Abstract
Bone replacement might have been practiced for centuries with various materials of natural origin, but had rarely met success until the late 19th century. Nowadays, many different bone substitutes can be used. They can be either derived from biological products such as demineralized bone matrix, platelet-rich plasma, hydroxyapatite, adjunction of growth factors (like bone morphogenetic protein) or synthetic such as calcium sulfate, tri-calcium phosphate ceramics, bioactive glasses, or polymer-based substitutes. All these substitutes are not suitable for every clinical use, and they have to be chosen selectively depending on their purpose. Thus, this review aims to highlight the principal characteristics of the most commonly used bone substitutes and to give some directions concerning their clinical use, as spine fusion, open-wedge tibial osteotomy, long bone fracture, oral and maxillofacial surgery, or periodontal treatments. However, the main limitations to bone substitutes use remain the management of large defects and the lack of vascularization in their central part, which is likely to appear following their utilization. In the field of bone tissue engineering, developing porous synthetic substitutes able to support a faster and a wider vascularization within their structure seems to be a promising way of research.
Keywords: Synthetic, orthopedics, spine, cyst, dentistry, porosity, vascularization
Introduction
Bone defects can develop from different origins like infection, tumor, trauma, surgery, congenital etiology (Figure 1), and so on.1 For centuries, the idea of replacing missing bone tissue has emerged. Traces of orthopedic treatments have been found in Pre-Columbian and Egyptian civilizations.2,3 During the 17th century, Dutch surgeon Job Van Meekeren reported the first success in bone grafting. It consisted of the transplantation of a piece of bone from a dog’s skull into a cranial defect in a soldier. Nevertheless, the graft had to be removed under the orders of the Church. During the 19th century, Van Merren reported the first autogenic graft success, while cases of allogenic grafts were reported as well.4 Non osseous materials (wood, marble, etc.) were used during the same period, but the results were not really convincing until Dreesman used plaster of Paris (calcium sulfate) in 1892 and resulted in a success.5–7
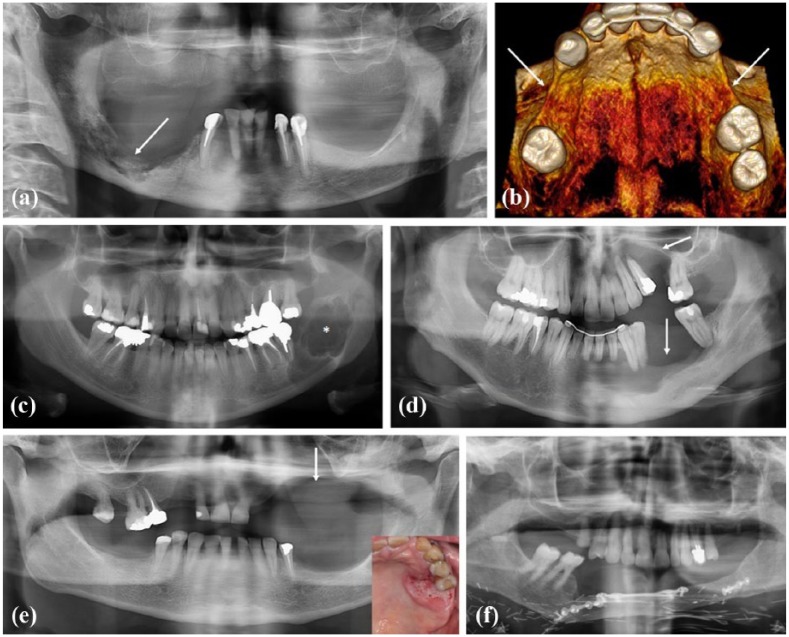
Figure 1.
Various origins of bone defects: (a) Panoramic X-ray. Bone defect of the mandible right body corresponding to an osteonecrosis of the jaw in relation to denosumab taking. (b) 3D-reconstructed view of upper jaw. Bilateral bone defect of premolar regions associated to tooth agenesis in a young adult presenting a WNT10A gene mutation. (c) Panoramic X-ray. Bone defect (radiolucency, *) of the mandible right ramus corresponding to an ameloblastoma, an odontogenic aggressive benign tumor. (d) Panoramic X-ray. Bone defect (radiolucency, arrows) of upper and lower jaws corresponding to a trauma. (e) Panoramic X-ray. Bone defect (arrow) of upper jaw after resection surgery of a gingival squamous cell carcinoma (clinical view, left corner). (f) Reconstruction of the mandible by autogenous bone (fibula) following an invasive squamous cell carcinoma of the gingiva.
In 2001, bone grafting represented 500,000 procedures per year in the United States, and more than 2 millions in the world,8–10 widely using autograft, which is qualified as the gold standard technique. To date, many different materials can be found to fill bone defects. These can be allogenic bone, xenogenic bone, or bone substitutes which are defined as “synthetic, inorganic or biologically organic combinations which can be inserted for the treatment of a bone defect instead of autogenous or allogenous bone.”11,12 The ideal material to replace bone tissue should meet precise specifications, such as being biocompatible, bioresorbable, osteoconductive, osteoinductive, structurally similar to bone, porous, mechanically resistant, easy to use, safe, and cost-effective.9 If a vast majority of the materials placed on the market are osteoconductive, very few offer osteoinductive properties.9,13 Regarding the specifications of an ideal material, the only one which seems to meet them is autologous bone. Indeed, autologous bone graft still is the gold standard technique for bone filling for many reasons.8,9,14–25 First of all, autologous bone meets the mechanical and biological requisites for a filling material. Moreover, its use avoids any immunogenicity or rejection problems23,24 and any disease transmission risk.24 Nevertheless, the technique shows many disadvantages as well, and the most important of them is certainly the comorbidity associated with the presence of a second surgical site: the donor site.15,18,24,25 Complications appear to be chronic pain in a range of 2.5% from 8% of cases, dysesthesia in 6% of cases, or infection in 2% of cases.26,27 For some surgical procedures that would not require a general anesthesia (e.g. hand surgery), the need to obtain autologous bone (from the iliac crest) makes this anesthesia mandatory, increasing surgical risks for the patient.16
The first alternative to autologous bone we can think of is the use of allogenic bone (Figure 2), but a risk of disease transmission exists.15 Although very rare cases are documented concerning HIV transmission (two cases have been reported since 1989, and the risk is estimated at 1/1.6 million)28,29 or hepatitis B and C viruses transmission (1 and 2 cases have been reported since 1989, respectively),28 transmission of other kind of viruses should not be excluded.28,30,31 Moreover, the high cost of such materials should be considered.23 Indeed, an allogenic bone graft has to be treated and sterilized before it is stored and clinically used, representing a significant cost. On the contrary, an autogenous bone graft procedure allows to overcome any storage issue and is practiced during a single operative time. Another alternative to autograft could be the use of xenogenic bone; however, the same limitations persist with a risk of immunogenicity problems23 and disease transmission8 even if the risk is estimated to be very low32 and mostly concerns the porcine endogenous retrovirus (PERV) and the bovine spongiform encephalopathy (BSE).8 Furthermore, the medical team needs to deal with the acceptance of this technique by patients, especially regarding their beliefs.26,33 Thus, to avoid all these limitations, the use of synthetic bone substitutes is becoming increasingly popular.34,35 However, not all bone substitutes, from biological or synthetic origin, can be used for every application. The aim of our review is to specify the properties of the clinically available most used bone substitutes, according to the literature, to precise some of their clinical use, and to discuss about characteristics that should be developed in order to use them for large bone defects filling.
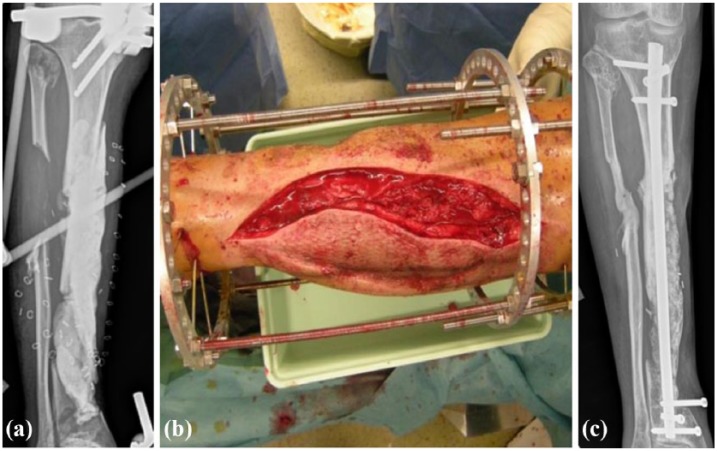
Figure 2.
(a) Radiographical view of a right fibula bone loss, after a road traffic accident in a 24 year-old woman, with an external fixator. (b) Surgical procedure with the use of allogeneic bone chips. (c) Radiographical view 4 months later (courtesy of Dr D. Brinkert).
Bone substitutes
Bone substitutes will be classified in two main categories: bone substitutes derived from biological products and synthetic bone substitutes.
