency call parameters that could be reliably measured by hand were measured manually using PRAAT software with spectrograms made using a fast Fourier transform length of 256 points with Gaussian window, time step of 1000 and window length of 0.05 s.
We measured eight acoustic variables to characterize prominent temporal and frequency features of primate vocalizations (call duration, fundamental frequency (F0) at the start and end of the call as well as the maximum F0; peak frequency—the frequency (Hz) with the maximum amplitude; time to maximum F0 and peak frequency from the start of the call and inter-call interval). We derived an additional four acoustic variables to further characterize fundamental and peak frequency change through each call (F0 drop: F0 max − F0 end; steepness of F0 drop: F0 drop/duration from F0 max − F0 end; position of the maximum F0: time to maximum F0/call duration; position of the peak frequency: time to peak frequency/call duration). For duration measures, we used the standard cursor function along the fundamental frequency, which was clearly visible in all recordings. We calculated F0 measures using the ‘Pitch Listing’ function, which measures the F0 at less than 0.01 s intervals. We used the spectral slice function in PRAAT as a double check for accuracy of the ‘Pitch Listing’ function. Maximum F0 was defined as the highest fundamental frequency in the call. Start and end F0 measures were taken within the first or last 0.05 s of the visible F0 band for the call, respectively. Changes in F0 across each call were measured by two variables: F0 drop was the drop in F0 from the maximum F0 to the end F0. Slope steepness was the rate of decrease in F0 from the maximum to the end F0. We measured two variables that captured the time point in the call in which maximum F0 and peak frequency occurred. Here, each time point was calculated as a proportion of call length. To measure time to peak frequency, the ‘Intensity Listing’ function was used together with the cursor function to first determine the position of the peak frequency within the call. Inter-call interval was the duration between two calls within the same unbroken sequence of calls of the same call type, measured from the end of the last call to the beginning of the next call.
2.5. Statistical analyses
Where required for assumptions of statistical tests, appropriate variable transformations were conducted to obtain symmetrical distributions prior to the analysis [46]. We log-transformed call duration, maximum F0 and position of maximum F0. We square root-transformed frequency drop, position of peak frequency and inter-call interval. The position of maximum F0, position of peak frequency, F0 start and F0 end were used to construct our key variables and remained untransformed. High correlations were found between max F0, start and end F0 as well as between F0 drop and slope steepness (Pearson's correlation: r > 0.7). We thus discarded slope steepness, start and end F0, and kept the remaining six variables in the analysis with variance inflation factor less than 2, showing acceptably small correlational propensity.
2.5.1. Context effects on acoustic structure
To determine whether hoos emitted in the three different contexts could be acoustically differentiated, we conducted a permuted discriminant function analysis, permuting contexts within subjects (‘pDFA’, [46]). This procedure has been recommended to account for non-independence of calls due to repeated recordings from the same subjects. Calls with sufficient quality to measure target acoustic variables totalled N = 271 calls from 29 chimpanzees (table 1).
To derive the discriminant functions, we only included hoos from individuals that contributed to each context (pDFA1: N = 11 callers, pDFA2: N = 11 callers), using one randomly selected call per individual per context. Thus, 33 calls were used to derive the discriminant functions: N = 3 adult females, N = 5 adult males and N = 2 subadult males (table 1). All the remaining calls were then cross-classified using the derived discriminant functions. We ran two pDFAs: the first included only acoustic parameters that described single calls and the second also included a parameter that described call bout information (inter-call interval). Given that hoos in rest contexts are almost always emitted as single calls, rest hoos were omitted from the second pDFA. Calls used for cross-classification, in pDFA1, were 129 calls from the same individuals used to make the discriminant functions and 109 calls from 17 additional individuals. In pDFA2, for cross-validation, there were 23 additional calls from the same individuals used to make the discriminant functions and 55 calls from 14 additional individuals. To avoid that the result would unduly depend on a particular random selection, we created 100 such random selections and averaged the result. We included the selected acoustic parameters (five for DFA1 and six for DFA2, including inter-call interval) that described the temporal and frequency distribution characteristics of each call and had a variance inflation factor of less than 2.
We based our assessment of the discriminability of the three contexts on the percentage correctly cross-classified calls and used 10 000 permutations to estimate the p-value for discriminability. The pDFAs were conducted in R v. 3.2.5 [47] using a function (provided by R. Mundry), which is based on the function lda of the R package MASS [48].
2.5.2. Controlling for age, sex, observer and recording methods on acoustic structure
To determine the influence of the context of call production in relation to other possible influencing factors on the acoustic structure of hoos, we conducted linear mixed models (LMMs; [49]). We conducted one model for each acoustic variable shown to be influential on the distribution of hoos across contexts in the pDFA (those with a discriminant function score greater than 1.0 or less than −1.0) (table 2). We used LMMs with a Gaussian error structure and identity link using R v. 3.2.5 [47] and the function glmer of the package lme4 [50]. Here, we used all hoos from each context (N = 271 calls and N = 29 subjects, table 1).
