Abstract
Background
To understand whether patient-reported experiences with lung cancer may create teachable moments (TM) for their relatives as evidenced by shifts in their risk perceptions, affective response, and self-image and in turn, motivation to quit smoking.
Methods
Patients at a comprehensive cancer center (n = 152) completed a survey within 6 months of lung cancer diagnosis to assess their cancer-related symptoms and openness and enumerated relatives who were smokers. Relative smokers (n = 218) then completed a survey assessing their risk perceptions, affective response, and self-image as a smoker related to the patient’s diagnosis (TM mechanisms), and their motivation to quit smoking. Cross-sectional mediation and moderation analyses were conducted to explore the links between patient-reported experiences, and relatives’ TM mechanisms, and motivation to quit smoking.
Results
Relative-reported affect was a significant mediator of the association between patient-reported symptoms and relative smoker’s desire to quit. Relatives’ self-image was a significant moderator of the association between patient-reported symptoms and relative smoker’s desire to quit, such that patients’ reported symptoms were associated with relatives’ desire to quit only when the relative smoker reported a generally positive self-image as a smoker. No evidence was found for moderated mediation. However, the link between symptoms and negative affect was moderated by perceptions of risk.
Conclusion
Whether smokers experience a family member’s lung cancer as a TM is influenced by multiple interrelated cognitive and affective factors that warrant further exploration. Clearer understanding of these factors could inform how to re-invigorate and sustain this motivation to promote concrete actions toward smoking cessation.
Background
The notion that a health event can be a cue for behavior change, often referred to as the ‘teachable moment (TM),’ has had considerable conceptual appeal [1–5]. As a result, innumerous observational and intervention studies have been timed to coincide with health events ranging from low threat (e.g., pregnancy, dental visits, and identification of abnormal test results) [1,6,7] to higher threat events (e.g., emergency room visits, hospitalization, cancer diagnosis, and heart attack) [8–10] as a means to promote relevant health behavior change. Results of these studies have been mixed, with some finding improvements in behavior and others finding null effects [2,6,9,11].
In relation to smoking cessation, McBride and colleagues [1] found evidence to suggest that the most influential cueing events (those that were most likely to prompt smoking cessation) appeared to tap into three cognitive domains: perceptions of personal risk, levels of disease-specific worry, and self-image. However, although each of these cognitive domains can be supported conceptually, the majority of studies evaluating TMs have evaluated the impact of the cueing event on risk perceptions and disease-related worry without considering shifts in self-image, or their effect on motivation for behavior change.
There is considerable conceptual rationale to support self-image as elemental to the TM. Indeed, various aspects of self-identity have been linked to motivation for health behaviors. For example, several seminal conceptual models including the theory of reasoned action and social cognitive theory suggest that the interpersonal or relational self can inspire motivation to comply with the desires of important others [12,13]. Moreover, social role theory suggests that relational expectations about role performance can motivate individuals to make efforts to comply; a sick person is expected to behave in a manner that is consistent with efforts to get better [14]. Accordingly, there is increasing conceptual discussion that a cancer diagnosis, when perceived as a traumatic experience, can prompt reconfigurations in the self-schema that, in turn, spurs motivation to adopt new behaviors [15]. Whether this can occur vicariously among family members has yet to be explored. Additionally, notions of the possible self, that is, one’s comparison of current self-image with what one hopes for or fears as a future self, also have been suggested to have motivational effects on health behaviors [16,17]. Thus, omission of this key element could account, at least in part, for observed inconsistencies in the association of cueing events with behavior change outcomes. For this study, we considered smoker self-image to be an individual’s cognitive generalizations and evaluations about the self (i.e., self-schemata) as it related to their smoking behavior [18].
Consideration of the impact of the TM on behavior change outcomes also has relied almost solely on evaluating an individual’s subjective responses to his or her own health events and own self-reported behavior changes. These subjective perceptions of the cueing event could be influenced by biases in attention given to aspects of threatening information [19]. For example, individuals may attend only to aspects of the cueing event that support behavioral inertia. Moreover, reports of behavioral intentions and change also could be subject to social desirability biases such that individuals experiencing events such as a disease diagnosis could feel compelled to report increased motivation to take behavioral actions [20]. Taken together, these biases could artificially inflate motivation and make it difficult to find associations of cueing events with health behavior change outcomes.
