Abstract
Neural progenitor cells in the developing dorsal forebrain give rise to excitatory neurons, astrocytes, and oligodendrocytes for the neocortex. While we are starting to gain a better understanding about the mechanisms that direct the formation of neocortical neurons and astrocytes, far less is known about the molecular mechanisms that instruct dorsal forebrain progenitors to make oligodendrocytes. In this study, we show that Sonic hedgehog (Shh) signaling is required in dorsal progenitors for their late embryonic transition to oligodendrogenesis. Using genetic lineage-tracing in mice of both sexes, we demonstrate that most oligodendrocytes in the embryonic neocortex derive from Emx1+ dorsal forebrain progenitors. Deletion of the Shh signaling effector Smo specifically in Emx1+ progenitors led to significantly decreased oligodendrocyte numbers in the embryonic neocortex. Conversely, knock-out of the Shh antagonist Sufu was sufficient to increase neocortical oligodendrogenesis. Using conditional knock-out strategies, we found that Shh ligand is supplied to dorsal progenitors through multiple sources. Loss of Shh from Dlx5/6+ interneurons caused a significant reduction in oligodendrocytes in the embryonic neocortex. This phenotype was identical to that observed upon Shh deletion from the entire CNS using Nestin-Cre, indicating that interneurons migrating into the neocortex from the subpallium are the primary neural source of Shh for dorsal oligodendrogenesis. Additionally, deletion of Shh from migrating interneurons together with the choroid plexus epithelium led to a more severe loss of oligodendrocytes, suggesting that the choroid plexus is an important non-neural source of Shh ligand. Together, our studies demonstrate that the dorsal wave of neocortical oligodendrogenesis occurs earlier than previously appreciated and requires highly regulated Shh signaling from multiple embryonic sources.
SIGNIFICANCE STATEMENT Most neocortical oligodendrocytes are made by neural progenitors in the dorsal forebrain, but the mechanisms that specify this fate are poorly understood. This study identifies Sonic hedgehog (Shh) signaling as a critical pathway in the transition from neurogenesis to oligodendrogenesis in dorsal forebrain progenitors during late embryonic development. The timing of this neuron-to-glia “switch” coincides with the arrival of migrating interneurons into the dorsal germinal zone, which we identify as a critical source of Shh ligand, which drives oligodendrogenesis. Our data provide evidence for a new model in which Shh signaling increases in the dorsal forebrain late in embryonic development to provide a temporally regulated mechanism that initiates the third wave of neocortical oligodendrogenesis.
Keywords: choroid plexus, forebrain, interneuron, neural progenitor, oligodendrocyte, Sonic hedgehog
Introduction
Neural circuit formation requires the production of many different neuronal and glial cell types with unique functions. A key question is how the genesis of these diverse cell types is coordinated and regulated during brain development. In the embryonic forebrain, neural progenitor cells produce excitatory neurons, astrocytes, and oligodendrocytes for the neocortex (Franco and Müller, 2013). Oligodendrocytes are glial cells that myelinate axons and play critical roles in the development and function of neocortical circuits. While much has been discovered about signaling mechanisms and transcription factor codes that dictate neuron and astrocyte cell fates in the neocortex, less is known about how oligodendrocytes are generated from neocortical progenitor cells.
Significant progress has been made toward mapping the developmental origins of neocortical oligodendrocytes. Oligodendrocyte precursor cells (OPCs) are produced sequentially from neural progenitors in multiple germinal zones in the ventral and dorsal forebrain (Richardson et al., 2006). The first wave of OPCs is produced in the medial ganglionic eminence (MGE) at around embryonic day (E) 12.5 in mice, followed by a second wave from the lateral ganglionic eminence (LGE) by ∼E15.5 (Kessaris et al., 2006). Some of these ventrally derived OPCs migrate into the dorsal forebrain, where they either remain as OPCs or differentiate into myelinating oligodendrocytes in the maturing neocortex (Kessaris et al., 2006; Tripathi et al., 2011). These ventral sources are largely replaced by a third wave of OPCs, which are locally produced from neural progenitors in the dorsal forebrain (Kessaris et al., 2006), so that by postnatal ages the vast majority of neocortical oligodendrocytes are dorsally derived (Kessaris et al., 2006; Tripathi et al., 2011; Crawford et al., 2016). Even though dorsal neural progenitor cells are the primary source of neocortical oligodendrocytes, the mechanisms by which these cells are specified to an oligodendrocyte fate are poorly understood.
Most of our knowledge about oligodendrocyte specification comes from studies in the ventral spinal cord, where Sonic hedgehog (Shh) signaling promotes oligodendrogenesis by inducing transcription factor cascades to instruct neural progenitor cell fates (Lu et al., 2000; Zhou et al., 2000; Nery et al., 2001). Shh ligand and the components of its signaling pathway are also highly expressed in the ventral forebrain, where Shh signaling is similarly required for oligodendrocyte production (Nery et al., 2001; Tekki-Kessaris et al., 2001; Fuccillo et al., 2004). Whether similar mechanisms are involved in producing oligodendrocytes in the embryonic dorsal forebrain, where Shh signaling is thought to be low, has not been tested. Several studies indicate that neural progenitors in dorsal regions of the spinal cord and forebrain can, independently of Shh signaling, generate oligodendrocytes through an Fgf (fibroblast growth factor)-dependent mechanism (Chandran et al., 2003; Cai et al., 2005). On the other hand, a recent study showed that progenitors in the postnatal ventricular–subventricular zone of the dorsal forebrain require Shh signaling to generate oligodendrocytes for the corpus callosum (Tong et al., 2015). Here we show that dorsal forebrain progenitors begin making OPCs already at embryonic ages and contribute the vast majority of neocortical oligodendrocytes even before birth. We therefore tested whether Shh signaling is required in these embryonic dorsal progenitors for proper neocortical oligodendrogenesis.
In this study, we show that Shh signaling is necessary for normal oligodendrocyte production from embryonic dorsal forebrain progenitors. We also show that elevated Shh signaling is sufficient to increase neocortical oligodendrogenesis. Using conditional knock-out approaches with multiple Cre driver lines, we provide evidence that Shh ligand is supplied to dorsal progenitors by migrating interneurons, as well as by the choroid plexus via CSF in the lateral ventricles. Together, these studies indicate that the third wave of neocortical oligodendrogenesis occurs earlier than previously appreciated and requires highly regulated Shh signaling from multiple embryonic sources.
Materials and Methods
Mice.
The following mice were obtained from The Jackson Laboratory: Emx1-Cre (B6.129S2-Emx1tm1(cre)Krj/J, stock no. 005628); R26NZG [FVB.Cg-Gt(ROSA)26Sortm1(CAG-lacZ,-EGFP)Glh/J, stock no. 012429]; Ai9 [B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, stock no. 007909]; Smo-floxed (Smotm2Amc/J, stock no. 004526); Shh-floxed (B6;129-Shhtm2Amc/J, stock no. 004293); Nestin-Cre [B6.Cg-Tg(Nes-cre)1Kln/J, stock no. 003771]; Dlx5/6-Cre [Tg(dlx6a-cre)1Mekk/J, stock no. 008199]; hGFAP-Cre (stock no. 004600). The Cux2-Cre line has been previously described (Franco et al., 2012; Gil-Sanz et al., 2015). Mice carrying the floxed Sufu allele (Sufufl) were kindly provided by Dr. Chi-Chung Hui (University of Toronto, Toronto, Canada) to S.J.P. and were genotyped as described previously (Pospisilik et al., 2010).
Emx1-Cre mice were crossed with R26NZG mice to generate double heterozygous animals. Emx1+/NZG;R26+/NZG animals were then crossed with Smo-floxed animals to generate Emx1+/NZG;R26+/NZG;Smo+/fl triple heterozygotes. The triple heterozygous animals were mated back to Smo+/fl animals to generate conditional knock-out mice of the informative genotype Emx1+/NZG;R26+/NZG;Smofl/fl (Smo cKO). Emx1+/NZG;R26+/NZG;Smo+/fl and Emx1+/NZG;R26+/NZG;Smo+/+ littermates were used as heterozygous (Smo Het) and wild-type (Smo WT) controls. Shh and Sufu conditional knock-out mice were generated using a similar breeding strategy with various Cre driver lines, as described in Results. Animals were maintained according to the guidelines from the Institutional Animal Care and Use Committee of the University of Colorado, Denver, or the University of California, San Francisco. Male and female mice were used equally throughout our experiments.
Expression plasmids.
A piggyBac (PB) transposase system was used for in utero electroporation experiments to permit stable integration of the reporter plasmid into electroporated progenitors (García-Moreno et al., 2014). We found this approach to be necessary to label the oligodendrocyte lineage, in line with previous reports that episomal plasmids are silenced or lost in glial lineages (García-Marqués and López-Mascaraque, 2013; Siddiqi et al., 2014). pPB-STOP-nc.mCherry has been described (García-Moreno et al., 2014). Briefly, it encodes a nuclear mCherry fluorophore driven by the CAG promoter, which is transcriptionally blocked by an intervening LoxP-flanked stop cassette. The entire CAG-LoxP-STOP-LoxP-mCherry region is flanked by PB recognition sites to facilitate genomic insertion by PBase. The PBase expression plasmid CMV-mPB was cloned by replacing the CAG promoter in mPB (Yusa et al., 2009) with the CMV promoter from pcDNA3.1+. CAG-Cre (pML78) has been described previously (Lewandoski et al., 1997).
