Abstract
Purpose of Review
To examine recent literature on dairy products, dairy fatty acids, and cardiometabolic disease. Primary questions of interest include what unique challenges researchers face when investigating dairy products/biomarkers, whether one should consume dairy to reduce disease risk, whether dairy fatty acids may be beneficial for health, and whether one should prefer low- or high-fat dairy products.
Recent Findings
Dairy composes about 10% of the calories in a typical American diet, about half of that coming from fluid milk, half coming from cheese, and small amounts from yogurt. Most meta-analyses report no or weak inverse association between dairy intake with cardiovascular disease and related intermediate outcomes. There is some suggestion that dairy consumption was inversely associated with stroke incidence and yogurt consumption was associated with lower risk of type 2 diabetes. Odd chain fatty acids (OCFAs) found primarily in dairy (15:0 and 17:0) appear to be inversely associated with cardiometabolic risk, but causation is uncertain. Substitution analyses based on prospective cohorts suggested that replacing dairy fat with vegetable fat or polyunsaturated fat was associated with significantly lower risk of cardiovascular disease.
Summary
Current evidence suggests null or weak inverse association between consumption of dairy products and risk of cardiovascular disease. However, replacing dairy fat with polyunsaturated fat, especially from plant-based foods, may confer health benefits. More research is needed to examine health effects of different types of dairy products in diverse populations.
Keywords: Dairy, Saturated fat, Yogurt, Cardiovascular disease, Odd chain fatty acids
Introduction
In an era where many food categories have been deemed healthy or unhealthy, dairy remains controversial. Its relationship with cardiometabolic disease, particularly cardiovascular disease (CVD), which includes coronary heart disease (CHD), stroke, heart failure, and peripheral artery disease, has long been a subject of investigation, and despite many publications, no consensus has been reached. Dairy products are based on milk from mammals and include milk itself, cream, butter, cheese, yogurt, frozen desserts, and whey and contain a varied collection of micro- and macronutrients, namely saturated fatty acids (SFAs) [1]. While researchers have recommended the reduction in total SFAs in the diet for the prevention of CVD [2], the relationship between dairy fat and disease is still unsettled. Thus, it is important to pose several questions: do dairy SFAs decrease CVD risk? Should one eat more dairy for a healthier heart? Is low fat or high fat preferred? The present review of dairy and dairy SFAs will consider these questions through a review of available evidence, outline several challenges in the field, and present gaps in knowledge that may be filled by future research.
Metabolism and Measurement of Dairy Products and Biomarkers
Dairy is a major part of the Western diet and composes 10% of daily calories in the USA [3]. Dairy products are diverse in both variety and composition, but all are derived from milk. The lipid profile of milk consists of dozens of types of acylglycerols, cholesterols, and fatty acids [4]. Saturated fats compose the majority of the total fat content in milk [5], although the specific composition of milk varies considerably according to animal feeding method, genetics, environment, lactation stage, and processing method [6, 7]. Table 1 lists the fatty acid composition of retail milk from the USA in 2008 [8]. Proposed biomarkers of dairy include fatty acids 14:0, 14:1, 15:0, 17:0, 17:1, and trans 16:1n-7 [9], but most research efforts have focused on the odd chain fatty acids (OCFAs) 15:0 (pentadecanoic acid) and 17:0 (heptadecanoic acid). Historically, OCFAs were of little scientific interest due to their relatively low physiological concentrations compared to even chain fatty acids (ECFAs) [10] and were used primarily as internal standards in chromatographic analyses [11]. In the body, the majority of OCFAs undergo β-oxidation to produce acetyl-CoA, NADH, and FADH2 [12]. Since β-oxidation involves shortening fatty acid chains two carbons at a time, metabolism of OCFAs ends with propionyl CoA, whereas ECFAs end with acetyl CoA [13]; whether or not this makes a difference in incidence of clinical endpoints has yet to be reported. In addition to fat, milk also typically contains about 3.5% protein, 80% of which is in the form of casein and 20% in whey protein [14]. Existing studies of milk protein consumption point to some potential benefit for metabolic health, but the data are inconclusive [15]. Carbohydrates dominated primarily by lactose constitute approximately 4.6% of milk by weight [16]. Although the cardiovascular effects of lactose are poorly characterized, lactose intolerance may affect individual willingness to consume dairy products [17].
Table 1.
