Abstract
Background
Prostate cancer remains the second leading cause of cancer related death in men. Immune check point blocking antibodies have revolutionized treatment of multiple solid tumors, but results in prostate cancer remain marginal. Previous reports have suggested that local therapies, in particular cryoablation might increase tumor immunogenicity. In this work, we examine potential synergism between tumor cryoabalation and check point blocking antibodies.
Methods
FVB/NJ mice were injected subcutaneously into each flank with either 1 × 106 or 0.2 × 106 isogenic hormone sensitive Myc-Cap cells to establish synchronous grafts. Mice were treated with four intraperitoneal injections of anti-PD-1 (10 mg/kg), anti-CTLA-4 (1 mg/kg), or isotype control antibody with or without adjuvant cryoablation of the larger tumor graft and with or without neo-adjuvant androgen deprivation with degarelix (ADT). Mouse survival and growth rates of tumor grafts were measured. The immune dependency of observed oncological effects was evaluated by T cell depletion experiments.
Results
Treatment with anti-CTLA-4 antibody and cryoablation delayed the growth of the distant tumor by 14.8 days (p = 0.0006) and decreased the mortality rate by factor of 4 (p = 0.0003) when compared to cryoablation alone. This synergy was found to be dependent on CD3+ and CD8+ cells. Combining PD-1 blockade with cryoablation did not show a benefit over use of either treatment alone. Addition of ADT to anti-PD1 therapy and cryoablation doubled the time to accelerated growth in the untreated tumors (p = 0.0021) and extended survival when compared to cryoablation combined with ADT in 25% of the mice. Effects of combining anti-PD1 with ADT and cryoablation on mouse survival were obviated by T cell depletion.
Conclusion
Trimodal therapy consisting of androgen deprivation, cryoablation and PD-1 blockade, as well as the combination of cryoablation and low dose anti-CTLA-4 blockade showed that local therapies with cryoablation could be considered to augment the effects of checkpoint blockade in prostate cancer.
Introduction
Prostate cancer is the second most common cause of cancer related death in males, killing one in every 36 American men [1]. While advances have been made in early detection and local treatments, few breakthroughs have been able to alter the course of disease once it forms subclinical or clinical metastases. In this setting, androgen deprivation therapy is considered frontline, but while many patients will experience initial tumor regression, their prostate cancer inevitably develops a castrate resistant phenotype. In an effort to treat patients in this progressive stage, multiple approaches have been undertaken [2]. These include the development of second and third generation anti-androgens, chemotherapy and testing of pathway targeted small molecules, as well as combing those with standard androgen ablation therapy in hormone naive metastatic disease [3]. The complexity of advanced tumors however, and their ability to adapt resistance often allows for cancer progression and the patient’s ultimate demise [4].
Recent advances in our understanding of, and ability to manipulate the immune system have resulted in a sea change in the treatment of aggressive malignancies such as melanoma, lung cancer, bladder cancer, and renal cell carcinoma. At the forefront of emerging immunotherapies have been checkpoint blockers targeting CTLA-4 and PD-1 [5]. CTLA-4 normally functions to bind CD28’s ligand B7, and does so with higher affinity than CD28, thus terminating the immune response by preventing continued T-cell activation. PD-1 binding to its ligands (PD-L1 and PD-L2 which can be expressed on antigen presenting cells and on the tumor, itself) down-regulates signaling from the T-cell receptor and results in T-cell anergy and apoptosis, thus limiting the immune response. As opposed to CTLA-4 which acts primarily at the level of lymphoid organs, PD-1 is considered to exert its effects in the tumor micro-environment [6].
Immunotherapy through checkpoint blockade has begun to be explored in metastatic prostate cancer but with modest efficacy [7–10]. Release of tumor antigens and recruitment of an inflammatory response in prostate cancer might be augmented by drug induced mutagenesis of prostate tumors, tumor vaccines, androgen deprivation or local ablative therapies [11–15]. This last mechanism, known as the abscopal effect, has been demonstrated clinically in anecdotal reports but has not been systematically studied [16].
Previous work has demonstrated that among ablative therapies, cryotherapy causes a particularly robust immune response. For example, Waltz et al. demonstrated in an immune re-challenge experiment with an androgen independent mouse model of prostate cancer that cryoablation of tumors synergizes with high dose (10 mg/kg) anti CTLA-4 antibody treatment [15]. Cryotherapy has also been reported to elicit distant anti-tumor effects in case reports of human malignancies including prostate cancer [17, 18]. Here we explore the effect of local tumor ablation with cryotherapy on distant tumor grafts and potential synergism with checkpoint blockade.
Materials and methods
Mice and tumor cells
All experimental procedures were approved by the Johns Hopkins Institutional Animal Care and Use Committee (IACUC). Immune –competent 7 to 11 weeks old male FVB/NJ mice (Jackson Laboratories, Bar Harbor, ME, USA) were used in all experiments. Two synchronous grafts were formed by subcutaneous (s.c.) injection of 1·106 and 0.2·106 Myc-Cap cells into the right and left flank, respectively (cells were obtained by courtesy of Dr. Simons) [19].