Bone substitutes derived from biological products
Demineralized bone matrix
Demineralized bone matrix (DBM) is bone that has been acid-treated in order to remove the mineral matrix, while maintaining the organic matrix and growth factors such as bone morphogenetic protein (BMP),24 insulin growth factor (IGF), transforming growth factor (TGF), or fibroblast growth factor (FGF).8 In proportion, 93% of a DBM is represented with collagen and 5% with growth factors.24 Since some growth factors are maintained, DBM can show osteoinductive capabilities36–40 and osteoconductive properties by the presence of a collagen structure.24,36 Nevertheless, a large rate of the osteogenic capacity of bone is lost during its processing.41 DBM has been clinically used since the early 1980s8 after Urist and collegues’42,43 work and is currently used in 50% of allografts performed in the United States,39 although evidence for or against its efficacy is still at low level.19,44 DBM shows no immunological rejections because the antigenic surface structure of the bone is destroyed during its demineralization by acid.24,45 The use of DBM avoids donor site morbidity, and studies showed a comparable pain intensity after the surgical procedure compared to autograft procedures.19 DBM is derived from human bone. It presents suitable availability, but this substitute is more expensive than an iliac crest bone autograft procedure,19 and its mechanical properties are quite low.8 Thus, DBM is only used for filling purposes and generally not as a stand-alone bone substitute.8,46
Platelet-rich plasma
Platelet-rich plasma (PRP) is generally used as a gel that is easily obtained with the patient’s blood.8 Blood is centrifuged through gradient density, and the resulting blood platelets are mixed with thrombin and calcium chloride.47 Hence, PRP includes an important concentration of platelets and fibrinogen,47 as well as growth factors such as platelet derived growth factors (PDGF), vascular endothelial growth factor (VEGF), IGF, and TGF.8,40,48–50 PRP is expected to show pro-coagulant effects due to platelets;48 however, there is no evidence in the literature of benefits for the addition of PRP to accelerate bone healing.40,51 Even if PRP shows limited infectious risks and adverse effects by its origin (autologous blood),52 it does not present any mechanical resistance and is not validated as a stand-alone bone substitute.8 PRP is rather used as a supplement to other materials.47,53–55
BMPs
Bone morphogenetic proteins (BMPs) are osteoinductive growth factors included in the transforming growth factor β (TGF-β) superfamily.8 They are produced by osteoblasts and are largely involved in the skeletogenic process,56 enabling ectopic bone formation.42 BMP play a role in the recruitment of osteoprogenitor cells in bone formation sites. Genetic engineering allows to synthetize recombinant human BMP (rhBMP-2 and rhBMP-7), which can be produced in large quantities39,57,58 and limit risks of contamination. rhBMP-2 and rhBMP-7 are allowed by the Food and Drug Administration (FDA) for clinical use.59,60 The history of the safety of BMP has been eventful: in 2009, a systematic review led by Agarwal et al.61 including 17 studies for 1342 patients concluded that the use of BMP-2 or BMP-7 did not lead to any adverse effect, whereas recent reviews reported complications up to 50%.26 After 2 years in 2011, Carragee et al.62 shared growing reportings linked to the utilization of BMP-2. These studies highlighted unpublished results regarding especially the use of BMP-2. Authors estimated that the risk of complications linked to BMP-2 is 10–50 times higher than the results that were showed in previous studies.63 Adverse effects then appeared: heterotrophic ossification, osteolysis, infection, and retrograde ejaculation.39,63 Moreover, paradoxical inhibitory effects of BMP-2 at high concentrations may appear64 and compromise a successful procedure. Thus, and due to the variability of the needed dosage which is patient- and site-dependant, the use of BMP is still surrounded by a blur. Moreover, BMPs require molecular carriers to deliver and maintain them at their intended osseous targets,39 their mechanical properties are not biomimetic of the native bone tissue, and their high cost makes their use prohibitive in most settings.8 However, excluding their adverse effects, BMPs appear to be promising regarding their results in nonunions resolutions,61 and the decrease in the operating time and blood loss during surgical procedures.65,66
Hydroxyapatite
Hydroxyapatite (HA) is part of the apatites family, which are crystalline compounds with crystalline hexagonal lattice. HA has the specific formula (Ca10(PO4)6(OH)2) and is the primary mineral component of teeth and bones.8 Thus, HA is extremely biocompatible67–69 and does not promote an inflammatory response.70 Natural HA is porous with a various porosity depending on the bone site that is extracted (for example 65% porosity and pores from 100 to 200 µm for trabecular bone71), which allows osteoconductive properties. Indeed, HA resorption is very slow72 and the material is usually maintained at least up to 3 years after implantation,69 allowing a slow bone ingrowth progress and cell colonization.69,71 Since HA offers very good mechanical properties with a compression resistance up to 160 MPa, it is likely to be utilized in small bone defects with low loading condition.69 Nevertheless, the use of HA alone may be deceiving.58,73 HA comes in both natural and synthetic forms,8 and HA-TCP (tri-calcium phosphate) ceramics are usually preferred to HA alone. Some composite materials containing HA and collagen exist as well, and their combination enhances osteoblasts differentiation and accelerates osteogenesis.74 HA-collagen composites have some mechanical advantages over HA used alone. Indeed, the ductile properties of collagen allow an increase in the poor fracture toughness of hydroxyapatite. Still, the effectiveness of this composite material has to be validated by further clinical studies.8
Coral
Corals have interconnected pores and a skeleton quite similar to cortical and spongy bones,75 and their use as bone substitute has been approved by the FDA in 1992.58 Coral-based substitutes are mainly calcium carbonate that can be transformed industrially into HA, or they can retain their original state which allows a better resorption by the natural bone.8 Coralline HA can be used as growth factors carrier, such as BMP, TGF-β, or FGF.58 It can be found in different aspects like granules or blocks. Despite its slow resorption, it does not induce adverse effects like inflammatory responses.58,76 Coralline HA is osteoconductive, can show an excellent bone-bonding capacity,77 avoids donor site morbidities,58 and is unlikely to promote disease transmissions or risks of deep infections.78
Synthetic bone substitutes
Calcium sulfate
The first therapeutic success using calcium sulfate (CaSO4) as a bone substitute was reported in 1892.5–8 However, this material also called “gypsum” or “plaster of Paris” and has only been FDA accepted in 1996.58 Calcium sulfate offers many advantages as it presents a structure similar to bone, it is osteoconductive,34,79 inexpensive,39,79 and available in different forms (hard pellets and injectable fluids).17,39 It does not generate allergic reactions.79 Moreover, calcium sulfate has a crystalline structure that is osteoconductive, onto which bone capillaries and perivascular mesenchymal tissue can invade.8,80 Calcium sulfate resorbs rapidly in 1–3 months.8,39,58,79 This resorption creates porosity while stimulating bony ingrowth.81 Nevertheless, the resorption of calcium sulfate is faster than the rate of new bone deposition,34,82 and thus, it is rather unsuitable as a material to support early functional rehabilitation.58,83 Calcium sulfate can be used as a support or a vehicle for local antibiotics or growth factors delivery.84–87 Although it presents many advantages, calcium sulfate also shows some disadvantages, in addition to its fast resorption. It is neither osteoinductive nor osteogenic, and in many cases, redness and swelling of the wound can persist after the procedures.17,58,79 Appearing in 4%–53% of cases,17,88,89 these kinds of complications are generally managed with local wound care, but just as other bone grafts, infections can appear as well and necessitate sometimes a further surgical intervention.89
Calcium phosphate cements
Calcium phosphate cements (CPCs) were invented in 1986 by Brown and Chow90 and were FDA approved for the treatment of non-load-bearing bone defect in 1996 (concerning tetracalcium phosphate and dicalcium phosphate dihydrate products).8 This bioresorbable material91 can stay in the body for long up to 2 years without resorption, depending on its formulation. It consists of a calcium phosphate powder which is mixed with a liquid to form a workable paste.8 Its isothermic hardening reaction varies from 15 to 80 min depending on the formulation,92 and this results in nanocrystalline HA, which makes CPC osteoconductive.8 The main advantage of CPC is the possibility to shape the paste to the complex bone cavity, avoiding gaps between the bone and the implant. Furthermore, some CPC are injectable and can be used in minimal invasive procedures such as vertebroplasty and kyphoplasty. Just like other substitutes (e.g. calcium sulfate and some HA-based grafts), CPC are brittle8 and can lead to some complications (13% overall complications according to Afifi et al.93 with 9% major complications and 5% infections). Because clinical outcomes seem not to be better and sometimes worse than the use of methylmethacrylate or autologous bone, CPC should be used selectively.93
β-tri-calcium phosphate ceramics
β-tri-calcium phosphate (β-TCP) (Ca3(PO4)2) has largely been used as a bone substitute94–96 for more than 25 years, mainly for orthopedics and dentistry applications,97 and is considered as the “gold standard” for synthetic bone.95 It is a biocompatible98,99 and bioresorbable material8,58,94,100,101 with properties similar to the inorganic phase of bone. β-TCP is osteoconductive96,98,102,103 due to its composition and its porosity,100 which depends on the processing condition. Indeed, its porous structure plays a role in its osteoconductive characteristics.104 β-TCP gradually resorbs, and although its resorption is unpredictable105 and slower than the resorption of calcium sulfate,39,106 β-TCP is meant to be completely resorbed in time58,100,107 by osteoclasts.108 β-TCP resorbs in approximately 13–20 weeks after implantation and is then completely replaced by remodeled bone.103,109 Furthermore, β-TCP with its interconnected pores may accelerate bone remodeling by facilitating the colonization of osteogenic cells and nutrients via an enhanced capillarity15 and seems to have the potential to influence angiogenesis.96 In vivo studies showed an incorporation of bone between 45% (in vertebral bodies of apes)110 and 70% (in piglets’ mandibles)111 6 months after implantation, and of 95% after 2 years.100 The use of β-TCP showed very few complications like infection or nonunion.100 Although its suitable mechanical resistance, it is still inferior to mechanical properties of cancellous bone39 or of a bone allograft.112 Therefore, β-TCP should be used selectively.39,112
Biphasic calcium phosphates (HA and β-TCP ceramics)
β-TCP is mostly used in association with HA.8,9,23,94,113,114 Synthetic HA can be made by the precipitation of calcium nitrate and ammonium dihydrogen phosphate.23 This association presents all the advantages of its two components (osteoconductivity,94,115–118 biocompatibility,94,113 safe and nonallergen use,113 and promotion of bone formation106). The major gain of using biphasic ceramics (HA and β-TCP mixture) concerns their resorption. Indeed, the resorption of β-TCP is faster than the resorption of HA,23,72,119 but mechanical properties of HA are slightly better than β-TCP’s (average compressive resistances are, respectively, of 160 and 100 MPa).23 Thus, the association of β-TCP and HA enables a faster and higher bone ingrowth rate than using HA alone94 while offering better mechanical properties than β-TCP alone.72,114 Indeed, 12 months after the implantation of the material, 60% of the β-TCP resorbs compared to only 10% for the HA.94 HA and β-TCP ceramics form a strong direct bond with the host bone.120 They can be found with different HA/β-TCP ratios and can be associated with bone marrow aspirate which then provides enhanced osteogenic properties to the material.121 Despite the improvement of mechanical properties of β-TCP by the incorporation of HA, the strength of HA and β-TCP ceramics is still lower than cortical bone compression strength, which is between 150 and 200 MPa.8 Different preparation methods are available, like a compact form, or a porous form with interconnected macropores equivalent to cancellous bone, which is preferred.122
A few studies mention the utilization of composite substitutes of calcium sulfate associated to β-TCP which would lead to very few complications.34,123 When applied to long bones, the return to full weight bearing and unrestricted activities of daily living is at a mean of 7.3 weeks34 against 14 weeks when using HA or β-TCP.23
Bioactive glasses
Developed for the first time by Hench et al.124 in the 1970s,125 bioactive glasses (or bioglasses) are originally silicates that are coupled to other minerals naturally found in the body (Ca, Na2O, H, and P). The original bioglass composition is 45% silica (SiO2), 24.5% calcium oxide (CaO), 24.5% sodium oxide (Na2O), and 6% phosphorous pentoxide (P2O5) in weight percentage.126 When subjected to an aqueous solution or body fluids, surface of bioglasses converts to a silica-CaO/P2O5-rich gel layer that subsequently mineralizes into hydroxycarbonate in a few hours.126–128 Bioglasses are biocompatible, osteoconductive,58,125,129 and—depending on their processing condition—offer a porous structure which promotes their resorption and bone ingrowth.130 The use of bioglasses does not induce an inflammatory response, and their resorption is complete in 6 months for silica-based bioglasses.131 More recently, phosphate- or borate-based bioglasses have been developed.132,133 Borate-based bioglasses, which are easily manufacturable, show a faster degradation than silica-based bioglasses, but this degradation rate can be controlled by adjusting its composition. This ability leads to a possible match with the bone regeneration rate.132 Phosphate-based bioglasses present a controllable solubility by manipulating their composition, and their structure makes them a specific and promising group of bioglasses for hard and soft tissue engineering.133 When implanted in bone tissues, these materials show a strong bond to bone and withstand removal from the implantation site.125,129,134 However, bioglasses are quite brittle58 and present low mechanical strength and decreased fracture resistance.125 Thus, their utilization should be selective125 or in association with other bone substitutes.
Polymer-based bone substitutes
Although natural polymers such as collagen exist and are slightly used alone rather than in combination with HA; for example, this section (synthetic bone substitutes) will be focused on synthetic polymers. They can be nondegradable (like poly(methylmethacrylate) or PMMA) or fully biodegradable, thus allowing a total bone replacement in time (e.g. polylactic acid (PLA)) without remaining foreign bodies.8 Initially used as graft extenders,135 researches focus on synthetic polymeric bone substitutes, especially in the field of tissue engineering. Polyesters like poly(ε-caprolactone) (PCL), for example, can be synthetized by mimicking the collagenic matrix, offering a structural porosity and osteoconductive properties.136,137 Most of the polymer-based bone substitutes are suitable to be used as bioactive molecules or growth factors carriers,138 potentially conferring osteogenetic properties.139 Since PCL is soluble in a wide range of organic solvents, it is a promising polymer for continuous researches in tissue engineering.8,140 Actual polymer-based bone substitutes can be found in different forms. Indeed, blocks of acrylic cement (with a similar composition of a prepolymerized PMMA powder mixed with a liquid monomer containing a large amount of methylmethacrylate monomer) can be fashioned into the desired shape,141 or methacrylate-based products can be used in injectable forms before their polymerisation.142 PMMA cements are of the most extended used materials for articular prosthesis fixation and vertebroplasty. However, according to a Cochrane review led by Handoll and Watts143 in 2008, they are materials which few would use to date for specific bone implantation after distal radial fracture, because they do not promote new bone growth and may rather inhibit it.143,144 Polymer-based bone substitutes are mainly scrutinized for their wide potential in tissue engineering, allowing their fabrication with macropores and micropores and in the shape of thick membranes (e.g. PCL or PLA).136,138 Clinicians should keep a close eye on outcomes of researches concerning polymer-based bone substitutes as scaffolds for regenerative medicine.