Fatty acid (FA) composition of retail milk samples in the USA [8]
| Variable (g/100 g of FA) | Mean | SEM |
|---|---|---|
| C4:0 | 4.15 | 0.017 |
| C6:0 | 2.13 | 0.008 |
| C8:0 | 1.19 | 0.006 |
| C10:0 | 2.59 | 0.015 |
| C12:0 | 2.87 | 0.018 |
| C14:0 | 9.53 | 0.039 |
| C14:1 | 0.82 | 0.007 |
| C15:0 | 0.89 | 0.004 |
| C16:0 | 28.08 | 0.078 |
| C16:1 | 1.48 | 0.011 |
| C17:0 | 0.52 | 0.002 |
| C18:0 | 11.68 | 0.078 |
| C18:1, trans-6 | 0.32 | 0.002 |
| C18:1, trans-9 | 0.29 | 0.002 |
| C18:1, trans-10 | 0.55 | 0.007 |
| C18:1, trans-11 | 1.48 | 0.013 |
| C18:1, trans-12 | 0.54 | 0.004 |
| C18:1, cis-9 | 23.58 | 0.074 |
| C18:2, cis-9, cis-12 | 3.19 | 0.019 |
| C20:0 | 0.09 | 0.001 |
| C18:3 | 0.38 | 0.004 |
| C18:2, cis-9, trans-11 | 0.55 | 0.004 |
| C18:2, trans-10, cis-12 | ND | – |
| Other | 3.09 | 0.021 |
ND, not detected (< 0.01% of total fatty acids); SEM, standard error of the mean
Reprinted with permission from O’Donnell-Megaro AM, Barbano DM, Bauman DE. Survey of the fatty acid composition of retail milk in the United States including regional and seasonal variations. Journal of Dairy Science. 2011;94 (1):59–65
Analysis of dairy products and biomarkers presents several challenges to researchers, the most important being the accurate measurement of exposure. Reliable measurements of food intake and nutrients have been a long-standing challenge in the field of nutritional epidemiology. For most large-scale observational studies, semiquantitative food frequency questionnaires (FFQs) are the preferred method of assessment for intake. FFQs such as the Willett [18] and the Block [19] have been validated for both accuracy and reproducibility. Dairy intake as reported by FFQs tends to exhibit a relatively high degree of correlation with diet records. Byers et al. reported a Spearman correlation of 0.53 for dairy products compared with 0.41 for fruits, 0.41 for vegetables, and 0.39 for meats [20]; van Liere et al. reported a correlation of 0.67 for dairy [21]; the EPIC group of Spain reported a correlation of 0.90 [22]. Regarding biomarkers, 15:0 and 17:0 remain the most popular candidates [23]. Assessment of these OCFAs typically consists of analyzing blood samples with gas chromatography-mass spectrometry after storage at − 80 °C. The concentration of OCFAs found in the adipose tissue is highly correlated with dairy intake assessed by diet record (Spearman’s r = 0.59 and 0.45 for 15:0 and 17:0, respectively) [23, 24], although trace amounts may also be found in beef, fish, and other animal ruminants [23], leading to doubts as to whether OCFAs may be used as a valid biomarker in populations consuming heavy amounts of these meats [25]. As such, caution must be exercised when deciding whether the nominal exposure of interest in biomarker studies is dairy products (which contain a multitude of micro- and macronutrients) or dairy fats (a single component derived from multiple potential sources). Recent publications have also speculated on the possibility of de novo synthesis of OCFAs in humans via α-oxidation, the details of which are beyond the scope of this review [26–28]. Roberts et al. observed conversion of 16:0 to 15:0 during adipocyte differentiation, raising the possibility that plasma OCFA concentrations may be regulated [29]. Weitkunat et al. further reported that gut-derived propionate may be used to synthesize OCFAs in the liver [30].
Dairy Products and Cardiometabolic Disease
Numerous observational and experimental studies of dairy intake and cardiovascular disease or related secondary outcomes have been conducted, most having been published after 2010. In the USA, the vast majority of dairy intake consists of fluid milk (51%) and cheese (45%), which presents challenges since cheese is most often consumed as an ingredient in other foods [31]. Thus, results from prospective studies of total dairy intake most likely represent a combination of milk and cheese intake and also capture correlations of CVD with other components consumed with cheese such as pizza and sandwiches.
Total Dairy
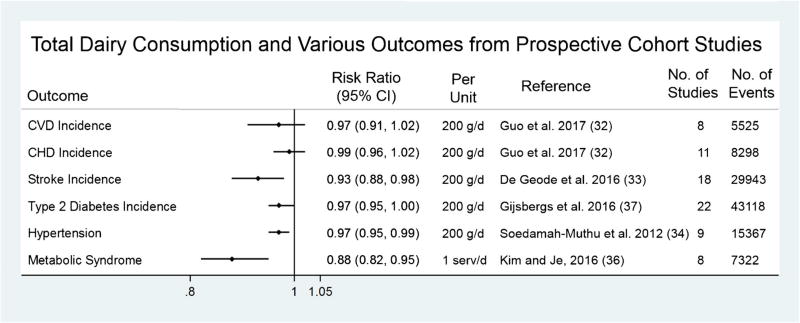
Figure 1 summarizes the results of several meta-analyses with various cardiometabolic endpoints. The most recent meta-analysis of dairy products by Guo et al. covered all cohort studies through September 2016 [32]. Using data from 29 studies accruing 28,419 cases of CHD and 25,416 cases of composite CVD, the authors found an inverse association between total dairy intakes for CVD (relative risk (RR) = 0.98, 95% confidence interval (CI) = 0.97, 0.99 per 20 g/day) but not CHD (RR = 0.99, 95% CI = 0.98, 1.01 per 20 g/day), although there was a large degree of heterogeneity in both outcomes. The results were similar for stroke: de Geode et al. indicate that a 200-g/day increase in milk intake was associated with a RR = 0.93, 95% CI = 0.88, 0.98; this association appeared to be stronger in East Asian (RR = 0.82, 95% CI = 0.75, 0.90) compared to Western countries (RR = 0.98, 95% CI = 0.95, 1.01) [33]. Soedamah-Muthu et al. report a similar magnitude of association for total dairy intake and hypertension in a meta-analysis of nine cohort studies (RR = 0.97, 95% CI = 0.95, 0.99 per 200 g/day) [34], although a publication using Mendelian randomization techniques did not support this finding (OR = 1.04, 95% CI = 0.88, 1.24 comparing high vs. low consumer allele) [35]. Kim and Je found an inverse association of dairy intake with metabolic syndrome (RR = 0.85, 95% CI = 0.73, 0.98 per 1 serving/day) [36], and a small benefit was observed for type 2 diabetes mellitus (T2DM) (RR = 0.97, 95% CI = 0.95, 1.00 per 200 g/day) [37].