Many of the TM studies have focused and continue to focus on tobacco use because of its ongoing importance as a risk factor [5,21]. In this report, we use data from an observational study, described elsewhere [22], that evaluated uptake of genetic susceptibility testing by smokers who were relatives of lung cancer patients, to extend the literature on TMs in two important ways. First, we characterize a patient’s reported symptoms and willingness to discuss their lung cancer experiences as a potential cueing event that could increase their relative smokers’ motivation to quit smoking. Second, we consider all three domains of the TM, that is, relative-smokers’ assessments of the cueing event’s impact on their own: perceived risk for lung cancer, a range of positive and negative affective responses they may have had to the patient’s diagnosis, and their self-image as a smoker.
In considering the effects of the cueing event on relative smokers’ desire to quit, we considered that the patient’s lung cancer diagnosis might have sufficient potency to prompt a TM via at least two different mechanisms. First, the patient’s cancer experience might be associated with increases in relative smokers’ TM responses (i.e., perceived risk, affect, or self-image). These responses in turn could operate as mediators via their association with increased motivation to quit smoking. Alternatively, the relative smokers’ TM responses could moderate the effect of the patient’s diagnosis on their motivation to quit. For example, the relative smoker’s level of disease worry could influence whether the patient’s cancer experience influences the relative smoker’s motivation to quit. Second, we considered whether relative’s TM responses to the patient’s cancer experience simultaneously served as moderators and mediators in the link between cueing events and motivation to quit by testing for moderated mediation. For example, a relative smoker’s perceived risk for lung cancer could influence whether the patient’s cancer experience influences the relative smoker’s emotional response and, in turn, their motivation to quit smoking. We also considered that the relative’s frequency of contact with the patient also could play an important role in cueing their TM responses.
Methods
Study sample
Patients and their relatives were recruited for this observational study in tandem with a larger multi-site smoking cessation trial [23,24]. Eligible patients were receiving care for stage IIIB/IV lung cancer in the Thoracic Oncology Clinic at the H. Lee Moffitt Cancer Center and Research Institute. Eligible patients were aged 18 years and over, diagnosed with lung cancer, intended to continue care at Moffitt Cancer Center, and had at least one person in their social network who smoked. Recruitment procedures were approved by the National Human Genome Research Institute and the University of South Florida and Duke University Medical Center Institutional Review Boards.
Patients with lung cancer were identified through their providers and approached by a recruiter during their visits to the clinic. Patients were asked if they were willing to be contacted for a brief telephone survey about their general well-being and to identify relatives who smoke. Patients who agreed were contacted within a week by a trained interviewer to complete a brief survey. As part of the survey, the patient enumerated relatives who were current smokers and was asked for permission to contact these relative smokers.
Relative smokers were sent a letter to inform them of the study, and they were provided a toll-free number to call to decline participation. Relatives who did not decline were contacted by a survey interviewer and asked to complete a 30-min telephone survey. In keeping with requirements of the smoking cessation intervention evaluated in the parent project, relative smokers were eligible if they reported smoking at least 100 cigarettes in their lifetime and 7 or more cigarettes per day in the prior 7 days.
Measures
Patient-reported cueing event characteristics
Symptoms
Patients were asked to rate how they felt over the past week on a 4-point scale (1-Excellent to 4-Poor); the frequency in the past week of three commonly reported lung cancer symptoms [25]: shortness of breath, poor appetite, and fatigue on a 4-point scale (1-not at all to 4-very much); and the degree of pain they experienced in the prior week ranging from 0-no pain to 10-‘pain as bad as you can imagine.’ These items were standardized and summed to arrive at a symptoms scale (α = 0.74). Patients’ reports of how they felt in the past week were significantly correlated with relatives’ ratings of patient health (r = 0.36, p < 0.001) and quality of life (r = 0.42, p < 0.001), further validating the use of patient report.
Openness
Patients were asked to rate their level of willingness to discuss their cancer experience with other family members using the 9-item openness scale [26]. For each statement (e.g., ‘I talk as little as possible about my illness because I don’t want to make my family uneasy’), patients were asked about their level of agreement (1-‘strongly agree’ to 4-‘strongly disagree’). Items were coded so that higher scores indicate more openness (α = 0.78).
Relative smokers’ teachable moment measures
Perceived risk
Relative smokers were asked to respond to questions related to risk on a 7-point scale (1-certain not to happen to 7-certain to happen). One item assessed their risk of acquiring lung cancer if they quit smoking, and two items assessed their risk of acquiring lung cancer or other health problems if they continue to smoke. The mean was taken of these two latter items to create a scale score (α = 0.83). Higher scores indicate greater risk.
