Abstract
Background
Hypertension diagnosed by blood pressure (BP) measured in the clinic is associated with rapid kidney function decline (RKFD) and incident chronic kidney disease (CKD). The extent to which hypertension defined using out-of-clinic BP measurements is associated with these outcomes is unclear.
Methods
We evaluated the association of any masked hypertension (daytime SBP/DBP ≥ 135/85 mmHg, night-time SBP/DBP ≥ 120/70 mmHg or 24-h SBP/DBP ≥ 130/80 mmHg) with RKFD and incident CKD among 676 African-Americans in the Jackson Heart Study with clinic-measured SBP/DBP less than 140/90 mmHg who completed ambulatory BP monitoring in 2000–2004. RKFD was defined as a decline in estimated glomerular filtration rate (eGFR) at least 30% and incident CKD was defined as development of eGFR less than 60 ml/min per 1.73 m2 with an at least 25% decline in eGFR between 2000–2004 and 2009–2013.
Results
The mean age of participants was 57.6 years, 28.8% were men and 52.7% had any masked hypertension. After a median follow-up of 8 years, 13.8 and 8.6% of participants had RKFD and incident CKD, respectively. In unadjusted analyses, masked hypertension was associated with an increased odds for incident CKD [odds ratio (OR) 2.20, 95% confidence interval (CI) 1.22, 3.97]. This association remained statistically significant after adjustment for demographic characteristics, baseline eGFR and albumin-to-creatinine ratio (OR 1.95, 95% CI 1.04, 3.67) but was eliminated after propensity score adjustment (OR 1.62, 95% CI 0.87, 3.00). There was no association between masked hypertension and RKFD.
Conclusion
Masked hypertension may be associated with the development of CKD in African-Americans.
Keywords: African-Americans, ambulatory blood pressure monitoring, chronic kidney disease, estimated glomerular filtration rate, masked hypertension, rapid kidney function decline
Ambulatory blood pressure monitoring (ABPM) can provide additional prognostic information on cardiovascular disease (CVD) risk to blood pressure (BP) measured in the clinic setting [1]. One phenotype identified using ABPM in conjunction with clinic-measured BP is masked hypertension. Initially, masked hypertension was defined by not having high clinic-measured BP (SBP <140 mmHg and DBP <90 mmHg) but having high daytime BP (SBP/DBP ≥ 135/85 mmHg) measured by ABPM [2]. Using this definition, the prevalence of masked hypertension has been reported to be 15–30% [1,3]. In 2013, the European Society of Hypertension/European Society of Cardiology recommended extending the definition of masked hypertension to include high daytime BP (mean SBP/DBP ≥ 135/85 mmHg), night-time BP (mean SBP/DBP ≥ 120/70 mmHg) and/or 24-h BP (mean SBP/DBP ≥ 130/80 mmHg) [3]. In a prior analysis of the Jackson Heart Study (JHS), a cohort comprised exclusively of African-Americans, the prevalence of masked hypertension, defined using daytime, night-time and 24-h BP, was 52.2% [4]. In two cohorts of African-Americans with established chronic kidney disease (CKD), the prevalence of masked hypertension, defined using 24-h ambulatory BP, was 27.8 and 26.0% [5,6].
Masked hypertension has been associated with an increased risk for cardiovascular target organ damage, including left ventricular (LV) hypertrophy, increased LV mass index [7], LV wall thickness, carotid intima–media thickness and pulse wave velocity [7-11]. It has also been associated with increased risk for cardiovascular and cerebrovascular events [12,13]. However, the effect of masked hypertension on the risk for CKD is less clear as prior studies have been cross-sectional or were conducted among people with established CKD [5,14,15]. In addition, there are few data on the association of masked hypertension on kidney outcomes among African-Americans. Determining the association of masked hypertension with kidney function decline and the development of CKD may lead to interventions aimed at reducing the burden of CKD. In this study, we examined the association between masked hypertension and rapid kidney function decline (RKFD) and incident CKD in the JHS.
METHODS
The JHS is a single-site, prospective, cohort study of risk factors for CVD among African-Americans residing in the Jackson, Mississippi metropolitan area. Details of the JHS have been described previously [16]. Briefly, the JHS enrolled 5306 participants from four groups in the community: participants enrolled in the Atherosclerosis Risk in Communities Study (31%), randomly selected community-dwelling adults (17%), family members of the participants (22%) and volunteers (30%). Recruitment was restricted to adults 35–84 years old except for family members where those at least 21 years old were eligible for enrollment [17]. Different age criteria were employed for the recruitment of family members to facilitate a JHS Family Study, which was designed to identify genes influencing the risk factors for heart, lung and blood disorders. Enrollment of families was restricted to the relatives of those who had already become JHS participants [18]. In addition to completing a baseline clinic visit (exam 1) in 2000–2004, participants returned for two additional clinic visits, exam 2 (October 2005–December 2008) and exam 3 (February 2009–January 2013). The institutional review boards at the participating institutions (Jackson State University, Tougaloo College and University of Mississippi Medical Center) approved the study protocol. Written informed consent was obtained from all participants at each study visit.
Analysis population
Participants who underwent ABPM at exam 1 (n = 1148) were included in the current analysis. We excluded participants with an incomplete ABPM recording (n = 102; defined below), clinic-measured SBP/DBP at least 140/90 mmHg (n = 202), unknown hypertension status due to missing clinic-measured BP (n = 5), self-report of being on dialysis (n = 2) or unknown dialysis status (n = 4) at exam 1, or missing serum creatinine measurements at exam 1 (n = 9). We also excluded participants who did not attend exam 3 (n = 140) and those who were missing a serum creatinine measurement from this visit (n = 8). After these exclusions, data from 676 participants were available for the analysis of RKFD. For the analysis of incident CKD, an additional 25 participants with reduced estimated glomerular filtration rate (eGFR) (eGFR <60 ml/min per 1.73 m2) at exam 1 were excluded, leaving 651 participants (Fig. 1).