Fig. 1.
Summary of recent meta-analyses of total dairy consumption with cardiometabolic endpoints from prospective cohort studies. CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; g/d, grams per day; MetS, metabolic syndrome; RR, risk ratio; serv/d, servings per day
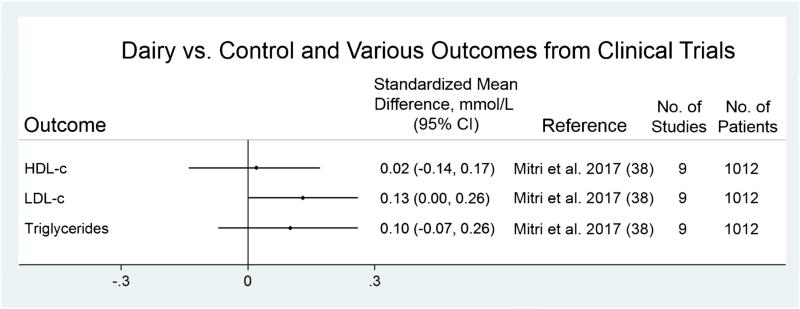
Although no randomized trials have been conducted for clinical endpoints, a meta-analysis of nine trials for lipid profile (Fig. 2) found no significant association for the effects of high dairy intake (≥ 3 servings/day) vs. low dairy (< 3 servings/day) on high-density lipoprotein cholesterol (HDL-c), or triglycerides, and a small significant increase in low-density lipoprotein cholesterol (LDL-c) [38], and another systematic review of eight trials conducted among overweight/obese adults reported seven neutral studies and one report of improved inflammatory marker profile among those randomized to dairy consumption [39].
Fig. 2.
Summary of dairy intervention vs. controls with cardiovascular risk factors from clinical trials. CI, confidence interval; CVD, HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; mmol/L, millimoles per liter
Subgroups of Dairy
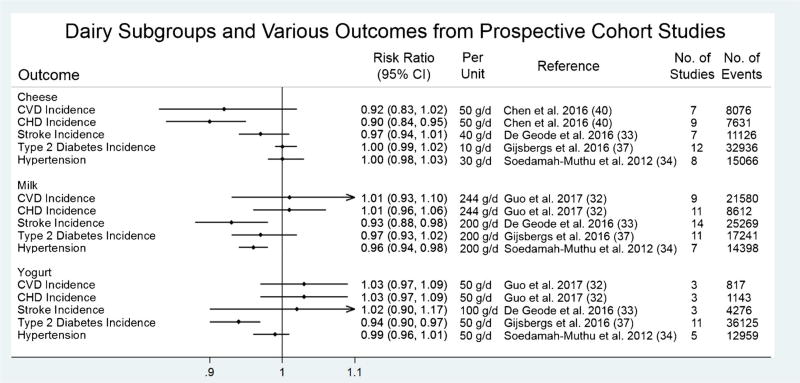
Figure 3 summarizes meta-analyses of cheese, milk, yogurt, and butter with various cardiometabolic outcomes. There was a modest inverse association between consumption of cheese and CHD (RR = 0.90, 95% CI = 0.84, 0.95 per 50 g/day) [40], but no significant association for yogurt and CVD (RR = 1.03, 95% CI = 0.97, 1.09 per 50 g/day) [32]. A large recent study not included in previous meta-analyses reported that yogurt and cheese consumption were strongly associated with lower risk of overall and CVD-specific mortality [41]. Although questions of type of cheese (high fat vs. low fat) remain unanswered, the finding that cheese seems to be inversely associated with CVD is surprising, given that cheese is seldom eaten by itself and is usually included in mixed dishes such as pizza, burgers, pasta, and other grain-based dishes [42]. These foods constitute a typical Western dietary pattern [43] that has been shown to increase risk of CHD [44, 45] and is associated with unhealthy lifestyle factors such as lower multivitamin use, smoking, low physical activity, and heavy drinking [46]. Yogurt is also a food of particular interest, given its potential to stimulate gut microbiota [47] and existing studies which suggest benefit for body weight and type 2 diabetes [37, 48]. Probiotic bacteria in yogurt and cheese, mainly members in the Lactobacillus and Bifidobacterium genera [49], have also shown favorable effects on immunity, inflammation, diarrhea prevention, and cardiovascular risk factors in clinical trials [50]. Thus, specific attention should be given to cheese and yogurt in future studies.
Fig. 3.
Summary of recent meta-analyses of cheese, milk, and yogurt with cardiometabolic endpoints from prospective cohort studies. CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; g/d, grams per day; RR, risk ratio
Total Dairy Fat Intake and Cardiometabolic Disease
Fat composes the majority of the energy content and weight of dairy (Table 1). Among fat subtypes, even chained saturated fatty acids 14:0, 16:0, and 18:0 constitute the majority of milk fat content. A case-control study conducted by Biong et al. indicated no significant association of 14:0 concentration in adipose tissue and later myocardial infarction [51]. Goldbohm et al. reported no association of milk fat intake with CVD mortality in men but a slight positive association of milk fat intake and CHD mortality (RR = 1.11, 95% CI = 1.01, 1.21) in a cohort of 120,852 participants [52]. de Oliveira Otto et al. reported that higher intake of dairy SFAs was associated with a lower CVD risk (RR = 0.79, 95% CI = 0.68, 0.92 for 5 g/day) but that higher intake of meat SFAs was associated with a higher risk (RR = 1.26, 95% CI = 1.02, 1.54 for 5 g/day) [53]. However, these results can be misleading because it is virtually impossible to separate the effects of SFAs from dairy or meats from other components of these foods.