Affective response
Relative smokers reported their level of worry about acquiring lung cancer and about having a smoking-related health problem in their lifetime (1-not at all worried to 5-very worried). These two items were averaged to create a worry scale score (α = 0.90). Higher scores indicate greater worry. Relative smokers also reported on the extent to which they had experienced each of 10 emotions drawn from the Positive and Negative Affect Scale (5 positive, e.g., energized; and 5 negative, e.g., guilty) [27] since learning of the patient’s diagnosis (1-very slightly or not at all to 5-extremely). Five items were averaged to create a positive affect scale (α = 0.62; higher scores indicate more positive affect), and five items were averaged to create a negative affect scale (α = 0.76; higher scores indicate more negative affect). The negative affect scale was highly correlated with the 10-item Centers for Epidemiologic Studies-Depression (CES-D) scale [28], as an assessment of depressive symptoms (r = 0.60, p < 0.001), validating our decision to focus on affect. The Positive and Negative Affect Scale measures negative affect indicative of subjective distress and positive affect indicative of pleasurable engagement with the environment [29].
Self-image as a smoker
Three questions were created to assess relative smokers’ self-image. Relatives were asked how they felt about themselves as a smoker, how their views about themselves as a smoker changed since learning about the patient’s lung cancer, and how strongly they agreed that people important to them think they should quit smoking. These three items were recoded and averaged to create a scale score ranging from 1 to 7 (α =0.63), with higher scores indicating more positive feelings about oneself as a smoker (See the Appendix for items).
Frequency of contact with the cancer patient
Relative smokers reported the frequency with which they spoke with the patient on the phone and saw the patient in person (e.g., daily, weekly and monthly). These two items were recoded and averaged to create a frequency of contact scale from 0 (Never) to 5 (Live with Patient) (α = 0.76).
Dependent variable: relative-reported motivation to quit smoking
Relatives were asked to rate on a scale from 1 to 7 how much they wanted to quit in the next 6 months (1-not at all to 7-very much).
Analysis
We tested bivariate associations among patient-reported symptoms and openness, relative-reported TM variables, and relative-reported desire to quit smoking. To better understand the role of TM variables in the association between patient-reported symptoms and the relative’s desire to quit smoking, we conducted multivariate mediation and moderation analyses, using multi-level models with relatives nested within families. Mediation analyses tested whether TM variables mediated the association between patient-reported symptoms and the relative’s desire to quit using the Baron and Kenny [30] method. We also used the MacKinnon [31] method and the Sobel [32] test, to calculate the mediated effect and test its significance. Moderation was tested by including interaction terms between centered TM variables and patient-reported symptoms when predicting the relative’s desire to quit smoking. Significant interactions were followed up by centering groups at one standard deviation (SD) above and below the mean, as specified by Aiken and West [33]. Finally, we tested for potential moderated mediation based on the mediation and moderation results. All models controlled for relative’s age, gender, and number of cigarettes smoked in the past 7 days.
Results
Sample
Recruitment efforts identified 482 patients seen at the clinical site from January 2005 thru July 2006. Patients who could be reached for the baseline survey (n = 336) identified 539 relatives they thought to be cigarette smokers. A total of 296 (55%) relative smokers completed the baseline survey; 218 met eligibility criterion of smoking 100 cigarettes in their lifetime and more than 7 cigarettes per day in the prior 7 days and had complete data for patient-reported cancer experiences (Figure 1). The final sample comprises 218 relative smokers of 152 patients.
Figure 1.
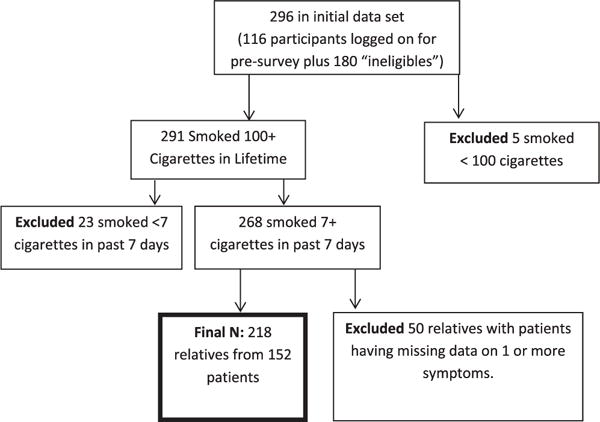
Sample flow chart
Patient and relative characteristics
Of the 152 patients surveyed, 64% were female, with a mean age of 63 years (SD = 9.8; age range 39–88 years). Two-thirds of the patients had received their diagnosis more than 3 months before the survey. On average, patients rated their health as fair (2.5 on a scale from 1, Excellent, to 4, Poor). Patients reported an average of 1.25 (SD=0.89) symptoms (0–3) and moderate levels of openness (2.7 on a 4-point scale, SD=0.49) and in willingness to discuss their cancer experiences with family members.
