Abstract
Data on the public’s reactions to online tailored colorectal cancer (CRC) risk estimates is sparse. We assessed among 560 men and women aged 50–75 with no CRC screening history reactions to online tailored CRC estimated comparative risk (i.e., self vs. other their age and sex). Assessed were reactions to estimate (i.e., repeating back estimate, match between perceived comparative risk and estimate, accuracy and usefulness of estimate, emotional reactions), risk appraisals and screening intentions. 73% of the sample accurately repeated back their estimate; the match between perceived comparative risk and the estimate was lowest among those informed of being at higher risk. Higher estimates were viewed as less useful and evoked more negative emotions. Viewing the estimate as more useful and experiencing more negative emotions were related with higher risk appraisals and, in turn, screening intentions. These data indicate that adults at higher comparative risk resist accepting a higher risk status.
Keywords: Colorectal Cancer, Cancer Screening, Risk Perceptions, World-wide Web
Introduction
Colorectal cancer (CRC) is the 3rd leading cause of cancer death among men and women in the United States (U.S.) (Lin et al., 2016). Fortunately, screening for CRC via guaiac and immunochemical-based fecal occult blood tests (FOBT/FIT), sigmoidoscopy (SIG) and colonoscopy saves lives (Lin et al., 2016). Thus, major health organizations such as the American Cancer Society and the U.S. Preventative Services Task Force (USPSTF) have put forth screening guidelines (Smith et al., 2016; U.S. Preventative Services Task Force, 2016). For example, among asymptomatic men and women at average risk who are between the ages of 50 to 75, the USPSTF screening guidelines include FOBT/FIT everyone to three years, SIG every five or ten years with annual FOBT, colonoscopy every ten years, or virtual colonoscopy every five years. As of 2015, about 38% of the U.S. population was not following the USPSTF guidelines (White et al., 2017).
Many interventions to promote CRC screening target health organizations and clinic settings (Sabatino et al., 2012; Senore et al., 2015). These interventions involve sending print or phone reminders and/or having medical staff (e.g., primary care provider) encourage patients to screen. To this end, one general practice gave patients who were off-schedule the ability to view their tailored CRC risk estimate online (Sequist et al., 2011); patients who reviewed their risk estimate were more likely to get screened than patients who did not view their risk (30% vs. 15%). These findings align with a recent meta-analysis showing a positive link between heightened perceived CRC risk and screening (Atkinson et al., 2015; see also Edwards et al., 2013). These results reinforce the utility of giving patients CRC risk online and suggest that conveying tailored CRC risk via the internet more broadly may encourage screening. Indeed, web-based approaches have high reach and potential impact. For example, based on Pew Internet reports, as of 2016, 72% of internet users looked online for health information within the past year (http://www.pewinternet.org/2013/01/15/health-online-2013/), and numerous materials on CRC screening exist online (Fleisher et al., 2012; John et al., 2016). However, to the best of our knowledge, publicly available web-based educational screening materials do not provide tailored CRC risk estimates using existing CRC risk algorithms to increase screening (Waters et al., 2009).
Conveying tailored CRC risk online to promote screening requires individuals to extract key data (e.g., risk estimates, risk factors), and understand, accept and apply the data to their personal risk appraisals. A challenge is that individuals informed of being at higher risk may feel threatened and engage in motivated reasoning/defensive processes to reduce this threat. Should this occur, it can reduce the potency of risk feedback to promote screening. This reasoning borrows from the Extended Parallel Processing Response Model (EPPM, Witte, 1994); according to the EPPM, defensive reasoning is common, especially when people see few if any effective ways to avert the threat (i.e., response efficacy) or lack confidence in being able to avert the threat (i.e., self-efficacy).
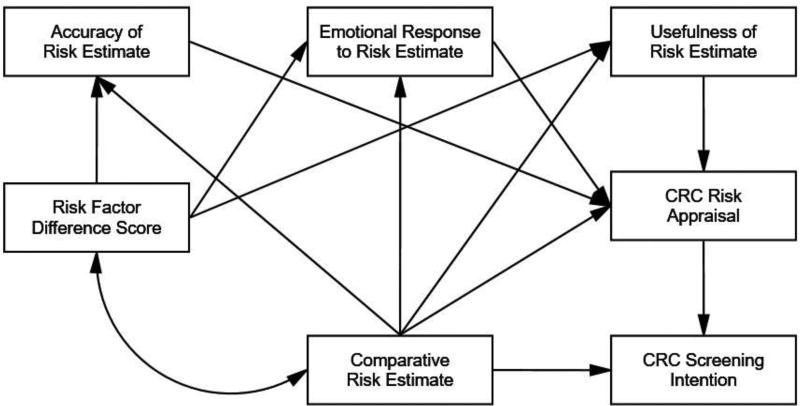
Our theoretical model, shown in Figure 1, captures self-reported outcomes related to the processing and acceptance of tailored comparative CRC risk feedback and risk appraisals (i.e., thoughts and feelings about risk) and their effects on screening. We hypothesized that receipt of higher comparative risk estimates (i.e., how one’s risk compares to others) as well as having a greater number of risk increasing than risk decreasing risk factors (measured by risk factor difference score) will instigate defensive reasoning processes. To this end, McQueen and colleagues (McQueen et al., 2013) detail defensive processes concerning CRC that occur in four sequential steps: preattention, focal attention, comprehension, and elaboration and assessment. Each step has strategies people can use to lessen perceived CRC threat. These include (a) attention avoidance (e.g., purposefully being unaware of risk information) in the preattention step, (b) blunting (e.g., mental disengagement and avoiding comprehension of CRC risk) for the focal attention step, (c) suppression (e.g., avoiding inferences of personal risk) for the comprehension step, and (d) counter-arguments (e.g., acknowledging relevance of risk but downplaying severity of threat) for the elaboration and assessment step.
