Abstract
Ethanol (EtOH) is one of the most frequently abused drugs with heavy health, economic and societal burdens. Although moderate to low EtOH may have some neuroprotective effects, heavy EtOH consumption associated with high blood alcohol level (BAL) can be quite detrimental. The brain is particularly vulnerable to the damaging effects of high BAL, leading to neuronal loss, cognitive and behavioral deficits. Although the exact causes of these detriments are not fully elucidated, it is believed that damage to the cholinergic system is at least partially responsible for the cognitive impairment. Thus, high BAL may result in selective apoptotic damage to the cholinergic neurons. Donepezil (DON), a centrally acting, reversible and non-competitive acetylcholinesterase (AChE) inhibitor, approved for use in Alzheimer’s disease (AD), may also attenuate EtOH-induced cognitive impairment. Cognitive effects of DON might be due to an anti-apoptotic activity as some AChE inhibitors have been shown to have this property. The aim of this study was to determine whether DON might protect against EtOH-induced toxicity and whether such protection might be apoptotically mediated. We exposed the human neuroblastoma-derived, SH-SY5Y cells to a relatively high concentration of EtOH (500 mM) for 24 h and evaluated the effects of two concentrations of DON (0.1 and 1.0 μM) on alcohol-induced toxicity and caspase-3, an apoptotic marker. We found a dose-dependent protection of DON against EtOH-induced toxicity as well as dose-dependent attenuation of EtOH-induced increases in caspase-3 levels. Thus, DON may inhibit apoptosis as well as alcohol-induced toxicity.
Keywords: Neurotoxicity, Neuroprotection, Acetylcholinesterase Inhibitor, Apoptosis, Caspase-3, SH-SY5Y Cells
Introduction
Alcohol or ethanol (EtOH) is one of the most frequently abused drugs with heavy health, economic and societal burdens (Goodwin et al., 1970; Tharp et al., 1974; Sacks et al., 2010; Antonelli et al., 2018). Moderate to low EtOH (two drinks for men and one for women) may reduce stress and increase feelings of well-being with some beneficial effects on cardiovascular and neuronal systems (Collins et al., 2008; 2010; Tizabi et al., 2018). However, heavy EtOH consumption is quite detrimental to overall health (Churchill et al., 2008; Collins and Neafsey, 2011; Krenz et al., 2012; Su et al., 2017; Tizabi et al., 2018) and particularly to the central nervous system (Moonat et al., 2010; Collins and Neafsey, 2011; Natarajan et al., 2015). High blood EtOH levels lead to: neuronal loss, brain shrinkage associated with reduced brain volume, enlarged ventricles and increased cerebral spinal fluid levels (Pfefferbaum et al. 1992; Maier and West, 2001; Moonat et al., 2010; Collins and Neafsey, 2011; Natarajan et al., 2015). These changes can result in behavioral deficits and cognitive dysfunction referred to as “alcoholic dementia” (Goodwin et al. 1970; Tharp et al., 1974; Weissenborn and Duka 2000). Although various neurotransmitter systems have been implicated, the cholinergic transmission may be a key player in cognitive dysfunctions induced by alcohol (Arendt 1988; Gawel et al 2016; Mulholland et al 2018). Indeed, “cholinergic hypothesis” of dementia has been proposed. This hypothesis is based on observations that loss of basal forebrain cholinergic neurons and the consequent reduction of acetylcholine (ACh) significantly contribute to the cognitive impairments such as those associated with Alzheimer’s disease (AD) or EtOH related dementias (Bartus et al., 2000; Sarter and Bruno, 2004; Cutuli et al. 2013). In AD models, studies have shown a marked neuroprotective effect of donepezil (DON), a centrally acting reversible and noncompetitive AChE inhibitor (Yamamoto et al., 2010). Indeed, DON is one of the very few drugs that is approved as a first-choice therapy for the treatment of mild to moderate AD (Small et al., 1997; Brodaty et al., 1999; Giacobini, 2000).