Of the 218 relative smokers, 50% reported being in the patient’s immediate family, of whom 67% were either the son or daughter of the patient. Fifty-nine percent of the relative smokers were women, and they reported an average age of 43 years (SD = 11.9). Just under half (47%) reported seeing the patient at least monthly since the diagnosis. Relative smokers reported smoking an average of 17 (SD = 10.3) cigarettes per day and a relatively strong desire to quit smoking (5.9 (SD = 1.7) on a 7-point scale). Relative smokers on average perceived themselves to be at moderately high risk for acquiring lung cancer if they continued to smoke (5.5 (SD = 1.1) on a 7-point scale) and perceived their risk would be lower if they quit smoking (4.3 (SD = 1.1) on a 7-point scale). Levels of worry about smoking-related conditions also were relatively high (3.7 (SD = 1.2) on a 5-point scale). Relatives reported experiencing a balance of positive and negative affect since learning of the patient’s diagnosis (3.0 (SD = 0.78) vs. 2.6 (SD = 1.0), respectively, on 5-point scales). Relatives tended to feel badly about themselves as a smoker (1.8 (SD = 1.0) on a 7-point scale where higher scores indicated positive self-image).
Association of the patient-reported experiences with TM constructs
As shown in Table 1, patient-reported symptoms and openness were significantly and positively associated with the relative’s reported desire to quit smoking (rs=0.15, and 0.14, p< 0.05, respectively). Of the TM constructs, only relative-reported positive and negative affect were significantly correlated with the number of patient-reported symptoms (−0.21 and 0.18, p< 0.01, respectively). None of the TM constructs (perceived risk, affective responses, or self-image) were associated with patient-reported openness. Patient-reported symptoms and openness were significantly and negatively correlated (−0.17, p < 0.05); the more symptoms the patient reported, the less open they were in discussing the cancer with their relatives. Thus, going forward, we focus on patient-reported symptoms as the indicator of the cueing event for relative smokers with the expectation that the patient’s experience of symptoms aligned with their suffering, and in turn, could prompt a relative’s TM responses.
Table 1.
Means, SD, and correlations of all study variables (n = 218 relatives, 152 patients)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary outcome: relative smoker reported | ||||||||||
| 1. Desire to quita | 1.00 | |||||||||
| Cueing events: patient reported | ||||||||||
| 2. Symptomsb | 0.15* | 1.00 | ||||||||
| 3. Opennessc | 0.14* | −0.17* | 1.00 | |||||||
| Teachable moment constructs: relative smoker reported | ||||||||||
| 4. Risk if quit smokingd | 0.07 | 0.03 | 0.05 | 1.00 | ||||||
| 5. Risk if continue to smoked | 0.31*** | 0.05 | −0.02 | 0.38*** | 1.00 | |||||
| 6. Worrye | 0.62*** | 0.13 | 0.05 | 0.24*** | 0.51*** | 1.00 | ||||
| 7. Positive affectf | −0.01 | −0.21** | 0.09 | −0.10 | −0.13* | −0.13* | 1.00 | |||
| 8. Negative affectf | 0.31*** | 0.18** | −0.01 | 0.12 | 0.24*** | 0.48*** | −0.36*** | 1.00 | ||
| 9. Positive self-imageg | −0.54*** | 0.02 | −0.01 | −0.16* | −0.36*** | −0.62*** | 0.14* | −0.51*** | 1.00 | |
| Additional moderator: relative smoker reported | ||||||||||
| 10. Contact frequencyh | 0.12 | 0.00 | 0.11 | 0.09 | 0.08 | 0.11 | 0.09 | 0.17* | −0.13 | 1.00 |
| Means (SD) | 5.9 (1.67) | −0.07 (3.43) | 2.1 (0.49) | 4.3 (1.07) | 5.5 (1.05) | 3.7 (1.18) | 3.0 (0.78) | 2.6 (1.00) | 1.8 (1.03) | 2.5 (1.17) |
SD, standard deviation.
p = 0.05.
p = 0.01.
p = 0.001.
Responses on a scale from 1 (Not at all) to 7 (Very much).
Patients were asked about how they were feeling on a scale from 1 (Excellent) to 4 (Poor), their experience with three symptoms (shortness of breath, poor appetite, and fatigue) on a scale from 1 (Not at all) to 4 (Very much), and their pain on a scale from 0 (No pain) to 10 (Pain as bad as you can imagine). These five items were standardized and summed to create the symptoms scale.
Scale scored from 1 to 4. Higher responses indicate more openness.
Responses on a scale from 1 (Certain not to happen) to 7 (Certain to happen).
Responses on a scale from 1 (Not at all worried) to 5 (Very worried).
Scale scored from 1 (Very slightly or not at all) to 5 (Extremely).