Each model tested the same set of fixed and random effects on each acoustic variable. Call context was our main variable of interest and was considered to be the test predictor. We added age (in years) and sex (male/female) as control predictors, given that we included calls from both males and females, adults and subadults in the model. We also added observer and recording device as control predictors, given that these varied across contexts. Two observers (C.C. or T.G.) recorded calls using different recording devices (Sennheiser directional microphone, video recorder internal microphone or external Sennheiser microphone attached to the video recorder). Since for two contexts we only had recordings of one observer, we were unsure whether the analysis would suffer from confounding effects or if we would be able to disentangle the respective influence of observer and context on call features. To address this question, we conducted simulations, which revealed that the LMM model used is indeed able to tease apart the effects of observer and context, revealing unbiased estimates (electronic supplementary material, figure S2).
Because subjects usually contributed calls to more than one category, subject identity was included as a random effect. No random slopes could be fitted, as all combinations of fixed and random effects had at least one instance when fewer than two different values of the fixed effects occurred per level of the random effects [51,52]. We tested the significance of the individual fixed effects by comparing the full model (comprising all fixed and random effects) with a respective reduced model (not comprising the test predictor) using likelihood ratio tests [53]. Model estimates were only considered if the full-null model comparison was significant. Model stability was assessed for all models by excluding the random effects one at a time and comparing the estimates for these data with those for the full dataset. This showed no influential subjects or events. Finally, given that we needed to run two models, one for each key acoustic variable, the LMMs conducted constituted multiple tests of the same calls. Thus, p-values were subjected to Bonferroni corrections, and significance was considered to be reached with p = 0.017.
Finally, to further examine the influence of call context on call bout information, particularly inter-call interval, we ran an LMM that included the two contexts that showed variation in inter-call interval: travel and alert contexts. Given that only single hoos are emitted in rest contexts, rest hoos had no inter-call interval and thus were excluded from this analysis. Here, we additionally included control predictors, sex and age. Owing to reduced power and complete separation issues, we did not include observer or recording device in this model.
3. Results
3.1. Context specificity of hoo types
Examining single calls from all three contexts together (alert, rest and travel, see figure 1 for spectrograms) showed reasonable discrimination of the hoo types based on five uncorrelated temporal and frequency parameters (pDFA: correct classification for cross-classified calls = 70.6%; expected correct classified calls = 35.8%; p = 0.001; figure 2 and table 2). Results from a single cross-validated DFA showed correct classification scores per context as follows: resting = 84.7%, travel = 83.0% and alert = 40.0%. Discriminant function loadings (table 2) and cross-classification scores showed that rest hoos discriminated well from travel hoos, having longer call duration. Alert hoos could be partially discriminated from the other two contexts having a higher maximum fundamental frequency. However, a subset of alert hoos did not discriminate well (figure 2).
Figure 1.
Spectrograms of the three hoo variants, including hoo sequences. (a) Alert hoos: two hoo sequences emitted by an adult male and female, respectively. (b) Travel hoos: four hoo sequences, emitted by two adult males and two adult females, respectively. (c) Five rest hoos, emitted by two adult males, two adult females and finally one adult male, respectively. Time and frequency scales are equivalent across spectrograms. Audio recordings are included in the electronic supplementary material.
Figure 2.
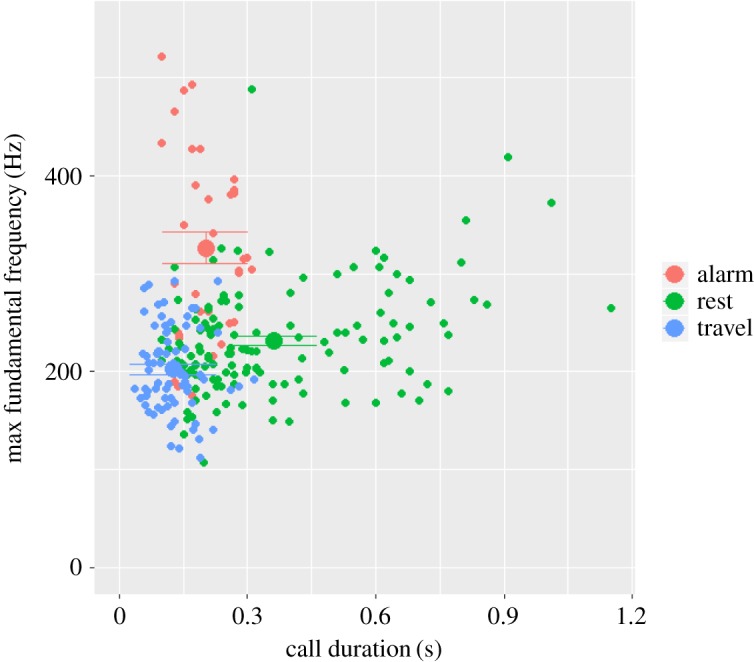
Classification of three hoo variants emitted in different behavioural contexts, delineated by two acoustic variables highly influential in permuted discriminant function analysis: call duration and maximum fundamental frequency (table 2). Group centroids with 95% confidence interval are shown.
