Abstract
Aim:
The present study designed to evaluate the toxic effect of anatase TiO2 NPs on BALF biochemical changes and liver alteration in rats.
Background:
Titanium dioxide (TiO2) nanoparticles (NPs) are utilized in food color additives and cosmetics worldwide. Humans uptake these nanoparticulate by different routes and may exhibit potential side effects, lags behind the rapid development of nanotechnology.
Methods:
Sixty-three mats rats were used. Included by the control group and the experimental groups treated twice a week with 0.5, 5, 50, 1.5, 15, 150 mg/kg of nano-TiO2 (size 21 nm), for four consecutive weeks. Animals were sacrificed at 4 days, a month and three months post-instillation. The levels of tumor necrosis factor - α (TNF-α) and macrophage inflammatory protein- 2 (MIP-2) were measured in the lung homogenate and in the bronchoalveolar lavage fluid (BALF) supernatants by enzyme-linked immunosorbent assay (ELISA) and histopathological examination of liver tissue was performed.
Results:
The results showed that TiO2 NP induces many alterations in the liver structure after 4 days, a month and reduced after 3 months from intratracheal instillation. This included liver heavy infiltration of inflammatory cells, an increase of collagen density in portal triads, beginning of fibrosis formation and Glisson capsule thickness increase and TiO2 NPs reached the liver tissue after a month from exposure at all doses especially low doses (0.5, 1.5, 5) mg/kg of TiO2 NPs.
Conclusion:
The immune system was strongly responded in the groups treated with high doses (15, 50, 150) mg/kg of TiO2 NP leading to raising the concentration of α-TNF, and MIP-2 in BALF while they decrease in tissue homogenate.
Key Words: Titanium dioxide effects, Liver histopathological alteration, MIP-2 changes, TiO2 effects on TNF-α
Introduction
Titanium dioxide nanoparticles (nano-TiO2)(<100 nm) are widely utilized in the cosmetics, pharmaceutical, and paint industries as a coloring material due to its high stability, anticorrosion, and photocatalysis. In addition, more and more nanoparticles are released into the environment with the increasing development and application of nanotechnology. With the small size and large surface area, nanoparticles can be an active group or exert intrinsic toxicity. It is therefore important to illuminate the effects of various nanoparticles on organs health as well as the pathogenic mechanisms convoluted (1). The function of surface charge on cytotoxicity in prevalent, the effect on exact cellular targets, modes of poisonous action, cellular uptake, and intracellular location of NPs, effects of serum and in the cells kind differences are addressed (2). Uptake of NPs in non-phagocytic cells appears an optimum at a particle volume of around 50 nm, and for phagocytes inconclusive, increased charge, either plus or minus, favors uptake in both cell types, while the constant amount is similar, positively charged particles are possessed up more extensively with increasing hydrophilicity, the uptake reduced, this can be attributed to the type of proteins exist (3).
The bio-distribution of NPs is particular through body’s biological barriers that show in different evident ways. For intravascular transport of NPs, the barrier apparent in the form of: (i) immune clearance in the spleen and liver, (ii) penetration through the endothelium into target tissues, (iii) permeation through the tissue interstitium, (iv) endocytosis in target cells, (v) expansion through cytoplasm and (vi) finally entree into the nucleus (4). Nano-TiO2 was retaining in various important organs, involving the liver, kidney, spleen, and brain after inserting the blood through several exposure paths, but the coefficient of the objective organs was modified partly from animal models.
Through the premier inflammatory phase, macrophages and neutrophils remove foreign material from the wound. They also produce soluble moderator that expand the wound-healing restraint by inducing T cells and another inflammatory cell (5). Monocytes foster progression of fibrotic disease during discrimination into M2a-like macrophages and fibrocytes that product different fibroblast stimulatory cytokines and growth factors, such as PDGF, TGF-β1, FGF2 (fibroblast growth factor 2), CCL18, insulin-like growth factor–binding protein 5, and Galectin-3 (5).
The TiO2 nanoparticles, induce drastic impact at the hepatocyte level, influence the nucleus and several organelles ultrastructural of organelles (mitochondria especially), also in the lipid metabolism. The TiO2 nanoparticles are accumulated in the lipid droplets, as well as in some mitochondria. The TiO2-Ag nanoparticles, altered the lipid metabolism and induced slightly, reversible modifications at the ultrastructural level, dilatation of the rough endoplasmic reticulum, the nucleus envelope (6).
BALF is usually sampled during bronchoscopy in individuals with suspected lung cancer, it contains a varied diversity of cellular material such as macrophages and neutrophils, a large number of proteins produced by epithelial and inflammatory cells, and tumor cells if present (7). Proximal biofluids have much reduced active range of protein abundances and in some cases are in direct interaction with the site of the disease (8). It is possible that nano-TiO2 can induce cell damage connected to exposure size and dose (9).
Zhu et al.(10) confirmed that nano-TiO2 of different size and types showed diverse levels of cytotoxicity on CHO cells and 293T cells with the sequence as 10–20 nm > anatase 50–60 nm anatase >50–60 nm rutile. TiO2 can be classified into three types: anatase, rutile, and amorphous. The photoactivity of anatase-type TiO2 was greater than that of rutile, whereas amorphous does not show photocatalytic activity. Because anatase-type TiO2 has the greatest toxicity to cells among the three types, we need further research to examine its toxicity in mouse liver.