Scale scored from 1 to 7. Higher scores indicate more positive feelings about self as a smoker.
Scale scored from 0 (Never) to 5 (Live with Patient).
Association of TM constructs with desire to quit smoking among relative smokers
Relative-smokers’ reported perceived risk if the relative continued to smoke, worry about smoking-related health conditions, negative affect since the patient’s diagnosis, and self-image all were significantly associated with their desire to quit smoking. Stronger perceived risk of continuing to smoke, greater worry, more negative affect, and more negative self-image as a smoker each were associated with greater reported desire to quit smoking in the coming 6 months (Table 1).
Notably, there were significant associations among the TM constructs. Having a positive self-image as a smoker was strongly and negatively associated with worry (−0.62, p< 0.001). Positive self-image as a smoker also was negatively associated with perceived risk of lung cancer with continued smoking (−0.36, p< 0.001) and with perceived risk after quitting smoking (−0.16, p< 0.05). Worry was strongly and positively associated with perceptions of higher risk of lung cancer with continued smoking (0.51, p< 0.001) and greater negative affect since the patient’s diagnosis (0.48, p< 0.001). Relatives’ reports of more negative affect were associated with perceptions of greater risk of continued smoking (0.24, p< 0.001).
TM constructs as mediators and moderators of the association between cueing event and relative-smoker’s desire to quit smoking
We first tested whether any of three TM constructs operated as mediators of the association between patient-reported symptoms and relative smokers’ desire to quit smoking. Negative affect was found to significantly mediate the association between patient-reported symptoms and relative smoker’s desire to quit. More severe patient symptoms were associated with greater relative negative affect, and in turn, more negative affect was associated with a great desire to quit smoking. As shown in Figure 1, about a third of the effect of the patient’s cancer experience (severity of symptoms) on the relative’s increased desire to quit smoking was mediated by the relative reported negative affect. Risk perception and self-image were not significant mediators of the link between symptoms and desire to quit.
We next tested whether any of the TM constructs, as well as frequency of contact, moderated the association of patient-reported symptoms with relative smoker’s desire to quit. Only self-image was a significant moderator of the association between patient-reported symptoms and relative smoker’s desire to quit (γ = 0.04, p = 0.05). As shown in Figure 2, patients’ reported symptoms were associated with relatives’ desire to quit only when the relative smoker reported a generally positive self-image as a smoker (γ = 0.12, p = 0.001). Among relatives with a more positive self-image as a smoker, more severe patient symptoms were associated with a stronger desire to quit. We also found frequency of contact to be a marginally significant moderator (γ = −0.05, p = <0.10) of the association between patient-reported symptoms and relative’s desire to quit. Among those who had less frequent contact with the patient, more severe patient symptoms were associated with a stronger desire to quit smoking (γ = 0.13, p = 0.01).
Figure 2.
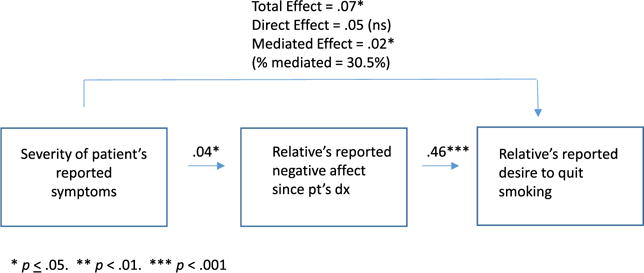
Relative’s negative emotional response as mediator of the cueing effect on their desire to quit
Lastly, we considered whether the mediating role of negative affect in the association between patient-reported symptoms and relative desire to quit was moderated by the other TM constructs. While we found no evidence of moderated mediation, we did find that relative smoker’s perceptions of risk of acquiring lung cancer if they quit smoking were a significant moderator of the association between patient-reported symptoms and relative’s reports of negative affect (γ = 0.04, p= 0.02). As shown in Figure 3, among relatives who perceived a higher risk of acquiring lung cancer if they quit smoking, more severe patient symptoms were associated with greater negative affect (γ = 0.08, p=0.002). There was only an association between patient reported symptoms and negative affect among relatives who thought that quitting would not be efficacious in lowering risk (Figure 4).
Figure 3.
