Abstract
The vascular hypothesis of Alzheimer’s disease (AD) considers cerebral hypoperfusion as a primary trigger for neuronal dysfunction. We have previously reported that red blood cells (RBCs) bind amyloid, which are the characteristic deposits found in AD brains, and interact with amyloid on the vasculature [1–3]. Oxidative stress triggered by these RBC/amyloid interactions could impair oxygen delivery. Recent literature has implicated copper bound amyloid-β peptide (CuAβ) and the associated production of reactive oxygen species (ROS) as one of the primary factors contributing to AD pathology. In this work, we have investigated CuAβ generated RBC oxidative stress. Aβ1–40 peptide with a stoichiometric amount of copper bound was produced and compared to the metal-free form of the peptide. Different aggregation states of the peptides were isolated and incubated with RBCs for 15 h. Interestingly, CuAβ stimulated a pronounced increase in red cell oxidative stress as indicated by increased hemoglobin (Hb) oxidation, increased formation of fluorescent heme degradation products, and a decrease in RBC deformability. These findings demonstrate a potential role for CuAβ in promoting vascular oxidative stress leading to impaired cerebral oxygen delivery, which may contribute to neurodegeneration associated with AD.
Keywords: Copper–amyloid, Deformability, Heme degradation, Hemoglobin, Oxidative stress
1 Introduction
Alzheimer’s disease (AD) is a progressive degenerative disorder of the central nervous system that ultimately results in the loss of cognitive function. At present, 5.1 million Americans have been diagnosed with AD. One of the hallmarks of AD is the existence of amyloid plaques, which are composed primarily of Aβ peptide in a fibrillar conformation, within the postmortem brain of disease victims. Even though these plaques reflect neuronal loss, it has not been established that these plaques cause AD pathology. In fact, the decline of cognitive functions actually precedes plaque formation. Therefore, the relationship between amyloid plaques and AD pathology is not established and needs to be further studied.
Several competing hypotheses have been proposed to explain AD pathology. For example, toxicity has been ascribed to the insoluble amyloid plaques [4]. Conversely, the lethal form of Aβ has been suggested as an intermediate species referred to as proto fibrils (or oligomers) that form along the aggregation pathway [5, 6]. Additionally, copper dyshomeostasis leading to ROS generation via Fenton-like chemistry has been suggested as the primary cause of AD [7, 8]. Finally, there is a suggestion that disease onset occurs within the vasculature, eventually leading to a breach of the blood–brain barrier [9, 10]. A combination of these hypotheses inspired the study described herein.
Vascular damage due to Aβ or CuAβ toxicity would lead to inefficient oxygen delivery resulting in brain hypoperfusion, followed by mild cognitive impairment and eventually neurodegeneration. In support of this, AD subjects commonly suffer from vascular inflammation and 95% have cerebral amyloid angiopathy (CAA), which is described as a vascular lesion made up of Aβ deposits on the blood vessels [11]. These deposits promote degeneration of the vessel wall, eventually causing cerebral microbleeds, which could be a source of metal dyshomeostasis [9]. Interestingly, it has recently been shown that vascular lesions from subjects with both AD and CAA had elevated levels of copper and Aβ [11].
Since blood flowing through the capillaries of AD subjects constantly encounters Aβ deposits found on the vasculature, we are interested in determining whether or not the red cell contributes to AD pathology. In this study, RBC oxidative stress following incubation with Aβ and CuAβ was measured by (a) determining Hb oxidation or metHb within the hemolysate, (b) measuring the level of fluorescent heme degradation products, and (c) evaluating the ability of the cell to deform. Hb oxidation and the presence of elevated levels of heme degradation products reflect a pool of un-neutralized ROS within the red cell. Impaired deformability suggests oxidative damage to the cytoskeleton.