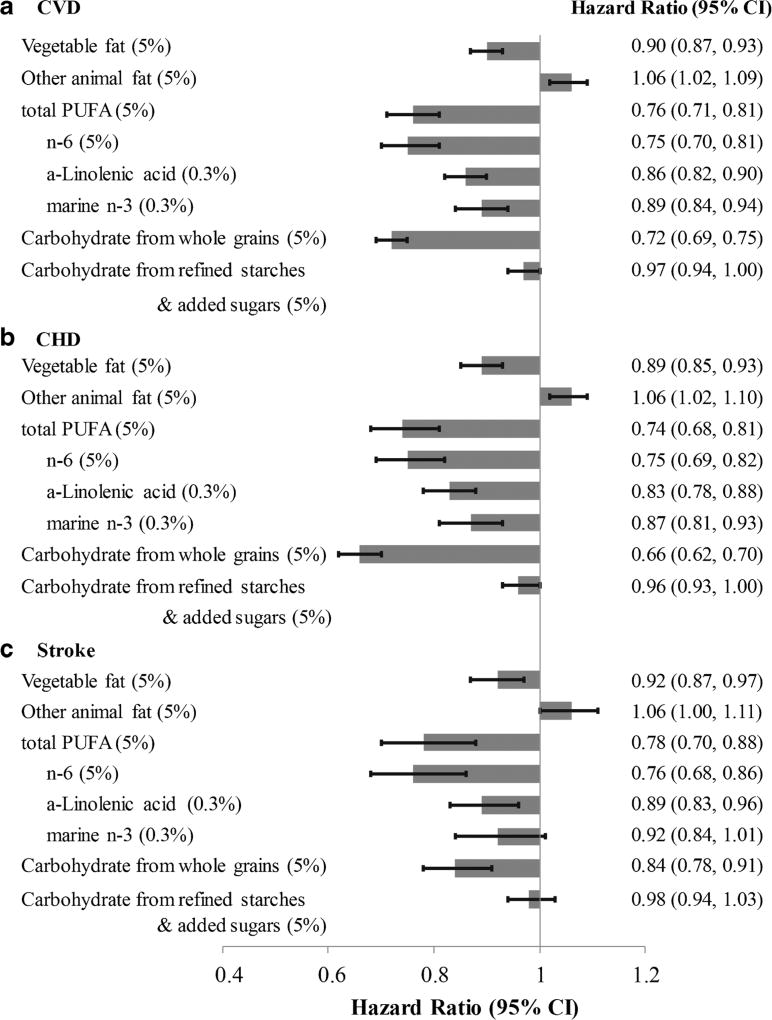
Chen et al. [54] found that dairy fat intake was not significantly associated with incident CVD, CHD, or stroke, but that substitution of 5% daily calories from dairy fat with polyunsaturated fat or vegetable fat was associated with a 24% (RR = 0.76, 95% CI = 0.71, 0.81) and 10% reduction (RR = 0.90, 95% CI = 0.87, 0.93) in CVD, respectively (Fig. 4). On the other hand, substitution of dairy fat with animal fat (primarily from red meat) was associated with an increased risk of CVD (RR = 1.06, 95% CI = 1.02, 1.09). These results are in line with the general observation that replacement of saturated fat with polyunsaturated fat, especially from plant sources, is associated with a reduced risk of CHD [55].
Fig. 4.
From Chen et al.: RR (95% CIs) for CVD (A), CHD (B), and stroke (C) associated with isocaloric substitutions of vegetable fat, other animal fat, PUFA, and carbohydrate for dairy fat in the NHS I, II, and HPFS. The 95% CIs are represented by horizontal lines, and gray bars represent overall estimates. Reproduced with permission from Frank B. Hu [54]. From Chen M, Li Y, Sun Q, Pan A, Manson JE, Rexrode KM, et al. Dairy fat and risk of cardiovascular disease in three cohorts of US adults. Am J Clin Nutr. 2016 Nov;104(5):1209–1217
Odd Chain Fatty Acids and Cardiometabolic Disease
Although the number of studies relating circulating OCFAs to cardiometabolic disease is sparse, existing data point to a possible protective effect. A meta-analysis of 13 studies found a non-significant association of 15:0 with CVD (RR = 0.94, 95% CI = 0.77, 1.15), but an inverse association for 17:0 (RR = 0.82, 95% CI = 0.68, 0.99) comparing top vs. bottom tertiles [56]. No meta-analysis has been published for T2DM, but the largest study from the EPIC-InterAct cohort reported a negative association for 15:0 (RR = 0.79, 95% CI = 0.73, 0.85 per standard deviation (S.D.)) and 17:0 (RR = 0.67, 95% CI = 0.63, 0.71 per S.D.) [57]. Similar results were observed in a case-control study from Northern Sweden [58], the Melbourne Collaborative Cohort Study [59], the Nurses’ Health Study and Health Professionals’ Follow-up Study [60], and the Insulin Resistance Atherosclerosis Study [61]. The EPIC-Norfolk cohort confirmed protective associations only for 17:0 [62]. Data from the Cardiovascular Health Study [63], EPIC-Potsdam [64], and Multi-Ethnic Study of Atherosclerosis [65] suggested non-significant inverse associations.