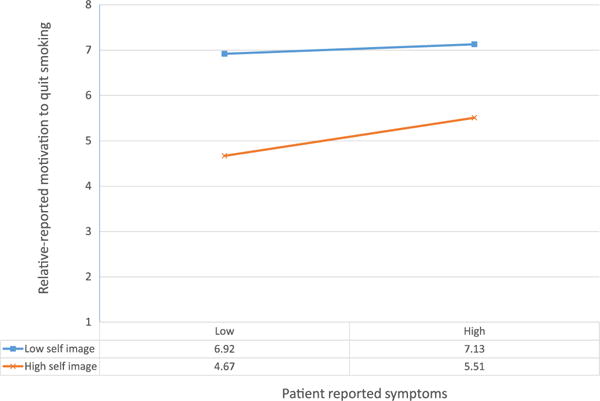
Relative’s self-image as a smoker moderates the association of patient-reported symptoms with relative’s motivation to quit smoking (interaction, p = 0.044)
Figure 4.
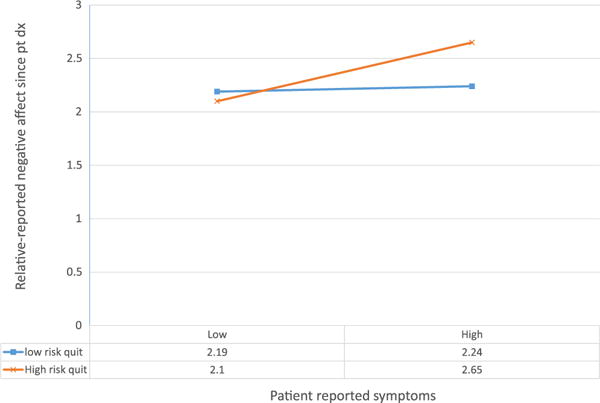
Relative’s perception of the efficacy of quitting for lowering risk moderates the association of relative’s negative affect with patient-reported symptoms (interaction, p = 0.033)
Conclusions
Our results provide preliminary evidence that a patient’s lung cancer experience can have a cueing effect on relative smoker’s motivation to quit smoking. In our sample, as found previously [34], this appeared to occur primarily via the influence of the patient’s symptom experience on relative smokers’ negative emotions. However, our results also suggested that the cueing effect on negative affect is heightened if the relative smoker perceives that smoking cessation will not lower the risk of lung cancer. Although we did not find the moderated mediation on motivation to quit, future TM research should give consideration to more complex conceptualizations of how events might influence motivation for behavior change.
The role of risk perceptions in creating at TM also merits further consideration. Prior research that has explored the association of qualitatively different categories of risk perceptions – objective number of risk factors [34] or risk of cancer recurrence [35] – with motivation for behavior change has found inconsistent results for the role of risk in the TM. Moreover, these results support the need for conceptual specificity in considering which risk perceptions are most likely to be influenced by the cueing event.
These results also support the need for further exploration of the role of smoker’s self-image on the power of the cueing event. Our results raise questions about whether patients’ negative lung cancer experiences may be most attention-getting and motivational for cessation among those who view themselves most positively as a smoker. It is noteworthy that this group was in the minority among relative smokers based on our measure. It is conceptually plausible that continuing to smoke in the context of a relative’s lung cancer diagnosis may challenge the relative smoker’s self-esteem as a smoker. This may have been influenced by other family members’ perspective as fully 82% of relative smokers reported agreement at level 7 on a 7-point scale that ‘important people thought that they should quit.’ Deeper understanding of these interpersonal influences and their association with motivation to quit smoking warrants further exploration.
These results should be considered with several caveats. The study was not designed to test the TM. The sample size was relatively small, which may have limited our ability to detect effects, particularly moderated and mediated effects, which require larger sample sizes than those to detect similar size main effects. For example, four times the sample size is necessary to detect an interaction effect compared with a main effect at equal size and power. Additionally, although both patients and relative perspectives were represented, each was surveyed only once Thus with cross-sectional analyses, directionality of the associations cannot be determined. Moreover, we do not have information on the relative’s level of motivation prior to the patient’s diagnosis. Findings related to self-image also need replication. Data collection was conducted as part of a randomized controlled intervention trial that permitted us relatively few items to assess the construct of self-image. We focused solely on the relative smoker’s self-image as a smoker (negative versus positive). It is possible that other aspects of self-schemata (e.g., role identity – son, daughter) or feared future self (e.g., a future with lung cancer) may also play a role. However, these preliminary findings lend credence to the notion that the role of self-image as a TM is key to fully understanding the effect of cueing events on motivation to quit smoking.
In conclusion, our results suggest that a family member’s lung cancer experiences can, via influences on negative affect, prompt motivation to quit smoking. However, we know well that desire to quit does not necessarily translate into successful cessation. In a related study, Schnoll and colleagues found that relatives of cancer patients were more likely to enroll in a smoking cessation program than relatives of orthopedic patients, but no more likely to quit smoking [36]. Schnoll suggests that additional strategies may be required to sustain the impact of the TM. Bastian and colleagues found that relative smokers who completed more telephone counseling and requested nicotine replacement were most likely to be successful at cessation [25]. Bastian and colleagues and others [37] have raised questions about the appropriate timing of interventions suggesting that soon after the diagnosis, when the TM might be most potent, may not be optimal. This period is characterized by stress and sadness that may make it counter-intuitive to give up a long-held coping strategy. However, other results suggest that the salience of a family member’s cancer diagnosis may have longer-lasting influences on motivation related to health habits [38].