Therefore, this study included the investigation on the inflammation effects caused from several doses of TiO2 nanoparticles with a specific size (21 nm) in lung and in which time reached the liver tissue after the intratracheal instillation in an animal model and investigating the immune response of the body after the instillation processes by measuring the alteration of some cytokines in biofluid such as tissue homogenize and BALF.
Methods
Animals and Treatments
Sixty-three mats rats (8 weeks age) were housed in cages kept in standard conditions in animals’ room. Rats were divided into seven groups (9 rats each). The control group was treated with 0.9% w/w NaCl solution. The experimental groups were treated with 0.5, 5, 50, 1.5, 15, 150 mg/kg of nano-TiO2 (size 21 nm) (11). All groups repeated the exposure (twice a week, for four consecutive weeks) by 0.1 ml/100 g (B.W) intratracheal instillation. Animals were sacrificed at 4 days, a month and three months post-instillation; animals weighted then lungs lavage to collect bronchoalveolar lavage fluid (BALF). The liver was removed and weighted for further study.
Collection of Broncho-Alveolar Lavage Fluid (BALF)
To determine the cytokines in BALF, the left lung clamped, and the right lung was lavaged 3 times with a 3 ml of 0.9% sterile buffer saline (physiological buffer solution PBS) using a 3mL Monoject syringe. Lavage fluid was collected in EDTA tubes. Tubes were centrifuged at 4°C and 1500 rpm for 3 minutes to isolate alveolar macrophages from the BALF (12). BALF supernatant was collected for immunological assay.
Cell viability assay
Cell viability was assessed according to Shani (13). 0.25 ml of Trypan blue solution was dissolved in 0.15 ml PBS, the staining solution was mixed with 0.1 ml of cell suspension. The specimens were examined under a light microscope by using an Improved Double Neubauer Ruling Counting Chamber. Viable cells do not stain while dead cells are stained with blue stain. Cells per unit volume were calculated according to the formula:
Percentage of cells viability = (viable cells No.)/(viable cells + dead cells )×100
Preparation of lung homogenates
Lungs sample incised and weighted, immediately freeze in -80˚C for later used. The samples were thawed and hold at 2-8ﹾC. PBS was added, then the samples were homogenized thoroughly by homogenizer, and centrifuged at 2000-3000 rpm for 20 minutes, the supernatants collected carefully and frozen for later, used in an immune assay.
Measurements of inflammatory mediators
The levels of tumor necrosis factor - α (TNF-α) and macrophage inflammatory protein- 2 (MIP-2) were measured in the lung homogenate and the BAL fluid supernatants enzyme-linked immunosorbent assay (ELISA) according to the manufacturer of ALISA Kits (Asiagene, Chania).
Histopathological Examination
Histopathological examinations were performed by using standard laboratory procedures. The liver was removed from experimental animals and rinsed thoroughly for 1 minute in normal saline. Then, the tissue was fixed in a Carnoy's fluid for 60 minutes and transferred to 95% percent or absolute alcohol for 1 hours. Thereafter, processed to paraffin embedding routine. Sections of 5-7 µm were stained with hematoxylin and eosin stain as well as Van Geison stain and PAS then examined under light microscope for histopathological alteration and collagen contents (14). Fibrosis was determined acquiring to the Metavir scoring system, staged on a 0–4 scale: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and few septa extending into lobules; F3, numerous septa extending to adjacent portal tracts or terminal hepatic venules; and F4, cirrhosis (15).
Statistical analysis
Statistical analysis of all data was carried out using the ANOVA test with differences less than 0.5 (p<0.05) considered to be statistically significant. This calculation was carried out according to the Statistical Package for Social Science (SPSS version 20) and the least significant difference (L.S.D) at a level less than (0.05) was also used.
Results
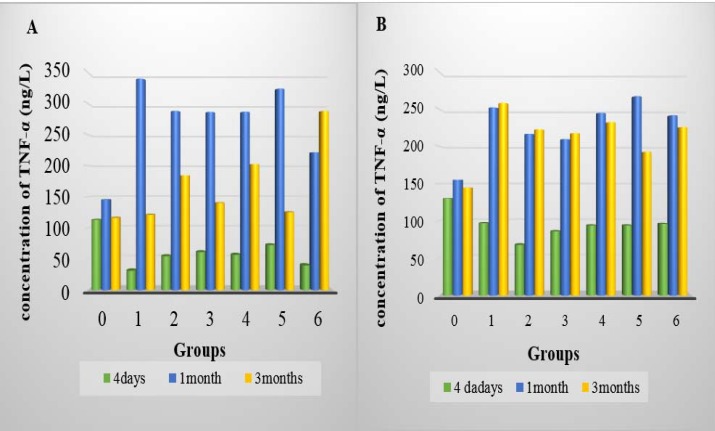
In all doses at 4-day post-instillation, the total granular cells represent more than 75 % but less than 90% in BALF that statistically showed a significant decrease compared to control groups (p <0.05), whereas the percentage was increased for the rest period of the study. The results showed that the TNF-α concentration after 4 days of TiO2 intra-tracheal instillation was significantly decreased (P<0.05) in both lung homogenate and in BALF of all groups compared to control group, there are significant differed between high and low doses and the 1.5 mg/kg reported the less value comparing to other doses in BALF while the 0.5 mg/kg reported less value in homogenate (table1). While it was significantly increased (p<0.05) in both homogenate and BALF of all groups after a month and 3 months post-instillation (table 1). The TNF-α concentration was significantly changed during the study, it was reported the highest concentration after a month of instillation in the homogenate tissue of all treated groups, while the concentration was increased after a month and 3 months of instillation in BALF of all treated groups compared with the concentration 4 days post-instillation (p<0.05) (figure1).