FIGURE 1.
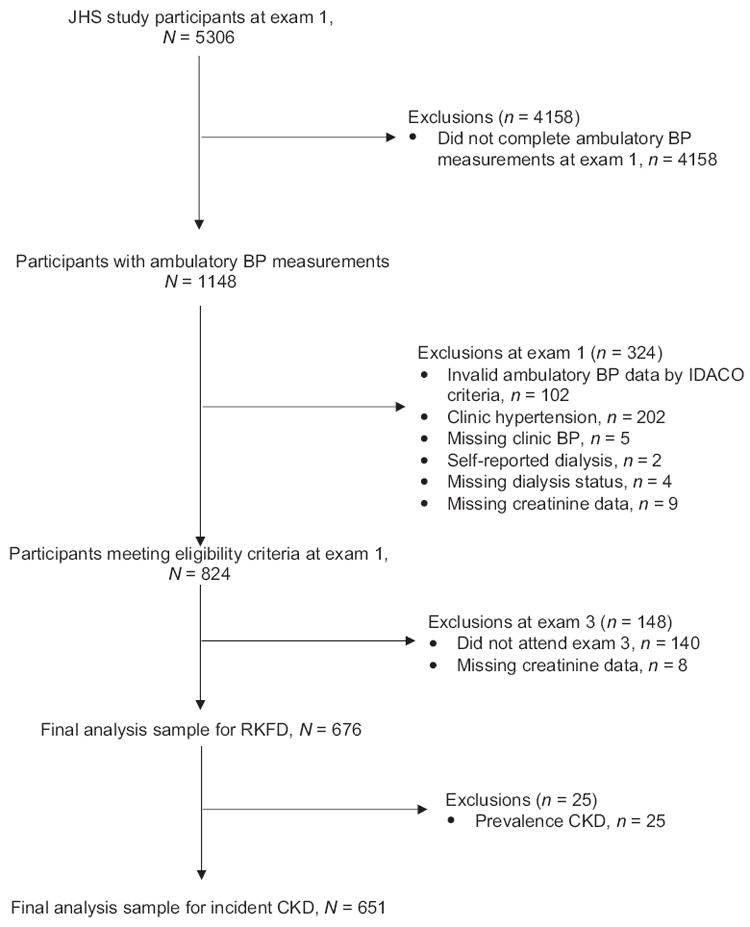
CONSORT flow diagram for the analysis of masked hypertension and rapid kidney function decline and incident chronic kidney disease. BP, blood pressure; CKD, chronic kidney disease; RKFD, rapid kidney function decline.
Data collection
Exam 1 data were collected during an in-home interview and a clinic exam [19]. Information on age, sex, education, cigarette smoking, alcohol use and physical activity was collected during in-home interviews. A modified Baecke questionnaire was used to record the duration, frequency and intensity of physical activity during living, work, home life and sports [20]. Ideal health status for physical activity was defined using American Heart Association criteria as at least 75 min of vigorous physical activity or at least 150 min of moderate or combined moderate and vigorous physical activity per week.
During exam 1, trained technicians measured BP, height and weight, recorded the names of prescription and over the counter medications taken in the previous 2 weeks, and collected fasting blood and urine samples. A 24-h urine collection was requested from all participants. Beginning in October 2002, random spot urine samples were also collected during exam 1.
Biochemical testing for fasting glucose, serum and urine creatinine were performed using an enzymatic method on a Vitros 950 or 250 Ortho-Clinical Diagnosis analyzer (Raritan, New Jersey, USA). A lipid profile was assayed by oxidase method on a Roche COBAS Fara analyzer (Roche Diagnostics, Indianapolis, Indiana, USA). Hemoglobin A1c (HbA1c) was measured with a TOSOH high performance liquid chromatography system. Urinary albumin was measured on a Dade-Behring BN 11 nephelometer (Dade Behring, Newark, Delaware, USA). Among participants for whom 24-h urine sample was not collected, the random spot urine sample was used to estimate the urinary albumin-to-creatinine ratio (UACR) [21]. Albuminuria was defined as a UACR at least 30 mg/g. Diabetes was defined as a fasting (≥8 h) plasma glucose at least 126 mg/dl, HbA1c at least 6.5% or use of antidiabetes medication.
Clinic blood pressure measurement
During exam 1, clinic BP was measured with a Hawksley random-zero sphygmomanometer (Hawksley and Sons Ltd, Langing, UK) and an appropriately sized cuff [22]. After the participant had rested for at least 5 min in a seated upright position with their back and arms supported, feet flat on the floor and legs uncrossed, two BP measurements were recorded in the right arm. The average of the two measurements recorded 1 min apart was used to define clinic BP. Quality control was conducted by JHS Coordinating Center and included monitoring digit preference for each staff member and by comparing the mean BP level measured within and between study staff. Other quality control measures included technician certification, recertification and procedural checklists [23]. As previously described, the random-zero sphygmomanometer used for BP measurements in Exam 1 was calibrated to the semiautomatic oscillometric device (Omron HEM-907XL; Omron Healthcare Inc., Lake Forest, Illinois, USA) [24] used for BP measurements at JHS Exams 2 (2005–2008) and 3 (2009–2013). To calibrate BP across two devices, a comparability substudy was conducted. This substudy included 2115 participants for which BP was assessed simultaneously by random-zero sphygmomanometer and the Omron HEM-907XL device using a Y-connector. As described elsewhere [25], the random-zero BP measurements were calibrated to the semiautomatic oscillometric device using robust regression. The calibrated clinic BP values were used for the primary analyses with non-calibrated BP used in sensitivity analysis.