In utero electroporation and injection. In utero electroporations were performed as described previously (Franco et al., 2011). Briefly, timed pregnant mice (E12.5 or E14.5) were anesthetized and their uterine horns exposed. One microliter of endotoxin-free plasmid DNA was injected into each embryo's lateral ventricles at the following concentrations: CAG-Cre, 1 mg/ml; pPB-STOP-nc.mCherry, 0.5 mg/ml; CMV-mPB, 0.3 mg/ml. For E12.5 electroporations, four pulses separated by 950 ms were applied at 40 V. For E14.5 electroporations, five pulses separated by 950 ms were applied at 45 V. Embryos were allowed to develop in utero for the indicated time. For quantification of electroporated cells that were oligodendrocyte-marker-positive, only electroporated cells in the ventricular zone, subventricular zone, and intermediate zone were displayed on graphs. Cells in the cortical plate were not included in the graphs because they were all neurons in both control and Smo knock-out brains, and therefore did not contribute to the phenotype being analyzed. In utero injections were performed similarly to in utero electroporations without the electric pulses. One microliter of 5 mm vismodegib (GDC-0449; Selleckchem, catalog no. S1082) was injected into each embryo's lateral ventricles at E14.5 and again at E15.5.
Tissue preparation.
Embryonic brains were fixed in 4% paraformaldehyde (PFA) for 1 h at room temperature (RT). Postnatal mice were transcardially perfused with 4% PFA and brains postfixed in 4% PFA for 1 h at RT. For 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich) labeling, pregnant dams were treated with 50 μg/g BrdU by intraperitoneal injection 4 h before collection of embryos and brain dissection. Brains were sectioned coronally either at 20 μm with a cryostat or at 75–100 μm with a vibrating microtome.
Immunohistochemistry.
Free-floating sections were blocked with 10% donkey serum and 0.2% Triton-X in 1× PBS for 2 h at RT. After 2 h, the blocking solution was removed and sections were incubated with primary antibodies in 10% donkey serum in 1× PBS for 1 h at RT, and then washed at RT with 1× PBS three times for 5 min each. After washing, sections were incubated with secondary antibodies in 10% donkey serum in 1× PBS for 1 h at RT, and then washed again with 1× PBS three times for 5 min each. Sections were mounted on slides with ProLong Diamond Antifade Mountant (Invitrogen). Images were captured using a LSM780 Zeiss laser-scanning confocal microscope. Antibodies used for immunostaining were as follows: guinea pig anti-Ascl1 (1:1000; Jane Johnson, University of Texas Southwestern; Kim et al., 2008), rabbit anti-Olig2 (1:500; Millipore, RRID:AB_2299035), goat anti-Sox10 (1:200; Santa Cruz Biotechnology, RRID:AB_2195374), goat anti-Sox10 (1:200; R&D Systems; RRID:AB_442208), rat anti-PDGFRα (1:500; BD Pharmingen; RRID:AB_397117), chicken anti-β-gal (1:2000; Abcam; RRID:AB_307210), mouse anti-BrdU (1:50; BD Pharmingen; RRID:AB_395993). Donkey secondary antibodies conjugated to Alexa Fluor 488, Rhodamine Red-X, or Alexa Fluor 647 were purchased from Jackson ImmunoResearch and used at 1:500.
Experimental design and statistical analysis.
For all immunostainings, ≥3 histological sections at three distinct rostrocaudal levels from each of three different animals (nine sections total for each condition) were analyzed in the mediolateral part of the neocortex comprising primarily the presumptive somatosensory cortex. Biological replicates are individual animals. Confocal, single-plane optical sections were used for quantification. Cells were analyzed in contiguous columns spanning the entire neocortex from ventricle to pial surface. Percentages of marker-positive cells were quantified from these columns. Absolute numbers of marker-positive cells were also quantified from these columns and then divided by the area of a column to get cell density (cells/mm2). Normalized cell counts are cell densities normalized to control. Values in graphs are mean ± SEM for biological replicates. For independent two-group experiments (WT vs KO and control vs treated), an unpaired two-tailed Student's t test was used to determine statistical significance. For analysis involving ≥3 independent groups, a one-way ANOVA was used followed by Tukey's post hoc test. Values were considered statistically significant at p ≤ 0.05.
Results
Emx1+ progenitors produce neocortical oligodendrocytes at late embryonic stages
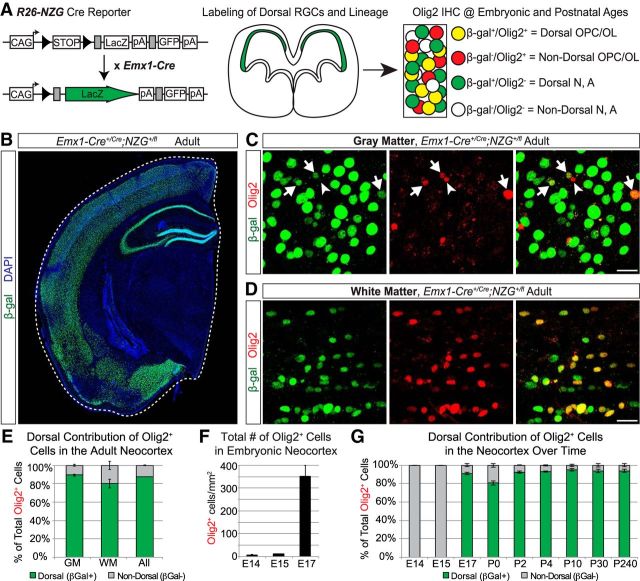
Neocortical oligodendrocytes are generated by neural progenitors in both the ventral and dorsal forebrain, with most arising from dorsal progenitors that express the transcription factor Emx1 (Gorski et al., 2002; Kessaris et al., 2006; Tripathi et al., 2011; Crawford et al., 2016). The lineage output of these Emx1+ dorsal progenitors can be genetically traced using the Emx1-Cre transgenic mouse line (Gorski et al., 2002) together with Cre-reporter lines. We crossed Emx1-Cre mice to the R26-NZG reporter line (Yamamoto et al., 2009) and stained brain sections at various ages for β-gal to identify the recombined Emx1 lineage cells and for Olig2 to label oligodendrocytes and OPCs (Fig. 1A). As expected, adult Emx1;NZG brains exhibited β-gal expression throughout the neocortex and hippocampus, but not in ventral forebrain regions (Fig. 1B). Consistent with previous reports (Tripathi et al., 2011; Crawford et al., 2016), we found that the vast majority of Olig2+ cells in the mature neocortex were β-gal+, and therefore derived from the Emx1+ dorsal lineage (Fig. 1C–E). In the somatosensory cortex, 89% of Olig2+ cells in the gray matter and 80% of Olig2+ cells in the white matter were recombined (Fig. 1E).
Figure 1.
Most Olig2+ cells in the neocortex are dorsally derived beginning at embryonic ages. A, Schematic of the genetic lineage-tracing approach to distinguish dorsally derived cells in the neocortex from ventrally derived cells. Emx1-Cre mice were crossed to R26-NZG reporter mice to permanently label all Emx1+ progenitors and their offspring (β-gal+) and identify oligodendrocyte lineage cells by immunostaining for Olig2. OPC, oligodendrocyte precursor cell; OL, Oligodendrocyte; N, neuron; A, astrocyte. B, Representative image of β-gal+ cells in the adult forebrain, showing the Emx1-Cre recombination pattern in dorsally derived, but not ventrally derived, structures. C, Examples of Olig2+ (red) cells in the gray matter of the adult somatosensory cortex. Arrows denote dorsally derived (β-gal+) Olig2+ cells and the arrowhead denotes a ventrally derived (β-gal−) Olig2+ cell. D, Examples of Olig2+ (red) cells in the white matter of the somatosensory cortex that are β-gal+ (green) and therefore dorsally derived. E, Graph of the average (±SEM among biological replicates) percentage of Olig2+ cells in the adult neocortex that were dorsally derived (β-gal+) versus not dorsally derived (β-gal−). Quantification was performed in the somatosensory cortex, separately in the white matter (WM), gray matter (GM), or combined (All). F, Quantification (average ± SEM among biological replicates) of total number of Olig2+ cells in the somatosensory cortex (white and gray matter combined) at early embryonic ages. Total numbers of Olig2+ cells were counted per mm2. G, Graph of the average (±SEM among biological replicates) percentage of Olig2+ cells in the somatosensory cortex (white matter and gray matter combined) that were dorsally derived (β-gal+) versus not dorsally derived (β-gal−) at different time points in embryonic (E) and postnatal (P) development. Scale bar, 20 μm.
We next wanted to determine at what age this dorsal source of Olig2+ cells arises during neocortical development. Previous studies showed that OPCs from the MGE first appear in the ventral subpallium at E12.5 and start to migrate into the dorsal pallium by E14.5, representing the first wave of OPCs to appear in the dorsal forebrain (Kessaris et al., 2006; Ono et al., 2008). However, at E14.5 only a few OPCs expressing platelet-derived growth factor receptor α subunit (PDGFRα; Kessaris et al., 2006) or Olig2 (Ono et al., 2008) can be found in the neocortex. Consistent with these reports, we found that Olig2+ cells in the dorsal pallium were rare at E13.5 or earlier (data not shown). At both E14.5 and E15.5, we observed a few Olig2+ cells in the dorsal pallium (Fig. 1F; ∼9 cells/mm2 at E14.5, ∼12 cells/mm2 at E15.5). All of these Olig2+ cells were located near the pallial–subpallial border (data not shown) and none of them were dorsally derived (Fig. 1G), indicating that they are likely ventrally derived OPCs migrating from the MGE/LGE. However, by E17.5 we observed a dramatic increase in the neocortical Olig2+ cell population (Fig. 1F; ∼350 cells/mm2 in the presumptive somatosensory cortex), in line with a previous report (Ono et al., 2008). Moreover, we found that at E17.5 the Emx1+ dorsal lineage was already the main contributing source of Olig2+ cells in the neocortex (Fig. 1G). The dorsal lineage continued to represent between 80 and 90% of all Olig2+ cells in the somatosensory cortex from E17.5 through adulthood (Fig. 1G). These data indicate that Emx1+ progenitors in the dorsal forebrain start producing Olig2+ cells during late embryonic development and are the primary source of Olig2+ cells from that point on.
