Abstract
We present a 47-year-old right-handed woman with a 15-year history of writer’s cramp who was provided with six sessions of cathodal transcranial direct current stimulation (tDCS) combined with observation of writing actions performed by a healthy subject and electromyographic (EMG) biofeedback training to decrease EMG activities in her right forehand muscles while writing for 30 min for 4 weeks. She showed improvement in dystonic posture and writing speed after the intervention. The writing movement and writing speed scores on a writer’s cramp rating scale decreased, along with writing time. Our findings demonstrated that cathodal tDCS combined with action observation and EMG biofeedback training might improve dystonic writing movements in a patient with writer’s cramp.
Keywords: Dystonic disorders, transcranial direct current stimulation, action observation, electromyographic biofeedback
Writer’s cramp (WC) is a task-specific focal hand dystonia characterized by abnormal postures and movements of the upper limb that is induced by excessive activities of the upper limb muscles [1] and the sensorimotor cortex [2] while writing. Decreased inhibition in the motor system is considered an important mechanism of WC that may affect increased activity of the cerebral motor cortex and increased muscle activities of the upper limb during writing. Noninvasive inhibitory transcranial stimulation, such as cathodal transcranial direct current stimulation (tDCS), has been applied to improve focal hand dystonia [3-5]. Traditional electromyographic (EMG) biofeedback training has been traditionally provided to decrease the EMG activities of the upper limb during writing in WC patients [6]. The combination of cathodal tDCS and EMG biofeedback training has the potential to further improve WC symptoms, but few studies have investigated the effects of combining the two methods on WC.
WC patients have impairment of movement representations, such as the mental rotation of the hands [7]. Action observation has been used to enhance an individual’s internal motor representation of the observed movement and motor learning [8]. However, there have been no prior reports concerning the effect of action observation on WC. WC patients also have higher corticospinal excitability during their observation of healthy writing movements compared to their observation of dystonic writing movements. This result is in contrast to healthy individuals, who have higher corticospinal excitability during their observation of dystonic writing movements compared to their observation of healthy writing movements [9]. This suggests that WC patients have maladaptive plasticity, in which sensorimotor circuits are abnormally reorganized. The combination of cathodal tDCS and action observations might improve the abnormal reorganization of motor representation in WC patients. Here, we tested cathodal tDCS in combination with action observations and EMG biofeedback training in a WC patient.
CASE REPORT
A 47-year-old right-handed woman with WC participated in this study. Written informed consent to participate and to have the results published was obtained from the patient. She first noticed her difficulty with writing 15 years prior to the study, and the difficulty increased over time. She had no other neurological or orthopedic disorders. She had been on medication for a few months since being diagnosed with WC, but the symptoms persisted, and she stopped taking the medication. She had not had any botulinum toxin injections or surgical treatments.
Her dystonic symptoms were limited to writing and progressed from the forearm to the shoulder. She showed dystonic posture of the fingers, wrist, elbow, and shoulder while writing with the thumb, index finger and middle finger in moderate flexion, with the wrist in moderate extension, with the elbow in moderate flexion, and with the shoulder in marked abduction, with poor flexible joint movement from the initiation of writing (Supplementary Video 1 in the online-only Data Supplement). The WC symptoms were assessed with a writer’s cramp rating scale (WCRS), comprising a writing movement score and a writing speed score [10], and we also measured her writing speed. The higher the WCRS score, the more severe the subject’s dystonic symptoms. In the assessment, she wrote four lines of 10 interconnected “l” letters plus a standard sentence, meaning “It is very nice weather today” in English at her preferred pace. The interval times between lines included the pre-movement time for writing the next line of letters or sentences. The pre-movement time in the reaction time task reflected the programming stages of motor planning [11]. Accordingly, we assessed the writing time as reflecting the ability to execute writing and the interval time between lines as possibly reflecting the ability to prepare for writing.
Her WCRS total score and the sub-scores of the writing movements, as well as the writing speed, writing time and interval time of the interconnected “l” and sentences in the pre-intervention assessment, are shown in Table 1. The patient reported difficulty imagining writing smoothly, with an increased difficulty as she continued writing, along with a sense of reluctance toward writing letters in daily life.
Table 1.
Changes in outcomes during the study period
| Items/sessions | 1 |
2 |
3 |
4 |
5 |
6 |
Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| WCRS | |||||||||||||
| Writing movement score | 12 | 8 | 8 | 6 | 8 | 4 | 6 | 4 | 6 | 8 | 4 | 6 | 6 |
| Writing speed score | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Interconnected “l” letters | |||||||||||||
| Writing time (s) | 44.4 | 49.7 | 38.3 | 31.1 | 27.9 | 35.6 | 32 | 31.9 | 33.9 | 34.4 | 36.4 | 35.3 | 32.2 |
| Interval time (s) | 5.5 | 6.1 | 5.3 | 4.4 | 5.7 | 5.3 | 4 | 3.8 | 5.2 | 4.6 | 4.5 | 5.6 | 4.7 |
| Sentences | |||||||||||||
| Writing time (s) | 80.4 | 82.6 | 64.7 | 63.3 | 56.7 | 70 | 63.7 | 69.3 | 64.4 | 69.3 | 63.8 | 69 | 63.8 |
| Interval time (s) | 10.1 | 6.1 | 5.2 | 4 | 4.3 | 5.6 | 4 | 4.1 | 4.4 | 4.3 | 4 | 4.2 | 3.8 |
WCRS: writer’s cramp rating scale.