We conducted a second discriminant function analysis that included call bout information, specifically the duration between calls. Since rest hoos are almost always produced singly (134/137 cases, 97.8%) while travel (79/94 cases, 84.0%) and alert hoos (38/40 cases, 95.0%) are almost always produced in bouts of more than one call, we included only travel and alert hoos in the second analysis, in cases when more than one call was emitted. Thus, we included N = 112 calls in which 22 were selected to create the discriminant functions (from the same 11 chimpanzees as for analysis 1, table 1; N = 5 calls were omitted where bout information could not be reliably measured). Calls from two different contexts (alert and travel) could be discriminated well with the additional acoustic variable encoding call bout information (pDFA: correct classification for permuted cross-classified calls = 95.34; expected correct classified calls = 54.6%; p = 0.001; figure 3 and table 2). Correct classification scores per context were 97.4% for alert hoos and 97.3% for travel hoos. Discriminant function loadings (table 2) and cross-classification scores showed that alert hoos had longer inter-call intervals that travel hoos.
Figure 3.

Influence of context on call interval in travel and alert contexts.
3.2. Acoustic differences in hoo types
We subjected the three acoustic variables (F0 max, call duration and inter-call interval) that were highly influential in the pDFA to further testing. Specifically, we conducted an LMM for each to determine the relative influence of test predictor, call context and control predictors on each acoustic variable. For each model, the full model was significant with respect to the null model (LMM: model significance against null model: F0 max as a response variable: χ2 = 45.07, d.f. = 2, p < 0.0001, three contexts, N = 271 calls from 29 subjects; call duration as a response variable: χ2 = 144.75, d.f. = 2, p < 0.0001, three contexts, N = 271 calls from 29 subjects; inter-call interval as a response variable: χ2 = 138.03, d.f. = 2, p < 0.0001; two contexts, N = 112 calls; 29 subjects). In both models, the acoustic variable tested was significantly influenced by the context of production of the call (table 3). We found no significant age or sex effects in either model. The observer had a significant influence on both F0 max (χ2 = 9.74, d.f. = 1, p = 0.002) and call duration (χ2 = 6.45, d.f. = 1, p = 0.011), while recording device effects were found for F0 max only (χ2 = 17.91, d.f. = 2, p = 0.0001; electronic supplementary material, figure S1). Here, it should be noted that the LMM separates variation attributed to the different predictors such that the influence of observer and device on the hoo acoustics cannot also account for context effects. In addition, our simulations showed that the influence of each predictor remained independent (electronic supplementary material, figure S2). The third model showed that the interval between calls is longer in alert than travel hoos (table 3 and figure 3).
Table 3.
The influence of behavioural context on hoo acoustic properties: LMM full model results. Analysis 1 includes hoos from rest, travel and alert contexts: N = 271 calls from 29 chimpanzees. Analysis 2 includes hoos from travel and alert contexts: N = 112 calls from 29 chimpanzees. LMM full versus null model results: maximum F0 model: χ2 = 45.01, d.f. = 2, p < 0.000. Call duration model: χ2 = 144.75, d.f. = 2, p < 0.000. Inter-call interval model: χ2 = 138.03, d.f. = 1, p < 0.000. s: directional microphone + audio recorder; cs: video recorder + external microphone; c: video recorder; tg: observer T.G. After Bonferroni correction, α level is set to p = 0.017. Bold: p < 0.017.
| acoustic variable | predictor variable | d.f. | χ2 | p | ß | s.e. | t |
|---|---|---|---|---|---|---|---|
| analysis 1 (three contexts) | |||||||
| maximum F0 (log) | intercept | 5.74 | 0.09 | ||||
| call context | 2 | 45.10 | <0.000 | ||||
| —rest hoo | −0.31 | 0.05 | −5.88 | ||||
| —travel hoo | −0.42 | 0.05 | −7.00 | ||||
| —alert hoo | 0 | 0 | 0 | ||||
| sex (male) | 1 | 0.71 | 0.40 | 0.00 | 0.04 | 0.85 | |
| age | 1 | 0.21 | 0.65 | 0.00 | 0.00 | −0.46 | |
| observer (tg) | 1 | 9.48 | 0.002 | −0.13 | 0.04 | −3.12 | |
| recording device (cs) | 2 | 17.91 | 0.0001 | −0.15 | 0.09 | −1.67 | |
| recording device (s) | 2 | 0.12 | 0.08 | 1.39 | |||
| call duration (log) | intercept | −1.62 | 0.18 | ||||
| call context | 2 | 144.75 | <0.000 | ||||
| —rest hoo | 0.63 | 0.12 | 5.24 | ||||
| —travel hoo | −0.28 | 0.14 | −2.00 | ||||
| —alert hoo | 0 | 0 | 0 | ||||
| sex (male) | 1 | 3.01 | 0.08 | 0.12 | 0.07 | 1.76 | |
| age | 1 | 0.00 | 0.96 | 0.00 | 0.00 | 0.04 | |
| observer (tg) | 1 | 6.35 | 0.01 | −0.24 | 0.10 | −2.53 | |
| recording device (cs) | 2 | 0.48 | 0.80 | −0.12 | 0.19 | −0.62 | |
| recording device (s) | 2 | −0.10 | 0.17 | −0.62 | |||
| analysis 2 (two contexts) | |||||||
| inter-call interval (sqrt) | intercept | 1.37 | 0.07 | ||||
| call context (travel hoo) | 2 | 138.03 | <0.000 | −0.71 | 0.04 | −16.55 | |
| sex (male) | 1 | 0.00 | 0.97 | 0.00 | 0.04 | −0.03 | |
| age | 1 | 3.34 | 0.07 | −0.00 | 0.00 | −1.85 | |
4. Discussion
4.1. Acoustic differences relating to the context
Clear acoustic differences were evident for hoos emitted in three different contexts, when callers were either resting, travelling or during alert contexts (seeing a snake). Hoos in two contexts, rest and travel, could be distinguished well from each other, with rest hoos having a longer duration than travel hoos. Around half of hoos emitted in alert contexts had a higher fundamental frequency than the other two contexts. However, the remaining alert hoos showed considerable overlap with hoos from rest and travel contexts. Adding information about the call bout (i.e. inter-call intervals in call sequences), however, increased discrimination considerably, in so far as alert hoos were emitted at lower rates compared to travel hoos, while rest hoos were almost exclusively emitted as single calls. Thus, even though chimpanzee hoos are quiet, inconspicuous calls that sound remarkably similar to each other, we were able to reliably identify different variants, especially when taking call sequences into account.