Table 1.
Cellular, biochemical parameters and inflammatory mediator in BALF and homogenates after 4 days, a month and 3 months from intratracheal instillation
| Groups | TiO2 NP (mg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| control | 0.5 | 1.5 | 5 | 15 | 50 | 150 | RLSD | |
| 4 days | ||||||||
| Cell viability% | 94.15±74 | 84.96±0.47*a | 75.91±1.83*b | 83.15±0.49*a | 81.94±1.42*b | 89.79±1.95*d | 87.18±1.57*d | 1.61 |
| BALF TNF-α (ng/L) | 130.33±11.31 | 97.33±15.5*a | 68.00±15.62*b | 86.67±12.02*a | 94.67±7.77*a | 94.33±22.62*a | 96.33±12.16*a | 6.73 |
| BALF MIP-2 (ng/L) | 81.67±8.48 | 147.67±26.53*a | 212.33±16.9*c | 138.33±18.38*a | 121.33±9.23*b | 141.67±18.61*a | 237.67±13.43*b | 5.83 |
| Homog TNF-α (ng/L) | 113.33±38.18 | 31.33±9.86*a | 54.67±22.03*a | 61.33±11.37*a | 57.33±26.87*a | 68.67±22.03*b | 40.00±3.46*a | 6.88 |
| Homog MIP-2 (ng/L) | 90.67±16.44 | 27.0±8.66*a | 54.67±10.61*b | 65.0±10.39*b | 64.67±7.09*b | 65.33±8.96*b | 50.33±4.24*b | 4.92 |
| 1 month | ||||||||
| Cell viability% | 98.82±0.087 | 92.77±0.25*a | 95.89±1.655*b | 95.71±1.339*b | 94.38±0.843*b | 95.096±0.616*b | 94.094±1.535*b | 1.71 |
| BALF TNF-α (ng/L) | 145.33±21.2 | 251.67±33.29*a | 216.33±16.52*a | 209.33±21.0*b | 244.0±39.34*a | 266.33±46.49*c | 241.67±46.51*a | 10.09 |
| BALF MIP-2 (ng/L) | 90.33±8.74 | 131.56±7.77*a | 129.67±9.19*a | 128.67±34.29*a | 128.33±20.21*a | 160.33±9.29*b | 135.56±14.85*a | 8.17 |
| Homog TNF-α (ng/L) | 115.67±5.67 | 339.33±31.39*a | 286.67±21.15*b | 286.0±21.63*b | 286.33±17.32*b | 323.0±42.03*a | 221.33±28.58*c | 26.28 |
| Homog MIP-2 (ng/L) | 88.33±8.74 | 136.56±16.26*a | 135.33±12.52*a | 130.33±32.71*a | 100.67±16.97*b | 130.56±24.82*a | 103.33±46.82*b | 10.51 |
| 3 months | ||||||||
| Cell viability% | 98.82±0.08 | 92.77±0.25*a | 95.08±1.25*b | 96.41±0.82*b | 94.38±0.84*c | 96.31±1.87*b | 94.09±1.53*c | 1.79 |
| BALF TNF-α (ng/L) | 144.33±25.42 | 262.67±15.04*a | 232.67±10.61a | 217.33±18.35*a | 215.0±21.23*a | 192.33±24.21*b | 225.33±35.35*a | 9.34 |
| BALF MIP-2 (ng/L) | 83.33±7.07 | 163.33±14.14*a | 132.33±5.65*b | 127.33±9.89*b | 140.33±13.31*c | 140.67±38.89*c | 127.33±6.93*b | 7.54 |
| Homog TNF-α (ng/L) | 116.33±12.5 | 121.0±12.5*a | 184.33±4.94*b | 140.0±42.21*a | 202.67±38.89*b | 225.67±4.24*b | 287.67±34.15*c | 7.83 |
| Homog MIP-2 (ng/L) | 81.33±9.19 | 153.33±18.23*a | 135.0±10.52*b | 129.67±14.2*b | 155.0±7.54*a | 137.67±38.89*b | 182.0±39.96*c | 8.11 |
Values are mean ± SD (n-3)
Represent a significant difference in comparison between treated groups and control (p<0.05)
The same letter means no significant between groups while different letters mean there was significant between groups
Figure 1.
A: TNF-α concentration in homogenate lung of all group after 4 days, 1 month and 3 months of intratracheal installation TiO2 in lung homogenate. B: TNF-α concentration in BALF of all group after 4 days, 1 month and 3 months of intratracheal installation TiO2 in BALF
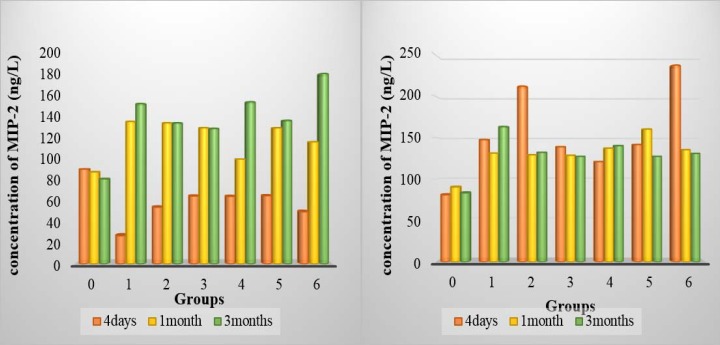
The mean difference of MIP-2 concentration in lung homogenate after 4 days of TiO2 intra-tracheal instillation was significantly decrease (p<0.05) compared with the control group and the 0.5 mg/kg dose was the more influence in the concentration, while the MIP-2 concentration in BALF of all groups was significantly increased compared to control group (p<0.05), both 0.5 and 150 mg/kg have the highest values. One month and three months post-instillation, the result showed significantly increased (p<0.05) in both homogenate tissue and BALF of all groups compared to the control group. (table 1 and figure 2)
Figure 2.