Six sessions of tDCS, combined with action observation and EMG biofeedback training, were conducted for 30 min, one or two times per week, for 4 weeks (Figure 1). She conducted action observation in the first half of each intervention session as well as EMG biofeedback training in the second half during tDCS. A 2 mA tDCS was delivered by a battery-driven constant current stimulator (DC Stimulator Plus; NeuroConn, Ilmenau, Germany) and a saline-soaked pair of surface sponge electrodes (7 cm × 5 cm; 35 cm2) for 30 min. The cathode was placed over the left sensorimotor cortex, and the anode was placed over the right sensorimotor cortex [4].
Figure 1.
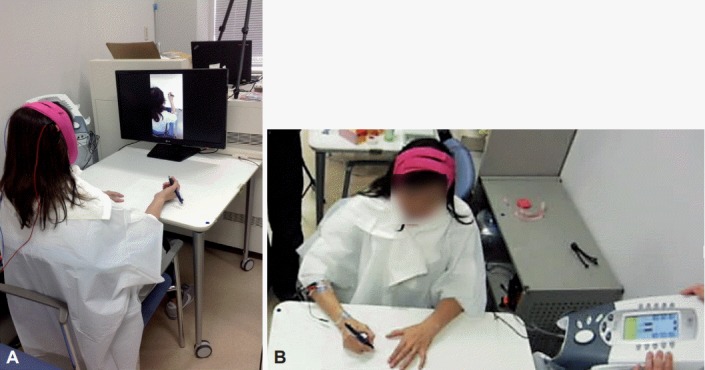
tDCS combined with action observation and EMG biofeedback training. A: The patient observing video clips of the writing movements of a healthy subject on the monitor while the patient underwent tDCS. B: The patient undergoing EMG biofeedback training during tDCS. tDCS: transcranial direct current stimulation, EMG: electromyographic.
For the action observation, she was instructed to watch a video clip showing a healthy subject, who looked like her, writing the same letters and sentences with the same pen in the same environment. This video clip was presented five times, during which the patient was not allowed to perform any writing movements.
The EMG biofeedback training was applied using an EMG biofeedback system (Intelect Advanced Combo; DJO Global, Vista, CA, USA). We selected the right flexor carpi radialis muscle (FCR) and extensor carpi radialis muscle (ECR) as the target muscles, since pronounced activities in her right FCR (50–80 μV) and ECR (110–150 μV) were confirmed while she wrote, previous to her first training session. We set the target EMG levels of the right FCR and ECR to 60 μV and 120 μV, respectively, based on the preliminary assessment. She was provided with auditory feedback when the EMG level during writing was above the target EMG level.
The patient completed all the treatment sessions, and no adverse effects were observed. As the treatment sessions progressed, the duration to keep her EMG level under the target level became longer during the EMG biofeedback training. The number of letters she wrote during the training increased from 71 in the first session to 309 in the final session.
Her writing speed improved in the post-intervention assessment, along with the degree of her dystonic writing posture, including the right thumb and index finger flexion, wrist extension, elbow flexion, and shoulder flexion (Table 1, Supplementary Video 1 in the online-only Data Supplement). From the pre-intervention assessment to the post-intervention assessment, her writing movement score and writing speed score on the WCRS decreased. A decrease also occurred in her writing time and interval time for the interconnected “l” letters and sentences (Table 1). In the post-intervention assessment, she reported that her feelings of reluctance to write letters while taking notes during her working and daily life had decreased in comparison to her feelings before the intervention.
DISCUSSION
Our patient showed a clinically significant improvement in her dystonic posture, her movements of the upper limb while writing and her writing speed after six sessions of the treatments. To the best of our knowledge, this is the first study to demonstrate the beneficial influence of inhibitory tDCS in combination with action observations and EMG biofeedback training in a WC patient.
The interval time between lines while writing sentences was particularly reduced in our patient. The interval time included the pre-movement time that reflects the programming stage of motor preparation [11] and seemed to reflect the ability to prepare writing movements. These results suggested that the combination of cathodal tDCS and action observation might improve the abnormal reorganization of motor representation and the programming in motor preparation of healthy writing movements in our patient.
