Abstract
Background:
Stool microscopy and concentration techniques are the two most important and necessary aspects of diagnostic parasitology. In an era when there is increased disease burden due to intestinal parasites, an early and appropriate diagnosis is warranted. Direct microscopy is usually labor intensive and tedious.
Materials and Methods:
Thirty-two fresh fecal specimens from patients presenting with eosinophilia and/or anemia (hemoglobin levels <10 g%), HIV-positive patients, and in patients clinically suspected of harboring parasites, were collected for the study. All the positive samples were processed by both the standard methodology, i.e., formalin-ethyl acetate sedimentation technique and Mini Parasep® SF method, by the standard operating procedure of our laboratory and the manufacturer's instruction. Stool pellet concentrates were subjected to saline/iodine wet mount, modified acid fast staining for intestinal coccidian parasites and trichrome staining for Blastocystis hominis. The average number of organisms counted in 0.5 ml of pellet was used for comparison of the two techniques.
Results:
The morphology of eggs was maintained in both the techniques; however, the wet mount prepared from the sedimentation technique had more background fecal debris in comparison to the Parasep® technique. The parasite yield was equal for both the techniques while Mini parasep had the advantage of less distortion of parasite morphology.
Conclusion:
We found that Parasep® offered a better parasitic yield, a better workflow capacity, and a reduced turnaround time, which would further benefit resource-restrained laboratories and those with a high sample turnover.
Keywords: Fine-needle aspiration cytology, fluid cytology, incidental findings, microfilaria
INTRODUCTION
Gastrointestinal parasites remain an overwhelming problem in tropical developing countries. It is estimated that some 3.5 billion people are affected, and that 450 million are ill as a result of these infections, the majority being children.[1] The severity varies from asymptomatic carriage to life-threatening gastrointestinal disease, as occurs in amebiasis and cryptosporidiosis.[2] In resource-poor settings, the impact of parasitic infections is of much greater significance in relation to morbidity, mortality, and economic impact.[2] The main process in diagnostic parasitology encompasses specimen collection/fixation, concentration, wet mount assessment, and microscopic examination of permanently stained smears. The detection of gastrointestinal parasites is through the retrieval of trophozoites and cysts, helminth eggs, and larvae, for which microscopic examination has been a useful tool. In order to enhance the parasitic recovery, it is essential to improve the detection rates of gastrointestinal parasites with the help of various concentration techniques. Moreover, such a diagnostic technique should be economical and highly sensitive and should be able to be performed by inexperienced technicians for the success in resource-restrained setups.[3]
Concentration of fecal specimens principally works on the basis of the differences in specific gravity of the solution and the parasites and removal of fecal debris in the background. It enables the detection of scanty organisms that might have been missed by employing only a direct wet smear. The closed concentration system allows safe, rapid, and reliable detection of intestinal parasites such as Ridley concentration system.[4]
We have employed a commercially available Mini Parasep® solvent-free (SF) technique using SF tubes and compared with standard concentration methods to analyze turnaround time and other stool specimen processes such as specimen collection/fixation and concentration.[2] A novel modification of the closed concentration system, the Mini Parasep® SF, comes with two-stage filtration matrix incorporated within the stool spoon. The vertical filter is anchored to a conical collection tube assembly, while the alcohol-based fixative is housed in a flat-bottomed tube having the screw-off cap.
Aim
The aim was to assess the single-vial, formalin-free fixative, Mini Parasep® technique in comparison to the current standard methodology, i.e., formalin-ethyl acetate sedimentation technique for isolation, comparison of parasitic morphology, and to find out turnaround time for each specimen.
MATERIALS AND METHODS
Thirty-two fresh fecal specimens, representing the number of positive specimens encountered in 6 months of parasitological investigations in patients presenting with eosinophilia and/or anemia (hemoglobin levels < 10 g%), HIV-positive patients, and in patients clinically suspected of harboring parasites, were collected for the study. All the positive samples were processed by both the standard methodology, i.e., formalin-ethyl acetate sedimentation technique and Mini Parasep® SF method, by the standard operating procedure of our laboratory and the manufacturer's instruction.