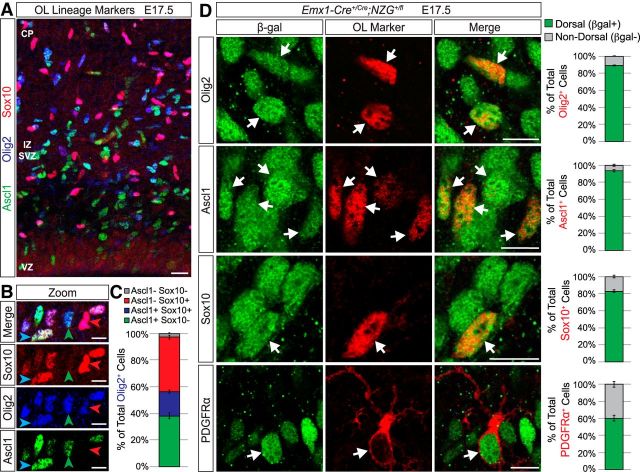
Because Olig2 is expressed by some immature astrocytes in the early postnatal neocortex (Marshall et al., 2005; Cai et al., 2007; Ono et al., 2008), we wanted to better define this Olig2+ population by comparing the expression of Olig2 to several other well established oligodendrocyte-lineage markers in E17.5 brains. Ascl1 is one of the earliest markers of the oligodendrocyte lineage, labeling pre-OPCs and OPCs before being downregulated as OPCs mature (Nakatani et al., 2013; Vue et al., 2014). Importantly, lineage-tracing studies using Ascl1-Cre and Ascl1-CreERT2 mice showed that neocortical cells derived from the Ascl1 lineage are oligodendrocytes and ventrally derived interneurons, but not astrocytes or excitatory neurons (Kim et al., 2008, 2011). In addition, Sox10 is an oligodendrocyte lineage-specific marker that comes on slightly later in the OPC stage and continues to be expressed in all OPCs and mature oligodendrocytes (Stolt et al., 2002; Nakatani et al., 2013). When we costained E17.5 brain sections for Olig2, Ascl1, and Sox10 (Fig. 2A,B), we found that nearly all Olig2+ cells in the presumptive somatosensory cortex expressed one or both of these markers (Fig. 2C). Only ∼3% of Olig2+ cells were negative for both Ascl1 and Sox10, which could represent a very small population of early astrocyte precursors (Marshall et al., 2005; Cai et al., 2007; Ono et al., 2008). These data suggest that the vast majority of Olig2+ cells in the E17.5 neocortex are oligodendrocyte-lineage cells. Moreover, when we analyzed Ascl1+ cells and Sox10+ cells in Emx1-Cre;NZG brains, we found that their dorsal/ventral lineage origins were very similar to what we observed for Olig2+ cells (Fig. 2D). At E17.5, the vast majority of Ascl1+ cells (94%) and Sox10+ cells (83%) in the presumptive somatosensory cortex were β-gal+ and, therefore, derived from the Emx1+ dorsal lineage (Fig. 2D). We also analyzed PDGFRα, which is specifically expressed only during the OPC stage (Hart et al., 1989; Hall et al., 1996). Most PDGFRα+ cells were also β-gal+ (Fig. 2D), but ∼40% were β-gal−; these most likely represent ventrally derived OPCs migrating into the neocortex (Kessaris et al., 2006). Together, our data indicate that the vast majority of neo cortical oligodendrocyte lineage cells are derived from dorsal Emx1+ progenitors, even during embryonic ages. We then wondered what signals are responsible for instructing this subset of dorsal progenitors to make oligodendrocytes instead of neurons or astrocytes.
Figure 2.
Most neocortical oligodendrocyte-lineage cells in different stages of maturation are dorsally derived at E17.5. A, Representative image of Ascl1 (green), Olig2 (blue), and Sox10 (red) expression in the presumptive somatosensory cortex at E17.5. Scale bar, 20 μm. B, Higher-magnification examples of Olig2+ cells that are Ascl1+Sox10+ (blue arrow), Ascl1+Sox10− (green arrow), and Ascl1−Sox10+ (red arrow). Scale bar, 10 μm. C, Graph of the average (±SEM among biological replicates) percentage of Olig2+ cells in the somatosensory cortex (white matter and gray matter combined) that are Ascl1+Sox10− (green), Ascl1+Sox10+ (blue), Ascl1−Sox10+ (red), or Ascl1−Sox10− (gray). D, Representative images of oligodendrocyte lineage cell markers (red) and β-gal (green) staining in the presumptive somatosensory cortex of Emx1;NZG mice at E17.5. The majority of Olig2+, Ascl1+, Sox10+, and PDGFRα+ cells were β-gal+ (white arrows). Graphs at right represent the average (±SEM among biological replicates) percentage of Olig2+, Ascl1+, Sox10+, or PDGFRα+ cells in the somatosensory cortex (white matter and gray matter combined) that were dorsally derived (β-gal+) versus not dorsally derived (β-gal−). OL, oligodendrocyte; CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. Scale bar, 10 μm.
The Shh pathway is required for dorsal OPC formation
In ventral regions of the spinal cord and forebrain, Shh signaling plays key roles in promoting oligodendrogenesis from neural progenitors (Lu et al., 2000; Zhou et al., 2000; Nery et al., 2001; Tekki-Kessaris et al., 2001; Fuccillo et al., 2004). We reasoned that Shh signaling might also be important for instructing a subset of progenitors to an oligodendrocyte fate in the embryonic dorsal forebrain. To begin to test this model, we used the Emx1-Cre driver to conditionally knock out the obligate Shh pathway component, Smoothened (Smo), specifically in dorsal forebrain progenitors (Fig. 3A). We also included the R26-NZG reporter to distinguish the Emx1 lineage of oligodendrocytes (β-gal+, dorsal) from those derived from the ventral telencephalon or diencephalon (β-gal−, nondorsal; Fig. 3A–E). We stained brains at E17.5 for Olig2 to label OPCs and oligodendrocytes. Homozygous Emx1;Smo mutants exhibited a dramatic decrease in Olig2+ cells compared with controls (Fig. 3B). Dorsally derived (β-gal+) Olig2+ cells were decreased by 90% in Emx1;Smo homozygous knock-outs (Fig. 3F). On the other hand, the number of nondorsal Olig2+ cells was unchanged in Emx1;Smo mutants (Fig. 3F), indicating that only the dorsally derived Olig2+ population was affected. Furthermore, we found that Emx1;Smo mutants exhibited a deficit in other oligodendrocyte lineage markers at E17.5 as well. Dorsally derived Ascl1+ cells were decreased by 80% in homozygous Emx1;Smo mutants (Fig. 3C,G), dorsally derived Sox10+ cells were decreased by 90% (Fig. 3D,H), and dorsally derived PDGFRα+ cells were decreased by 86% (Fig. 3E,I). As with Olig2+ cells, the number of ventrally derived Ascl1+ and Sox10+ cells was unaffected (Fig. 3G,H). However, the number of ventrally derived PDGFRα+ cells was increased in homozygous Emx1;Smo mutants (Fig. 3I), possibly as a compensatory homeostatic response to the decrease in dorsally derived mutant OPCs (Kessaris et al., 2006). Heterozygous Emx1;Smo mutants did not exhibit a statistically significant phenotype in any of the analyzed cell populations (data not shown). Together, these data indicate that Shh signaling received by Emx1+ progenitors is required for the generation of most, but not all, oligodendrocyte-lineage cells during late embryonic stages of neocortical development.
Figure 3.
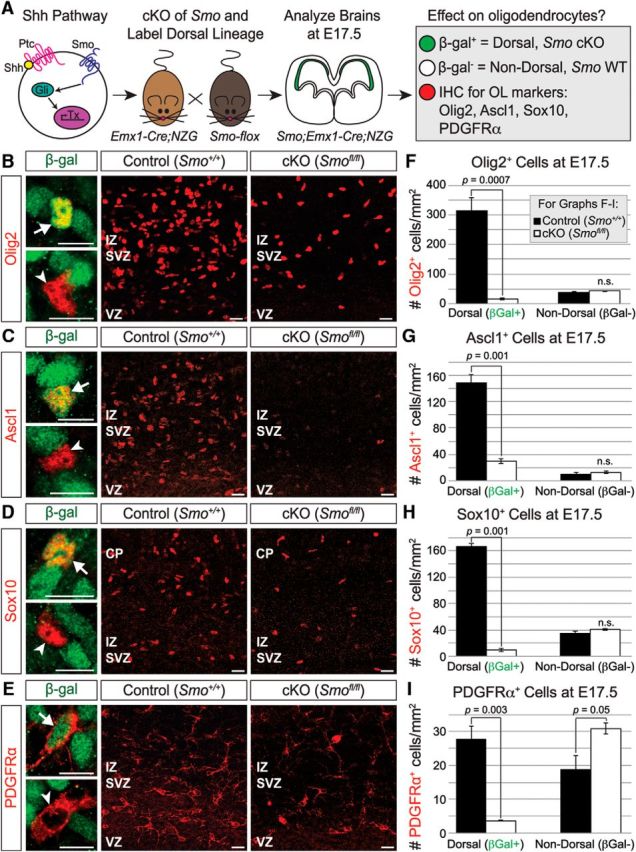
The Shh pathway is important for neocortical oligodendrogenesis. A, Schematic of experimental design. Shh binding to its receptor Patched (Ptc) allows the essential downstream effector Smo to activate Gli transcription factors and initiate downstream transcription. Smo was knocked out in just the dorsal progenitor population by crossing Smo-flox mice to Emx1-Cre;NZG mice. Brains were analyzed at E17.5 for the effect on neocortical oligodendrocytes. B–E, Sections were stained for β-gal (green) to identify dorsally derived cells, and for markers of the oligodendrocyte lineage (red): Olig2 (B), Ascl1 (C), Sox10 (D), and PDGFRα (E). High-magnification images on the left show examples of dorsally derived cells (β-gal+; arrows, top) and nondorsally derived cells (β-gal−; arrowheads, bottom). Scale bars, 10 μm. Overview images show loss of oligodendrocyte-lineage cells in Emx1;Smo mutant brains (right) compared with controls (middle). Scale bars, 20 μm. F–I, Conditional knock-out of Smo in the Emx1+ lineage resulted in a dramatic reduction in dorsally derived, but not nondorsally derived, oligodendrocyte-lineage cells in the E17.5 neocortex. Total numbers of β-gal+ (dorsal) and β-gal− (nondorsal) cells that expressed each marker were counted per mm2 in the presumptive somatosensory cortex in control and Emx1;Smo mutant neocortex at E17.5. Quantification is average ± SEM among biological replicates for Olig2+ cells (F), Ascl1+ cells (G), Sox10+ cells (H), and PDGFRα+ cells (I). SVZ/IZ, Subventricular zone/intermediate zone; VZ, ventricular zone.