Clinical use
Bone substitutes should be used selectively. According to the literature, here are some directions concerning their clinical use (Table 1).
Table 1.
Clinical use directions of some bone substitutes.
| Bone substitute | Clinical use |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spine fusion | OWTO | Contained bone defects | Hand surgery | Long bone fracture | Fracture nonunion | Periodontal defects | Sinus augmentation | Osteonecrosis of the jaw | Bone infections (drug carrier) | Cranioplasty | Vertebroplasty/kyphoplasty | ||
| Bone substitutes derived from biological products | DBM | + (except for anterior spinal fusion) | – | + | NI | NI | ++ | + | + | NI | NI | + | NI |
| PRP | NI | – | NI | NI | NI | NI | – | +/– | + | NI | NI | NI | |
| BMP | + | NI | – | NI | NI | NI | NI | NI | NI | NI | NI | NI | |
| HA | NI | + | NI | + (as a composite graft with calcium sulfate) | NI | NI | NI | NI | NI | + | + | NI | |
| Coral | – | NI | + | NI | NI | NI | - | NI | NI | NI | NI | NI | |
| Synthetic bone substitutes | Calcium sulfate | + | NI | + | + (as a composite graft with HA) | NI | + | NI | NI | NI | + | NI | – |
| CPC | NI | NI | NI | NI | + | NI | NI | NI | NI | + | + | +/– | |
| HA and β-TCP ceramics | ++ | ++ | ++ | + | + | NI | + | + | NI | + | NI | NI | |
| Bioactive glasses | NI | NI | NI | NI | NI | NI | + | + | NI | + | NI | NI | |
| Polymer-based substitutes | NI | + | – | NI | NI | NI | NI | NI | NI | ++ | ++ | ++ | |
OWTO: open-wedge tibial osteotomy; DBM: demineralized bone matrix; NI: no literature-related information are given in this review; PRP: platelet-rich plasma; BMP: bone morphogenetic protein; HA: hydroxyapatite; CPC: calcium phosphate cement; TCP: tri-calcium phosphate.
(+) gives good clinical outcomes; (++) gives good clinical outcomes and is largely used; (–) gives bad clinical outcomes; (+/–) both good and bad clinical outcomes are found in the literature.
Spine fusion
Spine fusions represent 200,000 procedures per year in the United States.145 To date, autograft and allograft are mainly used to promote spine fusion,146 although other materials seem to fit for this specific use.15 Indeed, DBM combined with marrow aspirate showed good results in posterolateral spine fusion,147 and DBM showed good results when used as a graft enhancer of autologous bone either in cervical fusion surgery37,39 or in lumbar fusion surgery.148–150 However, there is still no evidence for DBM to be used as a stand-alone material in spine fusion.37 DBM applied in anterior spinal fusion is currently not recommended in clinical practice146 because its results have shown a higher rate of graft collapse and pseudarthrosis when compared to autograft.151 Coralline HA has been studied for spine fusion as a graft enhancer.76,152 Since the host bleeding bone surface in this area is small, and knowing that coralline HA mixed with local bone and bone marrow needs adequate bleeding to bond to the bone surface, it appeared that coralline HA was inappropriate for intertransverse posterolateral fusion.76 Although calcium sulfate has been used as a graft expander for spine fusion,79 there is less evidence of its suitability than there is for β-TCP ceramics. The latter demonstrated efficacy for use as a bone graft extender in posterolateral spinal fusion.121,146,153 Moreover, β-TCP in a non injectable form showed good radiographic fusion in both single- and double-level lumbar fusion when mixed with local laminar autografts.154 Thus, many bone graft substitutes are suitable as bone graft extenders, but only osteoinductive proteins (such as rhBMP-2) provide evidence for use as both bone enhancers and bone substitutes58,146 in spine fusion. Products of tissue engineering (hydrogels or synthetic polymer composites) seem to have the potential to be used for spine fusion though warrants further investigation to be used in clinical practice.146
Open-wedge tibial osteotomy
Open-wedge tibial osteotomy (OWTO) is a classical way for treating medial knee osteoarthritis155 or varus deformity138 for example. In a review reporting 70 cases, β-TCP ceramics have been used as wedges101 and showed more than 96% osteointegration and 98.5% of the cases with an achieved bone healing.101 In accordance with other studies, β-TCP ceramics appear to be a bone replacement material with optimal biocompatibility, resorption characteristics, and bone conduction properties for OWTO,99,156,157 using indifferently granules or wedge preforms.158 Using β-TCP ceramics, the results seem to be more similar to those obtained with autologous bone after 6 months, but bone consolidation appears to be a bit longer, so β-TCP ceramics still have to be used selectively.112 In 2000, Hernigou and Ma141 obtained clinically satisfying results in OWTO when using acrylic cement wedges. In 2001, Koshino et al.69 reported a series of 10 cases using HA as a bone substitute for OWTO with good clinical outcomes. However, HA is assumed too frangible to be implanted in bone under mechanical stress or weight bearing,69,112 but the weak mechanical properties of porous HA might be eliminated once incorporation and bone ingrowth into the pores are achieved.159 From their retrospective review in 2015 concerning 83 patients having surgery, Giuseffi et al.160 concluded that allograft mixed with DBM and/or PRP was associated with nonunion.
Contained bone defects (benign tumors and cysts)
Since contained bone defects can occur in many types of bone, a wide range of substitutes has already been clinically used. However, a bone substitute that has been validated in a specific area is not necessarily expected to be validated in another area. Indeed, the setting is different, and the needed characteristics of the bone substitute are different.9 In some studies, calcium sulfate has successfully been used in filling contained bone defects,58,79,88,161–163 and results can be comparable to DBM-based allografts,39 with the advantage to be at lower cost.164 Calcium sulfate also showed good results in filling unicameral bone cysts in pediatrics, with a rate of healing mostly over 90%.162,165–167 While polymethylmethacrylate does not seem to be suitable for the filling of bone defects due to primary bone tumors, because it does not preserve bone stock and because the hardened cement does not share the same biomechanical properties as bone,34,168 the use of a calcium sulfate–calcium phosphate composite was associated with good clinical outcomes (rapid biological integration and early return to activities of daily living) in cavitary bone reconstruction, following intralesional curettage of primary benign bone tumors.34 Concerning PRP, there are major limitations in the literature in terms of low quality and heterogeneity, which hamper possible beneficial PRP treatments, despite positive preclinical findings on its biological potential to promote bone healing.169 Moreover, poor evidence mentions the efficacy of PRP in the treatment of traumatic bone cyst in the mandible.170 Coralline HA, in granules or blocks, seems to be suitable to fill contained bone defects.58 Although its slow resorption, it does not induce adverse effects.58 On the contrary, the use of BMP-2 (in the form of rhBMP-2) can lead to a poor healing rate with complications such as an exaggerated inflammatory response, pain, and limb swelling.171 Finally, β-TCP ceramics are largely used in this purpose9,23,165 and frequently associated with bone marrow aspirate.172,173 Using β-TCP, healing rates vary from 90% to 100% with very few complications which resolved uneventfully.166,172,173
Hand surgery (hand enchondroma and metacarpal fractures)
Enchondromas are the most common benign tumors of the hand16,174 appearing usually as solitary, cystic bone tumors.17 The literature is quite poor regarding the use of bone substitutes for bone filling in hands,17 as some authors advocate that a simple curettage without filling is a sufficient175,176 and a less-expensive option.176 Nevertheless, when bone substitutes are used, β-TCP ceramics seem to be suitable. Indeed, the application of this material gives the same good functional and radiological results compared to autologous bone.16
The use of a composite material with 60% calcium sulfate and 40% HA offered good clinical outcomes as well in terms of limited complications (53% redness and swelling lasting up to 10 postoperative days, 8% chronic regional pain syndrome treated successfully with intensive conservative treatment) and an effective return to normal daily activities after 2 months.17 Furthermore, the use of bone substitutes is especially helpful in the treatment of complicated metacarpal fractures in old multimorbid patients, in whom a general anesthesia or potential donor site morbidities should be avoided,16,17 allowing a reduced operating time and day-case surgery.16
Long bones fracture
Concerning tibial plateau fractures, it has been shown that CPC can provide similar and better mechanical support than autogenous iliac bone graft in the treatment of defects in unstable fractures, preventing subsidence.91 β-TCP ceramics have been used as well for many decades in long bone fractures, such as tibial plateau fractures.77 However, their use in distal radial fractures showed no significant benefits in terms of extra stability, compared to the use of internal fixation only, without bone substitute. Moreover, the occurrence of complications did also not show statistical significance.177 For distal radial fractures, some evidence about bone scaffolding that may improve anatomical outcomes compared with plaster cast immobilization alone exist, but are insufficient on functional outcome and safety.143
Fracture nonunion
There is actually no universally accepted definition of nonunion in the orthopedic literature.178 The FDA defines fracture nonunion as a fracture that is at least 9 months old in which there have been no signs of healing for 3 months.39 Other definitions mention a fracture in which more than 6 months have elapsed without any improvement toward union,179 distinguishing these cases from delayed unions. Nonunion appears in 10% of all fractures180 and are generally treated with an open reduction and internal fixation associated with an augmentation using an autologous bone graft.19 Since many synthetic bone substitutes are strictly osteoconductive, their biological role in fracture healing is limited,40 although calcium sulfate has already been used as a graft expander for the treatment of established nonunions with a healing rate of 88%.181 DBM is a popular bone substitute for the grafting of nonunions.19,39 It has been compared to autologous bone and led to good results in terms of consolidation (more than 80% success) and a decrease in the adverse effects (especially due to the presence of a donor site).19 However, the procedure cost was more expensive applying DBM (an average difference of US$190/case).19 Recently, biphasic calcium phosphate biomaterials have been use associated to autologous, expanded, bone marrow-derived mesenchymal stromal cells. The safety of their use in the treatment of fracture nonunions has been set, but bone healing obtained through this method still has to be determined to compare the efficacy of this strategy with that of current clinical standards such as autograft.182
Oral/periodontal procedures
Periodontal diseases are widespread pathologies with 50% of adults suffering from a severe attachment loss problem in France.183 Dental biofilm provokes an inflammatory response leading to the destruction of attachment tissues of teeth, while creating periodontal pockets whose depth is relative to the severity of the periodontal disease. To treat periodontal defect, the use of bone grafts seems to promote healing compared to open flap debridement alone.184 Not only the material but also the technique has a role to play as well. Bone grafts in combination with barrier membranes increase clinical attachment level and reduce probing depth compared to graft alone.184 Granules of β-TCP and HA ceramics can be used with significant pocket depth reduction and clinical attachment gain.114,185 Bioglasses also have shown good clinical outcomes with a consequent clinical experience.58,125,185,186 In comparison, other materials are not suitable for the treatment of periodontal defects, such as PRP, which does not demonstrate significant benefit,187–189 or coralline bone substitutes, which does not yield the desired outcomes.185 However, even if bone replacement grafts offer clinically satisfying results in terms of bone fill, histologic evidence of periodontal regeneration has only been reported for autogenous bone grafts and DBM.190 In situations like buccal bone defect filling after a dental implant placement, for example, DBM can also be used (Figure 3).