Two-level scoops/5 ml of stool was transferred to a mixing chamber, i.e., the Alcorfix® containing portion of the Parasep® tube which was then assembled to the sedimentation cone holding the vertical filtration device. The entire unit was vortexed briefly for 10–15 s to mix the contents, inverted to allow the contents to be filtered through the filter thimble, and then centrifuged at 400 g for 2 min [Figure 1]. The mixing chamber and the filter thimble were unscrewed carefully and then discarded. 3–5 ml or 2–3 G of stool, depending on the consistency, was transferred to a tube containing 7 ml of 10% formal saline and vortexed briefly for 20–30 s to mix the contents. The mixture was then strained through a sieve of 450–500 μ and collected into a conical 15 ml centrifuge tube. 4–5 ml of ethyl acetate was added to the mixture; the contents were mixed thoroughly and centrifuged at 1000 g for 3 min.
Figure 1.

Flow diagram depicting the use of Parasep® SF technique in the microbiology laboratory. (a) Unassembled Parasep® SF tube: flat-bottomed mixing chamber (1) and filter attached to the conical sedimentation with an inbuilt spoon (2); (b) the sedimentation cone scoop of fecal specimen is assembled to the mixing chamber and then vortexed with the sedimentation cone facing upward; (c) the Parasep® SF tube is then centrifuged after inverting at 400 g for 2 min; (d) sedimentation chamber after centrifugation; (e) wet mount preparation from the sediment
Examination of pellet by stool microscopy
The concentrate obtained from both the techniques after centrifugation and decanting was thoroughly mixed with a wooden applicator stick. 0.5 ml concentrate was pipetted onto dry, grease-free microscope slides in duplicate. 1 drop (25 μL) each of iodine solution and normal saline was added to the sediment, mixed, and examined under microscope for wet mount examination [Figure 2]. The concentrate containing coccidian parasites, Cryptosporidium, Cyclospora cayetanensis, Isospora oocysts, and Charcot Leyden crystals was similarly pipetted onto slides and spread in a uniform thin layer. A modified (Kinyoun) acid-fast stain was performed as per standard operating procedures. The concentrate containing Blastocystis hominis was similarly pipetted onto slides and spread in a uniform thin layer. A trichrome stain was performed as per standard operating procedures. The wet mount, modified acid-fast, and trichrome-stained slides were examined by two different microbiologists for better correlation of morphological appearance of different parasites and noting down morphological details of each parasite. The average number of organisms counted per 0.5 ml of fecal sediment pellet was used to compare the parasite detection efficiency of these two different techniques.
Figure 2.
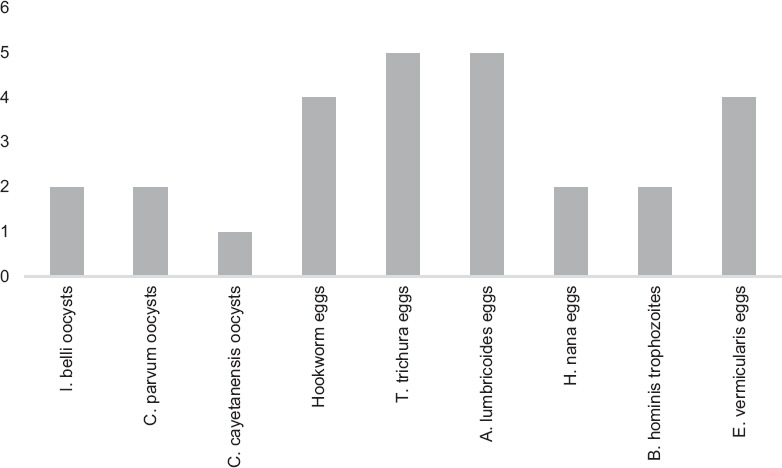
Isolation of different parasites using Mini Parasep® solvent-free method
RESULTS
Comparison of microscopic examination by two methods
The morphology of eggs was maintained in both the techniques; however, the wet mount prepared from the sedimentation technique had more background fecal debris in comparison to the Parasep® technique. Furthermore, a few eggs (Hymenolepis nana, Ascaris lumbricoides, and Trichuris trichiura) appeared distorted and entangled in the fecal debris and were difficult to appreciate with standard concentration method. The morphology of the coccidian oocysts (Cystoisospora belli, C. cayetanensis, and Cryptosporidium parvum) and Charcot Leyden crystals was retained well in both the techniques, with a better morphology being appreciated in the Mini Parasep® SF technique. However, many of the oocysts failed to retain the modified acid-fast stain and appeared as ghosts in standard stool concentration using formalin ether technique. The morphology of B. hominis trophozoites was retained in both the techniques, with a better appreciation of morphological structures of parasites in the Parasep® SF method.