Figure 1.
A Theoretical Model of Relations among Risk Estimate, Reactions to Risk Estimate, Risk Appraisals, and Colorectal Cancer Screening Intention.
We explore reactions to CRC risk estimates that align with these strategies. For blunting, we provide descriptive statistics of how many study participants repeated back exactly their tailored risk feedback. Inaccurately repeating back one’s risk estimate can be viewed as blunting since it can reflect inattention to risk feedback, which occurs relatively often. For example, in a study by Weinstein and colleagues, members of a health organization (n = 353) received tailored colon cancer risk estimates either as an absolute or absolute plus relative risk estimates (Weinstein et al., 2004). Overall, 12% and 36% of participants in the absolute and in the absolute plus relative risk conditions, respectively, recalled their estimates incorrectly. For suppression, which involves denouncing being at high risk, we also provide descriptive statistics of concordance (i.e., match) between tailored comparative risk and perceived comparative risk. Weinstein and colleagues found that only 45% and 39% of their sample had a match between their perceived risk and their absolute or relative risk estimate, respectively. We suggest suppression is evident when mismatches between perceived risk and risk estimates occurs more often for estimates of higher risk than for estimates of average and especially lower risk. Lastly, we assessed via self-report perceived usefulness and credibility of the risk estimate to capture counter-arguments, which entails strategies such as derogation of evidence. We hypothesize that individuals who receive an estimate of being at higher risk will view the estimate as less useful and credible.
Our model acknowledges that tailored risk estimates can evoke emotional reactions that can affect risk appraisals. According the affect heuristic (Slovic et al., 2004), people informed of being at higher risk should experience more negative affect (e.g., worry, anxiety). Thus, emotional reactions, along with how useful and credible the risk estimate is perceived, are deemed to mediate effects of the estimate on risk appraisals (See Figure 1). Specifically, individuals who view their risk estimate as accurate and credible and who experience stronger negative emotional reactions to the estimate, are hypothesized to report higher risk appraisals. In turn, higher risk appraisals should correlate with stronger CRC screening intentions.
In sum, herein we report how a sample of adults aged 50 to 75 who report no history of CRC screening reacted to how their CRC risk compared to others their age and sex (i.e., tailored comparative risk estimate) using on online risk algorithm (http://www.yourdiseaserisk.wustl.edu). We first report the proportion who repeated back accurately their risk estimate and the proportion who had a match (i.e., concordance) between their perceived comparative risk and their risk estimate. We then report overall fit of the hypothesized links in Figure 1 using path analysis. We focused on adults without a history of screening due to the sparsity of data available concerning their reactions to tailored online CRC risk estimates and the extent this strategy encourages screening (Han et al., 2015).
Methods
Procedures
Potential participants were recruited to take part in an online study to assess how CRC risk estimates, as well as framed educational messages, influences CRC screening. Potential participants were aged 50 to 75 who were panel members for the professional organization, Growth for Knowledge (GfK). After receipt of an invitation, panel members completed an eligibility screener; those who self-reported never having had CRC screening via colonoscopy/virtual colonoscopy, SIG, or FOBT/FIT were eligible and consented to the trial. Upon consent, the order of events was as follows: 1) assessment of CRC risk factors to compute a tailored comparative risk estimate, 2) receipt of risk estimate, 3) assessing reactions about the estimate, 4) review of one of two brochures on CRC screening that varied message framing (i.e., gain vs. loss frame), and 5) completing measures that included screening intentions. Of note, given their placement, the framed brochures were intended to influence screening intentions and could not affect reactions to the risk estimate. Because this paper focuses on reactions to and effects of the estimates, and the lack of framing effects on screening intentions, framing will not be discussed further. The Duke University Medical Center IRB approved this study.
Eligibility and recruitment
Potential participants were recruited from GfK’s Knowledge Networks online panel, a panel representative of the U.S. population, providing sampling coverage of 97% of the U.S. adult population via address-based sampling. A random sample of panelists who met the age criteria were approached and provided with a study description. Those interested completed a screener. Eligible participants with no history of screening and CRC, consented and then answered questions used to generate the tailored CRC risk estimate. Participants were given an incentive per GfK’s incentive structure plus an additional $15.00.