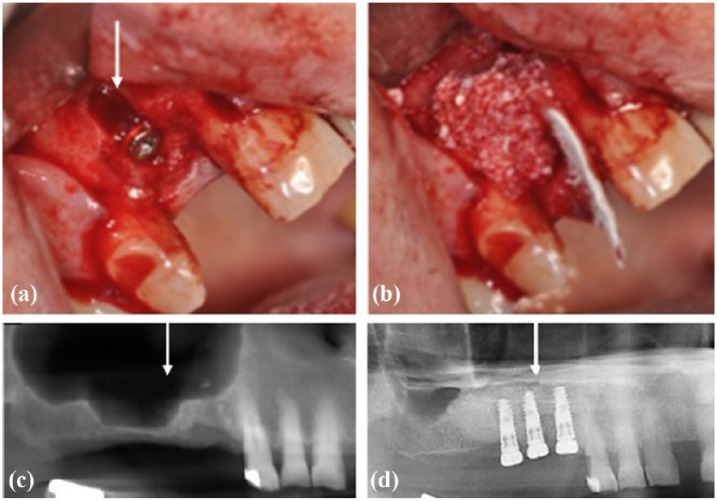
Figure 3.
Use of DBM in oral procedures: (a, b) DBM used to fill buccal bone defect (arrow) after implant placement. (c, d) Panoramic X-rays of a maxillary right sinus before (c) and after sinus floor elevation (d) filled with DBM and dental implants placement.
Concerning sinus elevation, some studies concluded the efficacy of PRP191 in terms of bone density at 6 months post-grafting,192 whereas others postulated that PRP did not improve the clinical outcome of sinus lift procedures using autogenous bone or bone substitutes.47,51,54,193 DBM can be used with significant results for sinus elevation (Figure 3) as injectable formulation,194 putty195,196 or powder form,196 showing no differences regarding dental implant stability and survival rate in a long-term follow-up.196 Moreover, using injectable formulation of DBM could allow practical advantages such as a decrease in operative time.194 The use of bioglasses or a mixture of β-TCP with autologous bone showed suitable results for this procedure;51 however, the available evidence neither supports nor refutes the superiority of autologous bone over other graft materials for sinus augmentation regarding implant survival or complications at the recipient site.197
Osteonecrosis of the jaw
Poor evidence mentions the efficacy of PRP in the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ).198,199 In some studies, however, the use of PRP seems to enhance wound healing and to reduce bone exposure and thus would be an effective treatment protocol to use in BRONJ subjects.47,200
Infections
Various bone substitutes can be used as drug carriers in the treatment of bone deep infections.201 Debridement, and implantation of antibiotic-loaded PMMA granules or beads mostly followed by an implant exchange, is currently the gold standard for this treatment.201,202 Most commonly used antibiotics are gentamicin, tobramycin, and vancomycin.201 Other bone substitutes have been used for this purpose, but clinical data in well controlled trials are still very limited:201 it is the case for β-TCP granules,203 porous HA blocks,202,204 calcium sulfate pellets,202,205 CPC,206,207 and bioglasses.208 However, the performance of some bone graft substitutes with antibiotics in one clinical site is not inevitably predictable of their performance in another site.201 Most infections occur during implantation time and that is why sterile techniques still remain of utmost importance.26,201
Cranioplasty
Cranioplasty is performed mostly after traumatic injuries, tumor removal, or decompressive craniectomies209 in order to protect the brain and achieve a natural appearance.209,210 Among other characteristics, the ideal material should then be easy to shape, radiolucent, resistant to infections, biocompatible, firm, and stable.209,210 Apart from some metals (titanium, tantrum, etc.), various bone substitutes are safely used for cranioplasties, such as CPC,211 HA,209,211,212 or DBM.213 But still, PMMA is the most extensively used cranioplasty material,209,210,214 even if there is still no consensus on which material is better for cranioplasty.209
Vertebroplasty and kyphoplasty
Vertebroplasty procedures were designed to stabilize vertebral body compression fracture and to alleviate pain in patients with various etiology such as hemangioma, spine tumor, or osteoporosis.215,216 Kyphoplasty is a variation of vertebroplasty that usually involves the use of a balloon to create a cavity within the cancellous bone and to elevate or expand the fractured vertebrae toward its original height. The cavity is then filled with bone cement to reinforce the vertebral body.215,217 The filling material plays a crucial role in the effectiveness of these treatments. It must be applicable in a flowable state due to the percutaneous surgical technique, have an adequate setting time to match the progress of surgeries, and have considerable mechanical strength to withstand cyclic and static complex loading patterns.215,217 The most popular bone cement used for this purpose is PMMA-based acrylic bone cement, but several disadvantages are mentioned, such as its heat generation during exothermic polymerization, its nonbiodegradability, and a lack of biologic potential to remodel or integrate into the surrounding bone.215,217 Good clinical results have been reported with PMMA for vertebroplasty and kyphoplasty (over 5° correction for 60% of reducible fractures, with an average of 95% pain reduction within the first week after surgery and improved activity levels for a majority of patients218,219). CPCs present interesting characteristics for their use as fillers in vertebroplasty and kyphoplasty. Indeed, they can be easily molded, injected into the defect area, offer the potential for resorption and replacement with new bone, and do not generate heat.215,217,220 However, there are still some questionings regarding their mechanical strength,221 and few evidence mentions their use other than in laboratory models.215,222 To date, it seems that few CPCs are yet readily available for use in vertebroplasty and kyphoplasty.215 Calcium sulfate has relatively higher mechanical strength than CPC and has been tested, but its fast degradation does not match with the bone formation process and would not allow to support spinal alignment while it is remodeling.215,217,223
Miscellaneous
Other surgical uses of bone substitutes are sometimes mentioned in the literature: β-TCP and HA ceramics have been used in hip arthroplasty,224,225 bioglasses in tympanoplasty,58 and PMMA in an original creation of a neo-rib for chest wall reconstruction.226
For bone defects that are not too large, autologous bone is often preferred. When it comes to large bone defects, the quantity of available autologous bone might not be sufficient, and a wide proportion of bone substitutes is then used as graft expanders,8,37,47,58,79 rather than as stand-alone grafts. Thus, it appears interesting to discuss about two particular properties—porosity and vascularization—that should be developed, leading to advances that would allow for a new generation of enhanced bone substitutes to be used for the treatment of large bone defects.
Vascularization, a requirement for bone regeneration
Currently, the use of bone substitutes is limited to relatively restricted bone defects, because they can become atrophic sequesters if they exceed a critical size (up to 60 cm3)136 and are not vascularized sufficiently.227,228 Thus, vascularization is vital for bone defects to heal, and there is a greater need for vascularization at sites where bone substitutes are used because the defects are larger.96 A lack of vascularization leads to osteonecrosis, which is not a specific disease entity, but the combination of conditions resulting in an impairment of blood supply to the bone tissue.229 It is also called avascular necrosis.229
Indeed, the bridging of bone defects with stable bone substitutes is limited by vascularization as angiogenesis must precede osteogenesis.106,230 The coupling of osteogenesis and angiogenesis is determinant in the bone healing environment,231 and osteogenesis, vascularization, and resorption kinetics must be in equilibrium to allow a harmonious bone remodeling process.232,233 Osteogenic cells will develop into the graft site through the existence of a vascular system234 that allows to understand why poor vascularity can impede effective osteosynthesis.39 Besides, studies showed that the presence of VEGF with resorbable carriers influences the ability to promote bone healing.106,136 Thus, the structure and the composition of bone substitutes must allow vascularization, by presenting an interconnected porosity and a favorable biochemical support. The latter may then accelerate bone remodeling by facilitating colonization and retention of osteogenic cells and nutrients through an enhanced capillarity.15 The establishment of a vascular network will provide nutrients, soluble factors, and minerals (e.g. calcium and phosphate) which are necessary for the bone healing process.136 A delayed healing and some nonunions are often attributed to a failure in restoring vasculature rather than a lack of osteogenic potential.235 That is why vascularization is one of the components of Giannoudis et al.’s236 diamond concept, which sets the main factors that affect bone regeneration.
To give the capacity to bone substitutes to allow the development of vascularization, pores appear to be essential in their structure.96 On one hand, the pore size directly plays a role in the bony ingrowth and can improve it when it is from about 8023,94,237,238 to 200 µm,58,71,102 ensuring a cell colonization, migration, and transport. Furthermore, porosity fraction in the material in the substitutes plays a role as well in bone ingrowth, allowing more cells to invade and offering a larger surface area that is believed to contribute to a higher bone-inducing protein adsorption.239 On the other hand, interconnected pores are a crucial characteristic.15,94 Indeed, dead-end pockets limit vascular supply to the in-growing bone.23 If 100- to 200-µm pores are enough to support cell migration, 300- to 500-µm pores appear to be recommended to allow the formation of capillaries.156,232,240,241 However, there is an equilibrium to be found between the decrease in compressive strength and an increased porosity, regarding the desired mechanical properties of bone substitutes.242
Nevertheless, even knowing that bone substitutes should be porous to allow vascularization, this biological process takes time. Thus, another approach to promote the quality and speed of bone regeneration is the ability to facilitate the development of a vascular network in the bone tissue during regeneration. For example, this could be achieved by adding growth factors (VEGF) to nanostructured implants,136 or by creating bone-like structured biodegradable synthetic scaffolds using techniques such as electrospinning.137,139 This network will provide the nutrients and minerals necessary for cells, conveying cellular waste243 and therefore avoiding the potential necrosis in the middle of bone defects of a moderate size.244 Being able to create polymer-based bone substitutes with a given porosity which will support biofunctionalization and promote the establishment of a vascular network136–139,243,244 are some of the major interests of current researches in bone tissue engineering. That is precisely why clinicians should keep a close eye on these researches.