In addition to causing a reduction in ACh, high EtOH’s damage may also involve neuronal death through apoptotic process (Guadagnoli et al., 2016; Heaton et al., 2003; Sun et al., 2017; Ieraci and Herrera 2018). Apoptosis, a mechanism of programmed cell death is essential for development and homeostasis, however, if over-activated, it can result in unintended neuronal death (Han et al. 2000; Kim and Zhao, 2005; Li and Liu, 2010). Overexpression of caspases, proteases acting through specific signaling pathways, can lead to mitochondrial dysfunction and eventual death of the neuron (Green and Reed, 1998; Thornberry and Lazebnik, 1998). Hence, caspases in general, and caspase-3 in particular, are measured as marker of apoptosis (Han et al. 2000; Kim and Zhao, 2005; Li and Liu, 2010).
SH-SY5Y, human neuroblastoma-derived cells are commonly used to investigate cellular/molecular mechanisms of drugs including EtOH-induced neurotoxicity and/or neurodegeneration (Ramlochansingh et al., 2001; Das and Tizabi, 2009; Tizabi et al., 2012; Xicoy et al. 2017). Exposed to high EtOH (500mM), cultured SHSY-5Y cells undergo apoptosis, which might be triggered via increased AChE activity (Sun et al., 2017). Since DON is a potent AChE inhibitor and has been shown to protect against various toxic agents in SH-SY5Y cells (Das and Tizabi, 2009; Sun et al., 2017), we undertook this study to evaluate possible protective effects of DON against EtOH-induced toxicity in these cells. Moreover, since caspase-3 has been implicated in EtOH-induced toxicity, the effects of DON on this protease was also evaluated. Our hypothesis was that DON will protect against EtOH-induced toxicity in SH-SY5Y cells and that at least part of this protection is mediated through caspase-3 reduction.
EtOH was obtained from EMD Chemicals Inc (Gibbstown, NJ) and donepezil hydrochloride (donepezil HCl), (RS)-2-[(1-benzyl-4-piperidyl)methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one was supplied by Ibaraki, Japan. Caspase-3 antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Western Blot and analytical reagents as well as MTT (3, (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) kit were purchased from Sigma Chemical Company (Sigma-Aldrich, St. Louis, MO). The SH-SY5Y human neuroblastoma cell line (originally derived from a female patient) were purchased from American Type Culture Collection (ATCC, Manassas, VA); caspase-3 antibody from Santa Cruz (Dallas, Texas) and the secondary antibody from Thermo Scientific (Rockford, IL).
SH-SY5Y cells were cultured in a 1:1 mixture of Dulbeccos Modified Eagle Medium (DMEM) and Ham’s F12 supplemented with 10 % fetal bovine serum, penicillin/streptomycin (100 IU/ml) at 37° C in 95% O2/5% CO2 humidified incubator. The cells were trypsinized when confluent (>90%) and plated in 96 well plates (approximately1.2 × 104 cells/well). Cells were allowed to adhere to bottom surface for 24 h. Then, fresh media containing various concentrations of drugs (EtOH, DON) were added to the carefully aspirated wells. In all cases, DON was added 2 h prior to alcohol. Control group consisted of cells that were maintained in media alone and without any drug treatment. All treatments were carried out for 24 h and the effects on cell viability was determined following the 24 h incubation. To determine the total caspase-3 levels, cells (approximately 1.9 × 105) were maintained in a 6 well culture plate to allow adequate protein harvesting. Here also, cells were pre-incubated with DON (0.1 and 1 μM), 2 h prior to the addition of EtOH. After 24 h incubation, the cells were harvested from the bottom of the wells for Western blot assay.