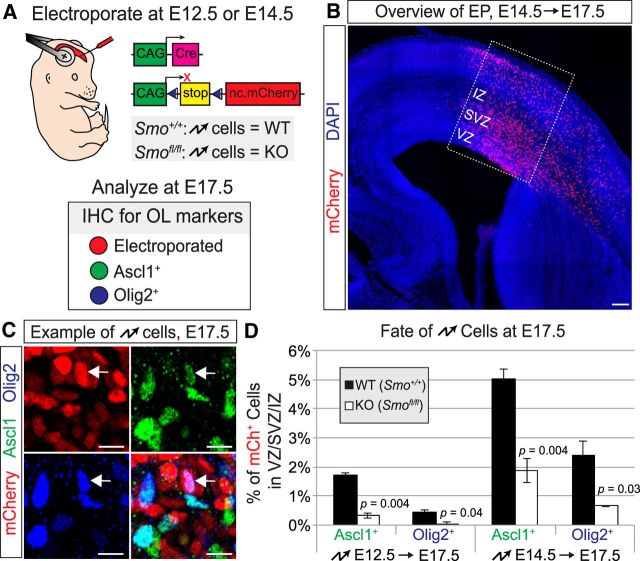
The Emx1-Cre line drives recombination in all dorsal progenitors and their offspring starting at E9.5 (Gorski et al., 2002). It also recombines a small subset of progenitors in the dorsal region of the LGE (Cocas et al., 2009), which is known to be an embryonic source of neocortical oligodendrocytes (Kessaris et al., 2006). To knock out Smo in a more temporally and spatially defined manner, we used in utero electroporation to target a limited number of dorsal progenitors during midneurogenesis (E14.5), just before significant numbers of dorsally derived oligodendrocyte-lineage cells are observed. We electroporated Cre together with a Cre-dependent fluorescent reporter plasmid into the presumptive primary somatosensory cortex of wild-type or Smo-flox embryos (Fig. 4A,B). Because episomal plasmids can be silenced or lost in oligodendrocyte and astrocyte lineages (García-Marqués and López-Mascaraque, 2013; Siddiqi et al., 2014), we used a PB transposase system (García-Moreno et al., 2014) in our electroporations to achieve stable integration of the reporter plasmid into the electroporated progenitors. We then allowed the embryos to mature to E17.5 and analyzed the electroporated cells for expression of Ascl1 and Olig2 (Fig. 4C,D). Consistent with our Emx1;Smo experiments, the percentage of electroporated cells expressing Ascl1 or Olig2 was significantly reduced in electroporated Smo-flox brains compared with wild-type controls (Fig. 4D). This reduction was not quite as complete as in the Emx1;Smo genetic mutants, possibly due to a delay in recombination or decrease in efficiency when Cre is expressed from a plasmid by electroporation. To account for this, we performed in utero electroporations at E12.5 and analyzed brains at E17.5. Fewer oligodendrocyte-lineage cells were labeled at this time point, suggesting that oligodendrocyte progenitors are more difficult to electroporate during early neurogenesis stages. Nevertheless, we found that knocking out Smo by electroporation at E12.5 caused reductions in Ascl1+ and Olig2+ cells similar to those we saw in Emx;Smo mutants (Fig. 4D). Together, these results confirm that Smo is required cell-autonomously in neural progenitors for normal production of oligodendrocytes in the embryonic dorsal forebrain.
Figure 4.
Smo is required cell-autonomously for normal dorsal oligodendrogenesis. A, Schematic of in utero electroporation approach to knock-out Smo in dorsal forebrain progenitors at E12.5 or E14.5. A Cre recombinase expression plasmid was electroporated together with a Cre-dependent nuclear mCherry reporter plasmid and a PBase expression plasmid (not depicted) into the somatosensory cortex of homozygous wild-type or Smo-flox embryos at E12.5 or E14.5. Brains were collected at E17.5 and stained for markers of the oligodendrocyte lineage. Electroporated cells were identified as mCherry+. B, Representative image of an E14.5 electroporation in an E17.5 brain. Boxed region represents the presumptive somatosensory cortex and quantification area. Scale bar, 100 μm. C, Example of electroporated brain section stained for Ascl1 and Olig2. Arrow denotes mCherry+-electroporated cell (red) expressing both Ascl1 (green) and Olig2 (blue). Scale bar, 10 μm. D, Quantification (average ± SEM among biological replicates) of the percentage of Cre-electroporated cells (mCherry+) that were Olig2+ or Ascl1+ in wild-type controls compared with Smo-flox mutants. Both populations were significantly decreased in Smo mutants. Abbreviations as in Figure 2.
Early dorsal forebrain progenitors are competent to generate Olig2+ cells in response to increased Shh signaling
Recent studies showed that restriction of Shh signaling at early stages of neocortical development is important for proper projection neuron specification (Yabut et al., 2015). Overactivation of Shh signaling in the developing dorsal forebrain, either by expression of constitutively active Smo or by knock-out of the Shh signaling antagonist Sufu, leads to fate changes in projection neuron subtypes (Yabut et al., 2015). Given our results above, we wondered whether overactivation of Shh signaling might increase oligodendrocyte production from dorsal progenitors. Therefore, we conditionally ablated Sufu in forebrain progenitors at E14.5 (Yabut et al., 2015) by crossing Sufu-flox mice (Pospisilik et al., 2010) to a GFAP-Cre line (Zhuo et al., 2001; Fig. 5A). We found that by E16.5, GFAP;Sufu mutants had increased numbers of Olig2+ cells in the neocortex compared with controls (Fig. 5B,C). Furthermore, the percentage of BrdU+ proliferating progenitors in the dorsal forebrain that were Olig2+ was also increased in the GFAP;Sufu mutant neocortex compared with controls (Fig. 5D,E). These data indicate that dorsal forebrain progenitors at mid-to-late neurogenesis can respond to increased Shh signaling by producing Olig2+ cells. We then asked whether earlier progenitors had the same competence to respond to Shh signaling. We crossed Sufu-flox mice to the Emx1-Cre line to knock out Sufu by E10.5 (Yabut et al., 2015). At E11.5, we did not observe any Olig2+ cells in either the wild-type control or the Emx1;Sufu mutant neocortex (data not shown). However, by E12.5, when control brains had very few Olig2+ cells in the neocortex, Emx1;Sufu mutants exhibited robust expression of Olig2 in the dorsal ventricular zone (Fig. 5F,G). This precocious generation of Olig2+ cells indicates that progenitors in the dorsal forebrain are competent to respond to Shh signaling across a wide developmental window, but are normally kept unresponsive in part by Sufu. Together with our Smo conditional knock-out approach, these results demonstrate that Shh signaling is both necessary and sufficient for the generation of oligodendrocytes from neural progenitors in the embryonic dorsal forebrain.
Figure 5.