Risk assessment and feedback
A risk assessment for CRC consisted of questions about: family history, BMI, CRC screening (reassessment), history of cancers, aspirin use, inflammatory bowel disease, use of multivitamin, calcium and vitamin D supplements, consumption of milk products, meat intake, alcohol consumption, physical activity, and estrogen replacement (see http://www.yourdiseaserisk.wustl.edu). Responses were used to create a tailored comparative risk estimate per the Your-Disease-Risk algorithm. The algorithm was evaluated for validity using CRC incidence in prospective cohort data, with data showing good agreement for CRC with a concordance statistic of .71 and .67 for men and women, respectively (Kim et al., 2004). This algorithm was used because biological samples are not needed to calculate CRC risk and hence practical for online application (Usher-Smith et al., 2016).
Participants then received the following message, “Based on your answers, on top of the next page you will get YOUR ESTIMATED RISK of getting colorectal cancer in the next 10 years compared to others your sex and age.” Mimicking the risk presentation format of the Your-Disease-Risk website, participants were given a risk estimate that took on one of seven levels “Very much below average”, “Much below average”, “Below average”, “Average”, “Above average”, “Much above average”, and “Very much above average.” The estimate was highlighted further by pointing to it on a colored vertical bar; the bar became increasingly green with lower risk estimates, increasingly red with higher risk estimates, and yellow for average risk. Participants were also informed of which factors increased and decreased their CRC risk. After receipt of this information, we assessed reactions to the risk estimate and risk appraisals.
Measures
Repeat of risk estimate
Participants were asked, “What were you told was your estimated risk of getting colorectal cancer compared to others your age and sex?” Response options were “Very much below average”, Much below average”, “Below average”, “Average”, “Above average”, “Much above average”, and “Very much above average.” Participants who repeated back exactly their estimate were deemed accurate (e.g., estimate was above average and person repeated back above average); all others were deemed inaccurate due to overestimation or underestimation.
Reactions to risk estimate
Perceived accuracy was assessed by, “How accurate was your estimated risk of getting colorectal cancer compared to someone your age and sex?” Responses were assessed on a six-point Likert scale from 1 = Extremely inaccurate to 6 = Extremely accurate. Emotional reactions towards the estimate were assessed on four 7-point bipolar scales: good/bad, not worried/worried, not anxious/anxious, and safe/unsafe. Items were summed and averaged (α = .94). Perceived usefulness was assessed by, “How personally useful did you find your estimated risk of getting colorectal cancer to be in relation to your health?” assessed on a 7-point Likert scale from 1 = Not at all useful to 7 = Extremely useful.
Risk appraisals
Consistent with the notion that risk appraisals capture emotions and cognitions about risk (Sheeran et al., 2014), this variable assessed these attributes using six items (see Table 1). Based on principal components analysis, all items loaded on one component that explained 60% of the variance, with loadings > .64. Thus, we created a composite risk appraisal score for each participant by using the Proc Score SAS procedure to derive a linearly transformed weighted average of the six-items. The weighted linear transformation was needed because not all measures used 7-point scales (e.g., absolute risk based on a 100-point scale).
Table 1.
CRC Risk Appraisal Measures
| Type of risk assessment | Question(s) and response options |
|---|---|
| Perceived comparative risk | “What do you think is your chance of getting colorectal cancer in the next 10 years compared to the average person your age and sex?” Response options were “Very much below average”, Much below average”, “Below average”, “Average”, “Above average”, “Much above average”, and “Very much above average.” |
| Perceived absolute risk | “What do you think is your chance of getting colorectal cancer in the next 10 years?” Response options were, “No chance”, “Very unlikely”, “Unlikely”, “Moderate chance”, “Likely”, “Very Likely”, and “Certain to happen.” This was followed by the same question asking for a numerical estimate from 0% = No chance to 100% = Certain to happen. |
| Worry/feelings about getting CRC | “How worried are you about getting colorectal cancer in the next 10 years.” Response options were “Not at all worried”, “Slightly worried”, “Somewhat worried”, “Very worried”, and “Extremely worried.” |
| Feelings/beliefs about risk conditional on screening. | “If I don’t get screened for colorectal cancer, I would feel likely to get colorectal cancer in my lifetime” (Weinstein et al., 2007). Response anchors were from 1 = Strongly disagree to 7 = Strongly agree. They were further asked, “How much, if at all, would you lower your chance of getting colorectal cancer if you get screened within the next six months?” Response anchors were 1 = Not at all to 7 = Completely. |
Note: All items were pooled to create a single risk appraisal score.
Screening intention
Immediately after reviewing the gain or loss frame brochure, participants were asked: (1) “Do you intend to get screened for colorectal cancer in the next six months?” (1 = Definitely no to 7 = Definitely yes); (2) “How do you feel about getting screened for colorectal cancer in the next six months? (1 = Very negative to 7 = Very positive); and (3) “Do you intend to talk to a doctor about colorectal cancer screening in the next six months.” (1 = Definitely no to 7 = Definitely yes). All items loaded on one component, explaining 88% of the variance, with loadings > .92. Items were summed and averaged (α = .92).