Determination of cell viability was performed by MTT colorimetric assay according to the manufacturer’s protocol. Briefly, the yellow MTT tetrazolium salt (0.5 mg/ml) was dissolved in DMEM and MTT (30 μL) was added to each well and incubated for 4 h at 37° C. The live cells cause a reduction of the yellow salt to insoluble purple formazan crystals. The wells were then aspirated, and 100 μL of 10% SDS (sodium dodecyl sulfate) was added to the wells to solubilize the crystals. The plates were then placed in an incubator at 37° C overnight and read spectrophotometrically at 570 nm with a background of 630 nm in a plate reader. Cell viability was determined by subtracting the test results from the background and is presented as a percentage of the control.
Following drug treatment: EtOH (500mM), DON (0.1 and 1uM) or their combination, cells were harvested and washed twice with cold PBS and subsequently lysed in 1x SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) loading buffer (120 μL per well in 6-well plate). The lysed cells were heated to 95–100°C for 10 min followed by cooling on ice and centrifugation at 10,960 ×g for 1 min at 4°C. The protein concentration was determined by Peirce BCA method. Samples were electrophoresed through 10% polyacrylamide gels, transferred onto PVDF membranes and immunoblotted with antibodies against total caspase-3 (1:250 dilution). Following incubation of the primary antibody, membranes were washed with tris-buffered saline (20 mM Tris/HCl, pH 7.5 and 150 mM NaCl) containing 0.1% Tween 20 and incubated in anti-rabbit horseradish peroxidase–conjugated secondary antibody (1:10,000). Following secondary antibody incubation, membranes were washed with tris-buffered saline, incubated in SuperSignal West Dura Extended Duration Substrate (Thermo Scientific) followed by developing and image analysis.
Data is expressed as mean ± SEM. Statistical differences within and between treatment groups were determined by one-way ANOVA followed by post-hoc Newman–Keuls Multiple comparison test, where P < 0.05 was considered statistically significant. Data were analyzed using Graphpad Prism 3 (Graphpad Software, Inc., San Diego, CA).
Compared with the control group, cell viability was dose-dependently reduced by EtOH treatment (Figure 1). Thus, the highest concentration of EtOH (500 mM) resulted in approximately 60% reduction (p<0.01) in cell viability. Hence, this concentration of EtOH was used in all subsequent experiments. As depicted in Figure 2, pretreatment with DON resulted in reversal of EtOH-induced toxicity, where the lower concentration of DON (0.1 μM) resulted in 80% protection (p<0.01) and the higher concentration of DON (1.0 μM) completely blocked the alcohol effect (P<0.01). Neither concentration of DON had any effect of its own (Fig 2). Thus, there was a dose-dependent inhibition of Et-OH-induced toxicity by DON where at 1.0 μM concentration, total block of Et-OH toxicity was obtained. EtOH treatment resulted in 100% increase in caspase-3 levels, which was significantly reduced (to 25% p<0.05) by the lower concentration of DON (0.1 μM) and completely blocked by the higher concentration of DON (1.0 μM, p<0.01) (Figure 3). Hence, parallel to the inhibition of the toxic effects of Et-OH, DON also dose-dependently inhibited Et-OH induced increases in caspase-3 levels. In this case also, total block of Et-OH-induced increase in caspse-3 was obtained with 1.0 μM DON.
Figure 1.
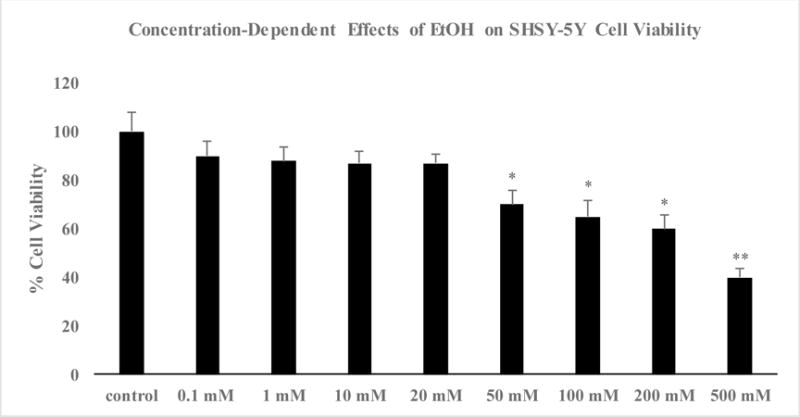
Effect of different concentrations of ethanol (EtOH) on cell viability as determined by MTT assay in SH-SY5Y cells. Cells were incubated for 24 h. Values are mean ± SEM of 3 independent experiments. In every experiment, each sample was run in replicates of 6. *P<0.05, **P<0.01 compared to control.