Conclusion
During the past decades, a plethora of materials have been used as bone substitutes. Some are derived from biological products, others are synthetic. But all of them present advantages and disadvantages and should mainly be chosen selectively. Many surgical procedures call out bone substitutes, such as spine fusion, filling of bone defects, and sinus augmentation, each one being suitable for specific substitutes among others. The main limitations to the use of bone substitutes are large defects and the central osteonecrosis which is likely to appear following their utilization. To avoid this phenomenon, current researches are focusing on the ability to create synthetic scaffolds with a desired porosity and to promote a faster and wider vascularization.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Poirier J, Ribadeau Dumas JL, Catala M, et al. Histologie: les tissus. Médecine 1ère année. 2ème éd Paris: Masson, 2002. [Google Scholar]
- 2. Urist MR, O’Conner BT, Burwell RG. Bone graft, derivatives and substitutes. Cambridge: Butterworth-Heinemann, 1994. [Google Scholar]
- 3. Donati D, Zolezzi C, Tomba P, et al. Bone grafting: historical and conceptual review, starting with an old manuscript by Vittorio Putti. Acta Orthop 2007; 78: 19–25. [DOI] [PubMed] [Google Scholar]
- 4. Mainard D, Delagoutte J-P. Les substituts osseux. In: Mainard D, Merle M, Delagoutte J-P, et al. (eds) Actualités en biomatériaux. Paris Romilliat, 1990, pp. 128–176. [Google Scholar]
- 5. Dreesman H. Über knochenplombierung. Beitr Klin Chir 1892; 9: 804–810. [Google Scholar]
- 6. Pietrzak WS. Musculoskeletal tissue regeneration: biological materials and methods. Human Press, Totowa, 2008, pp. 163–166. [Google Scholar]
- 7. Peltier LF, Bickel EY. The use of plaster of Paris to fill defects in bone. Ann Surg 1957; 146(1): 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campana V, Milano G, Pagano E, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci: Mater Med 2014; 25: 2445–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faour O, Dimitriou R, Cousins CA, et al. The use of bone graft substitutes in large cancellous voids: any specific needs? Injury 2011; 42: S87–S90. [DOI] [PubMed] [Google Scholar]
- 10. Greenwald AS, Boden SD, Goldberg VM, et al. Bone-graft substitutes: facts, fictions and applications. J Bone Joint Surg Am 2001; 83: 98–103. [DOI] [PubMed] [Google Scholar]
- 11. Schlickewie W, Schlickewie C. The use of bone substitutes in the treatment of bone defects—the clinical view and history. Macromol Symp 2007; 253: 10–23. [Google Scholar]
- 12. Pryor LS, Gage E, Langevin CJ, et al. Review of bone substitutes. Craniomaxillofac Trauma Reconstr 2009; 2(3): 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury 2008; 36(Suppl. 3): S20–S27. [DOI] [PubMed] [Google Scholar]
- 14. Bloemers FW, Blokhuis TJ, Patka P, et al. Autologous bone versus calcium-phosphate ceramics in treatment of experimental bone defects. J Biomed Mater Res B Appl Biomater 2003; 66(2): 526–531. [DOI] [PubMed] [Google Scholar]
- 15. Gunzburg R, Szpalski M, Passuti N, et al. The use of bone substitutes in spine surgery: a state of the art review. Springer Science+Business Media, Berlin Heidelberg, 2002, 129 pp. [Google Scholar]
- 16. Hung YW, Ko WS, Liu WH, et al. Local review of treatment of hand enchondroma (artificial bone substitute versus autologous bone graft) in a tertiary referral centre: 13 years’ experience. Hong Kong Med J 2015; 21(3): 217–223. [DOI] [PubMed] [Google Scholar]
- 17. Liodaki E, Kraemer R, Mailaender P, et al. The use of bone graft substitute in hand surgery: a prospective observational study. Medicine 2016; 95(24): e3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delloye C. Is there still a place for bone allografts in orthopedic surgery in 2011? Bull Mem Acad R Med Belg 2011; 166: 317–326. [PubMed] [Google Scholar]
- 19. Pieske O, Wittmann A, Zaspel J, et al. Autologous bone graft versus demineralized bone matrix in internal fixation of ununited long bones. J Trauma Manag Outcomes 2009; 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drosos GI, Kazakos KI, Kouzoumpasis P, et al. Safety and efficacy of commercially available demineralized bone matrix preparations: a critical review of clinical studies. Injury 2007; 4: S13–S21. [DOI] [PubMed] [Google Scholar]
- 21. Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 2002; 84-A: 454–464. [DOI] [PubMed] [Google Scholar]
- 22. Keating JF, McQueen MM. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br 2001; 83: 3–8. [DOI] [PubMed] [Google Scholar]
- 23. Saikia KC, Bhattacharya TD, Bhuyan SK, et al. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J Orthop 2008; 42(2): 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tilkeridis K, Touzopoulos P, Ververidis A, et al. Use of demineralized bone matrix in spinal fusion. World J Orthop 2014; 5(1): 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haute Autorité de Santé (HAS). Substituts osseux. Révision de catégories homogènes de dispositifs médicaux. Saint-Denis La Plaine: HAS, 2013. [Google Scholar]
- 26. Offner D, Wagner Q, Keller L, et al. Complications d’une autogreffe osseuse, et comparaison avec une allogreffe osseuse ou l’utilisation de BMPs (Bone Morphogenetic Proteins): une revue systématique de la littérature. Le Journal de l’Orthopédie 2017; 18(65): 3032–3043. [Google Scholar]
- 27. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma 1989; 3(3): 192–195. [DOI] [PubMed] [Google Scholar]
- 28. Zimmermann G, Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011; 42(Suppl. 2): S16–S21. [DOI] [PubMed] [Google Scholar]
- 29. Khan SN, Cammisa FPJ, Sandhu HS, et al. The biology of bone grafting. J Am Acad Orthop Surg 2005; 13(1): 77–86. [PubMed] [Google Scholar]
- 30. Delloye C, van Cauter M, Dufrane D, et al. Local complications of massive bone allografts: an appraisal of their prevalence in 128 patients. Acta Orthop Belg 2014; 80: 196–204. [PubMed] [Google Scholar]
- 31. Mroz TE, Joyce MJ, Steinmetz MP, et al. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg 2008; 16(10): 559–565. [DOI] [PubMed] [Google Scholar]
- 32. Laurencin CT, El-Amin SF. Xenotransplantation in orthopaedic surgery. J Am Acad Orthop Surg 2008; 16(1): 4–8. [DOI] [PubMed] [Google Scholar]
- 33. De Vries RBM, Oerlemans A, Trommelmans L, et al. Ethical aspects of tissue engineering: a review. Tissue Eng Part B Rev 2008; 14(4): 367–375. [DOI] [PubMed] [Google Scholar]
- 34. Evaniew N, Tan V, Parasu N, et al. Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics 2013; 36(2): e216–e222. [DOI] [PubMed] [Google Scholar]
- 35. Kirkpatrick JS, Cornell CN, Hoang BH, et al. Bone void fillers. J Am Acad Orthop Surg 2010; 18(9): 576–579. [DOI] [PubMed] [Google Scholar]
- 36. Jones CB. Biological basis of fracture healing. J Orthop Trauma 2005; 19: S1–S3. [DOI] [PubMed] [Google Scholar]
- 37. Chung HJ, Hur JW, Ryu KS, et al. Surgical outcomes of anterior cervical fusion using deminaralized bone matrix as stand-alone graft material: single arm, pilot study. Korean J Spine 2016; 13(3): 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han B, Tang B, Nimni ME. Combined effects of phosphatidylcholine and demineralized bone matrix on bone induction. Connect Tissue Res 2003; 44: 160–166. [DOI] [PubMed] [Google Scholar]
- 39. Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012; 8(4): 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flynn JM. Fracture repair and bone grafting. OKU 10: orthopaedic knowledge update. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2011, pp. 11–21. [Google Scholar]
- 41. Wang JC, Alanay A, Mark D, et al. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur Spine J 2007; 16: 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Urist M. Bone: formation by autoinduction. Science 1965; 150: 893–899. [DOI] [PubMed] [Google Scholar]
- 43. Urist M, Mikulski A, Boyd S. A chemosterilized antigen-extracted autodigest alloimplant for bone banks. Arch Surg 1975; 110(4): 416–428. [DOI] [PubMed] [Google Scholar]
- 44. De Long WG, Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. J Bone Joint Surg Am 2007; 89: 649–658. [DOI] [PubMed] [Google Scholar]
- 45. Tuli SM, Singh AD. The osteoninductive property of decalcified bone matrix. An experimental study. J Bone Joint Surg Br 1978; 60: 116–123. [DOI] [PubMed] [Google Scholar]
- 46. Kinney RC, Ziran BH, Hirshorn K, et al. Demineralized bone matrix for fracture healing: fact or fiction? J Orthop Trauma 2010; 24(Suppl. 1): S52–S55. [DOI] [PubMed] [Google Scholar]
- 47. Albanese A, Licata ME, Polizzi B, et al. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing 2013; 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev 2008; 14: 249–258. [DOI] [PubMed] [Google Scholar]
- 49. El-Sharkawy H, Kantarci A, Deady J, et al. Platelet rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol 2007; 78: 661–669. [DOI] [PubMed] [Google Scholar]
- 50. Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004; 91: 4–15. [DOI] [PubMed] [Google Scholar]
- 51. Rickert D, Huddleston Slater JJR, Meijer HJA, et al. Maxillary sinus lift with solely autogenous bone compared to a combination of autogenous bone and growth factors or (solely) bone substitutes. A systematic review. Int J Oral Maxillofac Surg 2012; 41: 160–167. [DOI] [PubMed] [Google Scholar]
- 52. Sanchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 2003; 18: 93–103. [PubMed] [Google Scholar]
- 53. Ozdemir B, Okte E. Treatment of intrabony defects with betatricalciumphosphate alone and in combination with platelet-rich plasma. J Biomed Mater Res B Appl Biomater 2012; 100: 976–983. [DOI] [PubMed] [Google Scholar]
- 54. Cabbar F, Güler N, Kürkcü M, et al. The effect of bovine bone graft with or without platelet-rich plasma on maxillary sinus floor augmentation. J Oral Maxillofac Surg 2011; 69: 2537–2547. [DOI] [PubMed] [Google Scholar]
- 55. Arenaz-Búa J, Luaces-Rey R, Sironvalle-Soliva S, et al. A comparative study of platelet-rich plasma, hydroxyapatite, demineralized bone matrix and autologous bone to promote bone regeneration after mandibular impacted third molar extraction. Med Oral Patol Oral Cir Bucal 2010; 15: 483–489. [DOI] [PubMed] [Google Scholar]
- 56. Wu X, Shi W, Cao X. Multiplicity of BMP signaling in skeletal development. Ann N Y Acad Sci 2007; 1116: 29–49. [DOI] [PubMed] [Google Scholar]
- 57. Cochran DL, Schenk R, Buser D, et al. Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. J Periodontol 1999; 70(2): 139–150. [DOI] [PubMed] [Google Scholar]
- 58. Chai F, Raoul G, Wiss A, et al. Bone substitutes: classification and concerns. Rev Stomatol Chir Maxillofac 2011; 112(4): 212–221. [DOI] [PubMed] [Google Scholar]
- 59. Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog 2009; 25(6): 1539–1560. [DOI] [PubMed] [Google Scholar]
- 60. Burks MV, Nair L. Long term effects of bone morphogenetic protein-based treatments in humans. J Long Term Eff Med Implants 2010; 20(4): 277–293. [DOI] [PubMed] [Google Scholar]
- 61. Agarwal R, Williams K, Umscheid CA, et al. Osteoinductive bone graft substitutes for lumbar fusion: a systematic review. J Neurosurg Spine 2009; 11(6): 729–740. [DOI] [PubMed] [Google Scholar]
- 62. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011; 11(6): 471–491. [DOI] [PubMed] [Google Scholar]
- 63. Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int 2013; 4(Suppl. 5): S343–S352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Castro FPJ. Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech 2004; 17(5): 380–384. [DOI] [PubMed] [Google Scholar]