Statistical Analysis
Descriptive statistics were computed to describe sample characteristics and distributions of comparative risk. Associations among interval variables were based on the Pearson correlation; associations between ordinal variables were computed using the Mantel Haenszel (MH) chi-square with one degree of freedom. To examine relationships among constructs in Figure 1, we utilized path analysis, a special case of structural equation modeling. The path analysis estimated direct, indirect (or mediated), and total effects. Model fit was evaluated using the chi-square of the estimated model (χ2), goodness of fit index (GFI), normed fit index (NFI), incremental fit index (IFI), relative fit index (RFI), comparative fit index (CFI), and root mean square error of approximation (RMSEA). A nonsignificant chi-square value (i.e., p > .05) suggests a good overall model fit to the data. For GFI, NFI, IFI, RFI, and CFI, values > .90 indicate a good model fit; whereas RMSEA should be below .06 (Hu & Bentler, 1999). The path analysis was conducted using IBM AMOS, and the Sobel’s test was used for testing mediated effects. Although AMOS does not allow missing data for path analysis, the sample had less than 3% missing values; further, the Little’s test (Little, 1988) indicated that the missing pattern was missing completely at random (χ2(36) = 44.7, p =.15). Thus, missing values were imputed by their arithmetic means based on the non-missing data before running AMOS. An α = .05 was set for all hypothesis testing.
Results
Sample characteristics
Of a total of 6055 panel members approached for participation, 3333 completed the screener; of these, 619 (18.5%) responded, qualified, and received a tailored comparative risk estimate. Upon further review, 59 reported having been screened, found during the risk factor assessment, and were excluded from analyses. Thus, the final sample was 560.
Sample characteristics are shown in Table 2. Nearly half of the sample were men, with a mean age of 57.9 (SD = 6.2). About three-quarters of the sample were non-Hispanic white. Almost half (46.0%) of the sample had a high school education or less with the rest having some college education (28.4%) or being college graduates (25.5%). Most had health insurance and nearly 58.2% were married. Slightly less than half (46.9%) of the sample was working full-time.
Table 2.
Demographic Characteristics of the Participants (N = 560).
| Variable | n | Mean (SD) or Percent |
|---|---|---|
| Gender (Male) | 262 | 46.8% |
| Age | 560 | 57.9 (6.2) |
| Race | ||
| White, non-Hispanic | 430 | 76.8% |
| Black, non-Hispanic | 41 | 7.3% |
| Other, non-Hispanic | 19 | 3.4% |
| Hispanic | 53 | 9.5% |
| 2+ Races, non-Hispanic | 17 | 3.0% |
| Education | ||
| Less than high school | 55 | 9.8% |
| High school | 203 | 36.2% |
| Some college | 159 | 28.4% |
| Bachelor's degree or higher | 143 | 25.5% |
| Has health insurance | 478 | 85.4% |
| Married | 326 | 58.2% |
| Job Status | ||
| Work fulltime | 262 | 46.9% |
| Work part-time | 68 | 12.2% |
| Unemployed | 74 | 13.2% |
| Retired | 155 | 27.7% |
Note. Numbers have been rounded
Distribution of tailored comparative risk estimates
Table 3 shows the distribution of the seven comparative risk estimates. Among the demographic variables, only education was associated with the comparative risk estimates (χ2MH= 10.49, p < 0.003).
Table 3.
Distribution and Levels of Tailored Comparative Risk Estimates and Percent Reporting Back Accurately Their Estimate
| Level of Tailored Comparative Risk Estimate |
n | Percent at Each Level of Risk |
Percent Repeating Back Accurately their Tailored Risk Estimate (N = 557) |
|---|---|---|---|
| Very much below average | 3 | 0.5% | 100.0% (n = 3) |
| Much below average | 121 | 21.6% | 64.2% (n = 77) |
| Below average | 91 | 16.2% | 80.2% (n = 73) |
| Average | 37 | 6.6% | 83.8% (n = 31) |
| Above average | 180 | 32.1% | 81.6% (n = 146) |
| Much above average | 122 | 21.8% | 60.3% (n = 73) |
| Very much above average | 6 | 1.1% | 50.0% (n = 3) |
Note. Numbers have been rounded. Match between the tailored comparative risk estimate and repeating back the risk estimate is based on 557 observations.
Repeating back risk estimate
Table 3 shows the percent of participants who accurately repeated their tailored risk estimate (N = 557). Overall, 72.9% (n = 406) of the sample accurately repeated back their estimate. Education was positively associated with repeating back accurately the risk estimate; among those with high school education or less, 67.7% accurately reported back their risk estimate compared to 77.3% of participants with some college education and higher (χ2MH = 8.8, p < .003). Non-Hispanic whites were more likely to repeat back accurately their estimate compared to other race/ethnicity (75.9% vs. 62.8%, χ2(4) = 11.4, p < .03). Age, gender, health insurance, marital and employment status were unrelated to repeating back one’s risk estimate.