A: MIP-2 concentration in homogenate lung of all group after 4 days, 1 month and 3 months of intratracheal installation TiO2 in lung homogenate. B: MIP-2 concentration in BALF of all group after 4 days, 1 month and 3 months of intratracheal installation TiO2 in BALF
However, the result revealed a significant increase (p<0.05) in the MIP-2 concentration of homogenate tissue in the treated groups compared to the MIP-2 concentration after 4 days of instillation during experiment interval, while it was decreased in BALF of treated groups compared to the MIP-2 concentration after 4 days of instillation (p<0.05).
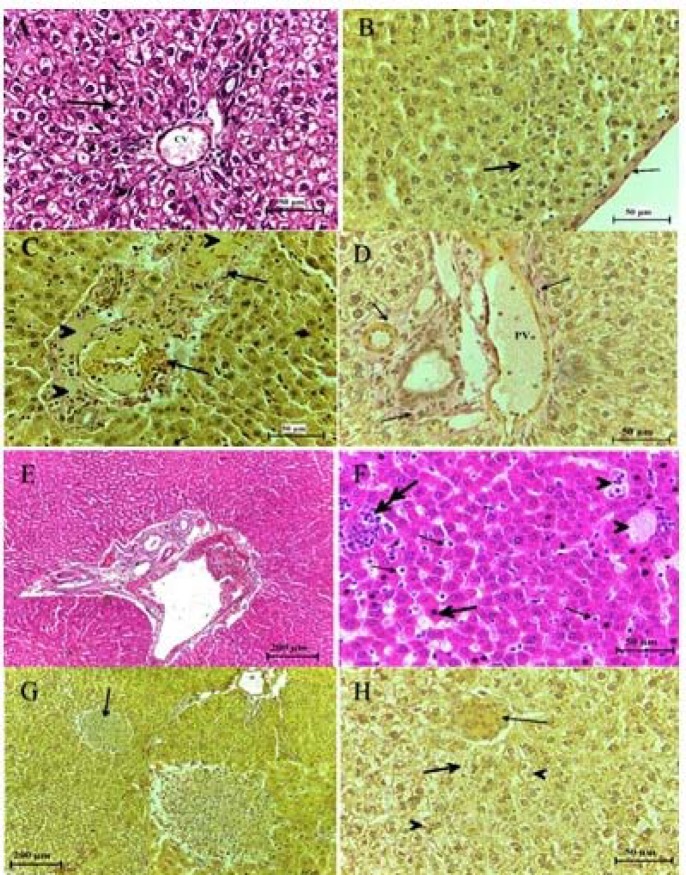
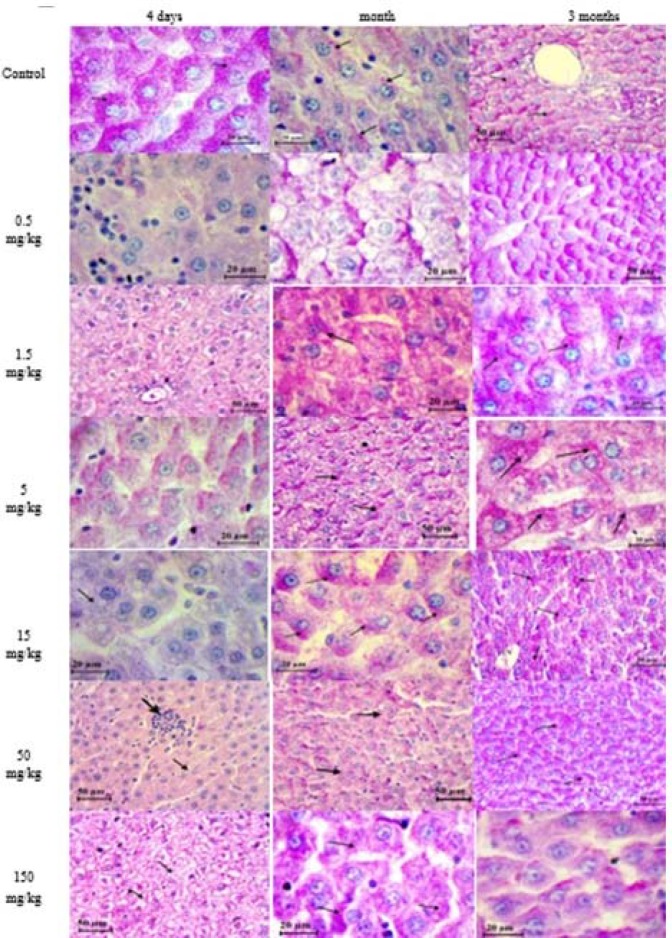
After 4 days from intra-tracheal instillation, the intensity of alteration was varied according to the dose of treatment comparing to control, including acute inflammation in the portal triads which was infiltrated with small basophilic inflammatory cells that destroyed the limiting plate of hepatocytes and extended into the lobule dilated, congested center veins, which continues from one portal triad to another portal triad may reveal the formation of fibrosis, necrosis occurring in hepatocyte which appeared shrink and more eosinophilic than normal, the numerous perisinusoidal stellate cell was detected, edema was occurring in some region, while some other hepatocytes were degeneration, increase the sinusoids space which was infiltrated with inflammatory cells and blood cells. Histiocytic forming was observed near the central vein. Increase density of collagen fiber was observed in portal triads and in the endothelial lining of central veins which was F1 in low doses and F2 in high doses. And Glisson capsule became thicker than normal, the vascular cytoplasm of hepatocytes was detected (figure 3).