Ambulatory blood pressure monitoring
Upon completion of exam 1, each participant was invited to undergo ABPM. ABPM was performed using a portable, noninvasive oscillometric device (Spacelabs 90207; Medifacts International Ltd, Rockville, Maryland, USA) with a cuff fitted to the participant’s nondominant arm. The device was programed to take BP measurements every 20 min.
Trained technicians instructed participants on the general procedures and function of the ABPM device to ensure compliance and successful collection of data. Participants returned to the clinic 24 h later for the removal of the ABPM device. The ABPM device was connected to a computer and the BP recordings were downloaded using commercially available software (Medicom, version 3.41) [16,19]. Consistent with the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO) criteria, we defined a complete ABPM recording as having at least 10 daytime (1000 to 2000 h) and at least five nighttime (0000 to 0600 h) BP measurements [26]. IDACO criteria were applied, rather than more stringent criteria, to include the maximum sample size available [27].
Masked hypertension
Masked daytime hypertension was defined as a mean daytime SBP/DBP at least 135/85 mmHg; masked night-time hypertension was defined as a mean night-time SBP/DBP at least 120/70 mmHg; and masked 24-h hypertension was defined as a mean SBP/DBP at least 130/80 mmHg using all BP readings taken over the ABPM recording period [3]. Participants with masked daytime, night-time or 24-h hypertension were categorized as having any masked hypertension.
Outcomes
The two outcomes included RKFD and incident CKD. Both endpoints were evaluated at exam 3 as serum creatinine was not measured at exam 2. Serum creatinine was measured using an enzymatic method at exam 1 and calibrated to the isotope-dilution mass spectrometry-traceable method used at exam 3 as previously described [28]. RKFD was defined as a decline in eGFR at least 30% from exam 1 to exam 3 [21]. Incident CKD was defined as a decline from eGFR at least 60 ml/min per 1.73 m2 at exam 1 to eGFR less than 60 ml/min per 1.73 m2 at exam 3 in conjunction with a decline in eGFR at least 25% over this time period [29]. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [30]. The percentage change in eGFR was calculated as 100 × (eGFR at exam 1 − eGFR at exam 3) divided by eGFR at exam 1. eGFR values more than 120 ml/min per 1.73 m2 were truncated at 120 ml/min per 1.73 m2 to avoid large changes among participants with high eGFR at exam 1 [31,32].
Statistical analysis
Baseline characteristics of the participants with and without any masked hypertension were calculated and compared using two-sample t tests for continuous variables and chi-square tests for categorical variables. The percentage of participants having RKFD and incident CKD was determined by masked hypertension status for any, daytime, night-time and 24-h masked hypertension. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) for RKFD and incident CKD comparing participants with versus without any masked hypertension in the following sequential models: Model 1, unadjusted; Model 2, adjusted for age, sex and education; Model 3, adjusted for age, sex, education, eGFR and UACR; and Model 4, adjusted for a propensity score. In Model 4, we used a propensity score to simultaneously adjust for age, sex, education, eGFR, UACR, BMI, diabetes, total cholesterol, high-sensitive C reactive protein, physical activity, current cigarette smoking, alcohol use and self-reported use of antihypertensive medication given the large number of covariates relative to the number of outcome events. The sequential modeling was repeated for masked daytime, night-time and 24-h hypertension, separately. All covariates were defined using exam 1 values. Different propensity scores were developed for each type of masked hypertension using logistic regression models, with the type of masked hypertension as the dependent variable (i.e. daytime, night-time, 24-h and any masked hypertension, separately) and all covariates as independent variables. Quintiles of the propensity score were included in Model 4 in place of the values of individual covariates. The above analyses were repeated using the noncalibrated BP values. Missing covariates at exam 1 (n = 141 participants did not have UACR and an additional n = 49 participants missing other covariates) were imputed using the Markov Chain Monte Carlo method [33]. Imputation was performed for each combination of outcomes and exposures analyzed. The imputation model included the outcome variable, the exposure variable and all the covariates used to create the propensity scores. Twenty (20) imputed datasets were generated for analysis. In a final analysis, ORs for RKFD and incident CKD were calculated stratified by diabetes status and use of antihypertensive medication at exam 1. All data analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Participant baseline characteristics
Among the 676 participants included in the analysis, the mean age was 57.6 ± 10.7 years, 28.8% were men and 52.7% had any masked hypertension (masked daytime hypertension, 29.9%; masked night-time hypertension, 48.1%; masked 24-h hypertension, 33.7%). Compared with participants without any masked hypertension, those with any masked hypertension were older, more likely to be men, have diabetes and self-report taking antihypertensive medication (Table 1). In addition, participants with any masked hypertension had higher HbA1c and fasting plasma glucose, and lower eGFR levels compared with their counterparts without any masked hypertension.
TABLE 1.