2 Methods
2.1 Sample Preparation
A 250 µM solution of Aβ1–40 peptide (BioSource) was prepared by dissolution of the lyophilized powder in Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+ and Mg2+. Aβ1–40 was used for this study because it is the dominant peptide fragment found in cerebrovascular plaques. The metallated form of Aβ1–40 was prepared by adding a stoichiometric amount of CuIISO4 (Sigma Aldrich). All samples were then incubated at 37°C while rotating for 0, 6, 12, 24, or 72 h. Harvesting different peptide conformers at various time points allows different intermediate aggregation states of Aβ and CuAβ to be examined. Peptide conformers were stored at −80°C.
RBCs were washed twice with 20-fold DPBS and centrifuged (~1,125 × g, 4°C, 10 min) for removal of plasma and buffy coat. This was repeated two times before final dilution to 50% HCT in DPBS. Each amyloid conformer (20 µM) was added to a freshly washed sample of RBCs. The samples were then gently vortexed to ensure proper mixing and incubated at 37°C for 15 h before final sample analysis. For all experiments, the control sample consists of washed RBCs without added amyloid, incubated at 37°C for 15 h. In addition, RBCs incubated with 20 µM CuIISO4 were examined to confirm that the observed oxidative stress was not from free metal. Since the results following addition of copper were very similar to the control, this data was averaged with the RBC control data. Each measurement was repeated two to three times and the mean value was used for sample analysis. The results are an average of six experiments, n = 6.
2.2 Sample Analysis
The percentage of oxyHb (λmax, 577 nm; ε, 15.4 M−1 cm−1) and metHb (λmax, 630 nm; ε, 3.7 M−1 cm−1) within the hemolysate was determined using a Perkin Elmer Lambda 35 UV–visible spectrometer. The amount of heme degradation was determined by measuring the fluorescence intensity of a 50 µM sample of total Hb (oxyHb + metHb) from hemolyzed RBCs using a Perkin Elmer LS 50B spectrofluorometer (λex, 321 nm; slit widths, 10 nm). The fluorescence intensity at 465 nm was used to determine the extent of heme degradation.
RBC deformability was measured using a microfluidic RheoScan-D slit-flow ektacytometer (Rheo Meditech) [12]. RBCs (6 µl) were suspended in 4% polyvinylpyrrolidone 360 solution (600 µL), supplied by Rheo Meditech within a microfluidic chip. The sample flows through a micro-channel under a range of shear stresses and the diffraction pattern from the laser that is directed through the deformed cells is analyzed by a microcomputer. Based upon the geometry of the elliptical diffraction pattern, an elongation index (EI) is calculated at each shear stress, EI = (L − W)/(L + W), where L and W are the length and width of the diffraction pattern.
2.3 Statistical Analysis
Origin 8.1 (Microcal Software, Northhampton, MA) was used for sample analysis of the data and plotted as mean values ± standard deviation. Analysis of variance (ANOVA) was used to test the significance of the results regarding the effect of Aβ and CuAβ conformers on RBCs.
3 Results and Discussions
3.1 Evidence of CuAβ Initiated Hb Oxidation and Membrane Associated Heme Degradation Products
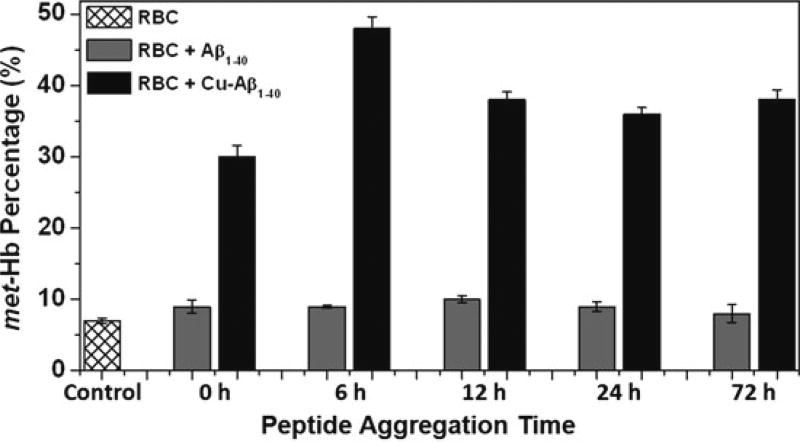
Hemolysate from various samples were analyzed for metHb in order to determine the degree of oxidative stress within the red cell. As shown in Fig. 19.1, Aβ conformers that lack coordinated copper result in only a minor increase in the amount of metHb in comparison to CuAβ. This increase in Hb oxidation for all aggregates is significant, p < 0.001; however, the dramatic increase for CuAβ is significantly higher than for Aβ, p < 0.0001. Interestingly, the 6 h CuAβ conformer, which has not yet formed large fibrils, exhibits the most oxidized Hb within the hemolysate, with the percentage of metHb increasing from 7 to 48%. In fact, this amount of metHb for the 6 h CuAβ conformer is significantly higher than the other CuAβ aggregates, p < 0.0001. This may suggest that an intermediate state of CuAβ is most toxic to the red cell.