We found hoo variant discrimination in spite of using a conservative acoustic analysis: we used relatively few acoustic variables (those that could be reliably measured for this quiet call). Also, we used only a subset of individuals to make the discriminant functions, fitting the hoos of remaining individuals according to those discriminant functions. Thus, discriminant functions only took some, not all, individual variation into account. Hence, it is highly likely that our acoustic analysis underestimates the context specificity of these calls. Indeed, in a previous playback experiment, we broadcast single rest or alert hoos, eliminating call-interval information. The broadcast hoos nonetheless produced different behavioural responses from chimpanzees depending on the hoo variant broadcast [42,43], suggesting that a single hoo is sufficient for receivers to extract contextual information.
While the distribution of hoos across contexts showed biases between observers and recording devices, the variables observer and recording device were controlled for in the LMMs, so that the demonstrated context effects cannot be accounted for by these variables.
A persistent hypothesis in animal communication is that call variation is a direct reflection of signaller arousal. In this study, we found no clear support for this, as acoustic differences were not consistently related to presumed differences in arousal state. Usually, high compared with low arousal contexts are linked to calls with a high fundamental frequency and rapid rates of emission [54]. Here, the snake context, arguably linked to a relatively high arousal state, elicited hoos with the highest fundamental frequency (alert hoos), but calls were emitted at slower rates than travel hoos (linked with a lower arousal state), suggesting that presumed states of arousal cannot fully explain acoustic characteristics of hoos.
Another key hypothesis in animal communication is that call variation can arise due to ecological adaptation. Calls, for example, that are required to reach receivers at varying distances or across varying habitats with varying patterns of acoustic degradation should be under selection to achieve maximum transmission integrity [55]. Here, however, in all three contexts, signallers and receivers of hoos are at similar (short) distances from each other and in the same habitat. Hence, ecological adaptation of acoustic variation found in these three hoo variants is expected to be minimal [55].
4.2. Social motivation to produce hoos
Although the contexts of hoo production differ, callers who emit hoos may share a similar motivation to remain together with receivers in the party. Indeed, previous playback and observational studies examining receiver behaviour in response to hoos emitted in alert, rest and travel contexts suggest that all hoo variants are connected with group cohesion, likely facilitating coordination between signaller and receiver [40,42,43]. Contact calls are common across animal species and are used to coordinate movement in and between animal groups [56]. Both male and female chimpanzees have highly differentiated relationships, showing preferences to associate with kin and non-kin bond partners [34–36]. These preferences probably confer benefits for both males [20] and females [18,29,35] suggesting a selective advantage, and a motivation, to remain associated. In a tropical forest environment, when visibility can be obscured beyond 20 m, vocal cues become vital for maintaining coordination. All three hoo variants are close-range calls unlikely to be audible over 150 m [42,43,57] (C.C. & T.G. 2009, personal observation). Thus, they seem designed to coordinate movement only with individuals close by [40]. Since chimpanzees most often travel with their cooperation partners (kin and bond partners [34,35]), it seems likely that hoos are targeted at these individuals.
Chimpanzees also have long-distance contact calls given while travelling, pant hoots, which promote cohesion. Given that pant hoots can be heard over 500 m [58], they probably promote cohesion of the entire group. Hoos, being quiet calls, can only promote cohesion within subgroups. Chimpanzees are subject to predation from leopards and lethal attacks from neighbouring chimpanzee communities [33,59,60], and thus, selection pressures may also have shaped the use of more ‘private’ calls, resulting in quiet hoos, particularly when individuals are potentially more vulnerable to attack, travelling in small parties.