Tumor growth and survival measurements
Tumor grafts were measured with calipers every two to three days starting on the 14th day after cells injection, graft volume was calculated by formula , with x denoting the longer diameter and y denoting shorter diameter of tumor graft. Mice were sacrificed when they became moribund or if tumor size reached 2000 mm3.
Cryoablation
In experimental groups where cryoablation was employed, it was performed on the larger graft only and was performed with the intent to treat the complete tumor area of that graft. Grafts were ablated two days after checkpoint inhibitor (or isotype control) treatment initiation. In the case of mice receiving androgen deprivation therapy, tumors were ablated 6 days after ADT initiation. Cryoablation was carried out using Cryocare machine (HeathTronics, Austin, TX, USA). Mice were anesthetized by 2.5% isoflurane gas anesthesia. Once anesthetized, skin overlaying the larger tumor graft was disinfected with alcohol pads and a single 8 mm cryoablation probe was inserted. In all cases, 2 freeze-thaw cycles were delivered with temperatures at the probe tip reaching a minimum of negative 40 °C for at least 30 s. Mice were provided all necessary post procedural care.
Immune check point blocking and androgen deprivation therapy
InVivoMAb Anti-mouse PD-1 (10 mg/kg, clone J43, Bioxcell, West Lebanon, NH, USA), InVivoMAb anti-mouse CTLA-4 antibody (1 mg/kg, clone 9H10, Bioxcell), or InVivoMAb hamster anti-mouse IgG control antibodies (clone N/A, catalog # BE 0091, Bioxcell) were administered every other day for a total of four doses by intraperitoneal (i.p.) injection. The first dose was administered upon the larger tumors reaching 100 to 200 mm3, except for mice allocated to groups receiving androgen deprivation therapy in which case first dose was given 4 days after initiation of androgen deprivation therapy. When employed, androgen deprivation was induced by degarelix (Ferring Pharmaceuticals, Saint Prex, Switzerland), administered in single s.c. dose of 25 mg/kg upon the larger tumor graft reaching a volume of 300–400 mm3.
Immuno-depletion
Depletion of all CD3+ lymphocytes, or CD8+ lymphocytes was performed as described in Belcaid et al [20]. Briefly, we utilized i.p. injection of InVivoMAb anti-mouse CD3 (clone 2.43, Bioxcell) and InVivoMAb anti-mouse CD8 (clone 145-2C11, Bioxcell) antibodies at doses of 100 μg and 200 μg, respectively. Induction of depletion was performed by using six doses of antibodies given every second day. Following the sixth dose, depletion was maintained by administering one dose of depleting antibody per week. The first dose of depleting antibody was given 5 days before larger tumors reached 100 to 200 mm3 or 300 to 400 mm3 in case of group treated with androgen deprivation therapy. Confirmation of depletion was obtained by flow cytometric analysis of blood after the second dose of antibodies was administered.
Flow cytometry
Two weeks after cryotherapy or the second dose of check point blocking antibody, tumors, spleens, and tumor draining lymph nodes were dissected from the mice. In order to obtain tumor infiltrating lymphocytes tumors were mechanically disassociated and digested for 1 h at 37 °C in RPMI media with 10 mg/ml of Collagenase IV and 1 mg/ml of DNase. After digestion, white blood cells were isolated from tumors by using anti-CD45 antibodies conjugated with magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer’s instructions. Lymph nodes and spleens were disrupted mechanically, washed and suspended as single cell solution in media. Tumors, spleens and lymph nodes from 2 mice, from the same group, were pooled in one sample and all experiments were conducted utilizing at least 6 mice.
In order to stain for intracellular cytokines, lymphocytes were stimulated for 5 h with PMA (100 ng/ml), ionomycin (500 ng/ml), and Protein transport inhibitor cocktail (eBioscience, San Diego, CA, USA) at 37 °C. Cells were permeabilized and fixed with Intracellular Fixation and Permeabilization Buffer Set (eBioscience, San Diego, CA, USA). Data were acquired on FACS Aria II machine (BD Biosciences, San José, CA, USA). Staining was performed by using antibodies against CD4 (clone H129.19, BD Biosciences), CD8 (clone 53-6.7, BD Biosciences), IFNγ (clone XMG 1.2, eBioscience), TNFα (clone MP6-XT22, eBioscience), IL-2 (clone JES6-5H4, eBioscience), FoxP3 (clone FJK-16s, eBioscience). Staining for immune checkpoint molecules was done on a separate panel with antibodies to CD4 (clone H129.19, BD Biosciences), CD8 (clone 53-6.7, BD Biosciences), PD-1 (clone J43, eBioscience), Tim-3 (clone RMT3-23, eBioscience) and Lag-3 (clone C9B7W, eBioscience). Gating controls were prepared with Fluorescence Minus One (FMO) stains of spleenocytes. All data were analyzed with FlowJo software (TreestarInc, Ashland, OR, USA).