The concentration by Mini Parasep® SF method produced a yield of parasites comparable to that achieved by concentration by formalin-ethyl acetate sedimentation technique, with advantage being no distortion or loss of parasite morphology. The additional advantage of the Mini Parasep® SF technique is the concentration of the entire collected specimen at once, leaving no residue with increased pellet yield which provided additional pellets in order to do additional microscopy workup such as trichrome stains, acid-fast stains, and wet mount preparations.
DISCUSSION
Newer and rapid methods are required for early and correct diagnosis of intestinal parasites; newer concentration procedures such as Mini Parasep® SF technique, which uses Mini Parasep® SF concentrator tube, have consistent methodologies, which can be used even in the field setting with little training and had shown improved parasite recovery.[3] The integration of Alcorfix®, an alcohol-based fixative in the same device, the Parasep® tube, has led to the elimination of formalin and mercuric polyvinyl alcohol fixatives from the laboratory and sample collection site.
This study aimed to evaluate the Mini Parasep® SF technique for implementation in parasitology laboratories in resource-restrained settings in comparison to the current standard stool concentration methodology. Study done by Perry et al., shows that sedimention clarity, less debris, and background uniformity of material are important considerations for microscopic detection of parasites in concentrated specimens.[5] Some of the studies compared Midi Parasep and Midi Parasep Solvent Free (SF) faecal parasite concentrators methods and concluded that small numbers of parasites in sample can be detected using Midi Parasep concentrator with ethyl acetate which can be missed using the SF faecal parasite system.[6] As compared to the study by Zeeshan et al.,[7] our study had a wider spectrum of parasitic ova and cyst yield including most of the intestinal nematodes except Strongyloides and common protozoan parasites and Cryptosporidium, Cyclospora, and Cystoisospora. Protozoan ova and cysts of Entamoeba species and Strongyloides were not seen in our study, as seen in the study by Couturier et al.;[3] yet these two studies employed formalin-fixed and preserved samples, in contrast to ours where we used only fresh fecal samples, which are better representative of the sample as there are less chances of morphological distortion of parasites.
A significant advantage of Parasep® technique was the increased turnaround time and faster reporting. The mean time for processing using the Parasep® was 4 min/sample, whereas that for the conventional formal-ethyl acetate sedimentation technique was 10–15 min/sample. The workflow study analysis revealed a time benefit of over 7 min with one specimen. The majority of the time saved was related to reduced centrifugation time and to the fact that printing and labeling of multiple tubes and the step of transfer of specimens to subsequent filters became nonmandatory. This is advantageous for laboratories with moderate-to-high parasitology specimen load and in field research setting, when compared to the tedious and laborious concentration techniques. There was not much disadvantage of using Parasep® SF method except the cost in areas where the load of finding parasites is less, but at the same time, in cases where the load is high and turnaround time is important, cost will be similar to that of the standard concentration method.
CONCLUSION
This study highlights not only the advantages of Parasep® SF method such as better yield of parasites, accurate diagnosis of parasites, and thus appropriate patient care, but also the need to improve workflow processes of parasitology laboratories.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Control of Tropical Diseases. Geneva: WHO; 1998. [Google Scholar]
- 2.Cohen J, Opal SM, Powderly WG. Infectious Diseases. 3rd ed. London: Mosby-Elsevier; 2010. [Google Scholar]
- 3.Couturier BA, Jensen R, Arias N, Heffron M, Gubler E, Case K, et al. Clinical and analytical evaluation of a single-vial stool collection device with formalin-free fixative for improved processing and comprehensive detection of gastrointestinal parasites. J Clin Microbiol. 2015;53:2539–48. doi: 10.1128/JCM.00838-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52:712–20. doi: 10.1128/JCM.02877-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry JL, Matthews JS, Miller GR. Parasite detection efficiencies of five stool concentration systems. J Clin Microbiol. 1990;28:1094–7. doi: 10.1128/jcm.28.6.1094-1097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saez AC, Manser MM, Andrews N, Chiodini PL. Comparison between the midi parasep and midi parasep solvent free (SF) faecal parasite concentrators. J Clin Pathol. 2011;64:901–4. doi: 10.1136/jcp.2011.090639. [DOI] [PubMed] [Google Scholar]
- 7.Zeeshan M, Zafar A, Saeed Z, Irfan S, Sobani ZA, Shakoor S, et al. Use of “Parasep filter fecal concentrator tubes” for the detection of intestinal parasites in stool samples under routine conditions. Indian J Pathol Microbiol. 2011;54:121–3. doi: 10.4103/0377-4929.77358. [DOI] [PubMed] [Google Scholar]