Baseline characteristics for Jackson Heart Study participants with and without any masked hypertension
| Any masked hypertension
|
|||
|---|---|---|---|
| No, n = 320 | Yes, n = 356 | P value | |
| Participant characteristics | |||
| Age (years) | 56.1 ± 11.1 | 58.9 ± 10.1 | 0.001 |
| Male | 74 (23.1) | 121 (34.0) | 0.002 |
| Education ≥high school | 275 (86.5) | 295 (82.9) | 0.195 |
| BMI (kg/m2) | 31.2 ± 6.2 | 31.0 ± 6.3 | 0.693 |
| Diabetes | 47 (14.7) | 95 (26.8) | <0.001 |
| Self-reported use of antihypertensive medication use | 144 (46.0) | 205 (59.1) | 0.001 |
|
| |||
| Laboratory measures | |||
| HbA1c | 5.8 ± 1.0 | 6.2 ± 1.4 | <0.001 |
| Fasting plasma glucose (mg/dl) | 98.1 ± 24.9 | 102.7 ± 31.8 | 0.043 |
| Fasting total cholesterol (mg/dl) | 200.3 ± 39.9 | 199.8 ± 40.0 | 0.856 |
| Fasting HDL-C (mg/dl) | 54.7 ± 14.5 | 54.0 ± 14.9 | 0.545 |
| Fasting LDL-C (mg/dl) | 124.4 ± 34.8 | 124.7 ± 38.7 | 0.922 |
| Fasting triglycerides (mg/dl) | 106.8 ± 101.9 | 106.8 ± 67.7 | 0.993 |
| HsCRP > 3.0 mg/l | 152 (47.8) | 180 (50.6) | 0.474 |
| UACR2 ≥ 30 mg | 14 (5.5) | 25 (9.0) | 0.115 |
| eGFR (ml/min per 1.73 m2) | 95.9 ± 20.4 | 92.8 ± 19.3 | 0.038 |
|
| |||
| Behavioral factors | |||
| Current smoking | 25 (7.8) | 36 (10.2) | 0.277 |
| Alcohol use (past 12 months) | 152 (47.5) | 162 (45.6) | 0.627 |
| Physical activity meeting AHA guidelines | 74 (23.1) | 64 (18.0) | 0.097 |
|
| |||
| Clinic BP (mmHg) | |||
| SBP | 118.2 ± 10.5 | 125.0 ± 9.3 | <0.001 |
| DBP | 71.9 ± 6.7 | 74.5 ± 7.9 | <0.001 |
|
| |||
| ABPM, (mmHg) | |||
| Daytime SBP | 119.0 ± 7.7 | 133.2 ± 10.6 | <0.001 |
| Daytime DBP | 72.8 ± 6.0 | 81.3 ± 8.6 | <0.001 |
| Night-time SBP | 107.4 ± 7.3 | 126.4 ± 12.2 | <0.001 |
| Night-time DBP | 61.1 ± 5.1 | 72.8 ± 8.4 | <0.001 |
| 24-h SBP | 114.5 ± 6.8 | 130.7 ± 9.8 | <0.001 |
| 24-h DBP | 68.1 ± 5.0 | 78.0 ± 7.6 | <0.001 |
Results are presented as mean ± SD for continuous variables or number of participants (column %) for categorical variables. ABPM, ambulatory blood pressure monitoring; AHA, American Heart Association; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HsCRP, high-sensitivity c-reactive protein; UACR, Urine albumin–creatinine ratio.
Masked hypertension and rapid kidney function decline
During a median follow-up of 8.0 years (range: 5.9–12.2 years), eGFR on average declined 12.1% (median: 10.0%, interquartile range: 1.3–21.7%). Ninety-three participants (13.8%) experienced RKFD (Fig. 2, panel a). Overall, 15.5 and 11.9% of participants with and without any masked hypertension, respectively, experienced RKFD (P = 0.178). The unadjusted OR for RKFD comparing participants with versus without any masked hypertension was 1.36 (95% CI 0.87–2.11) (Table 2). No association was present between any masked hypertension and RKFD after full multivariable adjustment (OR, 1.07; 95% CI 0.67–1.70). The multivariable-adjusted ORs for RKFD associated with daytime, nighttime and 24-h masked hypertension were 1.23 (95% CI, 0.76–1.99), 1.24 (0.78–1.96) and 1.37 (0.86–2.18), respectively. Results were similar when using noncalibrated BP values (Supplemental Table 1, http://links.lww.com/HJH/A917).
FIGURE 2.
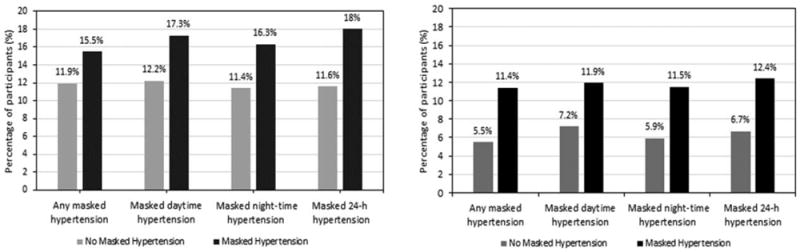
Percentage of participants experiencing rapid kidney function decline (panel a) and incident chronic kidney disease (panel b) by type of masked hypertension. Rapid kidney function decline was defined as a decrease in estimated glomerular filtration rate at least 30% from exam 1 to exam 3. Incident chronic kidney disease was defined as a decline in estimated glomerular filtration rate from at least 60 ml/min per 1.73 m2 at exam 1 to less than 60 ml/min per 1.73 m2 at exam 3 with at least 25% decline during this time period.
TABLE 2.