Statistical analysis
Statistical differences in Kaplan Meier survival curves were evaluated with log rank testing and hazard ratio estimation. Tumor growth curves were analyzed by fitting segmental linear (broken stick) model with two segments [21, 22]. Briefly, time to accelerated or fast growth is a model parameter defined as the “changepoint” or “breakpoint” and is the time at which tumor started to grow at a faster average rate. Pairwise Mann Whitney test was used to test differences in medians and F- test was used to compare standard deviations, for comparisons of means pairwise t test was used. Significance of linear trend between groups was evaluated with linear trend test. P values less than 0.05 were considered significant, all P values were two sided. Sample size was estimated by using Mead’s resource equation. Allocation was done by using complete randomization. All statistical analysis was done in Grapad Prism 6.07 software (GraphPad Software, La Jolla, CA, USA).
Establishing a mouse model to evaluate the abscopal effect
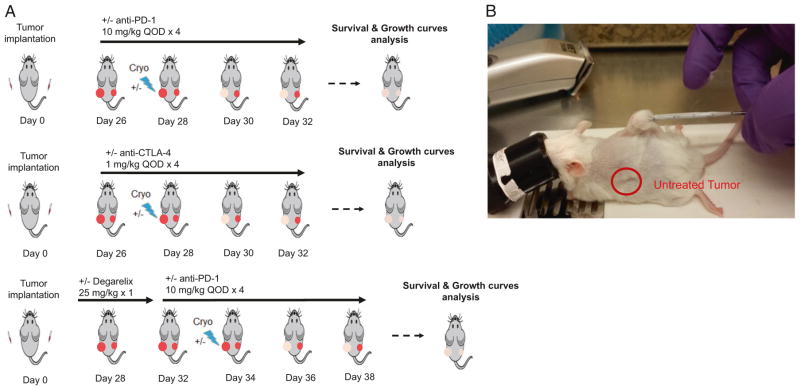
The Myc-CaP cell line was derived from primary prostate cancer developed in a Myc over-expressing transgenic mouse model and is isogenic on the FVB mouse background [19]. To establish a simple model with which to test for the abscopal effect, we subcutaneously injected both flanks of immune competent mice (Fig. 1). One flank was implanted with 1 million cells while other was implanted with 200,000 cells. Upon the larger tumor reaching a volume of 100 to 400 mm3 (approximately 3 to 4 weeks after injection), mice were allocated into 3 different treatment arms (Fig. 1a). Mice allocated to first arm received either anti-PD-1 antibody or isotype control, with or without cryoablation of larger tumor (Fig. 1b). The second experimental group was designed identically as the first but employing an anti-CTLA-4 antibody instead of anti-PD-1 antibody. In the third arm experimental group, a single dose of androgen deprivation therapy with degarelix was added to the treatment regimen of the first arm. In experimental groups where cryoablation was employed, this model would allow for straightforward assessment of growth of the distant untreated graft.
Fig. 1.
Scheme of study design. a Three different treatment regimens were studied. FVB/N mice were injected in each flank with 1 million and 200,000 isogenic Myc-CaP cells. Mice in the first treatment regimen received either anti-PD-1 antibody or isotype control, with or without cryoablation of larger tumor graft, in second arm synergism between anti-CTLA-4 therapy and cryoablation was tested, in third arm effect of neoadjuvant single dose androgen ablation therapy on anti-PD-1 and cryoablation was examined. Effects of treatment were measured in terms of mice survival and tumor growth. b Procedure of larger tumor graft cryoablation
Results
Cryoablation delays distant tumor growth but does not synergize with PD-1 blockade
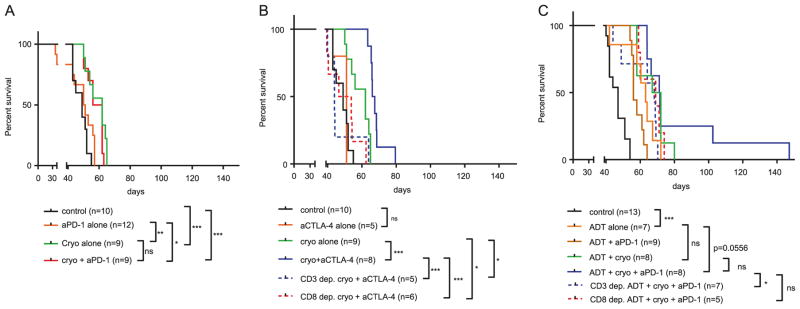
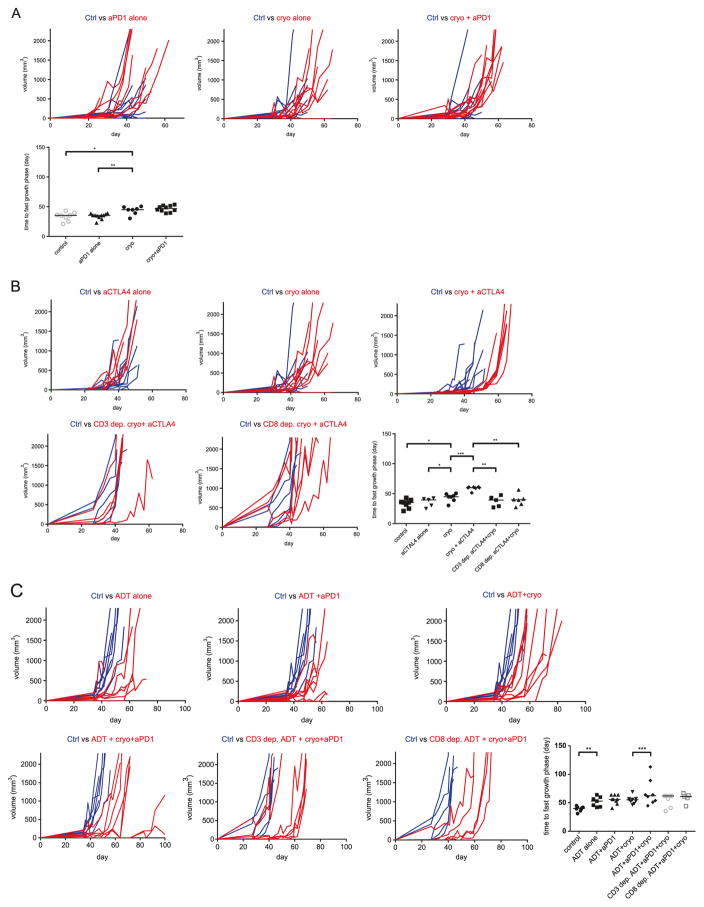