Fig. 19.1.
Percentage of metHb within hemolysate from red cells incubated with Aβ and CuAβ conformers as an indicator of RBC oxidative stress
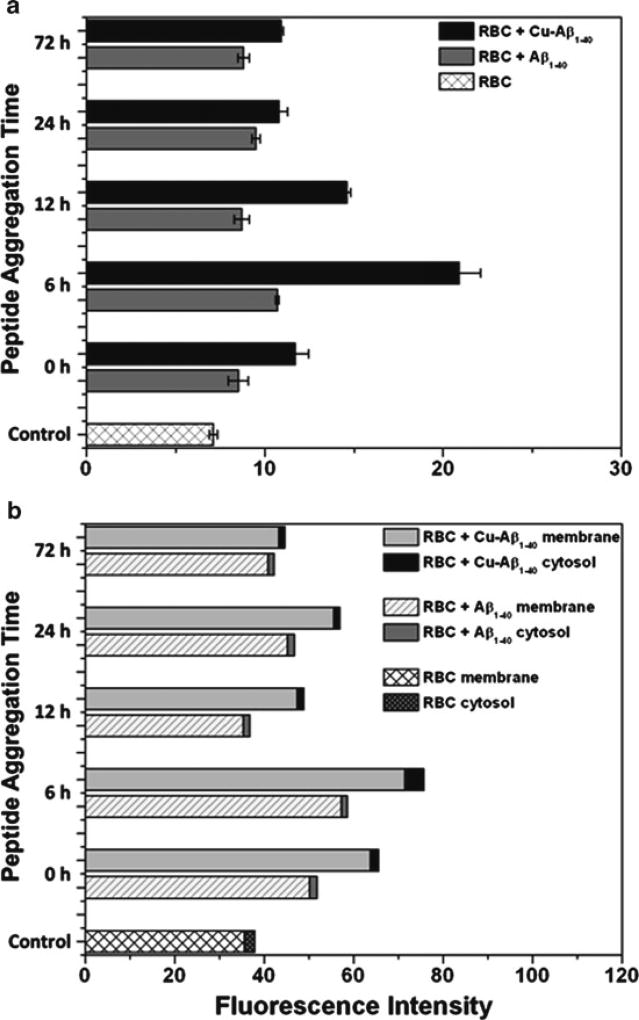
Previously, it was shown by our group that fluorescent heme degradation products are formed during Hb oxidation and slowly accumulate on the cell membrane [13, 14]. Higher levels of these fluorescent species provide an integrated measure of the oxidative stress experienced by the red cell. In the experiments described herein, an increase in heme degradation products was detected with addition of Aβ that is statistically significant, p < 0.001 (Fig. 19.2a). Consistent with the aforementioned results for Hb oxidation, a more dramatic increase in heme degradation was detected for CuAβ, p < 0.0001, than for the unmetallated Aβ conformers. The most significant effect was observed following incubation with the 6 h CuAβ conformer, which had triple the amount of heme degradation products in comparison to the control.
Fig. 19.2.