Concordance between perceived comparative risk and tailored risk estimate
Average perceived comparative risk was significantly lower than the average tailored estimated comparative risk (M = 3.3, SD = 1.3 vs. M = 4.2, SD = 1.5, respectively; p < .001), albeit positively related (r = .41, p < .0001). We examined where discrepancies occurred between the two. To this end, and consistent with Emmons and colleagues’ approach (Emmons et al., 2004), we operationalized concordance (i.e., match) at a group level of risk. If a participant received a tailored risk estimate of “Very much above average”, “Much above average”, or “Above average”, the person was deemed concordant for being at higher risk if they perceived their comparative risk as either “Very much above average”, “Much above average”, or “Above average”; otherwise they were deemed not concordant. The same logic was applied for concordance at lower and average risk. As operationalized, these data reveal whether a person’s perceived comparative risk matched their estimated risk grouping in general. Concordance was computed for the entire sample and subsample that accurately repeated back their estimate.
In the entire sample, 30.5% (n = 171) of participants had an exact match between their perceived comparative risk and their tailored comparative risk estimate (i.e., perceived risk was identical to what they were told). When participants were grouped based on their tailored risk estimate into above average, average and below average risk, percent concordant was 82.2% at below average risk, 67.6% at average risk, and 22.6% at above average risk; hence, concordance differed significantly overall (χ2MH = 129.1, p < .0001). Bonferroni adjusted contrasts (p = .0167) revealed no difference in concordance between below average and average risk (p < .05); concordance was significantly lower between above average risk and the other two risk levels (ps < .0001).
Among the subsample of participants (n = 406) who accurately repeated back their estimate, 42.1% (n = 171) had exact match between their perceived comparative risk and their tailored comparative risk estimate. When participants were grouped based on their tailored risk estimate into above average, average and below average risk, percent concordant was 85.6% at below average risk, 77.4% at average risk, and 24.4% at above average risk. Thus, concordance differed significantly (χ2MH =113.6, p < .0001), with the same pattern of resulting contrasts as with the full sample. None of the above findings in the full or subsample differed by any demographic variable.
Relations among path model variables
As shown in Table 4, significant relationships existed among the seven variables in the path model (Figure 1). On average, participants viewed the risk estimates as somewhat accurate (M = 4.0, SD = 1.3), did not experience a substantial amount of negative affect (M = 3.3, SD = 1.7), and found the information somewhat useful (M = 4.6, SD = 1.7). Consistent with hypotheses, reactions varied by risk estimate level. With increasing risk, the estimates were viewed as less useful (r = −.16, p < .001) and evoked greater negative affect (r = .54, p < .001). Further, participants who received a higher estimate reported a higher composite risk appraisal score (r = .34, p < .001) – thus, a single estimate was associated with a broader set of risk perception measures. Individuals with higher composite risk appraisals scores reported stronger negative emotions to feedback (r = .65, p < .001) and viewed estimates as more useful (r = .30, p<.001). However, there was no association between risk appraisals and perceived accuracy (r = .04, ns). Participants who had more risk increasing (M = 5.2, SD = 1.5) than risk decreasing risk factors (M = 6.4, SD = 1.6), as a difference score (M = −1.25, SD = 3.0), reported higher composite risk appraisals (r = .32, p < .001), viewed their risk estimate as less useful (r = −.20, p < .001), and felt more negative emotions (r = .53, p < .001).
Table 4.
Correlations among Comparative Risk Estimate, Reactions to Risk Estimate, Risk Appraisals and CRC Screening Intentions
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Comparative Risk Estimate | – | |||||
| 2. Risk Factor Difference Score | .86* | – | ||||
| 3. Accuracy of Risk Estimate | −.06 | −.08 | – | |||
| 4. Emotional Response to Risk Estimate | .54* | .53* | -.07 | – | ||
| 5. Usefulness of Risk Estimate | −.16* | −.20* | .27* | .06 | – | |
| 6. CRC Risk Appraisal | .34* | .32* | .04 | .65* | .30* | – |
| 7. CRC Screening Intention | .01 | .01 | .06 | .24* | .33* | .44* |
p < .001(2-tailed).
We hypothesized that higher perceived comparative risk and higher composite risk appraisals would correlate positively with screening intentions (M = 3.8, SD = 1.9). Indeed, participants reported stronger screening intentions when they had higher perceived comparative risk (r = .26, p < .01) and higher composite risk appraisal scores (r = .45, p < .0001).