Cryoablation of the larger tumor graft delayed onset of accelerated growth of the untreated tumor graft (median increase 9.83 days, 95% CI 3.2–18.81, p = 0.0205) and caused almost 8-fold decrease in mortality rates (HR = 0.126, 95% CI 0.0372–0.427, p = 0.0009) when compared to an untreated control group (Figs. 2a and 3a). Anti- PD-1 monotherapy did not show any effect in terms of delaying tumor growth nor did it improve survival when compared to control group. Combining PD-1 blockade with cryoablation did not show a synergistic effect when compared to cryoablation therapy alone (Fig. 2a).
Fig. 2.
Kaplan Meier curves for mice from all therapeutic groups. a Anti-PD-1 treatment did not synergize with cryoablation. b Low dose anti-CTLA-4 synergized with cryoablation in a CD3 and CD8 dependent fashion. c Combination of ADT and anti-PD-1 synergized with cryoablation in a CD3 and CD8 dependent fashion. Differences between mortality curves were tested by log rank test. Each treatment regimen was done with at least one experimental replicate. ns-p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 3.
Growth curves of smaller tumor grafts. a Anti-PD1 and cryoablation combination did not delay onset of tumor accelerated growth more than cryoablation monotherapy. b Low dose anti-CTLA-4 and cryoablation synergized and significantly delayed onset of accelerated tumor growth, in both CD3 and CD8 dependent manner, compared to cryoablation and anti-CTLA-4 monotherapies. c Combination of ADT and anti-PD-1 synergized with cryoablation and delayed onset of tumor fast growth phase in 25% (2/8) of mice in a CD3 and CD8 dependent fashion when compared to the ADT and cryoablation combination. Statistical comparisons in terms of medians were done by using MW test, F test was used to compare standard deviations of groups. Each treatment regimen was done with at least one experimental replicate. ns-p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001
Cryoablation synergizes with low dose anti-CTLA-4 treatment in a T cells dependent fashion
We next tested whether anti-CTLA-4 therapy might synergize with cryoablation to cause an abscopal effect. In a pilot experiment, we found that anti-CTLA-4 administered in concentrations of 10 mg/kg caused a delay in tumor growth and improvement in mouse survival (data not shown). Treatment with anti-CTLA-4 at doses of 1 mg/kg however, did not improve survival nor did it delay growth of tumor grafts (Figs. 2b and 3b). Cryoablation appeared to synergize with low dose (1 mg/kg) anti-CTLA-4, delaying the onset of accelerated growth in remaining graft for 14.7 days (95% CI 9.98–21.58, p = 0.0006) and decreasing mortality rate by factor of 4 (HR = 0.25, 95% CI 0.0782–0.784, p = 0.0003) when compared to cryoablation alone (Figs. 2b and 3b). Depletion of CD3+ or CD8+ cells in mice prior to treatment completely neutralized the beneficial effects of cryoablation and anti-CTLA-4 combinatorial therapy, with mortality rates being comparable to control mice (Fig. 2b). Depletion experiments also demonstrated that mice depleted of CD3+ or CD8+ cells and treated with combination of cryoablation and anti-CTLA-4 had significantly lower survival rates when compared to mice treated with cryoablation alone (p = 0.0251, p = 0.0480, respectively).