Odds ratios for rapid kidney function decline associated with any, daytime, night-time and 24-h masked hypertension among Jackson Heart Study participants
| Events/n at risk (%) | Odds ratio (95% confidence interval)
|
||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Any masked hypertension | |||||
| No | 38/320 (11.9) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 55/356 (15.5) | 1.36 (0.87, 2.11) | 1.26 (0.79, 1.99) | 1.20 (0.75, 1.91) | 1.07 (0.67, 1.70) |
|
| |||||
| Masked daytime hypertension | |||||
| No | 58/474 (12.2) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 35/202 (17.3) | 1.50 (0.95, 2.37) | 1.44 (0.89, 2.30) | 1.29 (0.79, 2.10) | 1.23 (0.76, 1.99) |
|
| |||||
| Masked night-time hypertension | |||||
| No | 40/351 (11.4) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 53/325 (16.3) | 1.52 (0.97, 2.36) | 1.41 (0.89, 2.23) | 1.35 (0.85, 2.14) | 1.24 (0.78, 1.96) |
|
| |||||
| Masked 24-h hypertension | |||||
| No | 52/448 (11.6) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 41/228 (18.0) | 1.67 (1.07, 2.61) | 1.59 (1.00, 2.52) | 1.46 (0.91, 2.35) | 1.37 (0.86, 2.18) |
Model 1: unadjusted. Model 2: adjusted for age, sex and education. Model 3: adjusted for age, sex and education, estimated glomerular filtration rate (eGFR) and urine albumin–creatinine ratio (UACR). Model 4: adjusted for quintiles of the propensity score for having masked hypertension. Propensity score quintiles were calculated for each type of masked hypertension, separately, using logistic regression models with the type of masked hypertension as the dependent variable and age, sex, education, eGFR, UACR, BMI, diabetes, high-sensitivity c-reactive protein, total cholesterol, physical activity, current cigarette smoking, alcohol intake and antihypertensive medication use as independent variables. RKFD, rapid kidney function decline.
Masked hypertension and incident chronic kidney disease
Fifty-six participants (8.6%) developed CKD during follow-up. Incident CKD occurred more often among participants with versus without any masked hypertension (11.4 versus 5.5%; P =0.008) (Fig. 2, panel b). The unadjusted OR for developing CKD comparing participants with versus without any masked hypertension was 2.20 (95% CI 1.22–3.97) (Table 3). The association remained statistically significant after adjustment for age, sex, education, eGFR and UACR (OR, 1.95; 95% CI 1.04–3.67). However, this association was eliminated after propensity score adjustment (OR, 1.62; 95% CI 0.87–3.00). When masked daytime, night-time and 24-h hypertension were analyzed separately, only the association of masked night-time hypertension with incident CKD was statistically significant after adjustment for age, sex, education, eGFR and UACR (OR, 1.86; 95% CI 1.01–3.42). The fully adjusted OR (95% CI) for incident CKD association with masked daytime, night-time and 24-h hypertension were 1.34 (0.74–2.42), 1.71 (0.95–3.09) and 1.55 (0.87–2.75), respectively. Results were similar when using noncalibrated BP values (Supplemental Table 2, http://links.lww.com/HJH/A917).
TABLE 3.
Odds ratios for incident chronic kidney disease associated with any, daytime, night-time and 24-h masked hypertension among Jackson Heart Study participants
| Events/n at risk (%) | Odds ratio (95% confidence interval)
|
||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Any masked hypertension | |||||
| No | 17/308 (5.5) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 39/343 (11.4) | 2.20 (1.22, 3.97) | 1.94 (1.05, 3.59) | 1.95 (1.04, 3.67) | 1.62 (0.87, 3.00) |
|
| |||||
| Masked daytime hypertension | |||||
| No | 33/457 (7.2) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 23/194 (11.9) | 1.73 (0.99, 3.03) | 1.52 (0.84, 2.72) | 1.55 (0.84, 2.85) | 1.34 (0.74, 2.42) |
|
| |||||
| Masked night-time hypertension | |||||
| No | 20/339 (5.9) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 36/312 (11.5) | 2.08 (1.18, 3.68) | 1.82 (1.01, 3.30) | 1.86 (1.01, 3.42) | 1.71 (0.95, 3.09) |
|
| |||||
| Masked 24-h hypertension | |||||
| No | 29/433 (6.7) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 27/218 (12.4) | 1.97 (1.13, 3.42) | 1.70 (0.96, 3.03) | 1.71 (0.94, 3.11) | 1.55 (0.87, 2.75) |
Model 1: unadjusted. Model 2: adjusted for age, sex and education. Model 3: adjusted for age, sex and education, estimated glomerular filtration rate (eGFR) and urine albumin–creatinine ratio (UACR). Model 4: adjusted for quintiles of the propensity score for having masked hypertension. Propensity score quintiles were calculated for each type of masked hypertension, separately, using logistic regression models with the type of masked hypertension as dependent variable and age, sex, education, eGFR, UACR, BMI, diabetes, high-sensitivity c-reactive protein, total cholesterol, physical activity, current cigarette smoking, alcohol intake and antihypertensive medication use as independent variables. CKD, chronic kidney disease.
Subgroup analysis
The propensity-score adjusted ORs for RKFD comparing participants with versus without any masked hypertension were 0.84 (95% CI 0.49–1.45) and 2.20 (95% CI 0.82–5.91) among participants taking and not taking antihypertensive medication, respectively (Table 4, P value for interaction =0.074). The propensity-score adjusted ORs for incident CKD comparing participants with versus without any masked hypertension were 1.38 (95% CI 0.66–2.88) and 3.24 (95% CI 0.91–11.5) among those taking and not taking antihypertensive medication, respectively (P value for interaction =0.187). The associations between any masked hypertension with RKFD and incident CKD were not statistically significantly different between participants with and without diabetes (each P value for interaction >0.6).
TABLE 4.