Taken together, the pressing challenge going forward is how to re-invigorate and sustain the motivational impact of the cueing event to promote smoking cessation. The short survival rates associated with lung cancer mean that the window of opportunity for involving patients in these efforts often will be limited. Our findings relating to affect suggest that techniques such as ‘ethical wills’, where patients write values, wisdom, hopes, and advice to family members, which have been shown to reduce a patient’s own suffering, also could arouse emotional responses in relatives that reinforce their motivation to take steps toward smoking cessation [39]. However, it is also likely that relatives will need coaching and support regarding alternatives to smoking for handling grief and negative emotions [25].
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Human Genome Research Institute and the National Cancer Institute grant 5U01-CA-92622. The authors have no conflicts of interest to report.
Appendix: Self-image items
On a scale from 1 to 7 where 1 is you feel bad about yourself because you smoke and 7 is you feel good about yourself because you smoke, what number best describes how you feel about yourself as a smoker?
Since you found out [PATIENT’S FIRST NAME, has or had] lung cancer, would you say you feel better about yourself as a smoker, worse about yourself as a smoker, or there’s been no change in how you feel about yourself as a smoker?
Using a scale from 1 to 7 where 1 is strongly disagree and 7 is strongly agree, please tell me how strongly you disagree or agree with the following statement. Most people important to you think you should quit smoking.
References
- 1.McBride CM, Lipkus IM, Emmons KM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 2.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehata NV, Reece GP. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 3.Coa KI, Smith KC, Klassen AC, et al. Capitalizing on the “teachable moment” to promote healthy dietary changes among cancer survivors: the perspectives of health care providers. Support Care Cancer. 2014 doi: 10.1007/s00520-014-2412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawson PJ, Flocke SA. Teachable moments for health behavior change: a concept analysis. Patient Educ Couns. 2009;76:25–30. doi: 10.1016/j.pec.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor KL, Sanderson Cox L, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56:125–134. doi: 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Poghosyan H, Sheldon LK, Cooley ME. The impact of computed tomography screening for lung cancer on smoking behaviors: a teachable moment? Cancer Nurs. 2012;35(6):466–475. doi: 10.1097/NCC.0b013e3182406297. [DOI] [PubMed] [Google Scholar]
- 7.Stevens VJ, Severson H, Lichtenstein E, Little SJ, Leben J. Making the most of a teachable moment: a smokeless-tobacco cessation intervention in the dental office. Am J Public Health. 1995;85(2):231–235. doi: 10.2105/ajph.85.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broderick JM, Hussey J, Kennedy MJ, O’Donnell DM. Testing the ‘teachable moment’ premise: does physical activity increase in the early survivorship phase? Support Care Cancer. 2014;22:989–997. doi: 10.1007/s00520-013-2064-4. [DOI] [PubMed] [Google Scholar]
- 9.Sabiston CM, Brunet J, Valliance JK, Meterissian S. Prospective examination of objectively assessed physical activity and sedentary time after breast cancer treatment: sitting on the crest of the teachable moment. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1324–1330. doi: 10.1158/1055-9965.EPI-13-1179. [DOI] [PubMed] [Google Scholar]
- 10.Sommers MS, Lyons MS, Fargo JD, et al. Emergency department-based brief intervention to reduce risky driving and hazardous/harmful drinking in young adults: a randomized controlled trial. Alcohol Clin Exp Res. 2013;37(10):1753–1762. doi: 10.1111/acer.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorin AA, Phelan S, Hill JO, Wing JR. Medical triggers are associated with better short-and long-term weight loss outcomes. Prev Med. 2004;39:612–616. doi: 10.1016/j.ypmed.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. [Google Scholar]
- 13.Bandura A. Social Learning Theory. Prentice Hall; Englewood Cliffs, N.J: 1977. [Google Scholar]
- 14.Williams SJ. Parsons revisited: from the sick role to…? Health: An Interdisciplinary Journal for the Social Study of Health. Illn Med. 2005;9(2):123–144. doi: 10.1177/1363459305050582. [DOI] [PubMed] [Google Scholar]