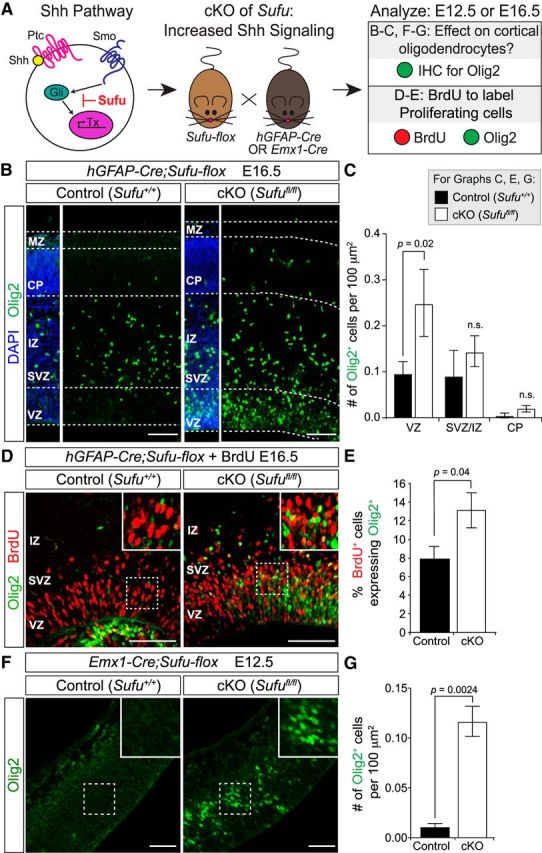
Overactivation of Shh signaling increases oligodendrocyte production from dorsal progenitors. A, Schematic of the genetic approach to upregulate Shh signaling in the embryonic brain. Sufu is expressed in the embryonic dorsal forebrain where it suppresses Shh downstream signaling by antagonizing Gli-mediated transcription. Sufu was ablated in forebrain progenitors by E14.5 by crossing Sufu-flox mice to GFAP-Cre mice, or by E10.5 by crossing to Emx1-Cre mice. Brains were collected at E12.5 (for Emx1;Sufu) or E16.5 (for GFAP;Sufu) and stained for Olig2 to determine the effects of Shh overactivation on neocortical oligodendrocyte production. In a separate cohort of GFAP;Sufu mice, BrdU was administered to the pregnant dams 4 h before embryo harvest, to label proliferating progenitors in S-phase. Sections were stained for BrdU and Olig2 to identify proliferating cells that were Olig2+. B, At E16.5, GFAP;Sufu mutants had increased numbers of Olig2+ cells in the neocortex compared with controls. Scale bar, 100 μm. C, Quantification (average ± SEM among biological replicates) of Olig2+ cells in control and GFAP;Sufu mutant neocortex at E16.5. Total numbers of Olig2+ cells were counted per 100 μm2 in the presumptive somatosensory cortex. DAPI staining was used to identify the histological ventricular zone (VZ), subventricular zone (SVZ), intermediate zone (IZ), cortical plate (CP), and marginal zone (MZ). Quantification was performed separately in the VZ, IZ/SVZ, and CP. Olig2+ cells were significantly increased specifically in the VZ. D, In E16.5 wild-type control brains, few BrdU+ proliferating cells in the VZ/SVZ were Olig2+, whereas GFAP;Sufu mutants exhibited more BrdU+Olig2+ cells. E, Graph of the average (±SEM among biological replicates) percentage of BrdU+ cells that were also Olig2+ in control and GFAP;Sufu mutant neocortex at E16.5. Scale bar, 100 μm. F, At E12.5, Emx1;Sufu mutants had increased numbers of Olig2+ cells in the presumptive somatosensory cortex compared with controls. Scale bar, 20 μm. G, Quantification of Olig2+ cells in control and Emx1;Sufu mutant neocortex at E12.5, as performed in C.
Multiple sources of Shh contribute to oligodendrogenesis in the dorsal forebrain
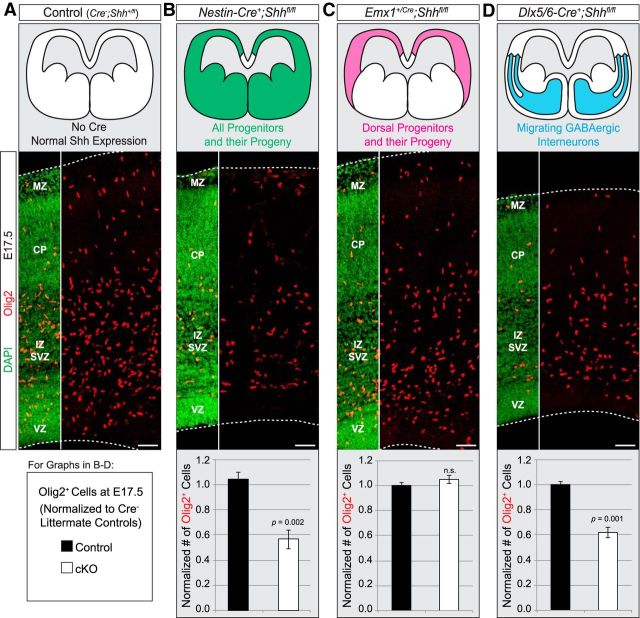
Our results indicate that Smo is required in Emx1+ progenitors for normal production of oligodendrocytes, suggesting that Shh signaling drives this fate. To test the role of Shh directly, we crossed Shh-flox mice (Lewis et al., 2001) to the Nestin-Cre line (Tronche et al., 1999) to conditionally knock out Shh throughout the entire neural tube starting at E10.5 (Graus-Porta et al., 2001). We found that Nestin;Shh mutant embryos exhibited a significant decrease in the number of Olig2+ cells in the dorsal forebrain at E17.5 compared with controls (Fig. 6A,B). Although Nestin;Shh mutants may also affect generation of ventrally derived OPCs, we already established that ∼90% of Olig2+ cells in the neocortex are dorsally derived by E17.5 (Figs. 1, 2). Thus, the large decreases in neocortical Olig2+ cells in these mutants are consistent with a role for Shh ligand in promoting neocortical oligodendrogenesis from dorsal neural progenitors. To determine more precisely the critical sources of the Shh ligand, we next crossed Shh-flox mice to several Cre driver lines with more restricted recombination patterns. According to several reports (Dahmane et al., 2001; Tekki-Kessaris et al., 2001; Komada et al., 2008), low levels of Shh are expressed in the embryonic neocortex, possibly by differentiating excitatory projection neurons. However, when we knocked out Shh using the Emx1-Cre driver, we saw no effect on the number of Olig2+ cells in the presumptive somatosensory cortex at E17.5 (Fig. 6C). This result suggests that neocortical progenitors and their postmitotic progeny (i.e., projection neurons, astrocytes, and oligodendrocytes) are not the critical source of Shh ligand. Interneurons that migrate into the neocortex from the MGE express high levels of Shh ligand (Flandin et al., 2011). We therefore used the Dlx5/6-Cre line to knock out Shh from migrating interneurons (Monory et al., 2006). Dlx5/6;Shh mutants exhibited a significant loss of Olig2+ cells in the E17.5 dorsal forebrain (Fig. 6D), demonstrating that Shh secreted by interneurons migrating into the dorsal forebrain is important for production of neocortical oligodendrocytes. Furthermore, the percentage decrease in Olig2+ cells in the Dlx5/6;Shh mutants was nearly identical to that in the Nestin;Shh mutants (Fig. 6B,D), indicating that infiltrating interneurons are the primary source of Shh ligand within the Nestin-Cre recombination domain.
Figure 6.
Interneurons are a major source of Shh ligand for dorsal oligodendrogenesis. A, Example of normal Olig2 (red) expression at E17.5 in the presumptive somatosensory cortex of control animals without Cre. DAPI was used to counterstain for nuclei to reveal histological regions. B, Crossing Shh-flox mice to the Nestin-Cre line conditionally knocked out Shh throughout the entire neural tube starting at E10.5, resulting in a significant decrease in the number of Olig2+ cells at E17.5 compared with controls. The number of Olig2+ cells was quantified in a standardized column of defined width that spanned the entire neocortical thickness. Graph is average (±SEM among biological replicates) cell counts in the standardized area, normalized to controls. C, Knocking out Shh in dorsal progenitors and their offspring starting at E9.5 with the Emx1-Cre driver did not affect the number of Olig2+ cells at E17.5. Quantification as in B. D, Shh-flox mice were crossed to the Dlx5/6-Cre line to knock out Shh from postmitotic interneurons that migrate into the neocortex from the subpallium. Dlx5/6;Shh mutants exhibited a decrease in Olig2+ cells compared with controls. Quantification as in B. Abbreviations as in Figure 5. Scale bar, 50 μm.
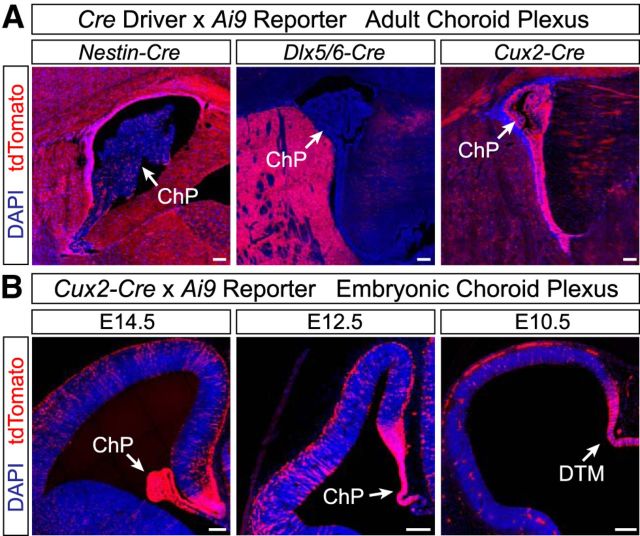
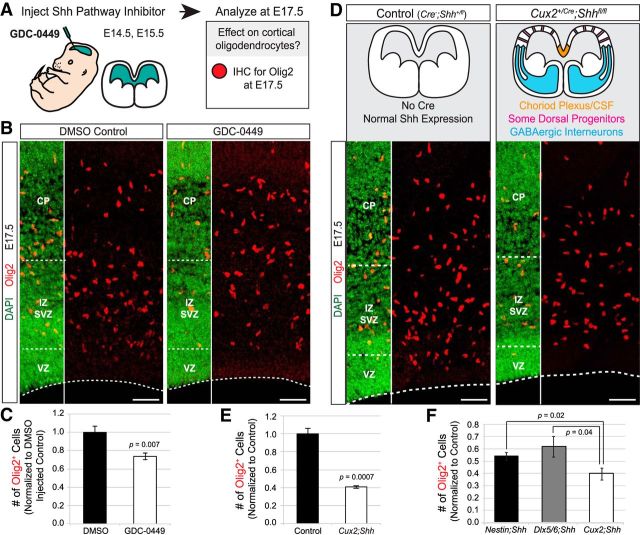
The decreases in Olig2+ cells in Nestin;Shh and Dlx5/6;Shh mutants were not as severe as those in the Emx1;Smo mutants (40–50% compared with ∼90%), raising the possibility of another important source of Shh ligand. Shh protein has been found in the embryonic CSF (Huang et al., 2010; Chau et al., 2015), suggesting it is secreted by the choroid plexus epithelium and/or cells lining the lateral ventricles. Neither the Nestin-Cre line nor the Dlx5/6-Cre line recombines in the choroid plexus (Fig. 7A), raising the possibility that the choroid plexus could be supplying Shh ligand to dorsal progenitors in these mutants via the CSF. As an initial test to determine whether Shh in the CSF could play a role in dorsal oligodendrogenesis, we injected the Shh pathway inhibitor GDC-0449 into the CSF of the lateral ventricles in utero at E14.5 and E15.5, then analyzed Olig2+ cells at E17.5 (Fig. 8A). We observed a ∼20% decrease in Olig2+ cells in GDC-0449-treated brains compared with vehicle-treated controls (Fig. 8B,C), supporting the idea that Shh in the CSF can affect the ability of dorsal progenitors to generate oligodendrocytes. However, the injected inhibitor could potentially diffuse into the tissue and block Shh signaling from multiple sources, not just the CSF. Therefore, to address this question more directly, we used the Cux2-Cre line that drives recombination in multiple forebrain regions, including the entire choroid plexus epithelium (Fig. 7A), even as early as E10.5 in the dorsal telencephalic midline (Fig. 7B). In addition to the choroid plexus, the Cux2-Cre line also drives recombination in migrating interneurons and upper-layer projection neurons (Franco et al., 2011, 2012; Gil-Sanz et al., 2015). Therefore, we hypothesized that if the choroid plexus is a source of Shh ligand, we would see a more severe loss of Olig2+ cells in Cux2;Shh mutants (interneurons plus choroid plexus) compared with Nestin;Shh or Dlx5/6;Shh mutants (interneurons but not choroid plexus). Indeed, Cux2;Shh mutants exhibited a 59% reduction in neocortical Olig2+ cells at E17.5 (Fig. 8D,E). A one-way ANOVA indicated a significant difference between the percentage reduction of Olig2+ cells in the three different Shh mutants: Cux2;Shh, Nestin;Shh, and Dlx5/6;Shh (F(3,16) = 48.62, p = 0.0002). A post hoc Tukey's test revealed that there was a significant difference between Cux2;Shh mutants compared with Nestin;Shh mutant (p = 0.02) and compared with Dlx5/6;Shh mutants (p = 0.04; Fig. 8F). This result suggests that the additional 20% reduction of Olig2+ cells observed in Cux2;Shh mutants (∼60%) compared with Nestin;Shh or Dlx5/6;Shh mutants (∼40% reductions) is the result of knocking out Shh from the choroid plexus. Together, these results demonstrate that Shh ligand is supplied to dorsal progenitors from multiple sources, including interneurons migrating into the neocortex as well as the choroid plexus epithelium via CSF.