Figure 2.
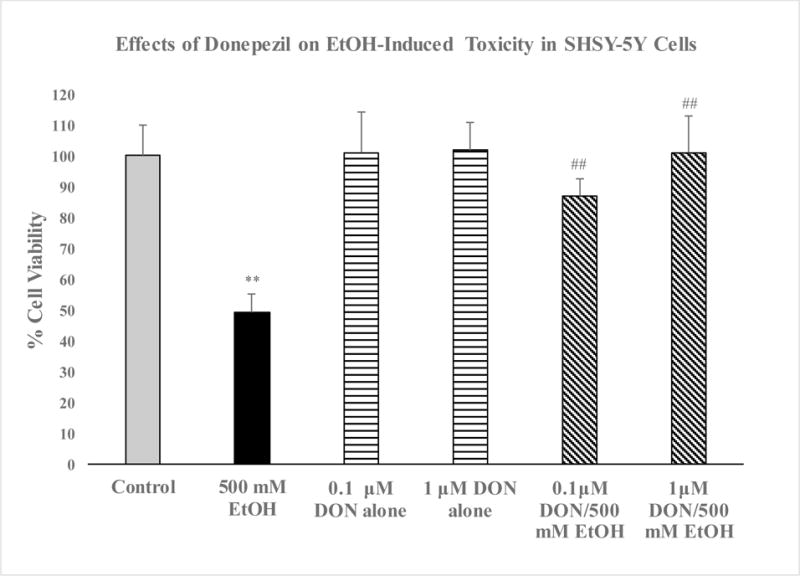
Effects of donepezil (DON), 0.1 and 1 μM on EtOH-induced cell death in SH-SY5Y cells. DON was applied 2 h before EtOH (500 mM) and cells were incubated for 24 h. Values are mean ± SEM of 5 independent experiments. In every experiment, each sample was run in triplicate. **P<0.01 compared to control, ##P<0.01 compared to EtOH.
Figure 3.
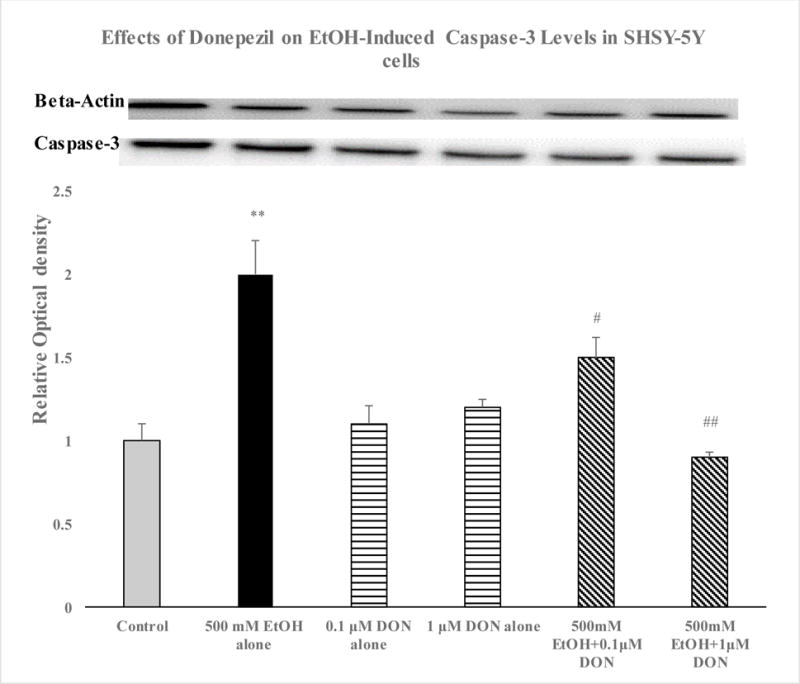
Effects of ethanol (EtOH) 500 mM and donepezil (DON 0.1 and 1 μM) alone or in combination on caspase-3 levels in SH-SY5Y cells. DON was applied 2 h before EtOH and cells were incubated for 24 h. Immunnoblots of beta-actin and total caspase-3 are included. Caspase-3 values were normalized per beta-actin protein. Values are mean ± SEM of 4 independent experiments. In every experiment, each sample was run in duplicate. **P<0.01 compared to control, #P<0.05, ##P<0.01 compared to EtOH.