The chimpanzee fission–fusion social structure creates an additional problem. In each context, whether travel, rest or alert, a different response is required from receivers in order to remain associated. We discuss each of these in turn. When travel hoos are emitted at the start of travelling, they often result in receivers, particularly bond partners of signallers, leaving feeding trees and joining in the travel [40] (C.C. & T.G. 2009, personal observation). Travel hoos sometimes elicit vocal replies, most often as either travel or rest hoos [40] (T.G. 2009, personal observation). With the high possibility of fission in chimpanzees in low visibility habitat, travel hoos seem to promote continued cohesion between specific individuals, especially at moments when fission is most likely, as one individual begins to travel. Thus, if the function of hoos is to maintain cohesion, receivers should travel when they hear travel hoos.
Alert hoos function to recruit others to a hidden non-predatory threat, such as snakes or snares [42,43]. Again, they promote cohesion, although alert hoos specifically do so within the vicinity of a threat. Unlike travel hoos, which also promote approach behaviour, alert hoos promote slow, hesitant approach behaviour, an important distinction when approaching a hidden potentially deadly threat.
Rest hoos are emitted principally when stationary and often elicit rest hoos as replies (C.C. & T.G. 2009, personal observation). Rest hoo production typically occurs when individuals are resting out of visibility of each other. Signallers may intermittently emit rest hoos, continuing to rest for some time following rest hoo emission (C.C. & T.G. 2009, personal observation). Sounds of chimpanzee movement can elicit further rest hoos from resting individuals. Since many primate vocalizations are individually distinctive [61], these vocalizations broadcast the continued presence of the signallers and, in addition, probably broadcast the behavioural intention [15,40,62] to remain, although this requires further testing. Thus, if the function of hoos is to maintain cohesion, when receivers hear rest hoos, unlike with travel hoos, they should stay in the vicinity of stationary signallers, and not travel.
It seems likely that these three hoo types announce a similar underlying motivational state (to stay together) that can explain why the differing contextual information should be expressed using variants of hoos. However, the question remains, why specifically encode the different contexts within the acoustics of the call type? Why have at least three hoo types?
In alarm call studies, species which have different behavioural escape responses to different predators, such as aerial versus ground predators, often emit acoustically different alarm calls to the different predator classes [5,11,63–65]. This suggests that when selection pressure is high, there may be selection for signallers to produce different signals in order to elicit different behavioural responses from receivers. We extend this logic to non-alarm contexts. If it is adaptive for chimpanzees to coordinate movement with preferred partners, but different contexts require different behaviour from receivers to maintain coordination, low visibility habitat and fission–fusion social structure may promote vocal encoding of context specificity, even in quiet contact-type calls.
Signalling theory states that signals evolve to change receiver behaviour, such that the outcome is favourable for both signaller and receiver [66,67]. To date, this idea has mainly been tested on contexts where signalling is expected to be under strong selection pressure, such as mate attraction [68], offspring begging calls [69,70] and predator contexts [9]. Here, we show a pattern consistent with selection acting on quiet coordination calls to promote different receiver responses in different contexts by encoding contextual differences in the acoustic properties of the calls. This suggests that relatively low-level selection pressures may be sufficient to promote acoustic signals that express specific motivations and elicit specific responses. In the case of hoos, emission is more likely if bond partners are present—in alert [41] and travel [40] contexts, and in travel contexts, bond partners are more likely to join in travel after hearing a travel hoo [40]. Since bond partners are primary cooperation partners [34], hoos may be designed to keep bond partners together to enable cooperation when it is needed. The extent to which coordination driven by benefits gained through cooperative activities explains the evolution of such context-specific call diversification requires further examination.
How much hoo variants are an expression of signallers' behavioural intentions, for example to stay or leave, requires further testing using an intentional framework [3,38,40,41,43,71,72]. If the hoos simply encode the signaller's emotional state, different acoustic properties would be expected, although a sharp distinction between emotion and intention is unwarranted and may not transpire in the acoustic properties of a signal [57]. A question requiring further testing is thus whether or not a degree of intentionality is required to evolve the context specificity described in the acoustic properties of chimpanzee hoos.
5. Conclusion
We conclude that even within a single acoustic call type, the hoo, variants of the call are context-specific and can be reliably discriminated using acoustic analysis. Previous playback and observational studies examining receiver behaviour in response to hoos suggest that all three hoo variants are connected with group cohesion, but nonetheless seem to elicit subtly different responses from receivers [40,42,43]. To maintain cohesion, receivers must respond differently to signallers in each context: in rest contexts, receivers must stay in the vicinity of signallers; in travel contexts, receivers must approach signallers; and in alert contexts, receivers must approach signallers with caution. For chimpanzees separated even by short distances in low visibility habitat, visual signals or non-specific vocal signals are likely to be unreliable in maintaining cohesion. One particularly interesting feature of the hoos is the low emotional arousal associated with their production, and that acoustic properties of the three hoo variants cannot be easily explained by emotional state. Relatively low-level selection pressures in the social domain may be sufficient to promote differentiated acoustic signals that encode specific motivations and elicit specific responses. One factor driving the evolution of call diversification may have been the demands of cooperative activities.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Roger Mundry for statistical support and for conducting simulations. S. Adue, J. Alyo, M. Gideon, J. Okuti and S. Amati for their hard work in the field, Budongo Conservation Field Station and the Ugandan Authorities (UWA, UNCST) for permission to conduct the study, Adriano Lameira and one anonymous reviewer for their helpful comments.