WC patients have a higher corticospinal excitability when observing healthy writing movements in comparison to when they observe dystonic writing movements. Healthy individuals, on the other hand, have higher corticospinal excitability during their observation of dystonic writing movements [9]. The opposing results of WC patients might be explained by a ‘like me’ effect, which causes more activation, attributable to observing actions by another agent rather than those attributed to the self [12]. In the present study, the patient observed healthy writing movements executed by a similar-looking person during cathodal tDCS over the left sensorimotor cortex. This treatment strategy, in combination with action observations, might have evoked a feeling in our patient that the observed movements executed by the healthy subject were similar to those executed by the patient herself, and this may facilitate the motor preparation of healthy writing movements.
In various noninvasive brain stimulations and neuroimaging studies, WC patients show increased excitability in the motor and sensory cortices at rest and during writing [1]. Our patient conducted EMG biofeedback training during cathodal tDCS over the sensorimotor cortex and showed improvements in the excessive EMG activity of the forearm muscles during the training, as well as improvements in WC symptoms and writing time, in the post-intervention assessment. Previous studies have reported that cathodal tDCS generates lasting excitability reductions after stimulations were induced by dopaminergic receptor (D2) activation [13], while EMG biofeedback training increases striatal dopaminergic receptor binding in WC patients [6]. It is possible that the repetition of the EMG biofeedback training during cathodal tDCS might have led to plastic changes in the dopaminergic system and motor system in our patient.
The present study did not have a sham tDCS condition’ thus, the possibility of a placebo effect cannot be excluded. This study examined only the combined effect of cathodal tDCS, action observation and EMG biofeedback training and cannot specify which components of the treatment improved the WC symptoms.
In conclusion, cathodal tDCS combined with action observation and EMG biofeedback training has the potential to improve dystonic writing movements in a WC patient. Further studies are needed to confirm the effectiveness of this method in studies with larger sample sizes.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.14802/jmd.18007.
Video 1.
Writing scene of the interconnected “l” letters in the pre-intervention and post-intervention assessments.
REFERENCES
- 1.Hallett M. Pathophysiology of writer’s cramp. Hum Mov Sci. 2006;25:454–463. doi: 10.1016/j.humov.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer’s cramp. Mov Disord. 1998;13:497–508. doi: 10.1002/mds.870130321. [DOI] [PubMed] [Google Scholar]
- 3.Cho HJ, Hallett M. Non-invasive brain stimulation for treatment of focal hand dystonia: update and future direction. J Mov Disord. 2016;9:55–62. doi: 10.14802/jmd.16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosset-Llobet J, Fàbregas-Molas S, Pascual-Leone Á. Effect of transcranial direct current stimulation on neurorehabilitation of task-specific dystonia: a double-blind, randomized clinical trial. Med Probl Perform Art. 2015;30:178–184. doi: 10.21091/mppa.2015.3033. [DOI] [PubMed] [Google Scholar]
- 5.Marceglia S, Mrakic-Sposta S, Fumagalli M, Ferrucci R, Mameli F, Vergari M, et al. Cathodal transcranial direct current stimulation improves focal hand dystonia in musicians: a two-case study. Front Neurosci. 2017;11:508. doi: 10.3389/fnins.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger HJ, van der Werf SP, Horstink CA, Cools AR, Oyen WJ, Horstink MW. Writer’s cramp: restoration of striatal D2-binding after successful biofeedback-based sensorimotor training. Parkinsonism Relat Disord. 2007;13:170–173. doi: 10.1016/j.parkreldis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Fiorio M, Tinazzi M, Aglioti SM. Selective impairment of hand mental rotation in patients with focal hand dystonia. Brain. 2006;129(Pt 1):47–54. doi: 10.1093/brain/awh630. [DOI] [PubMed] [Google Scholar]
- 8.Eaves DL, Riach M, Holmes PS, Wright DJ. Motor imagery during action observation: a brief review of evidence, theory and future research opportunities. Front Neurosci. 2016;10:514. doi: 10.3389/fnins.2016.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorio M, Zhang W, Bresciani MC, Rodi G, Bertolasi L, Gambarin M, et al. Corticospinal excitability during action observation in task-specific dystonia: a transcranial magnetic stimulation study. Neuroscience. 2010;171:117–124. doi: 10.1016/j.neuroscience.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Wissel J, Kabus C, Wenzel R, Klepsch S, Schwarz U, Nebe A, et al. Botulinum toxin in writer’s cramp: objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry. 1996;61:172–175. doi: 10.1136/jnnp.61.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klapp ST. Motor response programming during simple choice reaction time: The role of practice. J Exp Psychol Hum Percept Perform. 1995;21:1015–1027. [Google Scholar]
- 12.Schütz-Bosbach S, Mancini B, Aglioti SM, Haggard P. Self and other in the human motor system. Curr Biol. 2006;16:1830–1834. doi: 10.1016/j.cub.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Utz KS, Dimova V, Oppenländer K, Kerkhoff G. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of noninvasive brain stimulation in neuropsychology--a review of current data and future implications. Neuropsychologia. 2010;48:2789–2810. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1.
Writing scene of the interconnected “l” letters in the pre-intervention and post-intervention assessments.