Figure 3.
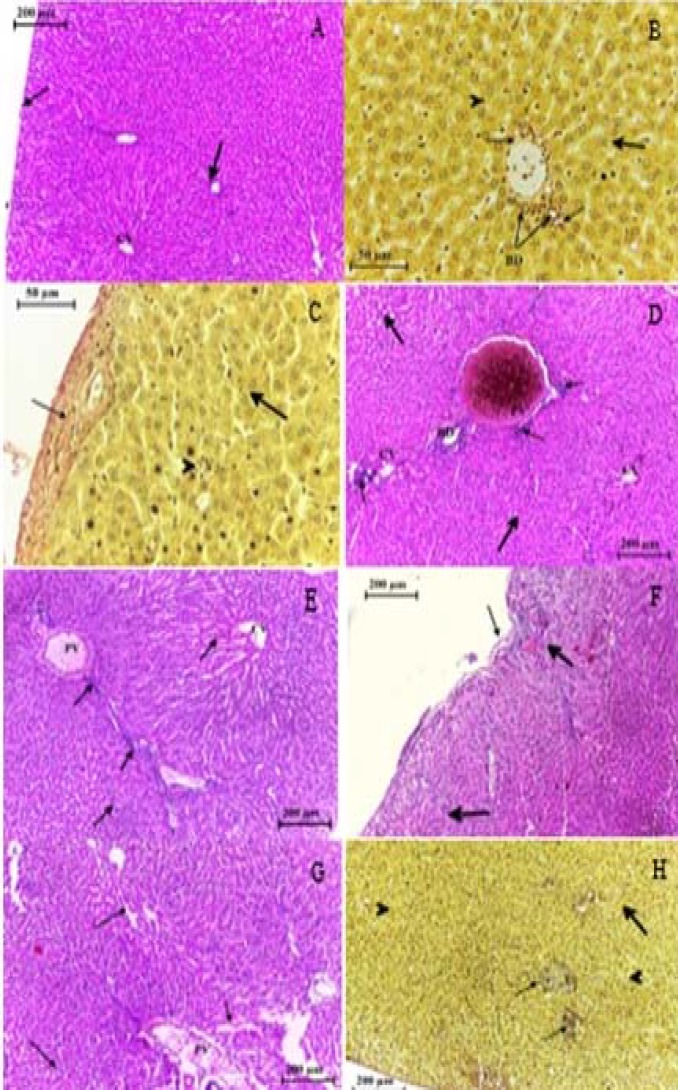
Histological structure of the liver after 4 days from intratracheal instillation control group (A, B), group treated with (0.5, 1.5, 5, 15,50, 150) mg/kg of TiO2 NP (C- H) respectively, showing the congested, dilated portal vein (PV) and sinusoid extended, inflamed and bleeding, around bile and veins, individual necrotic hepatocyte (head arrow). Histiocytic occurring, increase in density of collagen fiber around portal vein. H&E and Van Geison stains
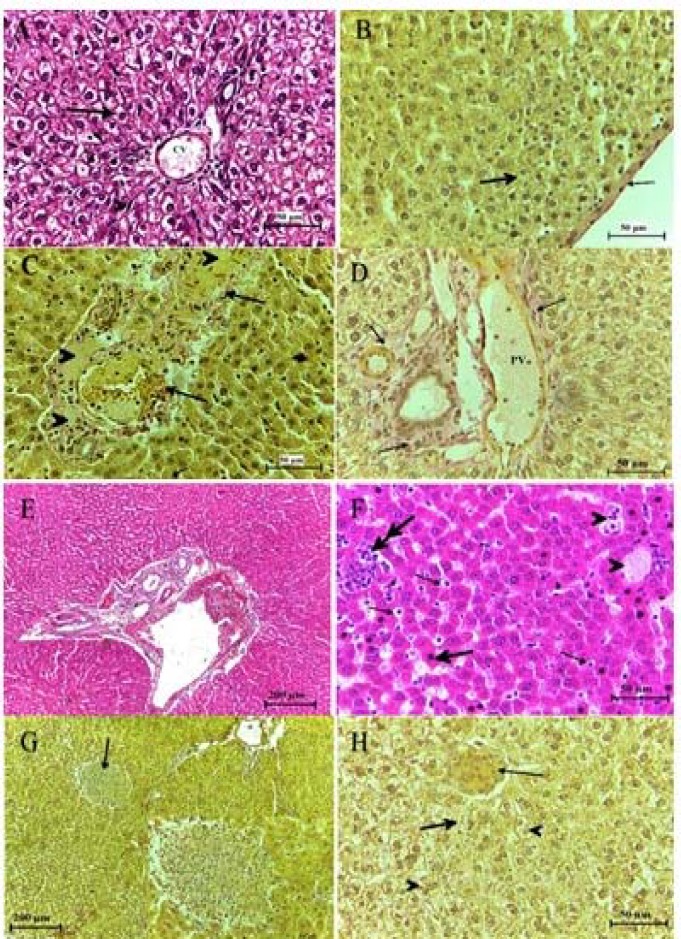
After a month from intra-tracheal instillation, the structure of the liver in the treated groups with low doses showed decreas in inflammatory cells infiltrated around the portal triads and nearby the central veins period, congested dilated blood vessel filled with lymphocyte, aggregation of blood cells detected in Glisson capsule and between sinusoids. Increase the thickness of Glisson capsule was detected due to a increase in the density of collagen fiber which extended into the sinusoids and between the portal triads structures which was F2 in low doses and F3 in high doses. Multi ducts formed with increased in blood vessels.