Path analysis
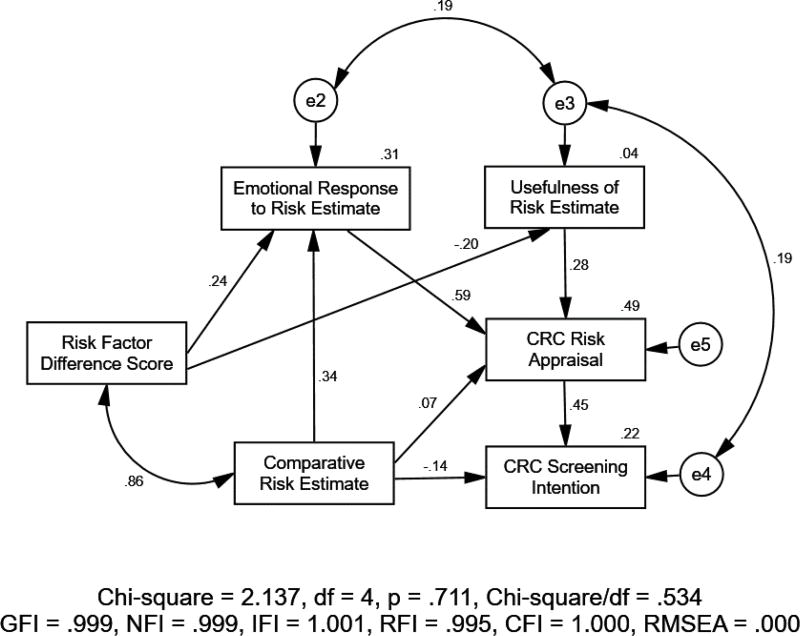
After initially fitting the hypothetical path model (Figure 1) to the data, four non-significant paths (three involving perceived accuracy of estimate, one from comparative risk estimate to perceived usefulness of estimate) were removed and two error correlations (one between emotional response to estimate and perceived usefulness of estimate, and another between perceived usefulness of estimate and CRC screening intention) were added based on the suggested modification indices produced by AMOS. The final model (Figure 2) with standardized estimates fit the data very well [χ2(4) = 2.137 (p = .711), RMSEA < .001, GFI = .999, NFI = .999 and CFI = 1.000]. Noteworthy, risk estimates had a negative relation with screening intentions (β = −0.14, p < .001). The difference score between risk increasing and decreasing factors, while being strongly related to the final risk estimate (r = .86, p < .001), was positively related to emotional reactions (β = 0.24, p < .001) while being negatively related to the perceived usefulness of the estimate (β = −0.20, p < .001); it had no relations with risk appraisals or screening intention. This suggests effects of providing risk factor information on screening intentions is mediated most strongly through effects of the risk estimate. The standardized total effects of the risk factor difference score and of the comparative risk estimate on screening intention were 0.039 and −0.021, respectively.
Figure 2.
The Final Parsimonious Path Model of Relations among Comparative Risk Estimate, Reactions to Risk Estimate, Risk Appraisals, and Colorectal Cancer Screening Intention with Standardized Estimates of Path Coefficients Significant at p < .05.
Discussion
Using a sample of adults who have never screened for CRC, findings suggest that acceptance of online CRC risk estimates poses challenges. First, about 27% of the sample did not repeat back their risk estimate accurately immediately after receipt. Second, irrespective of whether participants repeated back accurately their risk estimate, individuals at higher estimated risk were least likely to have a match between their perceived risk and their estimate; rather they perceived their comparative risk as lower. This pattern indicates that many adults express unrealistic optimism, believing they are at a lower risk than their risk factors warrant (Shepperd et al., 2015). Third, adults who received higher risk estimates viewed the estimate as less useful. Overall, findings point to the likely use among participants at higher risk of defensive strategies, such as blunting, suppression, unrealistic optimism and counter-arguing. Practically, this means that providing online CRC risk estimates to promote screening will not work well among people at higher risk, unless strategies are in place that can curb the defensiveness this group shows.
One such strategy may be self-affirmation. Self-affirmation exercises ask people to reflect upon cherished values, actions and personal attributes often prior to being exposed to potentially threatening information. Self-affirmations serve to maintain self-integrity and self-worth, thereby reducing a person’s motivation to defend against threats while enhancing acceptance of high health risk information (Sherman et al., 2000). Thus, increasing acceptance of potentially threatening CRC risk feedback may occur by providing an opportunity to self-affirm (Klein et al., 2010). Relatedly, based on the EPPM, messaging should aim to increase a person’s efficacy beliefs since doing so can also reduce defensive responses to high risk feedback. Finally, a simple strategy is to encourage individuals to be open-minded about their risk due to its relevance in health decisions (Jenkins & Sheeran, under review). Unfortunately, experimental tests of these strategies targeting individuals who have never screened are lacking and are needed.
We found that non-Caucasians and those with lesser education were least accurate in repeating back their risk estimate. This may reflect lower health literacy, as has been found among less educated and non-Caucasian samples (Martin et al., 2009). It is critical to address health literacy challenges. For example, among less (health) literate populations, and consistent with risk communication practices (Fagerlin et al., 2011), key facts should be conveyed concisely to avoid overwhelming the consumer with data. This study may not have achieved this end. Rather, the risk estimate, a graphic to display the estimate, and a listing of factors that increased and decreased risk were presented on a single page. Among the less health literate, the abundance of data may have caused interpretational challenges, resulting in less attention to the facts. Presenting the estimate only on one page may have helped. Further, users may need to be informed when they report an incorrect risk estimate. These are strategies to test in the future.