Association between any masked hypertension with rapid kidney function decline and incident chronic kidney disease stratified by antihypertensive medication use and by diabetes status at baseline
| RKFD
|
Incident CKD
|
|||
|---|---|---|---|---|
| Subgroups | Propensity score-adjusted OR (95% CI) | P value for interaction | Propensity score-adjusted OR (95% CI) | P value for interaction |
| Self-reported antihypertensive medication use | ||||
| Yes | 0.84 (0.49, 1.45) | 0.074 | 1.38 (0.66, 2.88) | 0.187 |
| No | 2.20 (0.82, 5.91) | 3.24 (0.91, 11.5) | ||
|
| ||||
| Diabetes status | ||||
| Yes | 1.20 (0.52, 2.80) | 0.606 | 1.54 (0.47, 5.07) | 0.949 |
| No | 0.94 (0.53, 1.65) | 1.61 (0.79, 3.29) | ||
Due to small number of cases in the stratified analyses, the propensity score was included in the model as a continuous variable. Propensity score was calculated for each type or masked hypertension using logistic regression models, with the any masked hypertension as the dependent variable and age, sex, education, estimated glomerular filtration rate (eGFR), urinary albumin–creatinine ratio (UACR), BMI, diabetes, high-sensitivity c-reactive protein (HSCRP), total cholesterol, physical activity, current cigarette smoking, alcohol intake and use of antihypertensive medication as independent variables. CI, confidence interval; CKD, chronic kidney disease; OR, odds ration; RKFD, rapid kidney function decline.
DISCUSSION
In the current study, having any masked hypertension was associated with an increased risk for incident CKD after adjustment for age, sex, education, eGFR and UACR. However, this association was eliminated after propensity score adjustment. The results were consistent for masked daytime, night-time and 24-h hypertension. No statistically significant association was present between any, daytime, night-time and 24-h masked hypertension and RKFD. Although there was a suggestion of a higher risk for RKFD and incident CKD among participants who were not taking antihypertensive medication, the differences in the ORs across subgroups defined by use of antihypertensive medication were not statistically significant. The association of masked hypertension and RKFD and incident CKD did not differ by diabetes status.
Masked hypertension has been associated with reduced eGFR and higher levels of urine protein excretion in cross-sectional studies [9] and an increased risk for end-stage renal disease, death or doubling of serum creatinine in longitudinal studies [6,34,35] of adults with established CKD. In the Ohasama study, a population-based cohort, prevalent CKD at baseline, defined as eGFR less than 60 ml/min per 1.73 m2 and/or testing positive for proteinuria using a dipstic were more common among those with versus without masked daytime hypertension defined by a daytime SBP/DBP at least 140/85 mmHg [14]. Over a median follow-up of 8.3 years and among participants without CKD at baseline, the risk for incident CKD increased with progressively higher baseline 24-h and night-time SBP but not with higher levels of daytime or clinic SBP. The association between night-time SBP and incident CKD remained statistically significant after adjusting for daytime SBP. Similar results were present with night-time DBP and incident CKD [36]. Although the Ohasama study did not specifically evaluate the risk for incident CKD among participants with masked hypertension, the current results are consistent with the Ohasama study, suggesting a possible association between any and night-time masked hypertension and the development of CKD. Other risk factors may be mediators between masked hypertension and CKD, indicated by the elimination of this association with propensity score adjustment in the current study. Risk factors for CKD, including smoking, obesity, physical activity and psychosocial factors [37], have been associated with masked hypertension. In JHS, male sex, smoking, diabetes, antihypertensive medication use and clinic BP were associated with an increased prevalence of masked hypertension [38]. In addition, a high prevalence of obesity and high levels of perceived stress might also explain the increased prevalence of masked hypertension in African-Americans [39,40].
Although few prospective studies have reported on masked hypertension and CKD, several cohort studies, including the JHS, have investigated the association between masked hypertension and incident CVD [10,41-45]. A meta-analysis of 7961 adults estimated the risk for CVD among participants with masked hypertension to be 2.09 (95% CI 1.55–2.81) times higher when compared with normotensive participants [41]. Similarly, in a pooled analyses of four population cohorts, Hansen et al. [10] showed that cardiovascular risk was higher in participants with masked hypertension, hazard ratio of 1.62 (95% CI 1.35–1.96), when compared with their counterparts with normotension. In the JHS, participants with any masked hypertension had a 2.49 (95% CI 1.26–4.93) times higher risk of CVD compared with their counterparts without masked hypertension. The risk for CVD was statistically significantly elevated among participants with masked daytime, night-time or 24-h hypertension [46]. Furthermore, night-time BP has been associated with cardiovascular mortality after adjustment for daytime BP [42]. As CVD and CKD share many risk factors, these studies provide further support for the plausibility of an association between masked hypertension and CKD. Considering that African-Americans have a high prevalence of masked hypertension and CKD and increased risk for incident CKD, the lack of association observed in the current study may indicate no association truly existing. However, this should be confirmed in other cohorts.
There are several strengths of the current study. The JHS enrolled a large community-based sample of African-Americans. This population has been well characterized, and we were able to control for multiple potential confounders. Despite these strengths, the results should be interpreted in the context of possible limitations, including the conduct of only a single ABPM procedure, which may have resulted in misclassification of participants’ masked hypertension status. However, this misclassification would most likely be nondifferential (i.e. not dependent on risk for future RKFD or incident CKD). Therefore, the association between masked hypertension and RKFD and incident CKD may be stronger than we report. Only a subset of JHS participants completed the ABPM procedure. Differences were present in demographic and clinical characteristics between participants who did and did not complete the ABPM procedure [38]. Although this may limit the generalizability of the study results, the association with RKFD and incident CKD for participants with versus without masked hypertension should remain internally valid. Clinic BP was measured in a single occasion; some participants may have different clinic BP if measured on a separate day. In addition, clinic BP measured using random-zero sphygmomanometer at Exam 1 was calibrated to an oscillometric device. However, results were similar when using non-calibrated BP values. Further, interarm differences in BP may have affected the current results. Specifically, the ABPM cuff was placed on each participant’s nondominant arm to minimize the effect of daily activities on readings and clinic BP was measured in the right arm. Too few participants had clinic measured BP in the hypertensive range to study the association of white-coat hypertension and renal outcomes. The JHS cohort enrolled African-Americans exclusively and the current results may not be generalizable to other racial/ethnic groups. In addition, serum creatinine levels were measured at only two time points separated by 8 years, limiting the ability to know the actual timing and rate of decline of kidney function.