The totality of evidence to date suggests an inverse association of 15:0 and to a larger extent, 17:0, with CVD and T2DM, but questions remain. Are OCFAs truly proxies for dairy intake or are they themselves a causal factor for cardiometabolic risk reduction? If so, is it plausible that small amounts of OCFAs could have large effect sizes? One salient discrepancy is that the effect sizes of dairy biomarkers are much larger than those of dairy intake measured from consumption data. Attenuated risk ratios could arise due to methodological issues associated with FFQs, such as within-person variation or non-differential misclassification [66]. Another explanation is the relationship between OCFAs and cardiometabolic outcomes could be confounded by healthy lifestyle [67]. In the EPIC-InterAct study, OCFAs showed the highest correlation with dairy intake, they were also significantly correlated with intakes of fruits/vegetables and nuts [57]. Rosell et al. note that 15:0 and 17:0 levels were associated with low alcohol consumption, high physical activity [68], and high fiber intake [30]. Finally, the possibility that OCFAs, particularly 17:0 [69], are imperfect markers of dairy or that they may be synthesized de novo and therefore regulated [13, 31] cannot be ruled out. One must also keep in mind that dairy fat contains only trace amounts of OCFAs by weight and that the majority of SFAs in milk is composed of 14:0, 16:0, and 18:0 [70], with 16:0 exhibiting the strongest positive associations with CHD [54].
High Fat or Low Fat?
The USDA currently recommends that Americans should consume fat-free and low-fat dairy instead of high-fat alternatives, citing that “increasing the proportion of fat-free milk consumed to meet Dairy Group recommendations would … decrease amounts of sodium, cholesterol, and saturated fatty acids” [31]. Current evidence of high-fat vs. low-fat dairy do not conclusively point to a benefit one way or the other [71]. Guo et al. report that neither low-fat (RR = 0.98, 95% CI = 0.95, 1.01 per 200 g/day) nor high-fat dairy (RR = 0.93, 95% CI = 0.84, 1.03 per 200 g/day) was significantly associated with CVD [32]; similarly, Gijsbers et al. indicate similar associations for low-fat (RR = 0.96, 95% CI = 0.92, 1.00 per 200 g/day) and high-fat dairy (RR = 0.98, 95% CI = 0.93, 1.04 per 200 g/day) with type 2 diabetes [37]. Current experimental data are also inconclusive. A meta-analysis of 21 randomized trials revealed that both low-fat and high-fat dairies significantly increase body weight, but that there were no differences for waist circumference, HOMA-IR, fasting glucose, LDL-c, HDL-c, systolic blood pressure, diastolic blood pressure, or C-reactive protein [72]. In a comprehensive Finnish study of 34,525 participants over 40 years, population-level shifts from high-fat to low-fat dairy sources were critical in dramatic decreases in CHD mortality (overall 83% decreased mortality rate) [73]. On the other hand, low-fat dairy, cheese, and yogurt may contribute to the prevention of T2DM according to a meta-analysis of eight prospective studies (RR = 0.88, 95% CI = 0.84, 0.93 per 200 g/day) [74]. Thus, while advocated by some, current evidence does not suggest that high-fat dairy (such as whole milk) is superior to low-fat dairy in terms of disease risk [75].
In light of the lack of robust evidence for the differential health effects of low vs. high-fat dairy, the primary motivation to recommend low-fat products therefore comes from the anticipated reduction in consumed SFAs. A great deal of controversy surrounds the issue of SFAs and cardiovascular disease, and much of the misunderstanding stems from poorly conducted studies that fail to consider replacement nutrient for saturated fat. In free-living populations, a person consuming more SFAs typically replaces something else from their diet, usually carbohydrates [76]. Thus, in most epidemiological analyses, saturated fat is often compared to total carbohydrates (comprised of refined starch and added sugars in significant amounts) [77]. When saturated fats are replaced with unsaturated fats, an inverse association with CVD is observed [55]. Consideration of these substitution studies led to the American Heart Association (June 2017) recommending the replacement of SFAs with unsaturated fats, especially polyunsaturated fat [2].
Conclusions
To date, several large meta-analyses found null or weak inverse associations between dairy consumption and risk of CVD. Currently, the 2015–2020 Dietary Guidelines for Americans recognize that low- or reduced fat dairy can be part of a healthy diet [32], based on dietary pattern analyses, observational evidence, and the fact that dairy products provide significant amounts of under-consumed nutrients in the US population such as calcium, potassium, and vitamin D. Current evidence does not support consuming high-fat dairy products to improve metabolic health. Despite some evidence that minor components of dairy fat such as odd chain fatty acids were associated with lower risk of diabetes and cardiometabolic risk factors, dairy fat as a whole is not considered an optimal type of fat due to high saturated fatty acid content, and there is some evidence that replacing dairy fat with polyunsaturated fat, especially from plant-based foods may confer health benefits. Whether fermented dairy products such as different types of cheese and yogurt have unique metabolic benefits requires further investigations.
Several methodological issues need to be considered when interpreting the current evidence. First, despite reasonable validity of FFQs in assessing dairy intake, measurement errors are inevitable, especially for individual types of dairy. Random errors may have attenuated the observed associations. Therefore, it is important to collect repeated measures of diet, which can be used to reduce within-person random errors and represent long-term dietary habits. It is also important to continue to identify reliable biomarkers of dairy intake. Second, sources of heterogeneity in population characteristics must be considered. For example, there is a biological reason to believe that dairy may have different health effects on individuals from East Asia compared to other countries, since lactose, a major component of dairy, cannot be metabolized by most East Asians [78]. Effect modifications by sex [79], obesity [80], and baseline hypertension [81] have also been observed. Third, studies should continue to report the relative risks of specific dairy products with health outcomes. Specific foods such as milk, cheese, and yogurt may show different effects on cardiometabolic health due to different amounts of nutrients, bioactive compounds, and fermentation methods for different dairy products. Therefore, more research is needed to examine health effects of different types of dairy products in diverse populations. Lastly, substitution analyses should be employed to investigate the replacement of dairy with other foods, especially plant-based items such as soy and nuts. It is more desirable to make practical dietary recommendations based on substitution analyses.