- 65. Tressler MA, Richards JE, Sofianos D, et al. Bone morphogenetic protein-2 compared to autologous iliac crest bone graft in the treatment of long bone nonunion. Orthopedics 2011; 34(12): e877–e884. [DOI] [PubMed] [Google Scholar]
- 66. Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic Protein-1 (Bone Morphogenetic Protein-7) in the treatment of tibial nonunions: a prospective, randomized clinical trial comparing rhOP-1 with fresh bone autograft. J Bone Joint Surg Am 2001; 83-A(Suppl. 1 Pt2): S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 67. Ghosh SK, Nandi SK, Kundu B, et al. In vivo response of porous hydroxyapatite and beta tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds. J Biomed Mater Res B Appl Biomater 2008; 86: 217–227. [DOI] [PubMed] [Google Scholar]
- 68. Nandi SK, Kundu B, Ghosh SK, et al. Efficacy of nano-hydroxyapatite prepared by an aqueous solution combustion technique in healing bone defects of goat. J Vet Sci 2008; 9: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Koshino T, Murase T, Takagi T, et al. New bone formation around porous hydroxyapatite wedge implanted in opening wedge high tibial osteotomy in patients with osteoarthritis. Biomaterials 2001; 22: 1579–1582. [DOI] [PubMed] [Google Scholar]
- 70. Okazaki A, Koshino T, Saito T, et al. Osseous tissue reaction around hydroxyapatite block implanted into proximal metaphysis of tibia of rat with collagen-induced arthritis. Biomaterials 2000; 21: 483–487. [DOI] [PubMed] [Google Scholar]
- 71. Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998; 19: 1473–1478. [DOI] [PubMed] [Google Scholar]
- 72. Spivak JM, Hasharoni A. Use of hydroxyapatite in spine surgery. Eur Spine J 2001; 10: S197–S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johnson KD, Frierson KE, Keller TS, et al. Porous ceramics as bone graft substitutes in long bone defects: a biomechanical, histological, and radiographic analysis. J Orthop Res 1996; 14: 351–369. [DOI] [PubMed] [Google Scholar]
- 74. Xie J, Baumann MJ, McCabe LR. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J Biomed Mater Res A 2004; 71(1): 108–117. [DOI] [PubMed] [Google Scholar]
- 75. Chiroff RT, White EW, Weber KN, et al. Tissue ingrowth of replamineform implants. J Biomed Mater 1975; 9: 29–45. [DOI] [PubMed] [Google Scholar]
- 76. Korovessis P, Koureas G, Zacharatos S, et al. Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur Spine J 2005; 14: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bucholz MP, Carlton A, Holmes RE. Interporous hydroxyapatite as a bone graft substitute in tibial plateau fractures. Clin Orthop 1989; 240: 53–62. [PubMed] [Google Scholar]
- 78. Khan SN, Tomin E, Lane JM. Clinical applications of bone graft substitutes. Orthop Clin North Am 2000; 31: 389–398. [DOI] [PubMed] [Google Scholar]
- 79. Beuerlein MJS, McKee MD. Calcium sulfates: what is the evidence? J Orthop Trauma 2010; 24: S46–S51. [DOI] [PubMed] [Google Scholar]
- 80. Blaha JD. Evolving technologies: new answers or new problems? Calcium sulfate bone void filler. Orthopedics 1998; 21: 1017–1019. [DOI] [PubMed] [Google Scholar]
- 81. Urban RM, Turner TM, Hall DJ, et al. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin Orthop Relat Res 2007; 459: 110–117. [DOI] [PubMed] [Google Scholar]
- 82. Hak DJ. The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg 2007; 15: 525–536. [DOI] [PubMed] [Google Scholar]
- 83. Podaropoulos L, Veis AA, Papadimitriou S, et al. Bone regeneration using beta-tricalcium phosphate in a calcium sulfate matrix. J Oral Implantol 2009; 35(1): 28–36. [DOI] [PubMed] [Google Scholar]
- 84. Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg B 1995; 77: 93–97. [PubMed] [Google Scholar]
- 85. Keating JF, Blachut PA, O’Brien PJ, et al. Reamed nailing of open tibial fractures: does the antibiotic bead pouch reduce the deep infection rate? J Orthop Trauma 1996; 10: 298–303. [DOI] [PubMed] [Google Scholar]
- 86. Ferguson JY, Dudareva M, Riley ND, et al. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint J 2014; 96-B(6): 829–836. [DOI] [PubMed] [Google Scholar]
- 87. Thomas MV, Puleo DA, Al-Sabbagh M. Calcium sulfate: a review. J Long Term Eff Med Implants 2005; 15(6): 599–607. [DOI] [PubMed] [Google Scholar]
- 88. Kelly CM, Wilkins RM, Gitelis S, et al. The use of a surgical grade calcium sulfate as a bone graft substitute. Results of a multicenter trial. Clin Orthop Relat Res 2001; 382: 42–50. [DOI] [PubMed] [Google Scholar]
- 89. Ziran BH, Smith WR, Morgan SJ. Use of calcium-based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton. J Trauma 2006; 63: 1324–1328. [DOI] [PubMed] [Google Scholar]
- 90. Brown WE, Chow LC. A new calcium phosphate water-setting cement, In: Brown WE. Ed., Cements Research Progress, Westerville, 1986, pp. 352–379. [Google Scholar]
- 91. Russell TA, Leighton RK; on behalf of the Alpha-BSM Tibial Plateau Fracture Study Group. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am 2008; 90: 2057–2061. [DOI] [PubMed] [Google Scholar]
- 92. Burguera EF, Xu HHK, Weir MD. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J Biomed Mater Res B Appl Biomater 2006; 77(1): 126–134. [DOI] [PubMed] [Google Scholar]
- 93. Afifi AM, Gordon CR, Pryor LS, et al. Calcium phosphate cements in skull reconstruction: a meta-analysis. Plast Reconstr Surg 2010; 126(4): 1300–1309. [DOI] [PubMed] [Google Scholar]
- 94. Galois L, Mainard D. Bone ingrowth into two porous ceramics with different pore sizes: an experimental study. Acta Orthop Belg 2004; 70: 598–603. [PubMed] [Google Scholar]
- 95. Galois L, Mainard D, Delagoutte JP. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop 2002; 26: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Malhotra A, Habibovic P. Calcium phosphates and angiogenesis: implications and advances for bone regeneration. Trends Biotechnol 2016; 34(12): 983–992. [DOI] [PubMed] [Google Scholar]
- 97. Shigaku S, Katsuyuki F. Beta-tricalcium phosphate as a bone graft substitute. Jikeikai Med J 2005; 52: 47–54. [Google Scholar]
- 98. Cheung HS, Haak MH. Growth of osteoblasts on porous calcium phosphate ceramic: an in vitro model for biocompatibility study. Biomaterials 1989; 10: 63–67. [DOI] [PubMed] [Google Scholar]
- 99. Daculsi G, Passuti N. Effect of the macroporosity for osseous substitution of calcium phosphate ceramics. Biomaterials 1990; 11: 86–87. [PubMed] [Google Scholar]
- 100. Gaasbeck RDA, Toonen HG, van Heerwaarden RJ, et al. Mechanism of bone incorporation of β-TCP bone substitute in open wedge tibial osteotomy in patients. Biomaterials 2005; 26: 6713–6719. [DOI] [PubMed] [Google Scholar]
- 101. Koerten HK, van der Meulen J. Degradation of calcium phosphate ceramics. J Biomed Mater Res 1999; 44: 78–86. [DOI] [PubMed] [Google Scholar]
- 102. Dehoux E, Madi K, Fourati E, et al. High tibial open-wedge osteotomy using a tricalcium phosphate substitute: 70 cases with 18 months mean follow-up. Rev Chir Orthop Reparatrice Appar Mot 2005; 91: 143–148. [DOI] [PubMed] [Google Scholar]
- 103. Bohner M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury 2000; 31(Suppl. 4): 37–47. [DOI] [PubMed] [Google Scholar]
- 104. Frayssinet P, Mathon D, Lerch A, et al. Osseointegration of composite calcium phosphate bioceramics. J Biomed Mater Res 2000; 50: 125–130. [DOI] [PubMed] [Google Scholar]
- 105. Nery EB, LeGeros RZ, Lynch KL, et al. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/βTCP in periodontal osseous defects. J Periodontol 1992; 63: 729–735. [DOI] [PubMed] [Google Scholar]
- 106. Geiger F, Beverungen M, Lorenz H, et al. Bone substitute effect on vascularization and bone remodeling after application of phVEGF165 transfected BMSC. J Funct Biomater 2012; 3: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Daculsi G, LeGeros RZ, Heughebaert M, et al. Formation of carbonate-apatite crystals after implantation of calcium-phosphate ceramics. Calcif Tissue Int 1990; 46: 20–27. [DOI] [PubMed] [Google Scholar]
- 108. LeGeros RZ, Parsons JR, Daculsi G, et al. Significance of the porosity and physical chemistry of calcium phosphate ceramics. Biodegradation-bioresorption. Ann NY Acad Sci 1988; 523: 268–271. [DOI] [PubMed] [Google Scholar]
- 109. Chazono M, Tanaka T, Komaki H, et al. Bone formation and bioresorption after implantation of injectable beta-tricalcium phosphate granules-hyaluronate complex in rabbit bone defects. J Biomed Mater Res A 2004; 70(4): 542–549. [DOI] [PubMed] [Google Scholar]
- 110. Eggli PS, Muller W, Schenk RK. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin Orthop Relat Res 1988; 232: 127–138. [PubMed] [Google Scholar]
- 111. Buser D, Hoffmann B, Bernard JP, et al. Evaluation of filling materials in membrane—protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin Oral Implants Res 1998; 9(3): 137–150. [DOI] [PubMed] [Google Scholar]
- 112. Gouin F, Yaouanc F, Waast D, et al. Open wedge high tibial osteotomies: calcium-phosphate ceramic spacer versus autologous bone graft. Orthop Traumatol Surg Res 2010; 96(6): 637–645. [DOI] [PubMed] [Google Scholar]
- 113. Bansal S, Chauhan V, Sharma S, et al. Evaluation of hydroxyapatite and beta-tricalcium phosphate mixed with bone marrow aspirate as a bone graft substitute for posterolateral spinal fusionIndian. J Orthop 2009; 43(3): 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bansal R, Patil S, Chaubey KK, et al. Clinical evaluation of hydroxyapatite and β-tricalcium phosphate composite graft in the treatment of intrabony periodontal defect: a clinico-radiographic study. J Indian Soc Periodontol 2014; 18(5): 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Matsumine A, Myoui A, Kusuzaki K, et al. Calcium hydroxyapatite implants in bone tumour surgery: a long term follow up study. J Bone Joint Surg Br 2004; 86: 719–725. [DOI] [PubMed] [Google Scholar]
- 116. Reddy R, Swamy MK. The use of hydroxyapatite as a bone graft substitute in orthopedic conditions. Indian J Orthop 2005; 39: 52–54. [Google Scholar]
- 117. Oh KJ, Ko YB, Jaiswal S, et al. Comparison of osteoconductivity and absorbability of beta-tricalcium phosphate and hydroxyapatite in clinical scenario of opening wedge high tibial osteotomy. J Mater Sci Mater Med 2016; 12: 179. [DOI] [PubMed] [Google Scholar]
- 118. Rojbani H, Nyam M, Ohya K, et al. Evaluation of the osteoconductivity of α-tricalcium phosphate, β-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J Biomed Mater Res A 2011; 98(4): 488–498. [DOI] [PubMed] [Google Scholar]
- 119. Lee KS, Chang JS, Kim JH, et al. The role of osteoclast in resorption of hydroxyapatite and β-tricalcium phosphate coating layer. Key Eng Mater 2009; 396–398: 81–84. [Google Scholar]
- 120. Schwarz F, Herten M, Ferrari D, et al. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate or a collagen coated natural bone mineral: as immunohistochemical study in dogs. Int J Oral Maxillofac Surg 2007; 36: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 121. Muschik M, Ludwig R, Halbhubner S, et al. B-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J 2001; 10: S178–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res 1981; 157: 259–278. [PubMed] [Google Scholar]
- 123. Fillingham YA, Lenart BA, Gitelis S. Function after injection of benign bone lesions with a bioceramic. Clin Orthop Relat Res 2012; 470(7): 2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hench LL, Splinter RJ, Allen WC, et al. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 1971; 5: 117–141. [Google Scholar]