Ethics
The study was approved by St Andrews University Ethics Committee, St Andrews, UK. This is a study requiring only behavioural observation and sound recording of vocalizations at a distance of at least 5 m from chimpanzees. Research was conducted under the permits of the Uganda Wildlife Authority (TDO/33/02) and the Ugandan National Council for Science and Technology for (NS 181). Budongo Forest Reserve is not a national park, although chimpanzees are endangered and are under the protection of the Uganda Wildlife Authority.
Data accessibility
Data and code are available in electronic supplementary material, data file and code file.
Authors' contributions
C.C. and T.G. conceptualized the paper, collected the data and extracted the acoustic variables. C.C. conducted the analyses and all authors contributed to writing the paper.
Competing interests
We have no competing interests.
Funding
The study was funded by the Leverhulme Trust, the British Academy, the Leakey Foundation, the European Union's Horizon 2020 research and innovation programme under grant agreement no. 679787, the Max Planck Society and the Swiss National Science Foundation (grant nos. P300PA_164678, CR13I1_162720 and 31003A_166458). We thank the Royal Zoological Society of Scotland for providing core support for the Budongo Conservation Field Station.
References
- 1.Wheeler BC, Fischer J. 2012. Functionally referential signals: a promising paradigm whose time has passed. Evol. Anthropol. 21, 195–205. (doi:10.1002/evan.21319) [DOI] [PubMed] [Google Scholar]
- 2.Scarantino A, Clay Z. 2015. Contextually variable signals can be functionally referential. Anim. Behav. 100, e1–e8. (doi:10.1016/j.anbehav.2014.08.017) [Google Scholar]
- 3.Sievers C, Gruber T. 2016. Reference in human and non-human primate communication: what does it take to refer? Anim. Cogn. 19, 759–768. (doi:10.1007/s10071-016-0974-5) [DOI] [PubMed] [Google Scholar]
- 4.Wheeler BC, Fischer J. 2015. The blurred boundaries of functional reference: a response to Scarantino & Clay. Anim. Behav. 100, e9–e13. (doi:10.1016/j.anbehav.2014.11.007) [Google Scholar]
- 5.Fischer J, Price T. 2016. Meaning, intention, and inference in primate vocal communication. Neurosci. Biobehav. Rev. 82, 22–31. (doi:10.1016/j.neubiorev.2016.10.014) [DOI] [PubMed] [Google Scholar]
- 6.Townsend SW, Manser MB. 2013. Functionally referential communication in mammals: the past, present and the future. Ethology 119, 1–11. (doi:10.1111/eth.12015) [Google Scholar]
- 7.Rendall D, Owren MJ, Ryan MJ. 2009. What do animal signals mean? Anim. Behav. 78, 233–240. [Google Scholar]
- 8.Seyfarth RM, Cheney DL, Marler P. 1980. Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim. Behav. 28, 1070–1094. (doi:10.1016/S0003-3472(80)80097-2) [Google Scholar]
- 9.Zuberbühler K. 2009. Chapter 8 survivor signals: the biology and psychology of animal alarm calling. Adv. Study Behav. 40, 277–322. (doi:10.1016/S0065-3454(09)40008-1) [Google Scholar]
- 10.Smith MJ, Harper DGC. 1995. Animal signals: models and terminology. J. Theor. Biol., 177, 305–311. (doi:10.1006/jtbi.1995.0248) [Google Scholar]
- 11.Furrer RD, Manser MB. 2009. The evolution of urgency-based and functionally referential alarm calls in ground-dwelling species. Am. Nat. 173, 400–410. (doi:10.1086/596541) [DOI] [PubMed] [Google Scholar]
- 12.Smith JM. 2017. The evolution of alarm calls. Am. Nat. 99, 59–63. (doi:10.1086/282349) [Google Scholar]
- 13.Graw B, Hollén LI, Bousquet CA, Furrer RD, le Roux A. 2014. Vocal complexity in meerkats and other mongoose species. Adv. Study Behav. 46, 281–310. (doi:10.1016/B978-0-12-800286-5.00006-7) [Google Scholar]
- 14.Cheney DL, Seyfarth RM. 2007. Baboon metaphysics. Chicago, IL: University of Chicago Press. [Google Scholar]
- 15.Silk JB, Seyfarth RM, Cheney DL. 2016. Strategic use of affiliative vocalizations by wild female baboons. PLoS ONE 11, 1–11. (doi:10.1371/journal.pone.0163978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McComb K, Semple S. 2005. Coevolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385. (doi:10.1098/rsbl.2005.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustison ML, le Roux A, Bergman TJ. 2012. Derived vocalizations of geladas (Theropithecus gelada) and the evolution of vocal complexity in primates. Phil. Trans. R. Soc. B 367, 1847–1859. (doi:10.1098/rstb.2011.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234. (doi:10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 19.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. (doi:10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 20.Duffy KG, Wrangham RW, Silk JB. 2007. Male chimpanzees exchange political support for mating opportunities. Curr. Biol. 17, 586–587. (doi:10.1016/j.cub.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 21.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210. (doi:10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 22.Wittig RM, Crockford C, Weltring A, Langergraber KE, Deschner T, Zuberbühler K. 2016. Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nat. Commun. 7, 13361 (doi:10.1038/ncomms13361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furrer RD, Manser MB. 2009. Banded mongoose recruitment calls convey information about risk and not stimulus type. Anim. Behav. 78, 195–201. (doi:10.1016/j.anbehav.2009.05.002) [Google Scholar]