Acknowledgments
Funding Information This research was supported by the National Institute of Health grants R01 HL060712 and F31 DK114938.
Dr. Frank Hu has received research support from the California Walnut Commission and an honorarium from Metagenics.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Dr. Edward Yu declares no conflict of interest.
References
- 1.Gaucheron F. Milk and dairy products: a unique micronutrient combination. J Am Coll Nutr. 2011;30(5 Suppl 1):400s–9s. doi: 10.1080/07315724.2011.10719983. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 3.Agriculture USDo. [cited 2017 July 26];Food Availability (Per Capita) Data System 2016. Available from: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/
- 4.Bainbridge ML, Cersosimo LM, Wright A-DG, Kraft J. Content and composition of branched-chain fatty acids in bovine milk are affected by lactation stage and breed of dairy cow. PLoS One. 2016;11(3):e0150386. doi: 10.1371/journal.pone.0150386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SR28 U. National Nutrient Database for Standard Reference, Release 28. US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. 2016;26 http://wwwarsusdagov/ba/bhnrc/ndl Accessed. [Google Scholar]
- 6.Stefanov I, Baeten V, Abbas O, Colman E, Vlaeminck B, De Baets B, et al. Analysis of milk odd- and branched-chain fatty acids using Fourier transform (FT)-Raman spectroscopy. J Agric Food Chem. 2010;58(20):10804–11. doi: 10.1021/jf102037g. [DOI] [PubMed] [Google Scholar]
- 7.Lock AL, Bauman DE. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids. 2004;39(12):1197–206. doi: 10.1007/s11745-004-1348-6. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell-Megaro AM, Barbano DM, Bauman DE. Survey of the fatty acid composition of retail milk in the United States including regional and seasonal variations. J Dairy Sci. 2011;94(1):59–65. doi: 10.3168/jds.2010-3571. [DOI] [PubMed] [Google Scholar]
- 9.Sofie Biong A, Berstad P, Pedersen JI. Biomarkers for intake of dairy fat and dairy products. Eur J Lipid Sci Technol. 2006;108(10):827–34. [Google Scholar]
- 10.Horning MG, Martin DB, Karmen A, Vagelos PR. Fatty acid synthesis in adipose tissue. II. Enzymatic synthesis of branched chain and odd-numbered fatty acids. J Biol Chem. 1961;236:669–72. [PubMed] [Google Scholar]
- 11.Tserng KY, Kliegman RM, Miettinen EL, Kalhan SC. A rapid, simple, and sensitive procedure for the determination of free fatty acids in plasma using glass capillary column gas-liquid chromatography. J Lipid Res. 1981;22(5):852–8. [PubMed] [Google Scholar]
- 12.Eaton S, Bartlett KB, Pourfarzam M. Mammalian mitochondrial β-oxidation. Biochem J. 1996;320(2):345–57. doi: 10.1042/bj3200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (c15:0) and heptadecanoic acid (c17:0) in health and disease. Molecules. 2015;20(2):2425–44. doi: 10.3390/molecules20022425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Research Council Committee on Technological Options to Improve the Nutritional Attributes of Animal P. Designing foods: animal product options in the marketplace. Washington (DC): National Academies Press (US); 1988. Factors affecting the composition of milk from dairy cows. Copyright (c) 1988 by the National Academy of Sciences. [Google Scholar]
- 15.McGregor RA, Poppitt SD. Milk protein for improved metabolic health: a review of the evidence. Nutr Metab. 2013;10(1):46. doi: 10.1186/1743-7075-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenness R. Lactation/edited by Bruce L Larson; written by Ralph R Anderson [et al] 1985. Biochemical and nutritional aspects of milk and colostrum. [Google Scholar]
- 17.Scrimshaw NS, Murray EB. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am J Clin Nutr. 1988;48(4):1142–59. doi: 10.1093/ajcn/48.4.1142. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87(1):43–7. [PubMed] [Google Scholar]
- 19.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 20.Byers T, Marshall J, Anthony E, Fiedler R, Zielezny M. The reliability of dietary history from the distant past. Am J Epidemiol. 1987;125(6):999–1011. doi: 10.1093/oxfordjournals.aje.a114638. [DOI] [PubMed] [Google Scholar]
- 21.van Liere MJ, Lucas F, Clavel F, Slimani N, Villeminot S. Relative validity and reproducibility of a French dietary history questionnaire. Int J Epidemiol. 1997;26(Suppl 1):S128–36. doi: 10.1093/ije/26.suppl_1.s128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spain EGo. Relative validity and reproducibility of a diet history questionnaire in Spain. I. Foods. Int J Epidemiol. 1997;26(Suppl 1):S91–9. doi: 10.1093/ije/26.suppl_1.s91. [DOI] [PubMed] [Google Scholar]
- 23.Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr. 1998;68(2):291–5. doi: 10.1093/ajcn/68.2.291. [DOI] [PubMed] [Google Scholar]
- 24.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131(3):828–33. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 25.Lankinen M, Schwab U. Biomarkers of dairy fat. Am J Clin Nutr. 2015;101(5):1101–2. doi: 10.3945/ajcn.114.104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foulon V, Sniekers M, Huysmans E, Asselberghs S, Mahieu V, Mannaerts GP, et al. Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase: a revised pathway for the alpha-oxidation of straight chain fatty acids. J Biol Chem. 2005;280(11):9802–12. doi: 10.1074/jbc.M413362200. [DOI] [PubMed] [Google Scholar]
- 27.Kondo N, Ohno Y, Yamagata M, Obara T, Seki N, Kitamura T, et al. Identification of the phytosphingosine metabolic pathway leading to odd-numbered fatty acids. Nat Commun. 2014;5:5338. doi: 10.1038/ncomms6338. [DOI] [PubMed] [Google Scholar]