Fluorescence data corresponding to the amount of heme degradation products within hemolysate from RBC samples incubated with Aβ and CuAβ conformers. Data plotted are from before (a) and after (b) fractionation of the membrane and cytosolic portions
In order to analyze the distribution of heme degradation products within the red cell, the samples were centrifuged to separate the membrane and cytosolic portions of the hemolysate. As shown in Fig. 19.2b, almost all of the heme degradation products were associated with the membrane. This indicates that the oxidative reactions promoted by CuAβ occur on the membrane, since it is known from our previous work that these fluorescent products are not transferred from the cytosol to the membrane [13]. Although oxidation of Hb can increase membrane binding, membrane associated degradation products are not produced by a reaction involving metHb [15]. Instead, these heme degradation products are formed at the same time as metHb by a reaction involving H2O2 and Fe(II)–Hb, not Fe(III)–Hb [16]. Therefore, the results described herein imply that CuAβ is most likely membrane bound. The high level of Hb oxidation produced by CuAβ greatly exceeds the membrane binding sites of Hb but likely involves Hb transiently bound to the membrane. Membrane-bound Hb is more prone to oxidation because it is less accessible to the cytosolic proteins that make up the antioxidant network.
3.2 Impaired Oxygen Delivery Due to Reduced Red Cell Deformability
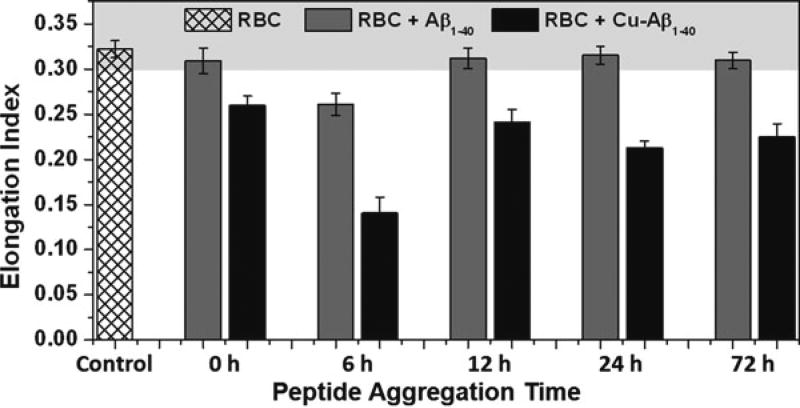
RBCs need to be sufficiently deformable in order to pass through the microcirculation and properly deliver oxygen to tissues. Since a source of oxidative stress bound to the membrane would be expected to have a direct effect on deformability, we investigated the effect of membrane-bound Aβ and CuAβ on deformability. To test this, the EI value was determined for each cell sample. Typical EI values from human subjects commonly vary between 0.3 and 0.35. Therefore, subjects with EI values within this range are considered to have healthy, highly deformable cells. Cells that exhibit lower EI values are considered rigid or less deformable.
As shown in Fig. 19.3, addition of CuAβ to red cells resulted in significantly reduced deformability (p < 0.001) in comparison to the control and unmetallated Aβ conformers. Again, incubation of RBCs with the 6 h CuAβ conformer resulted in a more dramatic change with the means significantly different than for the other CuAβ conformers, p < 0.0001. Interestingly, incubation of red cells with the unmetallated 6 h Aβ conformer also resulted in a significantly reduced EI value (p < 0.001). Yet, all other RBC samples incubated with unmetallated Aβ aggregates remained highly deformable and the overall means were not statistically significant. This result could have been caused by a trace amount of copper within the DPBS buffer that preferentially coordinates the 6 h Aβ conformer. More work is necessary to examine this possibility.
Fig. 19.3.