- 125. Krishnan V, Lakshmi T. Bioglass: a novel biocompatible innovation. J Adv Pharm Technol Res 2013; 4(2): 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hench LL, Wilson J. Surface-active biomaterials. Science 1984; 226: 630–636. [DOI] [PubMed] [Google Scholar]
- 127. Wallace KE, Hill RG, Pembroke JT, et al. Influence of sodium oxide content on bioactive glass properties. J Mater Sci Mater Med 1999; 10: 697–701. [DOI] [PubMed] [Google Scholar]
- 128. Neo M, Nakamura T, Ohtsuki C, et al. Ultrastructural study of the A-W GC-bone interface after long-term implantation in rat and human bone. J Biomed Mater Res 1994; 28: 365–372. [DOI] [PubMed] [Google Scholar]
- 129. Zhang H, Ye XJ, Li JS. Preparation and biocompatibility evaluation of apatite/wollastonite-derived porous bioactive glass ceramic scaffolds. Biomed Mater 2009; 4(4): 045007. [DOI] [PubMed] [Google Scholar]
- 130. De Aza PN, Luklinska ZB, Santos C, et al. Mechanism of bone-like formation on a bioactive implant in vivo. Biomaterials 2003; 24: 1437–1445. [DOI] [PubMed] [Google Scholar]
- 131. Moimas L, Biasotto M, Di LR, et al. Rabbit pilot study on the resorbability of three-dimensional bioactive glass fibre scaffolds. Acta Biomater 2006; 2: 191–199. [DOI] [PubMed] [Google Scholar]
- 132. Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater 2011; 7(6): 2355–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Knowles J. Phosphate based glasses for medical application. J Mater Chem 2003; 13: 2395–2401. [Google Scholar]
- 134. Hench LL, Paschall HA. Histochemical responses at a materials interface. J Biomed Mater Res 1974; 5: 49–54. [DOI] [PubMed] [Google Scholar]
- 135. Nandi SK, Roy S, Mukherjee P, et al. Orthopaedic applications of bone graft and graft substitutes: a review. Indian J Med Res 2010; 132: 15–30. [PubMed] [Google Scholar]
- 136. Wagner Q, Offner D, Idoux-Gillet Y, et al. Nanostructured implant combining mesenchymal cells and VEGF nanoparticles for enhanced engineered tissue vascularization. Nanomedicine 2016; 11: 2419–2430. [DOI] [PubMed] [Google Scholar]
- 137. Eap S, Ferrand A, Palomares CM, et al. Electrospun nanofibrous 3D scaffold for bone tissue engineering. Biomed Mater Eng 2012; 22(1–3): 137–141. [DOI] [PubMed] [Google Scholar]
- 138. Eap S, Keller L, Schiavi J, et al. A living thick nanofibrous implant bifunctionalized with active growth factor and stem cells for bone regeneration. Int J Nanomedicine 2015; 10: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ferrand A, Eap S, Richert L, et al. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan-based nanoreservoirs of growth factors. Macromol Biosci 2014; 14(1): 45–55. [DOI] [PubMed] [Google Scholar]
- 140. Porter JR, Henson A, Popat KC. Biodegradable poly(epsilon-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009; 30(5): 780–788. [DOI] [PubMed] [Google Scholar]
- 141. Hernigou P, Ma W. Open wedge tibial osteotomy with acrylic bone cement as bone substitute. Knee 2001; 8: 103–110. [DOI] [PubMed] [Google Scholar]
- 142. Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices 2006; 3(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 143. Handoll HHG, Watts AC. Bone grafts and bone substitutes for treating distal radial fractures in adults. Cochrane Database Syst Rev 2008; 2: CD006836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Carson JS, Bostrom MP. Synthetic bone scaffolds and fracture repair. Injury 2007; 38(Suppl. 1): S33–S37. [DOI] [PubMed] [Google Scholar]
- 145. Rajaee SS, Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine 2012; 37: 67–76. [DOI] [PubMed] [Google Scholar]
- 146. Gupta A, Kukkar N, Sharif K, et al. Bone graft substitutes for spine fusion: a brief review. World J Orthop 2015; 6(6): 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Vaccaro AR, Stubbs HA, Block JE. Demineralized bone matrix composite grafting for posterolateral spinal fusion. Orthopedics 2007; 30: 567–570. [DOI] [PubMed] [Google Scholar]
- 148. Kang J, An H, Hilibrand A, et al. Grafton and local bone have comparable outcomes to iliac crest bone in instrumented single-level lumbar fusions. Spine 2012, 37: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 149. Cammisa FP, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine 2004; 29: 660–666. [DOI] [PubMed] [Google Scholar]
- 150. Schizas C, Triantafyllopoulos D, Kosmopoulos V, et al. Posterolateral lumbar spine fusion using a novel demineralized bone matrix: a controlled case pilot study. Arch Orthop Trauma Surg 2008; 128: 621–625. [DOI] [PubMed] [Google Scholar]
- 151. An HS, Simpson JM, Glover JM, et al. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine 1995; 20: 2211–2216. [PubMed] [Google Scholar]
- 152. Mashoof AA, Siddiqui SA, Otero M, et al. Supplementation of autogenous bone graft with coralline hydroxyapatite in posterior spine fusion for idiopathic adolescent scoliosis. Orthopaedics 2002; 25: 1073–1076. [DOI] [PubMed] [Google Scholar]
- 153. Vaz K, Verma K, Protopsaltis T, et al. Bone grafting options for lumbar spine surgery: a review examining clinical efficacy and complications. SAS J 2010; 4(3): 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech 2006; 19: 424–429. [DOI] [PubMed] [Google Scholar]
- 155. Brouwer R, Jakma T, Bierma-Zeinstra S, et al. Osteotomy for treating knee osteoarthritis. Cochrane Database Syst Rev 2005; 1: CD004019. [DOI] [PubMed] [Google Scholar]
- 156. Meynet JC. Ostéotomie tibiale de valgisation par ouverture interne: place des substituts osseux. Ann Orthop Ouest 1998; 30: 171–174. [Google Scholar]
- 157. Romanet JP, Benoit J, Nordin JY. Utilisation de céramique d’hydroxyapatite de calcium comme substitut de greffe osseuse (Endobon*). Résultats sur 62 cas revus à 1 an. Rev Chir Orthop 1996; 87(Suppl. II): 17. [Google Scholar]
- 158. Van Hemert WLW, Willemms K, Anderson PG, et al. Tricalcium phosphate granules or rigid wedge preforms in open wedge high tibial osteotomy: a radiological study with a new evaluation system. Knee 2004; 11: 451–456. [DOI] [PubMed] [Google Scholar]
- 159. Holmes RE, Bucholz RW, Mooney V. Porous hydroxyapatite as a bone graft substitute in metaphyseal defects. J Bone Joint Surg 1986; 68-A: 904–911. [PubMed] [Google Scholar]
- 160. Giuseffi SA, Replogle WH, Shelton WR. Opening-wedge high tibial osteotomy: review of 100 consecutive cases. Arthroscopy 2015; 31(11): 2128–2137. [DOI] [PubMed] [Google Scholar]
- 161. Yu B, Han K, Ma H, et al. Treatment of tibial plateau fractures with high strength injectable calcium sulphate. Int Orthop 2009; 33(4): 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mik G, Arkader A, Manteghi A, et al. Results of a minimally invasive technique for treatment of unicameral bone cysts. Clin Orthop Relat Res 2009; 467(11): 2949–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mirzayan R, Panossian V, Avedian R, et al. The use of calcium sulfate in the treatment of benign bone lesions. A preliminary report. J Bone Joint Surg Am 2001; 83(3): 355–358. [DOI] [PubMed] [Google Scholar]
- 164. Kim JH, Oh JH, Han I, et al. Grafting using injectable calcium sulfate in bone tumor surgery: comparison with demineralized bone matrix-based grafting. Clin Orthop Surg 2011; 3: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Werier JM. Bone graft substitutes in oncology, paediatrics, and hip arthroplasty. Canadian Orthopaedic Association’s COA Bulletin, Ottawa: 2011, p. 95. [Google Scholar]
- 166. Joeris A, Ondrus S, Planka L, et al. ChronOS inject in children with benign bone lesions: does it increase the healing rate? Eur J Pediatr Surg 2010; 20: 24–28. [DOI] [PubMed] [Google Scholar]
- 167. Hou HY, Wu K, Wang CT, et al. Treatment of unicameral bone cyst: a comparative study of selected techniques. J Bone Joint Surg Am 2010; 92: 855–862. [DOI] [PubMed] [Google Scholar]
- 168. Campanacci M, Capanna R, Fabbri N, et al. Curettage of giant cell tumor of bone, reconstruction with subchondral grafts and cement. Chir Organi Mov 1990; 75(1 Suppl.): 212–213. [PubMed] [Google Scholar]
- 169. Roffi A, Di Matteo B, Krishnakumar GS, et al. Platelet-rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice. A systematic review. Int Orthop 2017; 41(2): 221–237. [DOI] [PubMed] [Google Scholar]
- 170. Subramaniam P, Kumar K, Ramakrishna T, et al. Bone regeneration with plasma-rich-protein following enucleation of traumatic bone cyst. Eur J Dent 2013; 7(3): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. MacDonald KM, Swanstrom MM, McCarthy JJ, et al. Exaggerated inflammatory response after use of recombinant bone morphogenetic protein in recurrent unicameral bone cysts. J Pediatr Orthop 2010; 30(2): 199–205. [DOI] [PubMed] [Google Scholar]
- 172. El-Adl G, Mostafa MF, Enan A, et al. Biphasic ceramic bone substitute mixed with autogenous bone marrow in the treatment of cavitary benign bone lesions. Acta Orthop Belg 2009; 75: 110–118. [PubMed] [Google Scholar]
- 173. Siegel HJ, Baird RC, 3rd, Hall J, et al. The outcome of composite bone graft substitute used to treat cavitary bone defects. Orthopedics 2008; 31(8): 754. [PubMed] [Google Scholar]
- 174. Payne WT, Merrell G. Benign bony and soft tissue tumors of the hand. J Hand Surg 2010; 35: 1901–1910. [DOI] [PubMed] [Google Scholar]
- 175. Schaller P, Baer W. Operative treatment of enchondromas of the hand: is cancellous bone grafting necessary? Scand J Plast Reconstr Surg Hand Surg 2009; 43: 279–285. [DOI] [PubMed] [Google Scholar]
- 176. Bachoura A, Rice IS, Lubahn AR, et al. The surgical management of hand enchondroma without postcurettage void augmentation: authors’ experience and a systematic review. Hand 2015; 10(3): 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Jakubietz MG, Gruenert JG, Jakubietz RG. The use of beta-tricalcium phosphate bone graft substitute in dorsally plated, comminuted distal radius fractures. J Orthop Surg Res 2011; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Fayaz HC, Giannoudis PV, Vrahas MS, et al. The role of stem cells in fracture healing and nonunion. Int Orthop 2011; 35: 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Russell AT, Taylor CJ, Lavelle DG. Fractures of tibia and fibula. In: Bucholz RW, Heckman JD, Court-Brown CM. (eds) Rockwood and Green’s: Fractures in adults, Lippincott Williams & Wilkins, Philadelphia: vol. 3, 1991, pp. 1915–1982. [Google Scholar]
- 180. Einhorn TA: Enhancement of fracture-healing. J Bone Joint Surg Am 1995; 77: 940–956. [DOI] [PubMed] [Google Scholar]
- 181. Borrelli J, Prickett WD, Ricci WM. Treatment of nonunions and On the contrary, allogenic osseous defects with bone graft and calcium sulfate. Clin Orthop Relat Res 2003; 411: 245–254. [DOI] [PubMed] [Google Scholar]
- 182. Gómez-Barrena E, Rosset P, Gebhard F, et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials. Epub ahead of print 19 March 2018. DOI: 10.1016/j.biomaterials.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 183. Bourgeois D, Bouchard P, Mattout C. Epidemiology of periodontal status in dentate adults in France, 2002–2003. J Periodontal Res 2007; 42(3): 219–227. [DOI] [PubMed] [Google Scholar]
- 184. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, et al. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol 2003; 8(1): 227–265. [DOI] [PubMed] [Google Scholar]
- 185. Bayerlein T, Mundt T, Mack F, et al. Bone graft substitutes in periodontal and peri-implant bone regeneration. Folia Morphol 2006; 65(1): 66–69. [PubMed] [Google Scholar]