In summary, the results of the current study indicate that masked hypertension, particularly masked night-time hypertension, may be associated with an increased risk for CKD but not RKFD. Currently, data are not available to indicate whether antihypertensive medication reduces the risk for adverse renal outcomes or CVD among people with masked hypertension. As the prevalence of masked hypertension and CKD is high among African-Americans, identifying modifiable risk factors for, and evaluating treatment of, this condition may help reduce the overall burden of CKD.
Supplementary Material
Acknowledgments
The authors thank the participants and data collection staff of the Jackson Heart Study. The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN26820 1300050C from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD) at the National Health Institute (NIH). This work was also supported by NIH National Institute of Diabetes, Digestive, and Kidney Disease grants R01 DK102134 (B.Y.) and R01HL117323 (P.M.). M.S. receives support through grants P60MD002249 and U54MD008176 from the NIMHD; 15SFDRN26140001 and P50HL120163 from the American Heart Association; and 1R01HL116446 from the NHLBI. P.M. receives support through grant 15SFRN2390002 from the American Heart Association. B.Y. is also supported in part by funding from Veterans Affairs Puget Sound Healthcare System. P.M. received an institutional grant from Amgen Inc. unrelated to the topic of the current article.
Abbreviations
- ABPM
ambulatory blood pressure monitoring
- BP
blood pressure
- CI
confidence interval
- CKD
chronic kidney disease
- OR
odds ratio
- RKFD
rapid kidney function decline
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the US Department of Health and Human Services. The US Department of Veteran Affairs does not endorse any of the statement or opinions advocated by this article.
Presentation: A poster based on this article was presented at the 2016 Annual Scientific Meeting and Exposition, American Society of Hypertension, Inc., 13–17 May 2016; New York, NY.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens. 2014;28:521–528. doi: 10.1038/jhh.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40:795–796. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SJ, Booth JN, 3rd, Bromfield SG, Seals SR, Spruill TM, Ogedegbe G, et al. Clinic and ambulatory blood pressure in a population-based sample of African Americans: the Jackson Heart Study. J Am Soc Hypertens. 2017;11:204–212.e5. doi: 10.1016/j.jash.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, et al. Masked hypertension and elevated nighttime blood pressure in ckd: prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016;11:642–652. doi: 10.2215/CJN.08530815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, et al. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7:1770–1776. doi: 10.2215/CJN.11301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotsis V, Stabouli S, Toumanidis S, Papamichael C, Lekakis J, Germanidis G, et al. Target organ damage in ‘white coat hypertension’ and ‘masked hypertension’. Am J Hypertens. 2008;21:393–399. doi: 10.1038/ajh.2008.15. [DOI] [PubMed] [Google Scholar]
- 8.Tomiyama M, Horio T, Yoshii M, Takiuchi S, Kamide K, Nakamura S, et al. Masked hypertension and target organ damage in treated hypertensive patients. Am J Hypertens. 2006;19:880–886. doi: 10.1016/j.amjhyper.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study) Circulation. 2001;104:1385–1392. doi: 10.1161/hc3701.096100. [DOI] [PubMed] [Google Scholar]
- 10.Hansen TW, Kikuya M, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25:1554–1564. doi: 10.1097/HJH.0b013e3281c49da5. [DOI] [PubMed] [Google Scholar]
- 11.Hanninen MR, Niiranen TJ, Puukka PJ, Kesaniemi YA, Kahonen M, Jula AM. Target organ damage and masked hypertension in the general population: the Finn-Home study. J Hypertens. 2013;31:1136–1143. doi: 10.1097/HJH.0b013e32835fa5dc. [DOI] [PubMed] [Google Scholar]
- 12.Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, et al. Cardiovascular prognosis of ‘masked hypertension’ detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342–1349. doi: 10.1001/jama.291.11.1342. [DOI] [PubMed] [Google Scholar]
- 13.Bjorklund K, Lind L, Zethelius B, Andren B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107:1297–1302. doi: 10.1161/01.cir.0000054622.45012.12. [DOI] [PubMed] [Google Scholar]
- 14.Kanno A, Metoki H, Kikuya M, Terawaki H, Hara A, Hashimoto T, et al. Usefulness of assessing masked and white-coat hypertension by ambulatory blood pressure monitoring for determining prevalent risk of chronic kidney disease: the Ohasama study. Hypertens Res. 2010;33:1192–1198. doi: 10.1038/hr.2010.139. [DOI] [PubMed] [Google Scholar]
- 15.Iimuro S, Imai E, Watanabe T, Nitta K, Akizawa T, Matsuo S, et al. Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol. 2013;8:721–730. doi: 10.2215/CJN.06470612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6-4–S6-17. [PubMed] [Google Scholar]
- 17.Mwasongwe S, Gao Y, Griswold M, Wilson JG, Aviv A, Reiner AP, et al. Leukocyte telomere length and cardiovascular disease in African Americans: the Jackson Heart Study. Atherosclerosis. 2017;266:41–47. doi: 10.1016/j.atherosclerosis.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JG, Rotimi CN, Ekunwe L, Royal CD, Crump ME, Wyatt SB, et al. Study design for genetic analysis in the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6-30–S6-37. [PubMed] [Google Scholar]
- 19.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Dubbert PM, Carithers T, Ainsworth BE, Taylor HA, Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6-56–S6-61. [PubMed] [Google Scholar]