- 28.Su X, Han X, Yang J, Mancuso DJ, Chen J, Bickel PE, et al. Sequential ordered fatty acid α oxidation and Δ9 desaturation are major determinants of lipid storage and utilization in differentiating adipocytes. Biochemistry. 2004;43(17):5033–44. doi: 10.1021/bi035867z. [DOI] [PubMed] [Google Scholar]
- 29.Roberts LD, Virtue S, Vidal-Puig A, Nicholls AW, Griffin JL. Metabolic phenotyping of a model of adipocyte differentiation. Physiol Genomics. 2009;39(2):109–19. doi: 10.1152/physiolgenomics.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weitkunat K, Schumann S, Nickel D, Hornemann S, Petzke KJ, Schulze MB, et al. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr. 2017 doi: 10.3945/ajcn.117.152702. ajcn152702. [DOI] [PubMed] [Google Scholar]
- 31.Health UDo, Services H. 2015–2020 dietary guidelines for Americans. Washington (DC): USDA; 2015. [Google Scholar]
- 32.Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32(4):269–87. doi: 10.1007/s10654-017-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Goede J, Soedamah-Muthu SS, Pan A, Gijsbers L, Geleijnse JM. Dairy consumption and risk of stroke: a systematic review and updated dose–response meta-analysis of prospective cohort studies. J Am Heart Assoc: Cardiovasc Cerebrovasc Dis. 2016;5(5):e002787. doi: 10.1161/JAHA.115.002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soedamah-Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60(5):1131–7. doi: 10.1161/HYPERTENSIONAHA.112.195206. [DOI] [PubMed] [Google Scholar]
- 35.Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ. 2017;356 doi: 10.1136/bmj.j1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Je Y. Dairy consumption and risk of metabolic syndrome: a meta-analysis. Diabet Med. 2016;33(4):428–40. doi: 10.1111/dme.12970. [DOI] [PubMed] [Google Scholar]
- 37.Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr. 2016;103:1111–24. doi: 10.3945/ajcn.115.123216. [DOI] [PubMed] [Google Scholar]
- 38.Mitri J, Barakatun N, Truong S, ElSayed N, Hamdy O. The effect of dairy consumption on lipid profile: a meta-analysis of randomized controlled trials. J Clin Lipidol. 2017;11(3):825–6. [Google Scholar]
- 39.Labonté M-È, Couture P, Richard C, Desroches S, Lamarche B. Impact of dairy products on biomarkers of inflammation: a systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am J Clin Nutr. 2013;97(4):706–17. doi: 10.3945/ajcn.112.052217. [DOI] [PubMed] [Google Scholar]
- 40.Chen GC, Wang Y, Tong X, Szeto IM, Smit G, Li ZN, et al. Cheese consumption and risk of cardiovascular disease: a meta-analysis of prospective studies. Eur J Nutr. 2016 doi: 10.1007/s00394-016-1292-z. [DOI] [PubMed] [Google Scholar]
- 41.Farvid MS, Malekshah AF, Pourshams A, Poustchi H, Sepanlou SG, Sharafkhah M, et al. Dairy food intake and all-cause, cardiovascular disease, and cancer mortality: the Golestan cohort study. Am J Epidemiol. 2017;185(8):697–711. doi: 10.1093/aje/kww139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Committee DGA. Scientific report of the 2015 dietary guidelines advisory committee. Washington (DC): USDA and US Department of Health and Human Services; 2015. [Google Scholar]
- 43.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 44.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161(15):1857–62. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 45.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72(4):912–21. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 46.Kerver JM, Yang EJ, Bianchi L, Song WO. Dietary patterns associated with risk factors for cardiovascular disease in healthy US adults. Am J Clin Nutr. 2003;78(6):1103–10. doi: 10.1093/ajcn/78.6.1103. [DOI] [PubMed] [Google Scholar]
- 47.Saulnier DMA, Spinler JK, Gibson GR, Versalovic J. Mechanisms of probiosis and prebiosis: considerations for enhanced functional foods. Curr Opin Biotechnol. 2009;20(2):135–41. doi: 10.1016/j.copbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(4):735–47. doi: 10.3945/ajcn.112.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heller KJ. Probiotic bacteria in fermented foods: product characteristics and starter organisms1–3. Am J Clin Nutr. 2001;73(2):374s–9s. doi: 10.1093/ajcn/73.2.374s. [DOI] [PubMed] [Google Scholar]
- 50.Plessas S, Bosnea L, Alexopoulos A, Bezirtzoglou E. Potential effects of probiotics in cheese and yogurt production: a review. Engineering in Life Sciences. 2012;12(4):433–40. [Google Scholar]
- 51.Biong AS, Veierod MB, Ringstad J, Thelle DS, Pedersen JI. Intake of milk fat, reflected in adipose tissue fatty acids and risk of myocardial infarction: a case-control study. Eur J Clin Nutr. 2005;60(2):236–44. doi: 10.1038/sj.ejcn.1602307. [DOI] [PubMed] [Google Scholar]
- 52.Goldbohm RA, Chorus AM, Galindo Garre F, Schouten LJ, van den Brandt PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr. 2011;93(3):615–27. doi: 10.3945/ajcn.110.000430. [DOI] [PubMed] [Google Scholar]
- 53.de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR, Jr, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Am J Clin Nutr. 2012;96(2):397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M, Li Y, Sun Q, Pan A, Manson JE, Rexrode KM, et al. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr. 2016;104:1209–17. doi: 10.3945/ajcn.116.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, et al. Saturated fat as compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66(14):1538–48. doi: 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang J, Zhou Q, Kwame Amakye W, Su Y, Zhang Z. Biomarkers of dairy fat intake and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2016:1–9. doi: 10.1080/10408398.2016.1242114. [DOI] [PubMed] [Google Scholar]