Androgen ablation therapy and anti-PD-1 treatment synergize with cryoablation in a T cells dependent fashion in a subset of mice
Androgen deprivation therapy (ADT) has been previously shown to correlate with an increased prostate cancer tumor lymphocytic infiltrate and to mitigate tolerance to prostate cancer antigens [14, 23–25]. We tested whether addition of neoadjuvant ADT could synergize with anti-PD-1 based treatment to improve oncologic efficacy (Figs. 2c, 3c). As expected ADT alone postponed the onset of fast growth phase for 12.6 days (95% CI 2.57–21.91, p = 0.0022) and caused 7.5-fold reduction in mortality rate (HR = 0.131, 95% CI 0.041–0.42, p = 0.0006). Combining ADT with anti-PD-1 therapy did not show additional beneficial effects when compared to ADT monotherapy. Adding ADT to cryoablation resulted in non-significant survival benefit (HR = 0.56, 95% CI 0.19–1.64, p = 0.1720) and also non-significant tumor growth delay of 2.82 days (95% CI-7 to 12.43, p = 0.5360) in comparison treatment with ADT alone (Fig. 2c). Trimodal therapy consisting of ADT, PD-1 blockade and cryoablation almost doubled the delay of tumor growth (p = 0.0021) and survival in 25% (2/8) of treated mice when compared to bi-modal therapy with ADT and cryoablation combination, however survival differences in these two experimental groups were not statistically different when all mice in each experimental group were considered (HR = 0.798, 95% CI 0.297–2.142, p = 0.6049) (Fig. 2c). In order to study the nature of survival prolongation in mice treated by trimodal therapy we depleted mice of CD3+ or CD8+ cells and treated them with ADT, anti-PD-1, and cryoablation combination therapy. In these immune-depleted mice, no mice experienced long term treatment response. Mice depleted of CD3+ cells demonstrated significantly lower survival when compared to non-depleted mice treated with identical therapy (HR = 4.015, 95% CI 1.098–14.68, p = 0.0356), however survival difference between mice depleted of CD8+ cells and non-depleted mice failed to reach statistical significance (HR = 2.202, 95% CI 0.5937– 8.165, p = 0.2378).
Characterization of immune response
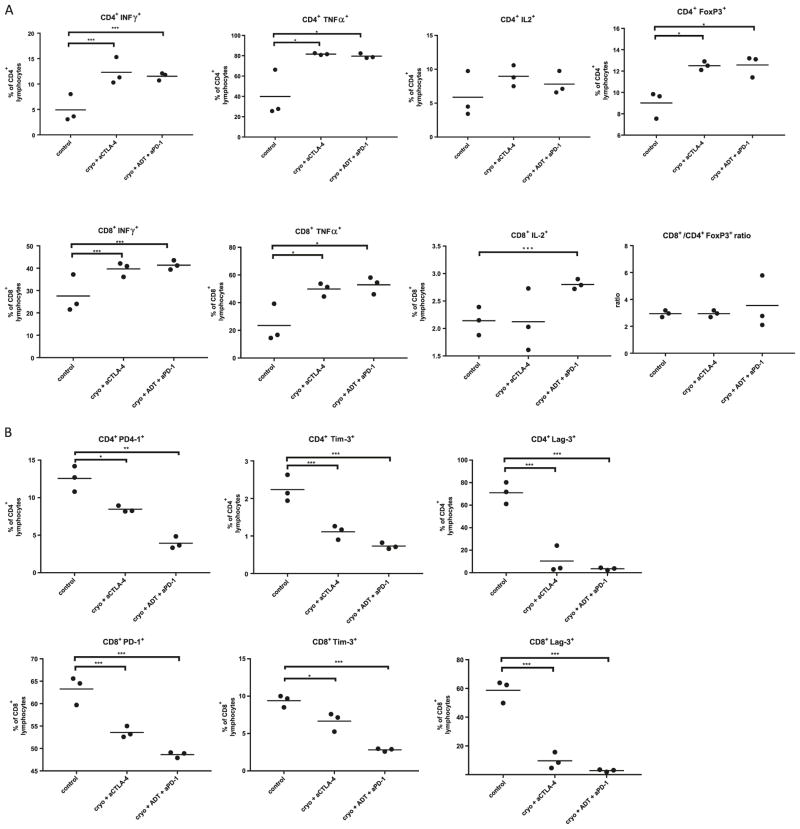
Systemic, regional and local immune responses could be crucial for the development of anti-cancer immunity [26]. Accordingly, we performed flow cytometric analyses on the spleen, tumor draining lymph node of the distant, untreated tumor graft, and lymphocytes within the distant untreated tumor graft. Tissues were harvested 2 weeks after tumor cryoablation utilizing experimental groups that showed the most robust treatment effects. We examined activation of CD4+ and CD8+ cells in the spleen by examining secretion of IFNγ, TNFα and IL-2 (Fig. 4a, Supplementary Table 1). Mice treated with trimodal therapy showed an average increase of 13.8% (95% CI 7.2–22.83, p = 0.05) and 6.61% (95% CI 4.5–9.63, p = 0.02) in CD8+IFNγ+ and CD4+IFNγ+ populations, respectively, when compared to control mice. Increased TNFα secretion was also noted in both CD4+ and CD8+ cells. Consistent with trimodal therapy, bimodal therapy with cryoablation and anti-CTLA-4 also caused an increase in TNFα+ and IFNγ+ populations. T regulatory cells (CD4+FoxP3+) were increased in bimodal and trimodal therapies by 3.49% (95% CI 2.03–4.45, p = 0.01) and 3.5% (95% CI 2.13–4.92, p = 0.02), respectively, when compared to control. Interestingly, this change did not reflect an alteration in the CD8/Treg ratio, which was equal in all experimental groups (Fig. 4a, Supplementary Table 1). Next, we studied expression of immune checkpoint molecules in splenic lymphocytes (Fig. 4b, Supplementary Table 1). Both bimodal and trimodal therapies demonstrated a decreased expression of PD-1 on CD8+ lymphocytes by 9.7% (95% CI 6.9–13.03, p = 0.008) and 14.63% (95% CI 12.26–18.13, p = 0.001), respectively. Similar changes were noted also on CD4+ cells. Expression of Lag-3 and Tim-3 on CD8+ and CD4+ cells was also significantly reduced in both therapeutic groups when compared to control.