- 186. Froum SJ, Weinberg MA, Tarnow D. Comparison of bioactive glass synthetic bone graft particles and open debridement in the treatment of human periodontal defects. A clinical study. J Periodontol 1998; 69: 698–709. [DOI] [PubMed] [Google Scholar]
- 187. Del Fabbro M, Bortolin M, Taschieri S, et al. Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J Periodontol 2011; 82: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 188. Piemontese M, Aspriello SD, Rubini C, et al. Treatment of periodontal intrabony defects with demineralized freeze-dried bone allograft in combination with platelet-rich plasma: a comparative clinical trial. J Periodontol 2008; 79: 802–810. [DOI] [PubMed] [Google Scholar]
- 189. Keceli HG, Sengun D, Berberoğlu A, et al. Use of platelet gel with connective tissue grafts for root coverage: a randomized-controlled trial. J Clin Periodontol 2008; 35: 255–262. [DOI] [PubMed] [Google Scholar]
- 190. Hanes PJ. Bone replacement grafts for the treatment of periodontal intrabony defects. Oral Maxillofac Surg Clin North Am 2007; 19(4): 499–512. [DOI] [PubMed] [Google Scholar]
- 191. Poeschl PW, Ziya-Ghazvini F, Schicho K, et al. Application of platelet-rich plasma for enhanced bone regeneration in grafted sinus. J Oral Maxillofac Surg 2012; 70: 657–664. [DOI] [PubMed] [Google Scholar]
- 192. Khairy NM, Shendy EE, Askar NA, et al. Effect of platelet rich plasma on bone regeneration in maxillary sinus augmentation (randomized clinical trial). J Oral Maxillofac Surg 2013; 42(2): 249–255. [DOI] [PubMed] [Google Scholar]
- 193. Esposito M, Grusovin MG, Rees J, et al. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database Syst Rev 2010; 17: CD008397. [DOI] [PubMed] [Google Scholar]
- 194. Irinakis T. Efficacy of injectable demineralized bone matrix as graft material during sinus elevation surgery with simultaneous implant placement in the posterior maxilla: clinical evaluation of 49 sinuses. J Oral Maxillofac Surg 2011; 69: 134–141. [DOI] [PubMed] [Google Scholar]
- 195. Sohn DS, Bae MS, Choi BJ, et al. Efficacy of demineralized bone matrix paste for maxillary sinus augmentation: a histologic and clinical study in humans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108: e30–e35. [DOI] [PubMed] [Google Scholar]
- 196. Aly LAA, Hammouda NI. Evaluation of implant stability simultaneously placed with sinus lift augmented with putty versus powder form of demineralized bone matrix in atrophied posterior maxilla. Future Dental Journal 2017; 3: 28–34. [Google Scholar]
- 197. Nkenke E, Stelzle F. Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: a systematic review. Clin Oral Impl Res 2009; 20(Suppl. 4): 124–133. [DOI] [PubMed] [Google Scholar]
- 198. Bocanegra-Perez S, Vicente-Barrero M, Knezevic M, et al. Use of platelet-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Int J Oral Maxillofac Surg 2012; 41: 1410–1415. [DOI] [PubMed] [Google Scholar]
- 199. Mozzati M, Gallesio G, Arata V, et al. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: a report of 32 cases. Oral Oncol 2012; 48(5): 469–474. [DOI] [PubMed] [Google Scholar]
- 200. Coviello V, Peluso F, Dehkhargani SZ, et al. Platelet-rich plasma improves wound healing in multiple myeloma bisphosphonate-associated osteonecrosis of the jaw patients. J Biol Regul Homeost Agents 2012; 26: 151–155. [PubMed] [Google Scholar]
- 201. Geurts J, Chris Arts JJ, Walenkamp GH. Bone graft substitutes in active or suspected infection. Contra-indicated or not? Injury 2011; 42(Suppl. 2): S82–S86. [DOI] [PubMed] [Google Scholar]
- 202. Lalidou F, Kolios G, Drosos GI. Bone infections and bone graft substitutes for local antibiotic therapy. Surg Technol Int 2014; 24: 353–362. [PubMed] [Google Scholar]
- 203. Silverman LD, Lukashova L, Herman OT, et al. Release of gentamicin from a tricalcium phosphate bone implant. J Orthop Res 2007; 25(1): 23–29. [DOI] [PubMed] [Google Scholar]
- 204. Takigami I, Ito Y, Ishimaru D, et al. Two-stage revision surgery for hip prosthesis infection using antibiotic-loaded porous hydroxyapatite blocks. Arch Orthop Trauma Surg 2010; 130(10): 1221–1226. [DOI] [PubMed] [Google Scholar]
- 205. Mousset B, Benoit MA, Bouillet R, et al. Biodegradable implants for potential use in bone infection. Int Orthop 1995; 19: 157–161. [DOI] [PubMed] [Google Scholar]
- 206. Ginebra MP, Canal C, Espanol M, et al. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev 2012; 64(12): 1090–1110. [DOI] [PubMed] [Google Scholar]
- 207. Espanol M, Perez RA, Montufar EB, et al. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater 2009; 5: 2752–2762. [DOI] [PubMed] [Google Scholar]
- 208. Lindfors NC, Hyvönen P, Nyyssönen M, et al. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010; 47(2): 212–218. [DOI] [PubMed] [Google Scholar]
- 209. Aydin S, Kucukyuruk B, Abuzayed B, et al. Cranioplasty: review of materials and techniques. J Neurosci Rural Pract 2011; 2(2): 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Cho YJ, Kang SH. Review of cranioplasty after decompressive craniectomy. Korean J Neurotrauma 2017; 13(1): 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Piitulainen JM, Kauko T, Aitasolo KM, et al. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg 2015; 83: 708–714. [DOI] [PubMed] [Google Scholar]
- 212. Gosain AK. Hydroxyapatite cement paste cranioplasty for the treatment of temporal hollowing after cranial vault remodeling in a growing child. J Craniofac Surg 1997; 8: 506–511. [DOI] [PubMed] [Google Scholar]
- 213. Plum AW, Tatum SA. A comparison between autograft alone, bone cement, and demineralized bone matrix in cranioplasty. Laryngoscope 2015; 125: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 214. Gladstone HB, McDermott MW, Cooke DD. Implants for cranioplasty. Otolaryngol Clin North Am 1995; 28: 381–400. [PubMed] [Google Scholar]
- 215. Lieberman IH, Togawa D, Kayanja MM. Vertebroplasty and kyphoplasty: filler materials. Spine J 2005; 5: 305S–316S. [DOI] [PubMed] [Google Scholar]
- 216. Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol 1994; 15: 83–86. [PMC free article] [PubMed] [Google Scholar]
- 217. He Z, Zhai Q, Hu M, et al. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: current status and future developments. J Orthop Translat 2015; 3: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Phillips FM, Ho E, Campbell-Hupp M, et al. Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine 2003; 28: 2260–2265. [DOI] [PubMed] [Google Scholar]
- 219. Garfin SR, Yuan HA, Reily MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 2001; 26: 1511–1515. [DOI] [PubMed] [Google Scholar]
- 220. Khairoun I, Magne D, Gauthier O, et al. In vitro characterization and in vivo properties of a carbonated apatite bone cement. J Biomed Mater Res 2002; 60: 633–642. [DOI] [PubMed] [Google Scholar]
- 221. Low KL, Tan SH, Zein SH, et al. Calcium phosphate-based composites as injectable bone substitute materials. J Biomed Mater Res B Appl Biomater 2010; 94: 273–286. [DOI] [PubMed] [Google Scholar]
- 222. Turner TM, Urban RM, Singh K, et al. Vertebroplasty comparing injectable calcium phosphate cement compared with polymethylmethacrylate in a unique canine vertebral body large defect model. Spine J 2008; 8(3): 482–487. [DOI] [PubMed] [Google Scholar]
- 223. Vlad MD, Sindilar EV, Marinoso ML, et al. Osteogenic biphasic calcium sulphate dihydrate/iron-modified alpha-tricalcium phosphate bone cement for spinal applications: in vivo study. Acta Biomater 2010; 6: 607–616. [DOI] [PubMed] [Google Scholar]
- 224. Blom AW, Wylde V, Livesey C, et al. Impaction bone grafting of the acetabulum at hip revision using a mix of bone chips and a biphasic porous ceramic bone graft substitute. Acta Orthop 2009; 80: 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. McNamara IR. Impaction bone grafting in revision hip surgery: past, present and future. Cell Tissue Bank 2010; 11: 57–73. [DOI] [PubMed] [Google Scholar]
- 226. Suzuki K, Park BJ, Adusumilli PS, et al. Chest wall reconstruction using a methyl methacrylate neo-rib and mesh. Ann Thorac Surg 2015; 100(2): 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kujala S, Raatikainen T, Ryhanen J, et al. Composite implant of native bovine bone morphogenetic protein (BMP) and biocoral in the treatment of scaphoid nonunions—a preliminary study. Scand J Surg 2002; 91: 186–190. [DOI] [PubMed] [Google Scholar]
- 228. Koo KT, Polimeni G, Qahash M, et al. Periodontal repair in dogs: guided tissue regeneration enhances bone formation in sites implanted with a coral-derived calcium carbonate biomaterial. J Clin Periodontol 2005; 32: 104–110. [DOI] [PubMed] [Google Scholar]
- 229. Fondi C, Franchi A. Definition of bone necrosis by the pathologist. Clin Cases Miner Bone Metab 2007; 4(1): 21–26. [PMC free article] [PubMed] [Google Scholar]
- 230. Glowacki J. Angiogenesis in fracture repair. Clin Orthop Relat Res 1998; 355: S82–S89. [DOI] [PubMed] [Google Scholar]
- 231. Gotz W, Reichert C, Canullo L, et al. Coupling of osteogenesis and angiogenesis in bone substitute healing—a brief overview. Ann Anat 2012; 194: 171–173. [DOI] [PubMed] [Google Scholar]
- 232. LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 2002; 395: 81–98. [DOI] [PubMed] [Google Scholar]
- 233. Axelrad TW, Kakar S, Einhorn TA. New technologies for the enhancement of skeletal repair. Injury 2007; 38: S49–S62. [DOI] [PubMed] [Google Scholar]
- 234. Muschler GF, Nitto H, Boehm CA, et al. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 2001; 19: 117–125. [DOI] [PubMed] [Google Scholar]
- 235. Schmidt-Bleek K, Schell H, Schulz N, et al. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res 2012; 347: 567–573. [DOI] [PubMed] [Google Scholar]
- 236. Giannoudis PV, Einhorn TA, Schmidmaier G, et al. The diamond concept—open questions. Injury 2008; 39(Suppl. 2): S5–S8. [DOI] [PubMed] [Google Scholar]
- 237. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005; 26: 5474–5491. [DOI] [PubMed] [Google Scholar]
- 238. Hulbert SF, Cooke FW, Klawitter JJ, et al. Attachment of prostheses to the musculoskeletal system by tissue in growth and mechanical inter locking. J Biomed Mater 1973; 7: 1–23. [DOI] [PubMed] [Google Scholar]
- 239. Yuan H, Kurashina K, de Bruijn JD, et al. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 1999; 20(19): 1799–1806. [DOI] [PubMed] [Google Scholar]
- 240. Lu JX. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J Mater Sci Mater Med 1999; 10: 111–120. [DOI] [PubMed] [Google Scholar]
- 241. Tsuruga E, Takita H, Itoh H, et al. Pore size of porous hydroxyapatite as the cell-substratum controls BMPinduced osteogenesis. J Biochem 1997; 121(2): 317–324. [DOI] [PubMed] [Google Scholar]
- 242. Le Huec JC, Schaeverbeke T, Clement D, et al. Influence of porosity on the mechanical resistance of hydroxyapatite ceramics under compressive stress. Biomaterials 1995; 16: 113–118. [DOI] [PubMed] [Google Scholar]
- 243. Guerrero J, Catros S, Derkaoui SM, et al. Cell interactions between human progenitor-derived endothelial cells and human mesenchymal stem cells in a three-dimensional macroporous plysaccharide-based scaffold promote osteogenesis. Acta Biomater 2013; 9: 8200–8213. [DOI] [PubMed] [Google Scholar]
- 244. Offner D, Wagner Q, Idoux-Gillet Y, et al. Hybrid collagen sponge and stem cells as a new combined scaffold able to induce the re-organization of endothelial cells into clustered networks. Biomed Mater Eng 2017; 28(S1): S185–S192. [DOI] [PubMed] [Google Scholar]