Figure 7.
The choroid plexus is recombined early embryonically in the Cux2-Cre line, but not in the Nestin-Cre or Dlx5/6-Cre lines. A, Representative images of recombination in Nestin-Cre;Ai9, Dlx5/6-Cre;Ai9, and Cux2-Cre;Ai9 adult animals. The choroid plexus is completely recombined in Cux2-Cre;Ai9 animals, very sparsely recombined in Nestin-Cre;Ai9 animals, and not recombined in Dlx5/6-Cre;Ai9 animals. B, Representative images of choroid plexus recombination in Cux2-Cre;Ai9 embryos. The choroid plexus is completely recombined in Cux2-Cre;Ai9 embryos, including in the dorsal telencephalic midline from which the choroid plexus derives, as early as E10.5. ChP, Choroid plexus; DTM, dorsal telencephalic midline. Scale bar, 100 μm.
Figure 8.
Shh secreted by the choroid plexus into the CSF is a potential non-neural source of Shh ligand for dorsal oligodendrogenesis. A, Shh pathway inhibitor GDC-0449 was injected into the lateral ventricles of embryos in utero at E14.5 and E15.5. Brains were collected at E17.5 and stained for Olig2 (red) at E17.5. DAPI was used to counterstain for nuclei (green) to reveal histological regions. B, GDC-0449-treated brains exhibited decreased numbers of Olig2+ cells in the presumptive somatosensory cortex compared with DMSO-treated controls. C, The number of Olig2+ cells was quantified in a standardized column of defined width that spanned the entire neocortical thickness. Graph is average (±SEM among biological replicates) cell counts in the standardized area, normalized to controls. D, To test whether the choroid plexus is an important source of Shh ligand for dorsal oligodendrogenesis, Shh-flox mice were crossed to the Cux2-Cre line to knock out Shh from the entire choroid plexus epithelium. The Cux2-Cre line also drives recombination in migrating interneurons and a subset of dorsal progenitors. E, Cux2;Shh mutants exhibited greatly diminished numbers of Olig2+ cells compared with Cre-negative littermate controls. Quantification as in C. F, The decrease in Olig2+ cells in Cux2;Shh mutants was significantly different compared with both Nestin;Shh and Dlx5/6;Shh mutants, as determined by a one-way ANOVA followed by Tukey's post hoc test. Quantification for F combined counts from Figures 6B,D and 8E. Abbreviations as in Figure 2. Scale bar, 50 μm.
Discussion
Much progress has been made in elucidating the molecular details of oligodendrocyte maturation and differentiation (Mitew et al., 2014). However, we know less about the early steps that initiate oligodendrocyte production from multipotent neural progenitor cells in vivo, especially in the developing neocortex. In this study, we focused on the mechanisms by which neural progenitors in the embryonic dorsal forebrain are instructed to produce neocortical oligodendrocytes. We show that (1) dorsal progenitors start making oligodendrocytes during late embryonic development; (2) Shh signaling through Smo is required in dorsal progenitors for normal oligodendrogenesis; and (3) Shh ligand is supplied to dorsal progenitors through multiple sources, including migrating interneurons and CSF secreted by the choroid plexus.
Developmental timing of the neuron-to-oligodendrocyte switch
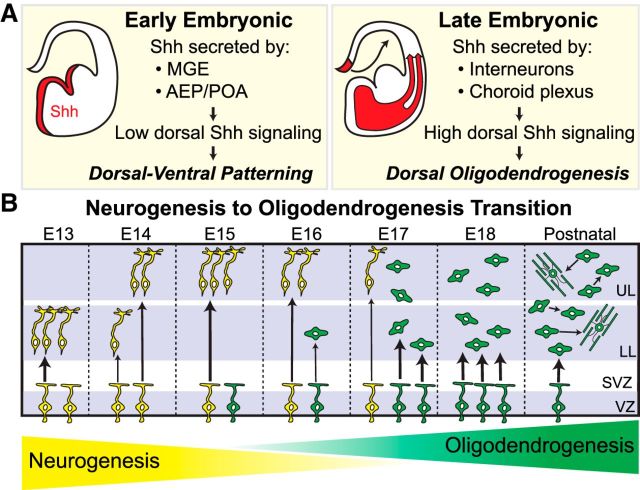
Neural progenitors in the developing forebrain can give rise to committed OPCs that populate the neocortex in three successive waves: first ventrally from Nkx2.1+ progenitors and then from Gsh2+ progenitors in the subpallium, and finally dorsally from Emx1+ progenitors in the pallium (Kessaris et al., 2006; Richardson et al., 2006). Our lineage-tracing experiments confirm previous reports that the dorsal Emx1+ wave is the primary source of Olig2+ cells for the mature neocortex (Gorski et al., 2002; Kessaris et al., 2006; Tripathi et al., 2011; Crawford et al., 2016). Our data extend these studies, showing that the Emx1+ lineage already contributes ∼90% of the total Olig2+ population in the neocortex as early as E17.5. Although Kessaris et al. (2006) reported first seeing Emx1-lineage OPCs at around birth, differences in the methods used in our study compared with theirs may account for different observations; e.g., expression levels of the Cre-reporter transgenes (CAG-driven β-gal vs R26-driven GFP) and timing of cell-type marker expression (Olig2 vs PDGFRα). Nevertheless, our data indicate that some Emx1+ progenitors in the dorsal forebrain begin generating OPCs at late embryonic ages, while others are still in the peak of neurogenesis. This is a significant finding, as it informs about the nature and timing of the neuron-to-glia “switch” that occurs among neural progenitors. A common theme throughout most of the CNS is that neural progenitors, as a population, start producing large numbers of glial cells only once neurogenesis winds down (Miller and Gauthier, 2007; Kessaris et al., 2008). Because we find that the number of Olig2+ cells in the neocortex increases dramatically between E15.5 and E17.5, we propose that this period represents a critical transition window in the developing neocortex, during which neurogenesis and oligodendrogenesis overlap across a population of heterogeneous progenitors (Fig. 9B). These findings raise important questions about the intrinsic and extrinsic mechanisms that initiate and promote this transition.
Figure 9.
Proposed model for the role of Shh signaling in dorsal forebrain development and neocortical oligodendrogenesis. A, During early embryonic ages, the primary sources of Shh ligand include ventral structures, such as the MGE, the anterior entopeduncular region (AEP), and the preoptic area (POA). In the dorsal forebrain, Shh signaling is maintained at low levels by weak ligand expression and antagonism by Sufu, which allows for normal dorsal-ventral patterning and neurogenesis. During later stages of embryonic development, Shh ligand reaches the dorsal forebrain via infiltrating interneurons and circulating CSF secreted by the choroid plexus, which increases downstream signaling via Smo and initiates oligodendrogenesis in a subset of dorsal progenitors. B, In the early embryonic forebrain, dorsal progenitors undergo neurogenesis to make excitatory neurons for the neocortex. The increase of Shh ligand in the dorsal forebrain, starting around midneurogenesis, upregulates downstream signaling in a subset of dorsal neural progenitors to instruct them toward an oligodendrocyte fate. During this period, neurogenesis and oligodendrogenesis overlap in a heterogeneous progenitor population, as the neocortex undergoes a neuron-to-glia transition that is complete by early postnatal ages. Yellow cells represent neurogenic progenitors and their neuronal offspring. Green cells represent oligodendrocyte progenitors and their OPC and oligodendrocyte progeny. UL, upper layers; LL, lower layers; SVZ, subventricular zone; VZ, ventricular zone.