Participants received information concerning their risk factors as well as a verbal risk estimate. The estimate was highly correlated with the risk factor difference score. Both had very similar effects on perceived composite risk appraisals and reactions to the estimate. Hence, a question is whether the provision of both reinforces effects on risk appraisals and ultimately screening intentions more so than conveying either one only. When both risk factors and the risk estimate are provided, participants may question the accuracy of either piece of information, potentially lessening the efficacy of risk feedback to modify risk appraisals. Unfortunately, we did not have participants evaluate the accuracy and comprehensiveness of their risk factors, which should be done in future research. For example, participants may have felt their risk estimate did not capture risk factors that they personally felt were important (Emmons et al., 1999).
There are several study limitations. Participants were recruited from a panel created to be representative of adults in the U.S.; how their reactions compare to representative samples of adults who do not participate in online studies is unknown. We provided comparative, but not absolute, CRC risk estimates as other studies have done (Han et al., 2015; Weinstein et al., 2004) preventing a test of which type of feedback is more influential, if any. Further, missing was a no risk feedback arm. People who do not receive a risk estimate could report higher or lower risk appraisals and screening intentions than those who do receive an estimate. Our study did not assess other key variables, such as health literacy. Hence, we could not test whether relations between race, education and repeating back accurately the risk estimate were mediated by health literacy. Lastly, findings do not provide definitive evidence of defensiveness to risk feedback and the sequences of those events; alternative explanations cannot be ruled out without the use of experimental designs.
In summary, the aim of this study was to simulate the receipt of and obtain reactions to online risk estimates to encourage screening among adults who have never screened, a high risk group in need of intervention. Our findings show that acceptance of tailored online risk estimates is suboptimal, especially among individuals who are at higher estimated comparative risk. In this group, processes of motivated reasoning/defensiveness are strongly implicated. To address these processes, several strategies that can enhance acceptance of CRC risk estimates were offered and deserve future testing. Our findings also replicate the positive link between risk appraisals and screening intentions (Atkinson et al., 2015), while highlighting key pathways through which a single CRC risk estimate influences screening intentions (Figure 1). Ultimately a strong test of the efficacy of tailored risk estimates to influence screening will require enhancing acceptance of risk estimates while taking into account the various modalities through which it can be delivered (e.g., clinic, web).
Acknowledgments
We thank Dr. Graham Colditz and Hank Dart for providing access to and helping with programming of their Your Disease Risk algorithm for colorectal cancer. We thank GfK for their responsiveness and collection of the online data. We thank Dr. Amy McQueen for her helpful comments pertaining to motivated reasoning/defensive processing of colorectal cancer risk estimates.
Funding Source
This work was supported by the National Institutes of Health grant R21-CA181256.
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Contributor Information
Isaac M. Lipkus, Duke University School of Nursing, 307 Trent Dr., Durham, NC 27710, USA, Isaac.Lipkus@Duke.edu
Constance Johnson, University of Texas Health School of Biomedical Informatics, 6901 Bertner Ave. Houston, TX 77030, USA, Constance.M.Johnson@uth.tmc.edu.
Sathya Amarasekara, Duke University School of Nursing, 307 Trent Dr., Durham, NC 27710, USA, Sathya.amarasekara@duke.edu.
Wei Pan, Duke University School of Nursing, 307 Trent Dr., Durham, NC 27710, USA, Wei.pan@duke.edu.
John Updegraff, Kent State University, Kent, OH 44242, USA, jupdegr1@kent.edu.
References
- Atkinson TM, Salz T, Touza KK, Li Y, Hay JL. Does colorectal cancer risk perception predict screening behavior? A systematic review and meta-analysis. Journal of Behavioral Medicine. 2015;38(6):837–850. doi: 10.1007/s10865-015-9668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons KM, Koch-Weser S, Atwood K, Conboy L, Rudd R, Colditz G. A qualitative evaluation of the Harvard Cancer Risk Index. Journal of Health Communication. 1999;4(3):181–193. doi: 10.1080/108107399126904. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Wong M, Puleo E, Weinstein N, Fletcher R, Colditz G. Tailored computer-based cancer risk communication: Correcting colorectal cancer risk perception. Journal of Health Communication. 2004;9(2):127–141. doi: 10.1080/10810730490425295. [DOI] [PubMed] [Google Scholar]
- Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: Ten steps to better risk communication. Journal of the National Cancer Institute. 2011;103(19):1436–1443. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher L, Kandadai V, Keenan E, Miller SM, Devarajan K, Ruth KJ, Weinberg DS. Build it, will they come? Unexpected findings from a study on a web-based intervention to improve colorectal cancer screening. Journal of Health Communication. 2012;17(1):41–53. doi: 10.1080/10810730.2011.571338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han PK, Duarte CW, Daggett S, Siewers A, Killam B, Smith KA, Freedman AN. Effects of personalized colorectal cancer risk information on laypersons’ interest in colorectal cancer screening: The importance of individual differences. Patient Education and Counseling. 2015;98(10):1280–1286. doi: 10.1016/j.pec.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jenkins KM, Sheeran P. Setting an open-mindedness goal improve persuasion: Evidence from three studies of fruit and vegetable consumption. Manuscript under review. [Google Scholar]
- John ES, John AM, Hansberry DR, Thomas PJ, Agarwal P, Deitch C, Chokhavatia S. Colorectal cancer screening patient education materials – how effective is outline health information? International Journal of Colorectal Disease. 2016;31(12):1817–1824. doi: 10.1007/s00384-016-2652-0. [DOI] [PubMed] [Google Scholar]
- Kim D. Validation of the Harvard Cancer Risk Index: a prediction tool for individual cancer risk. Journal of Clinical Epidemiology. 2004;57(4):332–340. doi: 10.1016/s0895-4356(03)00349-4. [DOI] [PubMed] [Google Scholar]
- Klein W, Lipkus IM, Scholl S, McQueen A, Cerully J, Haris PR. Self-Affirmation moderates effects of unrealistic optimism and pessimism on reactions to tailored risk feedback. Psychology and Health. 2010;25(10):1195–1208. doi: 10.1080/08870440903261970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Whitlock EP. Screening for colorectal cancer. Journal of the American Medical Association. 2016;315(23):2576. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198. doi: 10.2307/2290157. [DOI] [Google Scholar]
- Martin LT, Ruder T, Escarce JJ, Ghosh-Dastidar B, Sherman D, Elliott M, Lurie N. Developing predictive models of health literacy. Journal of General Internal Medicine. 2009;24(11):1211–1216. doi: 10.1007/s11606-009-1105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen A, Vernon SW, Swank PR. Construct definition and scale development for defensive information processing: An application to colorectal cancer screening. Health Psychology. 2013;32(2):190–202. doi: 10.1037/a0027311. [DOI] [PubMed] [Google Scholar]
- Sabatino SA, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers. American Journal of Preventive Medicine. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Senore C, Inadomi J, Segnan N, Bellisario C, Hassan C. Optimizing colorectal cancer screening acceptance: a review. Gut. 2015;64(7):1158–1177. doi: 10.1136/gutjnl-2014-308081. [DOI] [PubMed] [Google Scholar]
- Sequist TD, Zaslavsky AM, Colditz GA, Ayanian JZ. Electronic patient messages to promote colorectal cancer screening. Archives of Internal Medicine. 2011;171(7) doi: 10.1001/archinternmed.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychological Bulletin. 2014;140(2):511–543. doi: 10.1037/a0033065. [DOI] [PubMed] [Google Scholar]
- Shepperd JA, Waters EA, Weinstein ND, Klein WM. A Primer on unrealistic optimism. Current Directions in Psychological Science. 2015;24(3):232–237. doi: 10.1177/0963721414568341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DA, Nelson LD, Steele CM. Do messages about health risks threaten the self? Increasing the acceptance of threatening health messages via self-affirmation. Personality and Social Psychology Bulletin. 2000;26(9):1046–1058. doi: 10.1177/01461672002611003. [DOI] [Google Scholar]
- Slovic P, Finucane ML, Peters E, MacGregor DG. Risk as analysis and risk as Feelings: Some thoughts about affect, reason, risk, and rationality. Risk Analysis. 2004;24:311–322. doi: 10.1111/j.0272-4332.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- Smith RA, Andrews K, Brooks D, DeSantis CE, Fedewa SA, Lortet-Tieulent J, Wender RC. Cancer screening in the United States, 2016: A review of current American Cancer Society guidelines and current screening. CAL A Cancer Journal for Clinicians. 2016;66(2):95–114. doi: 10.3322/caac.21336. [DOI] [PubMed] [Google Scholar]
- Usher-Smith JA, Walter FM, Emery JD, Win AK, Griffin SJ. Risk prediction models for colorectal cancer: a systematic review. Cancer Prevention Research. 2015;9(1):13–26. doi: 10.1158/1940-6207.capr-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force. Screening for Colorectal Cancer. US Preventive Services Task Force Recommendation Statement. Journal of the American Medical Association. 2016;315:2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- Waters EA, Sullivan HW, Nelson W, Hesse BW. What is my cancer risk? How internet-based cancer risk assessment tools communicate individualized risk estimates to the public: Content analysis. Journal of Medical Internet Research. 2009;11(3) doi: 10.2196/jmir.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons KM. Colon cancer: Risk perceptions and risk communication. Journal of Health Communication. 2004;9(1):53–65. doi: 10.1080/10810730490271647. [DOI] [PubMed] [Google Scholar]
- White A, Thompson TD, White MC, et al. Cancer screening test use – United States. Morbidity and Mortality Weekly Reports. 2017;66:201–206. doi: 10.15585/mmwr.mm6608a1. http://dx.doi.org/10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K. Fear control and danger control: A test of the extended parallel process model (EPPM) Communication Monographs. 1994;61(2):113–134. doi: 10.1080/03637759409376328. [DOI] [Google Scholar]