The presence of Ito cells (stellate cells), in high doses the hepatocytes appeared with vascular cytoplasm. Histiocytic bodies formed in or near the portal triads (figure 4). The deposition of glycogen granules in hepatocyte returned to normal in all treated groups comparing to control group and previously, which detected by reaction with PAS. In addition, normal PAS staining of glycogen granules was also detected after three months post-instillation (figure 5).
Figure 4.
Histopathological observation in the liver section of control group (A, B) and of group treated with (0.5, 1.5, 5, 15, 50, 150) mg/kg of TiO2 NPs after a month from intra-tracheal instillation (C-H) respectively, showing hypertrophy in hepatocytes, aggregation of inflammatory cells (thick arrow) infiltrated in bile duct (thin arrow) and blood vessels (BV). (B)Ito cells detected between the endothelial lining sinusoids (thin arrows), histolytic appeared in some region while in other was hypertrophy in hepatocytes. H&E stain & Van Geison stain
Figure 5.
A section in the liver in control group and the group treated with (0.5, 1.5, 5, 15, 50, 150) mg/kg of TiO2 NP post-instillation, showing the negative reaction with PAS after 4 days while the normal reaction with PAS return in hepatocyte after one month and 3 months post-instillation glycogen granules deposition which was deeply pink (arrows
After 3 months from intra-tracheal instillation, the results confirmed that the structure of liver in control group was normal as described previously. Alterations in the liver structure were observed this including the necrotic in hepatocyte plates, dilated, congested portal veins, occasional inflammatory cells present in very small numbers, multi-duct formed from the bile duct. No change in the density of collagen fiber in portal triads was seen compared to previous period of the study while it was increased in Glisson capsule with invagination in some area of the capsule, with a present of inflammatory cells between sinusoids. Another section revealed congestion in central vein, increase in a number of Kupffer cell, Ito cells (satellite cells), ballooning hepatocyte with a dense basophilic nucleus. Vascular cytoplasm was frequently seen, the space of sinusoids between hepatocyte was increased, and bleeding sinusoids with aggregation of inflammatory cell especially around bile duct was also present. Forming of histiocytic body was noticed (figure 6).
Figure 6.
Histopathological observation in the liver section of control group (A,B) and of group treated with (0.5, 1.5, 5, 15, 50, 150) mg/kg of TiO2 NP (C-H) respectively after 3 months from intratracheal instillation, showing the alteration in liver structure including the hepatocytes plates necrotic (thin arrows) infiltrated of inflammatory cells in dilated sinusoids and dilated congested portal vein (PV) and central vein (CV). Increased collagen fiber on Gilson capsule. H &E stain and Van Geison stain
Discussion
Nano-TiO2 intra tracheal instillation caused an increase in both TNF-α and MIP-2 cytokines retrieved in BALF and decreased these cytokines in the tissue homogenate after 4 days post-instillation, while after a month and 3 months post-instillation the cytokines were increased in the tissue homogenate and decreased in BALF. MacSween & Whaley (16) reported that whether the decrease in cytokines caused a decrease in inflammatory cells or a decrease in inflammatory cells led to a decrease in the release of cytokines as the study demonstrated that cell viability increased after a month and three months that could explain the increasing in these cytokines. In addition, a high concentration of TNF-α and MIP-2 may result to raise macrophage and mature lymphocyte number in the lung. The results show that concentration of both TNF-α and MIP-2 cytokines were changed in interval time of the study thus, could be caused by the decreasing the viability of the cells at 4 days post-instillation led to decrease the TNF-α specially because it was very closed to the number of inflammatory cells while at a month and 3 months post-instillation the cells was increased resulted raise the concentration of the cytokines.
However, some studies demonstrated that ultrafine particles exposed to the respiratory tract could stimulate pulmonary inflammation and particle translocation influences compared with fine particles. Moon et al. (17) showed that the levels of pro-inflammatory mediators, like IL-1β, TNF-α and macrophage inflammatory protein-2 (MIP-2), in BALF and mRNA expression of TNF-α and IL-1β in lung tissue were raised post-exposure in mice. In mice, short-term administration of the Fas-activating antibody is characterized by an inflammatory response with up-regulation of MIP-2, TNF-α, monocyte chemotactic protein (MCP)-1, and IL-6 (18). Macrophage uptake and aggregation of large amounts with TiO2 accumulate can result in cytokine release and potential cytotoxicity in cells and tissues responsible for the rescue of TiO2 from circulation (19). Cho et al. (20) demonstrated the levels of TNF-α were significantly increased by TiO2 and ZnO NP whilst other NPs were comparable to control group because TiO2NP stimulated the greatest response with respect to IL-1β but only a slight rise in TNF-α.