The role of Shh in neocortical oligodendrogenesis
Early in mouse forebrain development (E9.5), Shh is highly expressed in ventral regions, where it is required to promote oligodendrogenesis (Nery et al., 2001; Tekki-Kessaris et al., 2001). Lower levels of Shh have also been reported in the embryonic dorsal forebrain (Dahmane et al., 2001; Tekki-Kessaris et al., 2001; Komada et al., 2008). Furthermore, loss-of-function mutants of Shh, Smo, or Ptc1 exhibit proliferation defects in dorsal progenitors (Komada et al., 2008; Dave et al., 2011), indicating a role for Shh signaling in the developing dorsal forebrain. Here, we show that Shh signaling via Smo is required in dorsal progenitors for normal production of neocortical oligodendrocytes. Previous studies indicate that the levels and timing of Shh signaling must be tightly regulated in the dorsal forebrain to prevent ventralization and progenitor mis-specification (Kohtz et al., 1998; Gaiano et al., 1999; Rallu et al., 2002; Yabut et al., 2015). In line with these studies, we find that overactivation of Shh signaling by conditional ablation of Sufu increases the percentage of dividing dorsal progenitors that are Olig2+, leading to precocious oligodendrogenesis in the neocortex. Based on these data, we propose a model (Fig. 9A) in which Shh signaling is maintained at low levels in the early dorsal forebrain to allow for normal dorsal-ventral patterning, but increases during later stages to initiate oligodendrogenesis. In line with this model, a recent study identified a dorsal domain in the postnatal telencephalon that exhibits high levels of Shh signaling, which is required for normal oligodendrocyte production for the postnatal corpus callosum (Tong et al., 2015).
Notably, Emx1;Smo mutants are not completely devoid of dorsally derived oligodendrocytes. Although we cannot rule out the possibility that these remaining dorsal oligodendrocytes escaped recombination and are therefore not mutants, we do not favor this interpretation given the early timing and robust recombination in Emx1-Cre mice. Instead, it is possible that a subset of dorsal progenitors can still generate neocortical oligodendrocytes in the absence of Shh signaling. Previous studies identified Shh-independent sources of oligodendrocytes in the spinal cord and forebrain (Chandran et al., 2003; Kessaris et al., 2004; Cai et al., 2005; Hashimoto et al., 2017). FGF signaling has emerged as a top candidate for promoting oligodendrogenesis independently of or together with Shh (Chandran et al., 2003; Gabay et al., 2003; Kessaris et al., 2004; Vallstedt et al., 2005; Abematsu et al., 2006; Bilican et al., 2008; Farreny et al., 2018). An interesting area for future study will be to determine whether Shh-dependent and Shh-independent sources of oligodendrocytes arise from separate progenitor lineages, and whether they ultimately have distinct functions in the mature brain.
Multiple sources of Shh ligand contribute to dorsal oligodendrogenesis
Our studies reveal that migrating interneurons are one source of Shh ligand critical for dorsal oligodendrogenesis, which is in line with previous studies demonstrating that interneurons migrating into the neocortex express high levels of Shh (Flandin et al., 2011). Furthermore, a recent study showed that interneurons secrete paracrine ligands, including the cytokine fractalkine. These ligands promote oligodendrogenesis from neocortical progenitors in vitro (Voronova et al., 2017). In vivo genetic ablation of interneurons before their migration leads to decreased numbers of neocortical oligodendrocytes (Voronova et al., 2017), indicating that migration of interneurons into the neocortex is a critical step for dorsal oligodendrogenesis. Notably, the developmental timing of interneuron infiltration into the dorsal germinal zone, beginning at E14.5 and increasing substantially by E16.5 (Anderson et al., 2001), coincides with the time at which we see dramatically increased oligodendrocyte production from dorsal progenitors (from E15.5 to E17.5).
Previous studies have shown that Shh protein is present in the embryonic CSF of the lateral ventricles (Huang et al., 2010; Chau et al., 2015), suggesting it is secreted by the choroid plexus and/or cells lining the ventricles. In line with this possibility, Shh is secreted into the fourth ventricle by the hindbrain choroid plexus to regulate hindbrain development (Huang et al., 2009, 2010). Additionally, CSF secreted by the postnatal forebrain choroid plexus can promote the oligodendrocyte lineage in ventricular–subventricular zone progenitors (Silva-Vargas et al., 2016). Our results from Cux2;Shh mutants indicate that, in addition to interneurons, the choroid plexus is also a likely source of Shh ligand for neocortical oligodendrogenesis.
Importantly, none of the Shh mutants in our study exhibited decreases in oligodendrocytes as severe as the decrease exhibited by the Emx1;Smo mutant. One possible explanation is that there is yet another source of Shh that remains unaffected in our mutants. Since the Nestin-Cre line drives recombination throughout the entire CNS starting at E10.5 (Graus-Porta et al., 2001), it is unlikely that this putative source of Shh is CNS-derived. Previous studies showed that Shh secreted from epithelial cells in hair follicles is transported to the perinatal hippocampus via platelets, where it controls stem-cell maintenance (Choe et al., 2015). It would be interesting to test whether this or another outside source of Shh is important for neocortical oligodendrogenesis. Alternatively, it is possible that other Hedgehogs, namely Indian hedgehog or Desert hedgehog, can drive dorsal oligodendrogenesis. Indeed, Indian hedgehog is required for OPC production in the zebrafish spinal cord (Chung et al., 2013) and is expressed in the mouse embryonic neocortex (Tekki-Kessaris et al., 2001) by migrating interneurons (Voronova et al., 2017).
Conclusion
Previous studies together with our data presented here have led to our working model of neocortical oligodendrogenesis (Fig. 9). We propose that the early embryonic dorsal forebrain requires low Shh signaling for proper dorsal-ventral patterning, which is facilitated in part by weak ligand expression and antagonism by Sufu. Starting at ∼E15, higher levels of Shh ligand are secreted by infiltrating interneurons and the choroid plexus, thereby increasing downstream signaling in a subset of dorsal neural progenitors to instruct them toward an oligodendrocyte fate (Fig. 9A). This provides a timing mechanism to initiate the transition away from neurogenesis and toward oligodendrogenesis, which then continues until the switch is complete by early postnatal ages (Fig. 9B). In the future, it will be important to determine how Shh signaling works in conjunction with other factors, such as fractalkine or Fgf signaling, to promote oligodendrogenesis. Additionally, it will be interesting to determine how the loss of embryonic Olig2+ cells in Emx1;Smo mutants subsequently affects oligodendrocytes and myelin in the mature brain. Further investigations into the developmental origins of neocortical oligodendrocytes will continue to expand our knowledge of brain development and neural progenitor behavior, as well as provide important information for understanding and combating neurological disease and demyelinating disorders.
Footnotes
This work was supported by the Children's Hospital Colorado Program in Pediatric Stem Cell Biology (S.J.F.), the Boettcher Foundation (S.J.F.), National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) Grant R01MH077694 (S.J.P.), NIH/NINDS Grant R01MH077694-S1 (H.G.G.), and NIH/National Cancer Institute Grant K01CA201068 (O.R.Y.). We thank Jane Johnson for the Ascl1 antibody, Fernando García-Moreno for the piggyBac plasmids, and Dailey Nettles for experiments that did not appear in the final version of the manuscript.
The authors declare no competing financial interests.