Deformability data for RBC samples incubated with Aβ and CuAβ conformers as an indicator of damage to the RBC membrane; typical range for healthy RBCs is shaded, EI≥0.3
4 Conclusions
Oxidative stress within the red cell that results from high ROS content will oxidize Hb to metHb, reducing the oxygen binding capacity of the RBC. In addition, a reduction in the deformation ability of the RBC will result in impaired delivery of oxygen. Following incubation of RBCs with different aggregate forms of Aβ peptide and CuAβ, we find that the copper-bound form overall results in increased RBC oxidative stress. This effect is most pronounced with the 6 h conformer, supportive of increased toxicity of the intermediate proto fibril. This enhanced red cell oxidative stress results in Hb oxidation and subsequent membrane bound heme degradation products as well as impaired RBC deformability. The suggested mechanism involves CuAβ associating with the membrane producing ROS. This could result from an electron transfer reaction that occurs when CuAβ is near deoxyHb yielding metHb and a reduced cuprous species that can readily react with O2 to form ROS. For these ROS to produce heme degradation and reduced deformability they need to form in close proximity to the membrane in order to avoid scavenging by cytosolic proteins. Overall, this attack on the membrane would lead to altered red cell function and impaired oxygen transport. Also, this same pool of ROS could leak out of the red cell and damage nearby cells or tissues.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Nagababu E, Usatyuk PV, Enika D, et al. Vascular endothelial barrier dysfunction mediated by amyloid-b proteins. J Alzheimers Dis. 2009;17:845–854. doi: 10.3233/JAD-2009-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravi LB, Poosala S, Ahn D, et al. Red cell interactions with amyloid-b1-40 fibrils in a murine model. Neurobiol Dis. 2005;19:28–37. doi: 10.1016/j.nbd.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Ravi LB, Mohanty JG, Chrest FJ, et al. Influence of b-amyloid fibrils on the interactions between red blood cells and endothelial cells. Neurol Res. 2004;26:579–585. doi: 10.1179/016164104225016227. [DOI] [PubMed] [Google Scholar]
- 4.Meyer-Luehmann M, Spires-Jones TL, Prada C, et al. Rapid appearance and local toxicity of amyloid-b plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lashuel HA, Hartley D, Petre BM, et al. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 6.Rauk A. Why is the amyloid b peptide of Alzheimer’s disease neurotoxic? Dalton Trans. 2008;14(10):1273–1282. doi: 10.1039/b718601k. [DOI] [PubMed] [Google Scholar]
- 7.Zatta P, Drago D, Bolognin S, et al. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol Sci. 2009;30:346–355. doi: 10.1016/j.tips.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Gaggelli E, Kozlowski H, Valensin D, et al. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 9.Stone J. What initiates the formation of senile plaques? The origin of Alzheimer-like dementias in capillary haemorrhages. Med Hypotheses. 2008;71:347–359. doi: 10.1016/j.mehy.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 11.Schrag M, Crofton A, Zabel M, et al. Effect of cerebral amyloid angiopathy on brain iron, copper, and zinc in Alzheimer’s disease. J Alzheimers Dis. 2011;24:137–149. doi: 10.3233/JAD-2010-101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S, Hou JX, Suh JS, et al. Validation and application of a micro fluidic ektacytometer (RheoScan-D) in measuring erythrocyte deformability. Clin Hemorheol Micro. 2007;37:319–328. [PubMed] [Google Scholar]
- 13.Nagababu E, Mohanty JG, Bhamidipaty S, et al. Role of the membrane in the formation of heme degradation products in red blood cells. Life Sci. 2010;86:133–138. doi: 10.1016/j.lfs.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rifkind JM, Ramasamy S, Manoharan PT, et al. Redox reactions of hemoglobin. Antioxid Redox Signal. 2004;6:657–666. doi: 10.1089/152308604773934422. [DOI] [PubMed] [Google Scholar]
- 15.Nagababu E, Rifkind JM. Formation of fluorescent heme degradation products during the oxidation of hemoglobin by hydrogen peroxide. Biochem Biophys Res Commun. 1998;247:592–596. doi: 10.1006/bbrc.1998.8846. [DOI] [PubMed] [Google Scholar]
- 16.Nagababu E, Rifkind JM. Reaction of hydrogen peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry. 2000;39:12503–12511. doi: 10.1021/bi992170y. [DOI] [PubMed] [Google Scholar]