TiO2 NP-induced some alterations in liver tissue after 4 days post-instillation and got more effects after a month and 3 months post-instillation included hepatocytes necrosis, increased density of collagen fiber in portal triads, fibrosis, histiocytosis, numerous branches of bile ducts, congested dilated blood vessels and change the thickness of Glisson capsule. The route of exposure, such as the intragastric, intraperitoneal, intratracheal, dermal, or intraarticular, does not seem to determine different toxic effects on liver function, both in terms of type and entity of manifestation. Particularly, hepatic fibrosis hydropic and fatty degeneration of hepatocytes, prominent vasodilatation, and focal ischemia were induced by TiO2 NP treatment, the inflammatory reaction in response to NP insult was also confirmed by significant increase of both mRNA and protein expression levels of different inflammatory cytokines and mediators in liver of treated mice (21). The liver, as a major detoxification tissue, is activated to eliminate the side effects stimulated by the mass ingested TiO2 particles. In addition, part of these particles should be excreted out by the kidneys. However, the small size and difficult clearance of 25 and 80nm TiO2 particles resulted in the long-time retention of nanoparticles in vivo and induced the damage of liver and kidneys after oral exposure to 5 g/kg TiO2 particles (22).
Ma et al. (1) stated that inflammatory responses and liver injury may be involved in exposure to the different size of TiO2 NP. The inflammatory responses, appeared mainly by leukocyte infiltration, are a defense mechanism in response to pathological factors or cell death, especially necrosis, while congestion indicates alteration of vasculature integrity (23). The pathological changes are irreversible, leading to the partial or overall failure of liver functions, such as lack of bile secretion, protein, and lipid metabolism, and detoxification processes (24). While the results obtained in Faddah et al. (19) study suggests that TiO2 NPs triggers oxidative stress, DNA damage and potentially changes the apoptotic genes and their proteins expression and enhances apoptosis and the expression of inflammatory cytokines and markers of myocardial infarction in heart tissue. The collagenolytic activity may play an important role in collagen accumulation associated with hepatic fibrosis and, furthermore, that mammalian collagenase initiates the collagen degradation in the recovery phase of hepatic fibrosis. The identification and characterization of collagenolytic enzymes are still a current topic in fibrosis research. MMP-1, -2, -8, -13, and MMP-14 are the only MMPs which are able to degrade native collagens (types I, II, III, and X) and they may well be responsible for key events in the degradation of ECM (25). Natarajan et al. (26) demonstrated that TiO2 nanoparticle exposure in primary hepatocytes results in increased ROS production.
A size-dependent induction of intracellular reactive oxygen species generation yields recognizes oxidative stress as a possible mechanism of cytotoxicity with subsequent release of inflammatory cytokines representing an additional mechanism for NP stimulated adverse influence (27). The present results are in agreement with the study of Duan et al. (28) in histology of the liver, the histopathological changes were observed in the liver tissue, i.e., the structure of hepatocytes was smutch in the large area, interstitial vessels were congested. Fatemeh et al. (29) used 1 ml of 30, 50 and 70 mg/kg doses of TiO2 nanoparticles, injection repeated every other day intraperitoneally for 21 days, showed that the central venous congestion tubular, the little hepatocyte necrosis (with penetrating lymphocytes in that region), the little increase of macrophages or the Kupffer cells. Also hydropic degeneration changes in the number of centrilobular vein hepatocytes of the liver, the relative macrophages or the Kupffer cells, infiltrating of sinusoids and centrilobular vein hepatocytes of the center of the liver and in the little necrosis or hepatocytes dots.
The proliferation of the bile ducts (ductular reaction) of the liver in response to different liver injuries is characterized by a raise in the number of intrahepatic bile ducts (30). Bile duct proliferation is a histological feature which is well recognized by all histopathologists and is seen in many forms of liver disease (31).
TiO2 NP induces many alterations in lung and liver structure after 4 days and a month from intratracheal instillation. This included decrease the inflammatory cell in lung due to the phagocytosis that occurring as a n elimination to the nanoparticles while in liver included heavy infiltration of inflammatory cells, increase of collagen density in portal triads, beginning of fibrosis formation the low concentration( 0.5, 1.5, 5) of TiO2 nanoparticles at 4 days post-instillation was in the first stage of fibrosis while the high concentration (15,50,150) of TiO2 nanoparticles was at the second stage of fibrosis, however the fibrosis was turn into the next stage of fibrosis for the rest of the study period and Glisson capsule thickness increased. The immune system was strongly responded in the groups treated with high doses (15, 50, 150) mg/kg of TiO2 NP leading to raise the concentration of α-TNF, and MIP-2 in BALF while they decrease in tissue homogenate.