- 21.Young BA, Katz R, Boulware LE, Kestenbaum B, de Boer IH, Wang W, et al. Risk factors for rapid kidney function decline among African Americans: the Jackson Heart Study (JHS) Am J Kidney Dis. 2016;68:229–239. doi: 10.1053/j.ajkd.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickson DA, Diez Roux AV, Wyatt SB, Gebreab SY, Ogedegbe G, Sarpong DF, et al. Socioeconomic position is positively associated with blood pressure dipping among African-American adults: the Jackson Heart Study. Am J Hypertens. 2011;24:1015–1021. doi: 10.1038/ajh.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyatt SB, Akylbekova EL, Wofford MR, Coady SA, Walker ER, Andrew ME, et al. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51:650–656. doi: 10.1161/HYPERTENSIONAHA.107.100081. [DOI] [PubMed] [Google Scholar]
- 24.Booth JN, 3rd, Abdalla M, Tanner RM, Diaz KM, Bromfield SG, Tajeu GS, et al. Cardiovascular health and incident hypertension in Blacks: JHS (The Jackson Heart Study) Hypertension. 2017;70:285–292. doi: 10.1161/HYPERTENSIONAHA.117.09278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdalla M, Booth JN, 3rd, Seals SR, Spruill TM, Viera AJ, Diaz KM, et al. Masked hypertension and incident clinic hypertension among Blacks in the Jackson Heart Study. Hypertension. 2016;68:220–226. doi: 10.1161/HYPERTENSIONAHA.115.06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Li Y, Dolan E, et al. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255–262. doi: 10.1097/mbp.0b013e3280f813bc. [DOI] [PubMed] [Google Scholar]
- 27.Bromfield SG, Booth JN, 3rd, Loop MS, Schwartz JE, Seals SR, Thomas SJ, et al. Evaluating different criteria for defining a complete ambulatory blood pressure monitoring recording: data from the Jackson Heart Study. Blood Press Monit. 2017 doi: 10.1097/MBP.0000000000000309. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Young BA, Fulop T, de Boer IH, Boulware LE, Katz R, et al. Effects of serum creatinine calibration on estimated renal function in African Americans: the Jackson Heart Study. Am J Med Sci. 2015;349:379–384. doi: 10.1097/MAJ.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bash LD, Coresh J, Kottgen A, Parekh RS, Fulop T, Wang Y, et al. Defining incident chronic kidney disease in the research setting: the ARIC Study. Am J Epidemiol. 2009;170:414–424. doi: 10.1093/aje/kwp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiramoto JS, Katz R, Peralta CA, Ix JH, Fried L, Cushman M, et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;60:225–232. doi: 10.1053/j.ajkd.2012.02.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y. Multiple imputation using SAS software. J Stat Soft. 2011;45:1–25. doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Zhang J, Li Y, Ma X, Ye Z, Peng H, et al. Masked hypertension, rather than white-coat hypertension, has a prognostic role in patients with nondialysis chronic kidney disease. Int J Cardiol. 2017;230:33–39. doi: 10.1016/j.ijcard.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:406–411. doi: 10.1038/sj.ki.5000081. [DOI] [PubMed] [Google Scholar]
- 36.Kanno A, Kikuya M, Asayama K, Satoh M, Inoue R, Hosaka M, et al. Night-time blood pressure is associated with the development of chronic kidney disease in a general population: the Ohasama Study. J Hypertens. 2013;31:2410–2417. doi: 10.1097/HJH.0b013e328364dd0f. [DOI] [PubMed] [Google Scholar]
- 37.Ogedegbe G, Agyemang C, Ravenell JE. Masked hypertension: evidence of the need to treat. Curr Hypertens Rep. 2010;12:349–355. doi: 10.1007/s11906-010-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz KM, Veerabhadrappa P, Brown MD, Whited MC, Dubbert PM, Hickson DA. Prevalence, determinants, and clinical significance of masked hypertension in a population-based sample of African Americans: the Jackson Heart Study. Am J Hypertens. 2015;28:900–908. doi: 10.1093/ajh/hpu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogedegbe G. Causal mechanisms of masked hypertension: socio-psychological aspects. Blood Press Monit. 2010;15:90–92. doi: 10.1097/MBP.0b013e3283380df5. [DOI] [PubMed] [Google Scholar]
- 40.Ford CD, Sims M, Higginbotham JC, Crowther MR, Wyatt SB, Musani SK, et al. Psychosocial factors are associated with blood pressure progression among African Americans in the Jackson Heart Study. Am J Hypertens. 2016;29:913–924. doi: 10.1093/ajh/hpw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24:52–58. doi: 10.1038/ajh.2010.203. [DOI] [PubMed] [Google Scholar]
- 42.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 43.Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Polo Friz H, et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension. 2009;54:226–232. doi: 10.1161/HYPERTENSIONAHA.109.129882. [DOI] [PubMed] [Google Scholar]
- 44.Angeli F, Reboldi G, Verdecchia P. Masked hypertension: evaluation, prognosis, and treatment. Am J Hypertens. 2010;23:941–948. doi: 10.1038/ajh.2010.112. [DOI] [PubMed] [Google Scholar]
- 45.Booth JN, 3rd, Diaz KM, Seals SR, Sims M, Ravenell J, Muntner P, et al. Masked hypertension and cardiovascular disease events in a prospective cohort of blacks: the Jackson Heart Study. Hypertension. 2016;68:501–510. doi: 10.1161/HYPERTENSIONAHA.116.07553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bromfield SG, Shimbo D, Booth JN, 3rd, Correa A, Ogedegbe G, Carson AP, et al. Cardiovascular risk factors and masked hypertension: the Jackson Heart Study. Hypertension. 2016;68:1475–1482. doi: 10.1161/HYPERTENSIONAHA.116.08308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