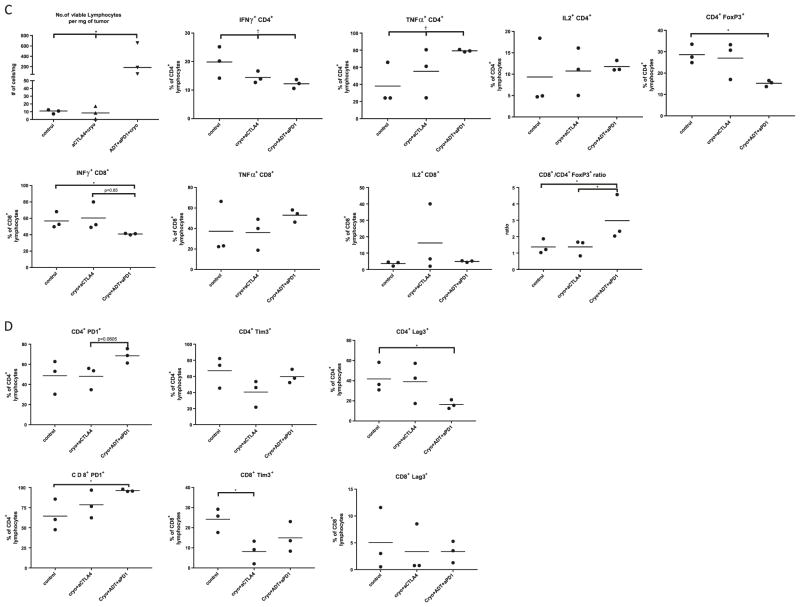
Fig. 4.
Characterization of immune response in most effective treatment groups. a Cytokine profile of CD4+ and CD8+ lymphocytes in spleen. b Expression of immune check point molecules on CD4+ and CD8+ lymphocytes in spleen. c Cytokine profile of CD4+ and CD8+ tumor infiltrating lymphocytes. d Expression of immune checkpoint molecules on tumor infiltrating lymphocytes. Data are presented as scatter plots with mean, each data point represents a sample made by combining 2 mice from same treatment group, means were compared by t test, significance of linear trend between means and treatment groups was tested by test for linear trend. *p < 0.05,**p < 0.01, ***p < 0.001 for t test. †p < 0.05, ††p < 0.01, †††p < 0.001, for test for linear trend
Changes in remaining tumor graft draining lymph node in general paralleled those in spleen, however differences were smaller and not statistically significant with the exception of a reduction of expression of Lag-3 and Tim-3 on CD8+ and CD4+ cells when compared to controls. (Supplementary Fig. 1A, B, Supplementary Table 2). Unlike TNFα+ CD4+ cells which increased with therapeutic efficacy, CD8+/Treg ratio decreased by the average of 0.9 (95% CI −1.7 to −0.17, p = 0.0484) per therapeutic group (Supplementary Fig. 1A).
In trimodal therapy treated animals, the tumor graft had a 19-fold higher (95% CI 5.88–58, p = 0.0001) lymphocyte infiltrate per mg of tumor when compared to control (Fig. 4c, Supplementary Table 3). Among CD4+ cells, treatment groups showed an increase in TNFα secretion with and a decrease in IFNγ secretion. The trimodal therapy group had a 13.9% (95% CI 8.8–17.2, p = 0.0253) decrease in Treg cells when compared to control mice and had a 2.1 (95% CI 1.28–3.34, p = 0.0143) increase in CD8/Treg ratio in comparison to control and bimodal therapy groups. Analyses of immune checkpoint expression (Fig. 4d, Supplementary Table 3) following trimodal therapy demonstrated a 25% (95 CI% 9.97–37.47, p = 0.04) decrease in Lag-3+ CD4+ cells when compared to control mice. The only significant difference between cryoablation and anti-CTLA-4 treated mice compared to control mice was a 16% (95% CI 8.44–23.65, p = 0.03) decrease in Tim-3+ CD8+ cells.