- 24.Crockford C, Boesch C. 2003. Context-specific calls in wild chimpanzees, Pan troglodytes verus: analysis of barks. Anim. Behav. 66, 115–125. (doi:10.1006/anbe.2003.2166) [Google Scholar]
- 25.Mitani JC, Watts DP. 1999. Demographic influences on the hunting behaviour of chimpanzees. Am. J. Phys. Anthropol. 109, 439–454. (doi:10.1002/(SICI)1096-8644(199908)109:<439::AID-AJPA2>3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- 26.Fedurek P, Slocombe KE, Hartel JA, Zuberbühler K. 2015. Chimpanzee lip-smacking facilitates cooperative behaviour. Sci. Rep. 5, 13460 (doi:10.1038/srep13460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uster D, Zuberbuhler K. 2001. The functional significance of Diana monkey ‘clear’ calls. Behaviour 138, 741–756. (doi:10.1163/156853901752233389) [Google Scholar]
- 28.Katsu N, Nakamichi M, Yamada K. 2016. Function of grunts, girneys and coo calls of Japanese macaques (Macaca fuscata) in relation to call usage, age and dominance relationships. Behaviour 153, 125–142. (doi:10.1163/1568539X-00003330) [Google Scholar]
- 29.Clay Z, Archbold J, Zuberbühler K. 2015. Functional flexibility in wild bonobo vocal behaviour. PeerJ 3, e1124 (doi:10.7717/peerj.1124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rendall D, Seyfarth RM, Cheney DL, Owren MJ. 1999. The meaning and function of grunt variants in baboons. Anim. Behav. 57, 583–592. (doi:10.1006/anbe.1998.1031) [DOI] [PubMed] [Google Scholar]
- 31.Mercier S, Neumann C, van de Waal E, Chollet E, de Bellefon JM, Zuberbühler K. 2017. Vervet monkeys greet adult males during high-risk situations. Anim. Behav. 132, 229–245. (doi:10.1016/j.anbehav.2017.07.021) [Google Scholar]
- 32.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, 507–508. (doi:10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 33.Samuni L, Preis A, Mundry R, Deschner T, Crockford C, Wittig RM. 2016. Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proc. Natl Acad. Sci. USA 114, 268–273. (doi:10.1073/pnas.1616812114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langergraber KE, Mitani JC, Vigilant L. 2007. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786–7790. (doi:10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langergraber K, Mitani J, Vigilant L. 2009. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 71, 840–851. (doi:10.1002/ajp.20711) [DOI] [PubMed] [Google Scholar]
- 36.Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbühler K, Deschner T. 2013. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 280, 20122765 (doi:10.1098/rspb.2012.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fedurek P, Schel AM, Slocombe KE. 2013. The acoustic structure of chimpanzee pant-hooting facilitates chorusing. Behav. Ecol. Sociobiol. 67, 1781–1789. (doi:10.1007/s00265-013-1585-7) [Google Scholar]
- 38.Sievers C, Wild M, Gruber T. 2017. Flexibility, inference and intentionality in animal communication. In Routledge Handbook of Philosophy of Animal Minds (eds Andrews K, Beck J), pp. 333–342. Abingdon, UK: Routledge. [Google Scholar]
- 39.Crockford C, Herbinger I, Vigilant L, Boesch C. 2004. Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology 110, 221–243. (doi:10.1111/j.1439-0310.2004.00968.x) [Google Scholar]
- 40.Gruber T, Zuberbühler K. 2013. Vocal recruitment for joint travel in wild chimpanzees. PLoS ONE 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crockford C, Wittig RM, Mundry R, Zuberbühler K. 2012. Wild chimpanzees inform ignorant group members of danger. Curr. Biol. 22, 142–146. (doi:10.1016/j.cub.2011.11.053) [DOI] [PubMed] [Google Scholar]
- 42.Crockford C, Wittig RM, Zuberbühler K.. 2014. An intentional vocalization draws others’ attention: a playback experiment with wild chimpanzees. Anim. Cogn. 18, 581–591. (doi:10.1007/s10071-014-0827-z) [DOI] [PubMed] [Google Scholar]
- 43.Crockford C, Wittig RM, Zuberbühler K. 2017. Vocalizing in chimpanzees is influenced by social-cognitive processes. Sci. Adv. 3, e1701742 (doi:10.1126/sciadv.1701742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds V. 2005. The chimpanzees of the Budongo forest: ecology, behaviour and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 45.Boersma P, Weenink D.2005. PRAAT: doing phonetics by computer (Version 4.3.01) [Computer program]. See www.praat.org.