References
- Abematsu M, Kagawa T, Fukuda S, Inoue T, Takebayashi H, Komiya S, Taga T (2006) Basic fibroblast growth factor endows dorsal telencephalic neural progenitors with the ability to differentiate into oligodendrocytes but not gamma-aminobutyric acidergic neurons. J Neurosci Res 83:731–743. 10.1002/jnr.20762 [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL (2001) Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128:353–363. [DOI] [PubMed] [Google Scholar]
- Bilican B, Fiore-Heriche C, Compston A, Allen ND, Chandran S (2008) Induction of Olig2 precursors by FGF involves BMP signalling blockade at the Smad level. PLoS One 3:e2863. 10.1371/journal.pone.0002863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M (2005) Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and shh signaling. Neuron 45:41–53. 10.1016/j.neuron.2004.12.028 [DOI] [PubMed] [Google Scholar]
- Cai J, Chen Y, Cai WH, Hurlock EC, Wu H, Kernie SG, Parada LF, Lu QR (2007) A crucial role for Olig2 in white matter astrocyte development. Development 134:1887–1899. 10.1242/dev.02847 [DOI] [PubMed] [Google Scholar]
- Chandran S, Kato H, Gerreli D, Compston A, Svendsen CN, Allen ND (2003) FGF-dependent generation of oligodendrocytes by a hedgehog-independent pathway. Development 130:6599–6609. 10.1242/dev.00871 [DOI] [PubMed] [Google Scholar]
- Chau KF, Springel MW, Broadbelt KG, Park HY, Topal S, Lun MP, Mullan H, Maynard T, Steen H, LaMantia AS, Lehtinen MK (2015) Progressive differentiation and instructive capacities of amniotic fluid and cerebrospinal fluid proteomes following neural tube closure. Dev Cell 35:789–802. 10.1016/j.devcel.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Huynh T, Pleasure SJ (2015) Epithelial cells supply Sonic Hedgehog to the perinatal dentate gyrus via transport by platelets. Elife 4. 10.7554/eLife.07834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AY, Kim S, Kim E, Kim D, Jeong I, Cha YR, Bae YK, Park SW, Lee J, Park HC (2013) Indian hedgehog B function is required for the specification of oligodendrocyte progenitor cells in the zebrafish CNS. J Neurosci 33:1728–1733. 10.1523/JNEUROSCI.3369-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocas LA, Miyoshi G, Carney RS, Sousa VH, Hirata T, Jones KR, Fishell G, Huntsman MM, Corbin JG (2009) Emx1-lineage progenitors differentially contribute to neural diversity in the striatum and amygdala. J Neurosci 29:15933–15946. 10.1523/JNEUROSCI.2525-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AH, Tripathi RB, Richardson WD, Franklin RJM (2016) Developmental origin of oligodendrocyte lineage cells determines response to demyelination and susceptibility to age-associated functional decline. Cell Rep 15:761–773. 10.1016/j.celrep.2016.03.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Sánchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Ruiz i Altaba A (2001) The sonic hedgehog-gli pathway regulates dorsal brain growth and tumorigenesis. Development 128:5201–5212. [DOI] [PubMed] [Google Scholar]
- Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ (2011) Sonic hedgehog and notch signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS One 6:e14680. 10.1371/journal.pone.0014680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farreny MA, Agius E, Bel-Vialar S, Escalas N, Khouri-Farah N, Soukkarieh C, Danesin C, Pituello F, Cochard P, Soula C (2018) FGF signaling controls shh-dependent oligodendroglial fate specification in the ventral spinal cord. Neural Dev 13:3. 10.1186/s13064-018-0100-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, Westphal H, Rubenstein JL (2011) Lhx6 and Lhx8 coordinately induce neuronal expression of shh that controls the generation of interneuron progenitors. Neuron 70:939–950. 10.1016/j.neuron.2011.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Müller U (2013) Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron 77:19–34. 10.1016/j.neuron.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U (2011) Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69:482–497. 10.1016/j.neuron.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Müller U (2012) Fate-restricted neural progenitors in the mammalian cerebral cortex. Science 337:746–749. 10.1126/science.1223616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M, Rallu M, McMahon AP, Fishell G (2004) Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development 131:5031–5040. 10.1242/dev.01349 [DOI] [PubMed] [Google Scholar]
- Gabay L, Lowell S, Rubin LL, Anderson DJ (2003) Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron 40:485–499. 10.1016/S0896-6273(03)00637-8 [DOI] [PubMed] [Google Scholar]
- Gaiano N, Kohtz JD, Turnbull DH, Fishell G (1999) A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nat Neurosci 2:812–819. 10.1038/12186 [DOI] [PubMed] [Google Scholar]
- García-Marqués J, López-Mascaraque L (2013) Clonal identity determines astrocyte cortical heterogeneity. Cereb Cortex 23:1463–1472. 10.1093/cercor/bhs134 [DOI] [PubMed] [Google Scholar]
- García-Moreno F, Vasistha NA, Begbie J, Molnár Z (2014) CLoNe is a new method to target single progenitors and study their progeny in mouse and chick. Development 141:1589–1598. 10.1242/dev.105254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sanz C, Espinosa A, Fregoso SP, Bluske KK, Cunningham CL, Martinez-Garay I, Zeng H, Franco SJ, Müller U (2015) Lineage tracing using Cux2-cre and Cux2-CreERT2 mice. Neuron 86:1091–1099. 10.1016/j.neuron.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR (2002) Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci 22:6309–6314. 10.1523/JNEUROSCI.22-15-06309.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Müller U (2001) Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31:367–379. 10.1016/S0896-6273(01)00374-9 [DOI] [PubMed] [Google Scholar]
- Hall A, Giese NA, Richardson WD (1996) Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development 122:4085–4094. [DOI] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Heldin CH, Westermark B, Raff MC (1989) PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development 105:595–603. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Jiang W, Yoshimura T, Moon K-H, Bok J, Ikenaka K (2017) Strong sonic hedgehog signaling in the mouse ventral spinal cord is not required for oligodendrocyte precursor cell (OPC) generation but is necessary for correct timing of its generation. Neurochem Int pii:S0197-0186(17)30470-9. 10.1016/j.neuint.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Huang X, Ketova T, Fleming JT, Wang H, Dey SK, Litingtung Y, Chiang C (2009) Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development 136:2535–2543. 10.1242/dev.033795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Liu J, Ketova T, Fleming JT, Grover VK, Cooper MK, Litingtung Y, Chiang C (2010) Transventricular delivery of sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A 107:8422–8427. 10.1073/pnas.0911838107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Jamen F, Rubin LL, Richardson WD (2004) Cooperation between sonic hedgehog and fibroblast growth factor/MAPK signalling pathways in neocortical precursors. Development 131:1289–1298. 10.1242/dev.01027 [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD (2006) Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 9:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Pringle N, Richardson WD (2008) Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos Trans R Soc Lond B Biol Sci 363:71–85. 10.1098/rstb.2006.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Battiste J, Nakagawa Y, Johnson JE (2008) Ascl1 (Mash1) lineage cells contribute to discrete cell populations in CNS architecture. Mol Cell Neurosci 38:595–606. 10.1016/j.mcn.2008.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE (2011) Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One 6:e18472. 10.1371/journal.pone.0018472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz JD, Baker DP, Corte G, Fishell G (1998) Regionalization within the mammalian telencephalon is mediated by changes in responsiveness to sonic hedgehog. Development 125:5079–5089. [DOI] [PubMed] [Google Scholar]
- Komada M, Saitsu H, Kinboshi M, Miura T, Shiota K, Ishibashi M (2008) Hedgehog signaling is involved in development of the neocortex. Development 135:2717–2727. 10.1242/dev.015891 [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Meyers EN, Martin GR (1997) Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol 62:159–168. 10.1101/SQB.1997.062.01.021 [DOI] [PubMed] [Google Scholar]
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP (2001) Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 105:599–612. 10.1016/S0092-8674(01)00369-5 [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH (2000) Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25:317–329. 10.1016/S0896-6273(00)80897-1 [DOI] [PubMed] [Google Scholar]
- Marshall CA, Novitch BG, Goldman JE (2005) Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci 25:7289–7298. 10.1523/JNEUROSCI.1924-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS (2007) Timing is everything: making neurons versus glia in the developing cortex. Neuron 54:357–369. 10.1016/j.neuron.2007.04.019 [DOI] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B (2014) Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276:29–47. 10.1016/j.neuroscience.2013.11.029 [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wölfel B, Dodt HU, Zieglgänsberger W, Wotjak CT, et al. (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51:455–466. 10.1016/j.neuron.2006.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H, Martin E, Hassani H, Clavairoly A, Maire CL, Viadieu A, Kerninon C, Delmasure A, Frah M, Weber M, Nakafuku M, Zalc B, Thomas JL, Guillemot F, Nait-Oumesmar B, Parras C (2013) Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J Neurosci 33:9752–9768. 10.1523/JNEUROSCI.0805-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G (2001) Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 128:527–540. [DOI] [PubMed] [Google Scholar]
- Ono K, Takebayashi H, Ikeda K, Furusho M, Nishizawa T, Watanabe K, Ikenaka K (2008) Regional- and temporal-dependent changes in the differentiation of Olig2 progenitors in the forebrain, and the impact on astrocyte development in the dorsal pallium. Dev Biol 320:456–468. 10.1016/j.ydbio.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, et al. (2010) Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140:148–160. 10.1016/j.cell.2009.12.027 [DOI] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G (2002) Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and hedgehog signaling. Development 129:4963–4974. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N (2006) Oligodendrocyte wars. Nat Rev Neurosci 7:11–18. 10.1038/nrn1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi F, Chen F, Aron AW, Fiondella CG, Patel K, LoTurco JJ (2014) Fate mapping by piggyBac transposase reveals that neocortical GLAST+ progenitors generate more astrocytes than Nestin+ progenitors in rat neocortex. Cereb Cortex 24:508–520. 10.1093/cercor/bhs332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F (2016) Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19:643–652. 10.1016/j.stem.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M (2002) Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev 16:165–170. 10.1101/gad.215802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD (2001) Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 128:2545–2554. [DOI] [PubMed] [Google Scholar]
- Tong CK, Fuentealba LC, Shah JK, Lindquist RA, Ihrie RA, Guinto CD, Rodas-Rodriguez JL, Alvarez-Buylla A (2015) A dorsal SHH-dependent domain in the V-SVZ produces large numbers of oligodendroglial lineage cells in the postnatal brain. Stem Cell Reports 5:461–470. 10.1016/j.stemcr.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, Clarke LE, Burzomato V, Kessaris N, Anderson PN, Attwell D, Richardson WD (2011) Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts. J Neurosci 31:6809–6819. 10.1523/JNEUROSCI.6474-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23:99–103. 10.1038/12703 [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J (2005) Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 45:55–67. 10.1016/j.neuron.2004.12.026 [DOI] [PubMed] [Google Scholar]
- Voronova A, Yuzwa SA, Wang BS, Zahr S, Syal C, Wang J, Kaplan DR, Miller FD (2017) Migrating interneurons secrete fractalkine to promote oligodendrocyte formation in the developing mammalian brain. Neuron 94:500–516.e9. 10.1016/j.neuron.2017.04.018 [DOI] [PubMed] [Google Scholar]
- Vue TY, Kim EJ, Parras CM, Guillemot F, Johnson JE (2014) Ascl1 controls the number and distribution of astrocytes and oligodendrocytes in the gray matter and white matter of the spinal cord. Development 141:3721–3731. 10.1242/dev.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabut OR, Fernandez G, Huynh T, Yoon K, Pleasure SJ (2015) Suppressor of fused is critical for maintenance of neuronal progenitor identity during corticogenesis. Cell Rep 12:2021–2034. 10.1016/j.celrep.2015.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Shook NA, Kanisicak O, Yamamoto S, Wosczyna MN, Camp JR, Goldhamer DJ (2009) A multifunctional reporter mouse line for cre- and FLP-dependent lineage analysis. Genesis 47:107–114. 10.1002/dvg.20474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa K, Rad R, Takeda J, Bradley A (2009) Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods 6:363–369. 10.1038/nmeth.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ (2000) Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 25:331–343. 10.1016/S0896-6273(00)80898-3 [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A (2001) hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31:85–94. 10.1002/gene.10008 [DOI] [PubMed] [Google Scholar]