Discussion
Developing novel approaches for the treatment of advanced prostate cancer is of paramount importance. The combination of local therapies and immune-checkpoint blockade could potentially increase the efficacy of treatment at distant tumor sites. Here we utilized a straightforward mouse model with which to assess potential abscopal effects in prostate cancer. We further demonstrate a possible T cell dependent synergy between cryoablation and checkpoint blockade in eliciting distant tumor responses. We find that oncological responses were uniform and not dependent on additional therapy when cryoablation was combined with anti-CTLA-4 therapy, and only effected a subset of the population and required neoadjuvant hormonal deprivation when cryoablation was combined with PD-1.
Previous animal models in which immune therapy of prostate cancer was assessed utilized an immune re-challenge design, and models that were not representative of prostate adenocarcinoma or tumor models engineered to have dominant artificial antigen [15, 27, 28]. Synchronous tumor model such as ours have been tested in stetting of studying abscopal effect in breast cancer [29]. Although we used cryoablation as a local ablative therapy we believe that other local ablative therapies such as external beam radiation or heat ablation by high frequency ultrasound might also harbor a potential for eliciting an abscopal effect [29].
Anti-CTLA-4 and anti-PD1 or PDL1 treatments represent the most heavily studied checkpoint directed immunotherapies [5, 6]. It is important to note that these immune checkpoints are functionally and spatially distinct [6]. The CTLA-4/B7 checkpoint functions at secondary lymphoid organs and regulates the amplitude of early activation of naïve T cells during the immune response. This is in contrast to the PD-1/PDL1/2 checkpoint, which operates in the tissue micro-environment and limits the activity of T cells in peripheral organs. These differences not only influence the efficacy of treatment with checkpoint blockade, but also influence differences in the toxicity profiles of these molecules (with CTLA-4 having a less favorable toxicity profile) [30]. The mechanistic differences of these therapies may explain our findings.
In mice treated with low-dose anti-CTLA-4 therapy, we found a uniform synergy of checkpoint blockade and cryotherapy. This result was abrogated by T cell depletion. In addition, T cell depleted mice treated with cryo-ablation and anti-CTLA4 therapy demonstrated survival and non-cryoablated tumor growths that were comparable to completely untreated control mice, suggesting a possible immunomodulatory role of cryoablation (as opposed to a result of simple decreased tumor burden). Combination of low dose anti-CTLA-4 and cryoablation was accompanied by increased activation of splenic T cells along with lower expression of other immune checkpoint molecules. However, the local tumor immune response was comparable to control mice regarding to the number of tumor infiltrating lymphocytes (TILs), the CD8+ cell cytokine profiles, and the CD8 +/Treg ratio. Despite, these mice had the lowest expression of Tim-3 on CD8+ cells. Since flow cytometry analyses were performed at a single time point, further characterization of tumor infiltrating lymphocytes at additional timepoints might yield valuable insights into the intratumoral immune response.
In mice treated with anti-PD1 therapy, ADT was required to see an oncological benefit, and as stated, only a quarter of mice had prolonged survival or delayed untreated tumor growth. ADT has been previously shown to cause a T cell infiltrate and to modulate the immune response in both anti and pro-inflammatory sense [14, 23–25, 31, 32]. Consistent with this, neoadjuvant ADT followed by cryoablation and PD-1 blockade increased systemic T cell activation in the spleen and markedly increased tumor infiltrating lymphocytes with favorable profiles including CD4+ TNFα secretion and the CD8+/Treg ratio when compared to control and low dose anti-CTLA-4 based bi-modal therapy. While degarelix, a GnRH antagonist, has not been widely investigated in the context of immunotherapy, our data and the work of others [31] support that it functions similarly to GnRH agonists in immune based treatment regimes. Since the oncologic benefit occurred in only a fraction of the treated mice, we hypothesize that neoadjuvant degarelix treatment may have acted as an inconsistently efficacious priming agent for the T cell dependent responses seen. Regardless, encouraged by the findings in this study, we have developed an ongoing phase II clinical trial of cryoablation combined with anti-PD-1 therapy and androgen deprivation in men with oligometastatic hormone naïve prostate cancer (NCT02489357).
There are many limitations to our study and areas which will benefit from further investigation. Among these is that we only studied complete tumor ablation as a method to elicit a distant immune response and were not able to investigate the immune response within the ablated graft or distant graft at multiple time points. It is possible that methodologies employing partial ablation of tumors might have resulted in a greater immune-oncological effect in distant tumors [33]. Further, examination of tumor infiltrating lymphocytes at multiple time points might have provided more insight into the nature of the immune mediated effect. In addition, we employed treatment regimens with check point blockade administered both before, during and after treatment with the intent of mitigating pre-existing immune suppression, boosting T cell activation upon antigen presentation and then allowing for T cell activation and expansion [34, 35]. It is possible that treatment with checkpoint blockade at all of these time points is not necessary or, conversely, that longer duration of checkpoint blockade would have allowed for a more complete response in experimental arms treated with anti-PD1 based therapy.
Results from immunotherapy trials in prostate cancer have not been as favorable as those seen in other malignancies, suggesting that effective immunotherapy in prostate cancer will likely require a multi-modal approach. Use of local therapies may be able to boost an anti-neoplastic immune response in prostate cancer, but may require additional adjuncts to treatment, particularly when using anti-PD1 based therapeutics. As optimization of combinatorial approaches to immunotherapy require study not only of which therapies best synergize but also their optimal dosing and timing of administration [34, 35], immunocompetent animal models will be critical for the better design and outcomes of human trials.