- 15.Bell K. Remaking the self: trauma, teachable moments, and the biopolitics of cancer survivor-ship. Cult Med Psychiatry. 2012;36:584–600. doi: 10.1007/s11013-012-9276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black MEA, Stein KF, Loveland-Cherry CJ. Older women and mammography screening behavior: do possible selves contribute? Health Educ Behav. 2001;28(2):200–216. doi: 10.1177/109019810102800206. [DOI] [PubMed] [Google Scholar]
- 17.Ouellette JA, Hessling R, Gibbons FX, Reis-Bergan M, Gerrard M. Using images to increase exercise behavior: prototypes of possible selves. Pers Soc Psychol Bull. 2005;31:610–620. doi: 10.1177/0146167204271589. [DOI] [PubMed] [Google Scholar]
- 18.Markus HR. Self-schemas and processing information about the self. J Pers Soc Psychol. 1977;35:63–78. [Google Scholar]
- 19.Kim S, Lee JH. Time course of attentional bias for health-related information in individuals with health anxiety. J Health Psychol. 2014 doi: 10.1177/1359105314557976. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Miller TM, Abdel-Maksoud MF, Crane LA, Marcus AC, Byers TE. Effects of social approval bias on self-reported fruit and vegetable consumption: a randomized controlled trial. Nutr J. 2008;7:118. doi: 10.1186/1475-2891-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flocke SA, Antognoli E, Step MM, Marsh S, Parran T, Mason MJ. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109–113. doi: 10.1186/1472-6963-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neill SC, White DB, Sanderson SC, et al. The feasibility of online genetic testing for lung cancer susceptibility: uptake of a Web-based protocol and decision outcomes. Genet Med. 2008;10(2):121–130. doi: 10.1097/GIM.0b013e31815f8e06. [DOI] [PubMed] [Google Scholar]
- 23.Bastian LA, Fish LJ, Peterson BL, et al. Proactive recruitment of cancer patients’ social networks into a smoking cessation trial. Contemp Clin Trials. 2011;32:498–504. doi: 10.1016/j.cct.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastian LA, Fish LJ, Peterson BL, et al. Assessment of the impact of adjunctive proactive telephone counseling to promote smoking cessation among lung cancer patients’ social networks. Am J Health Promot. 2013;27(3):181–190. doi: 10.4278/ajhp.101122-QUAN-387. [DOI] [PubMed] [Google Scholar]
- 25.Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Cancer. 1994;73:2087–2098. doi: 10.1002/1097-0142(19940415)73:8<2087::aid-cncr2820730813>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Mesters I, van den Borne H, McCormick L, Pruyn J, de Boer M, Imbos T. Openness to discuss cancer in the nuclear family: scale, development and validation. Psychosom Med. 1997;59:269–279. doi: 10.1097/00006842-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 28.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 29.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 30.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical consideration. J Pers Soc Psychol. 1986;5(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. Available at: http://www.public.asu.edu/~davidpm/classes/psy536/Baron.pdf. [DOI] [PubMed] [Google Scholar]
- 31.MacKinnon D. Analysis of mediating variables in prevention and intervention research. In: Cázares A, Beatty L, editors. Scientific Methods for Prevention Intervention Research. National Institute on Drug Abuse; Rockville, MD: 1994. pp. 127–153. (NIDA Monograph No. 139). [PubMed] [Google Scholar]
- 32.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 33.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage; Thousand Oaks, CA: 1991. [Google Scholar]
- 34.McBride CM, Puleo E, Pollak KI, Clipp EC, Woolford S, Emmons KM. Understanding the role of cancer worry in creating a “teachable moment” for multiple risk factor reduction. Soc Sci Med. 2008;66(3):790–800. doi: 10.1016/j.socscimed.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay JL, Ostroff J, Burkhalter J, Li Y, Quiles Z, Moadel A. Changes in cancer-related risk perception and smoking across time in newly-diagnosed cancer patients. J Behav Med. 2007;30(2):131–142. doi: 10.1007/s10865-007-9094-7. [DOI] [PubMed] [Google Scholar]
- 36.Schnoll RA, Wileyto EP, Leone FT, Langer C, Lackman R, Evans T. Is a cancer diagnosis a teachable moment for the patient’s relative who smokes? Cancer Causes Control. 2013;24:1339–1346. doi: 10.1007/s10552-013-0212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25(19):2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 38.Humpel N, Magee C, Jones SC. The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Support Care Cancer. 2007;15:621–630. doi: 10.1007/s00520-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 39.Gessert CE, Baines BK, Kuross SA, Clark C, Haller IV. Ethical wills and suffering in patients with cancer: a pilot study. J Palliat Med. 2004;7(4):517–526. doi: 10.1089/jpm.2004.7.517. [DOI] [PubMed] [Google Scholar]