The results of this study indicate that donepezil, a cholinesterase inhibitor may protect against alcohol-induced toxicity in neuroblastoma-derived SH-SY5Y cells. Moreover, the toxicity induced by the high alcohol concentration in these cells was associated with an increase in caspase-3, an apoptotic marker, which was dose-dependently blocked by donepezil. Thus, donepezil, which is an FDA-approved drug for AD, may be useful in countering alcohol toxicity as well.
Other in-vitro studies have shown that exposure of the same cells to high alcohol concentration causes increases in AChE activity (Sun et al., 2017). Thus, it may be suggested that a decrease in ACh content due to an increase in the activity of the degrading enzyme may lead to eventual cell death (Sun et al., 2017). It would be of significant interest to determine whether a similar phenomenon may occur in-vivo, which would implicate ACh reduction as a major player in alcohol-induced cellular damage or death. Moreover, since the role of ACh in cognitive function is well-established a mechanistic link between high alcohol and cognitive impairment may also be suggested. That donepezil may reverse these effects could indicate a novel intervention in alcohol-induced toxicity in general and its cognitive impairment, in particular. Clearly, in-vivo follow-up studies are warranted. In this regard, beneficial effects of donepezil on behavioral deficits induced by cholinergic depletion and the concomitant hippocampal and neocortical neurodegeneration have been reported (Cutuli et al. 2013). In the same study, it was shown that the elevated caspse-3 levels induced by cholinergic lesions were also attenuated by donepezil (Cutuli et al. 2013). More recently, the effectiveness of donepezil in reversing ethanol-induced cognitive impairments as well as morphological and epigenetic changes induced by intermittent ethanol exposure in adolescent rats have been reported (Gawel et al. 2016; Mulholland et al. 2018).
It is also of relevance to note that caspases, a group of intracellular proteases, are responsible for the deliberate disassembly of cells into apoptotic bodies, resulting in a “programmed” cell death (Thornberry and Lazebnik, 1998). Indeed, increases in caspase-3, a prominent protease has been proposed as marker of early neurodegeneration including processes leading to AD (Coleman and Yao 2003; D’Amelio et al., 2012). Conversely, down-regulation of caspase-3 levels is indicative of inhibition of neuronal apoptosis and hence neuroprotection (Han et al. 2000; Kim and Zhao, 2005; Li and Liu, 2010). Our findings are in concordance with studies that show EtOH can induce widespread apoptotic neurodegeneration as indicated via increased caspase activation (Sun et al., 2017; Ieraci and Herrera 2018). Here also, the fact that donepezil could block alcohol-induced increases in caspase-3 levels, suggests a novel mechanism in neuroprotective effect of donepezil.