Supplementary Material
Acknowledgments
AER and this work, was supported by Prostate Cancer Foundation Young Investigator Award. AER is supported by a DOD PRTA W81XWH-13- 1-0445 grant. Cryoablation probes were a generous gift from HeathTronics. We also thank to Mr. Robert Lee Blosser for his expert help with flow cytometry.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41391-018-0035-z) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest AER has served as an advisor and speaker for HeathTronics. AER and CGD are the principal and co-principal investigators of NCT02489357 who studied the combination of anti-PD-1 therapy, androgen deprivation, and cryoablation in oligometastatic newly diagnosed prostate cancer. AER and this work, was supported by Prostate Cancer Foundation Young Investigator Award. AER is supported by a DOD PRTA W81XWH-13- 1-0445 grant. AER has served as an advisor and speaker for Healthtronics. Cryoablation probes were a generous gift from Healthronics. The remaining authors declare that they have no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Antonarakis ES, Armstrong AJ. Emerging therapeutic approaches in the management of metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2011;14:206–18. doi: 10.1038/pcan.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;337:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small EJ, Sacks N, Nemunaitis J, Urba WJ, Dula E, Centeno AS, et al. Granulocyte macrophage colony-stimulating factor--secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–91. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 8.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–17. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 11.Franzese O, Torino F, Fuggetta MP, Aquino A, Roselli M, Bonmassar E, et al. Tumor immunotherapy: drug-induced neoantigens (xenogenization) and immune checkpoint inhibitors. Oncotarget. 2017;8:41641–69. doi: 10.18632/oncotarget.16335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleponis J, Skelton R, Zheng L. Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol Med. 2015;12:201–8. doi: 10.7497/j.issn.2095-3941.2015.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6:234–42. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–9. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ablin RJ, Soanes WA, Gonder MJ. Elution of in vivo bound antiprostatic epithelial antibodies following multiple cryotherapy of carcinoma of prostate. Urology. 1973;2:276–9. doi: 10.1016/0090-4295(73)90463-9. [DOI] [PubMed] [Google Scholar]
- 18.Soanes WA, Ablin RJ, Gonder MJ. Remission of metastatic lesions following cryosurgery in prostatic cancer: immunologic considerations. J Urol. 1970;104:154–9. doi: 10.1016/s0022-5347(17)61690-2. [DOI] [PubMed] [Google Scholar]
- 19.Watson PA, Ellwood-Yen K, King JC, Wongvipat J, Lebeau MM, Sawyers CL. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res. 2005;65:11565–71. doi: 10.1158/0008-5472.CAN-05-3441. [DOI] [PubMed] [Google Scholar]
- 20.Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkie KP, Hahnfeldt P. Mathematical models of immune-induced cancer dormancy and the emergence of immune evasion. Interface Focus. 2013;3:20130010. doi: 10.1098/rsfs.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone H. Approximation of curves by line segments. Math Comput. 1961;15:40–7. [Google Scholar]
- 23.Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;71:496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorrentino C, Musiani P, Pompa P, Cipollone G, Di Carlo E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res. 2011;17:1571–81. doi: 10.1158/1078-0432.CCR-10-2804. [DOI] [PubMed] [Google Scholar]
- 25.Tang S, Moore ML, Grayson JM, Dubey P. Increased CD8+T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012;72:1975–85. doi: 10.1158/0008-5472.CAN-11-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell. 2017;168:487–502. e415. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klyushnenkova EN, Riabov VB, Kouiavskaia DV, Wietsma A, Zhan M, Alexander RB. Breaking immune tolerance by targeting CD25+regulatory T cells is essential for the anti-tumor effect of the CTLA-4 blockade in an HLA-DR transgenic mouse model of prostate cancer. Prostate. 2014;74:1423–32. doi: 10.1002/pros.22858. [DOI] [PubMed] [Google Scholar]
- 28.Li F, Guo Z, Yu H, Zhang X, Si T, Liu C, et al. Anti-tumor immunological response induced by cryoablation and anti-CTLA-4 antibody in an in vivo RM-1 cell prostate cancer murine model. Neoplasma. 2014;61:659–71. doi: 10.4149/neo_2014_081. [DOI] [PubMed] [Google Scholar]
- 29.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–9. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pu Y, Xu M, Liang Y, Yang K, Guo Y, Yang X, et al. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumor relapse. Sci Transl Med. 2016;8:333ra347. doi: 10.1126/scitranslmed.aad5659. [DOI] [PubMed] [Google Scholar]
- 32.Kalina JL, Neilson DS, Comber AP, Rauw JM, Alexander AS, Vergidis J, et al. Immune modulation by androgen deprivation and radiation therapy: implications for prostate cancer immunotherapy. Cancer. 2017;9:13. doi: 10.3390/cancers9020013. https://doi.org/10.3390/cancers9020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 35.Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016;11:e0157164. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.