Acknowledgment
We thanks University of Basrah, Science of College, and Biotechnology, and Cell Researcher Unit.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Ma L, Zhao J, Wang J, Liu J, Duan Y, Liu H, et al. The Acute Liver Injury in Mice Caused by Nano-Anatase TiO2. Nanoscale Res Lett. 2009;4:1275–85. doi: 10.1007/s11671-009-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577–91. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kettler K, Veltman K, van de Meent D, van Wezel A, Hendriks AJ. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ Toxicol Chem. 2014;33:481–92. doi: 10.1002/etc.2470. [DOI] [PubMed] [Google Scholar]
- 4.Barua S, Mitragotri S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today. 2014;9:223–43. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol . 2013;8:241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corneanu G, Craciun C, Corneanu M, Lazau C, Grozescu I, Tripon S. the eukaryote cell interaction with doped tio2 nanoparticles. Romnet Net. 2010:20. [Google Scholar]
- 7.Sampsonas F, Kontoyiannis DP, Dickey BF, Evans SE. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2-year study. Cancer. 2011;117:3424–33. doi: 10.1002/cncr.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaaij-Visser TB, de Wit M, Lam SW, Jiménez CR. The cancer secretome, current status and opportunities in the lung, breast and colorectal cancer context. Biochim Biophys Acta. 2013;1834:2242–58. doi: 10.1016/j.bbapap.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Chang X, Zhang Y, Tang M, Wang B. Health effects of exposure to nano-TiO2: a meta-analysis of experimental studies. Nanoscale Res Lett. 2013;8:51. doi: 10.1186/1556-276X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu RR, Wang SL, Chao J, Lu D, Zhang R, Sun XY, et al. Bio-effects of Nano-TiO 2 on DNA and cellular ultrastructure with different polymorph and size. Materials Science & Engineering C. 2009;29:691–6. [Google Scholar]
- 11.Jasim FA, Suker DK, Albadran AI. TiO2 Nanoparticles Induce Lung Fibrosis and Proteinosis through Influence on Matrix Metalloproteinase Expression. Int J Sci. 2017;6:1–13. [Google Scholar]
- 12.Boyer L, Plantier L, Dagouassat M, Lanone S, Goven D, Caramelle P, et al. Role of nitric oxide synthases in elastase-induced emphysema. Lab Invest. 2011:353–62. doi: 10.1038/labinvest.2010.169. [DOI] [PubMed] [Google Scholar]
- 13.Shani WS. Practical immunology textbook. 1st ed. Bagdad, Iraq: 2012. [Google Scholar]
- 14.Drury RAB, Wallington EA, Carmeron Sir R. Carleton’s histological technique. 4th ed. London: Oxford University Press; 1967. pp. 129–33. [Google Scholar]
- 15.Bota S, Sirli R, Sporea I, Focsa M, Popescu A, Danila M, et al. A new scoring system for prediction of fibrosis in chronic hepatitis C. Hepat Mont. 2011;11:548–55. [PMC free article] [PubMed] [Google Scholar]
- 16.MacSween RNM, Whaley K. Muir’s textbook of pathology. 13th ed. British Government: Educational low-Priced Books Scheme; 1992. [Google Scholar]
- 17.Moon C, Park H-J, Choi Y-H, Park E-M, Castranova V, Kang JL. Pulmonary inflammation after intraperitoneal administration of ultrafine titanium dioxide (TiO2) at rest or in lungs primed with lipopolysaccharide. Journal of Toxicology and Environmental Health. Part A. 2010;73:396–409. doi: 10.1080/15287390903486543. [DOI] [PubMed] [Google Scholar]
- 18.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol. 2001;158:153–61. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faddah LM, Abdel Baky NA, Al-Rasheed NM, Al-Rasheed NM. Biochemical responses of nanosize titanium dioxide in the heart of rats following administration of idepenone and quercetin. Afr J Pharm Pharmacol. 2013;7:2639–51. [Google Scholar]
- 20.Cho W-S, Duffin R, Bradley M, Megson IL, MacNee W, Lee JK, et al. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part Fibre Toxicol. 2013;10 doi: 10.1186/1743-8977-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iavicoli I, Leso V, Fontana L, Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur Rev Med Pharmacol Sci. 2011;15:481–508. [PubMed] [Google Scholar]
- 22.Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168:176–85. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Miranda RR, Damaso da Silveira ALR, de Jesus IP, Grötzner SR, Voigt CL, Campos SX, et al. Effects of realistic concentrations of TiO2 and ZnO nanoparticles in prochilodus lineatus juvenile fish. Environ Sci Pollut Res Int. 2016;23:5179–88. doi: 10.1007/s11356-015-5732-8. [DOI] [PubMed] [Google Scholar]
- 24.Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007;84:415–30. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Roderfeld M, Hemmann S, Roeb E. Mechanisms of fibrolysis in chronic liver injury (with special emphasis on MMPs and TIMPs) Z Gastroenterol. 2007;45:25–33. doi: 10.1055/s-2006-927388. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan V, Wilson CL, Hayward SL, Kidambi S. Titanium dioxide nanoparticles trigger loss of function and perturbation of mitochondrial dynamics in primary hepatocytes. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0134541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharjee S, Ershov D, Fytianos K, van der Gucht J, Alink GM, Rietjens IM, et al. Cytotoxicity and cellular uptake of tri-block copolymer nanoparticles with different size and surface characteristics. Part Fibre Toxicol. 2012;9:11. doi: 10.1186/1743-8977-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan Y, Liu J, Ma L, Li N, Liu H, Wang J, et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials. 2010;31:894–9. doi: 10.1016/j.biomaterials.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Fatemeh MF, Mohammad F. The histological and biochemical effects of titanium dioxide nanoparticle (TiO2) on the liver in wistar Rat. Int res j boil sci. 2014;3:2278–3202. [Google Scholar]
- 30.Yoshioka K, Mori A, Taniguchi K, Mutoh K. Cell proliferation activity of proliferating bile duct after bile duct ligation in rats. Vet Pathol. 2005;42:382–5. doi: 10.1354/vp.42-3-382. [DOI] [PubMed] [Google Scholar]
- 31.Burt AD, MacSween RN. Bile duct proliferation--its true significance? Histopathology. 1993;23:599–602. doi: 10.1111/j.1365-2559.1993.tb01258.x. [DOI] [PubMed] [Google Scholar]