- 46.Mundry R, Sommer C. 2007. Discriminant function analysis with nonindependent data: consequences and an alternative. Anim. Behav. 74, 965–976. (doi:10.1016/j.anbehav.2006.12.028) [Google Scholar]
- 47.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 48.Venables WN, Ripley BD. 2002. Modern applied statistics with S. 4th edn New York, NY: Springer. [Google Scholar]
- 49.Baayen RH. 2008. Analyzing linguistic data. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 50.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-5.
- 51.Schielzeth H, Forstmeier W. 2009. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. (doi:10.1093/beheco/arn145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barr DJ, Levy R, Scheepers C, Tily HJ. 2013. Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 68, 255–278. (doi:10.1016/j.jml.2012.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobson AJ. 2002. An introduction to generalized linear models. London, UK: Chapman and Hall, CRC. [Google Scholar]
- 54.Owren MJ, Rendall D. 2001. Sound on the rebound: bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evol. Anthropol. 10, 58–71. (doi:10.1002/evan.1014) [Google Scholar]
- 55.Waser PM, Brown CH. 1986. Habitat acoustics and primate communication. Am. J. Primatol. 10, 135–154. (doi:10.1002/ajp.1350100205) [DOI] [PubMed] [Google Scholar]
- 56.Rendall D, Cheney DL, Seyfarth RM. 2000. Proximate factors mediating ‘contact’ calls in adult female baboons (Papio cynocephalus ursinus) and their infants. J. Comp. Psychol. 114, 36 (doi:10.1037/0735-7036.114.1.36) [DOI] [PubMed] [Google Scholar]
- 57.Gruber T, Grandjean D. 2017. A comparative neurological approach to emotional expressions in primate vocalizations. Neurosci. Biobehav. Rev. 73, 182–190. (doi:10.1016/j.neubiorev.2016.12.004) [DOI] [PubMed] [Google Scholar]
- 58.Kalan AK, Piel AK, Mundry R, Wittig RM, Boesch C, Kühl HS. 2016. Passive acoustic monitoring reveals group ranging and territory use: a case study of wild chimpanzees (Pan troglodytes). Front. Zool. 13, 34 (doi:10.1186/s12983-016-0167-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson ML, et al. 2014. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417. (doi:10.1038/nature13727) [DOI] [PubMed] [Google Scholar]
- 60.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï forest: behavioural ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 61.Zuberbühler K, Wittig RM. 2011. Field experiments with non-human primates: a tutorial. In Field and laboratory methods in primatology: a practical guide, 2nd edn (eds Setchell JM, Curtis DJ), pp. 207–224. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 62.Cheney DL, Seyfarth RM.. 2018. Flexible usage and social function in primate vocalizations. Proc. Natl Acad. Sci. USA 115, 1974–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seyfarth RM, Cheney DL, Marler P. 1980. Monkey responses to three different alarm calls: evidence of predator classification and semantic communication. Science 210, 801–803. (doi:10.1126/science.7433999) [DOI] [PubMed] [Google Scholar]
- 64.Fischer J. 1998. Barbary macaques categorize shrill barks into two call types. Anim. Behav. 55, 799–807. (doi:10.1006/anbe.1997.0663) [DOI] [PubMed] [Google Scholar]
- 65.Zuberbühler K. 2000. Causal cognition in a non-human primate: field playback experiments with Diana monkeys. Cognition 76, 195–207. (doi:10.1016/S0010-0277(00)00079-2) [DOI] [PubMed] [Google Scholar]
- 66.Laidre ME, Johnstone RA. 2013. Animal signals. Curr. Biol. 23, 829–833. (doi:10.1016/j.cub.2013.07.070) [DOI] [PubMed] [Google Scholar]
- 67.Krebs JR, Dawkins R. 1984. Animal signals: mind reading. In Behavioural ecology: an evolutionary approach, 2nd edn (eds Krebs JR, Davies NB), Sunderland, MA: Sinauer. [Google Scholar]
- 68.Ryan M, Cummings M. 2005. Animal signals and the overlooked costs of efficacy. Evolution 59, 1160–1161. [Google Scholar]
- 69.Price K. 1998. Benefits of begging for yellow-headed blackbird nestlings. Anim. Behav. 56, 571–577. (doi:10.1006/anbe.1998.0832) [DOI] [PubMed] [Google Scholar]
- 70.Haskell D. 1999. The effect of predation on begging-call evolution in nestling wood warblers. Anim. Behav. 57, 893–901. (doi:10.1006/anbe.1998.1053) [DOI] [PubMed] [Google Scholar]
- 71.Townsend SW, et al. 2017. Exorcising Grice's ghost: an empirical approach to studying intentional communication in animals. Biol. Rev. 92, 1427–1433. (doi:10.1111/brv.12289) [DOI] [PubMed] [Google Scholar]
- 72.Lameira AR. 2014. The forgotten role of consonant-like calls in theories of speech evolution. Behav. Brain Sci. 37, 559–560. (doi:10.1017/S0140525X1300407X) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available in electronic supplementary material, data file and code file.