- 57.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–8. doi: 10.1016/S2213-8587(14)70146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, et al. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18(7):503–10. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86(1):189–97. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 60.Yakoob MY, Shi P, Willett WC, Rexrode KM, Campos H, Orav EJ, et al. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation. 2016;133(17):1645–54. doi: 10.1161/CIRCULATIONAHA.115.018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santaren ID, Watkins SM, Liese AD, Wagenknecht LE, Rewers MJ, Haffner SM, et al. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr. 2014;100(6):1532–40. doi: 10.3945/ajcn.114.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw K-T, Wareham NJ, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk cohort. Am J Clin Nutr. 2010;92(5):1214–22. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- 63.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in US adults. Ann Intern Med. 2010;153(12):790–9. doi: 10.1059/0003-4819-153-12-201012210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Döring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. Am J Clin Nutr. 2011;93(1):127–42. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- 65.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, et al. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the multi-ethnic study of atherosclerosis (MESA) Am J Clin Nutr. 2013;97(4):854–61. doi: 10.3945/ajcn.112.045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willett W. Nutritional epidemiology. Oxford University Press; 2012. [Google Scholar]
- 67.Risérus U, Marklund M. Milk fat biomarkers and cardiometabolic disease. Curr Opin Lipidol. 2017;28(1):46–51. doi: 10.1097/MOL.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosell M, Johansson G, Berglund L, Vessby B, de Faire U, Hellenius ML. The relation between alcohol intake and physical activity and the fatty acids 14:0, 15:0 and 17:0 in serum phospholipids and adipose tissue used as markers for dairy fat intake. Br J Nutr. 2005;93(1):115–21. doi: 10.1079/bjn20041290. [DOI] [PubMed] [Google Scholar]
- 69.Jenkins BJ, Seyssel K, Chiu S, Pan P-H, Lin S-Y, Stanley E, et al. Odd chain fatty acids; new insights of the relationship between the gutmicrobiota, dietary intake, biosynthesis and glucose intolerance. Sci Rep. 2017;7:44845. doi: 10.1038/srep44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palmquist DL. Milk fat: origin of fatty acids and influence of nutritional factors thereon. In: Fox PF, McSweeney PLH, editors. Advanced dairy chemistry volume 2 lipids. Boston, MA: Springer US; 2006. pp. 43–92. [Google Scholar]
- 71.Huth PJ, Park KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr: Int Rev J. 2012;3(3):266–85. doi: 10.3945/an.112.002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benatar JR, Sidhu K, Stewart RA. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS One. 2013;8(10):e76480. doi: 10.1371/journal.pone.0076480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jousilahti P, Laatikainen T, Peltonen M, Borodulin K, Männistö S, Jula A, et al. Primary prevention and risk factor reduction in coronary heart disease mortality among working aged men and women in eastern Finland over 40 years: population based observational study. BMJ. 2016;352 doi: 10.1136/bmj.i721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, et al. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response Meta-analysis. PLoS One. 2013;8(9):e73965. doi: 10.1371/journal.pone.0073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.García Yu IA-L, Sánchez-Aguadero N, Recio-Rodríguez JI. Chapter 25 - Effect of the fat component of dairy products in cardiovascular health, vascular structure and function A2 - Watson, Ronald Ross. In: Collier RJ, Preedy VR, editors. Nutrients in dairy and their implications on health and disease. Academic Press; 2017. pp. 325–32. [Google Scholar]
- 76.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 77.Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obes Res. 2003;11(11):1325–32. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- 78.Mattar R, de Campos Mazo DF, Carrilho FJ. Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol. 2012;5:113–21. doi: 10.2147/CEG.S32368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dugan CE, Barona J, Fernandez ML. Increased dairy consumption differentially improves metabolic syndrome markers in male and female adults. Metab Syndr Relat Disord. 2014;12(1):62–9. doi: 10.1089/met.2013.0109. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Steffen LM, Vessby B, Basu S, Steinberger J, Moran AM, et al. Obesity modifies the relations between serum markers of dairy fats and inflammation and oxidative stress among adolescents. Obesity (Silver Spring) 2011;19(12):2404–10. doi: 10.1038/oby.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalmeijer GW, Struijk EA, van der Schouw YT, Soedamah-Muthu SS, Verschuren WMM, Boer JMA, et al. Dairy intake and coronary heart disease or stroke—a population-based cohort study. Int J Cardiol. 2013;167(3):925–9. doi: 10.1016/j.ijcard.2012.03.094. [DOI] [PubMed] [Google Scholar]