Although a link between ACh reduction and apoptosis has been suggested (Cutuli et al. 2013; Sun et al., 2017), it is likely that the actions of donepezil might not be solely due to its inhibition of AChE. This contention is based on a number of studies where direct interaction of donepezil with nicotinic receptors and neuroprotective effects of such interaction, at least against glutamate-induced toxicity, have been provided (Shen et al., 2010). Indeed, neuroprotective effects of nicotine against alcohol-induced toxicity and involvement of nicotinic receptors in such protection have been reported (Tizabi et al., 2005). Moreover, activation of nicotinic receptors may lead to an anti-apoptotic effect via PI3K/Akt/Bcl2 signal transduction mechanism (Takada-Takatori et al., 2009; Akaike et al., 2010).
In addition, few reports suggest that some of the neuroprotective effects of donepezil might be mediated via σ1 receptors, activation of which can result in intracellular modulation of Ca2+ and mobilization of phospholipase C (PLC)/protein kinase C (PKC) transduction pathways. Activation of this signaling pathway can lead to neuroprotection by preventing oxidative stress and hence dysfunction of mitochondrial or endoplasmic reticulum (Saxena et al., 2011; Shen et al., 2010). Thus, donepezil may have variety of actions in addition to AChE inhibition.
In summary, our findings indicate that high alcohol-induced cell death in SH-SY5Y cells is at least partially mediated via apoptosis and that donepezil, a cholinesterase inhibitor can block this toxicity by inhibiting apoptosis. Thus, donepezil may be useful in countering alcohol-induced toxicity.
Acknowledgments
Supported by R03AA022479 from NIAAA (YT), NIH/NIA R21AG047474 (ABC), Latham Trust Fund and NSF IOS-1355034 (T Hein) and VA-HBCU 5lK2RX001114-06 (T Huds). The authors wish to thank Ms. Collis Brown for her expert technical assistance.
References
- Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of Neuroprotective Effects of Nicotine and Acetylcholinesterase Inhibitors: Role of α4 and α7 Receptors in Neuroprotection. J Mol Neurosci. 2010;40:211. doi: 10.1007/s12031-009-9236-1. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Ferrulli A, Sestito L, Vassallo GA, Tarli C, Mosoni C. Alcohol addiction - the safety of available approved treatment options. Expert Opin Drug Saf. 2018;17:169–177. doi: 10.1080/14740338.2018.1404025. [DOI] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Sinden J, Schugens MM, Marchbanks RM, et al. Cholinergic-rich brain transplants reverse alcohol-induced memory deficits. Nature. 1988;332:448–450. doi: 10.1038/332448a0. [DOI] [PubMed] [Google Scholar]
- Brats RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Brodaty H. Realistic expectations for the management of Alzheimer’s disease. Eur Neuropsychopharmacol. 1999;9(Suppl 2):S43–S52. doi: 10.1016/s0924-977x(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Disatnik MH, Budas GR, Mochly-Rosen D. Ethanol for cardiac ischemia: the role of protein kinase c. Therapeutic Advances in Cardiovascular Disease. 2008;2:469–483. doi: 10.1177/1753944708094735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer’s disease. Neurobiol Aging. 2008;24:1023–7. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2008;33:206–19. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms. Mol Neurobiol. 2010;41:420–5. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ. Neuroinflammatory pathways in binge alcohol-induced neuronal degeneration: oxidative stress cascade involving aquaporin, brain edema, and phospholipase A2 activation. Neurotox Res. 2011;21:70–8. doi: 10.1007/s12640-011-9276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutuli D, De Bartolo P, Caporali P, Tartaglione AM, Oddi D, D’Amato FR, Nobili A, D’Amelio M, Petrosini L. Neuroprotective effects of donepezil against cholinergic depletion. Alzheimers Res Ther. 2013;24(5):50. doi: 10.1186/alzrt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends Neurosci. 2012;35:700–709. doi: 10.1016/j.tins.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Gawel K, Labuz K, Gibula-Bruzda E, Jenda M, Marszalek-Grabska M, Filarowska J, Silberring J, Kotlinska JH. Cholinesterase inhibitors, donepezil and rivastigmine, attenuate spatial memory and cognitive flexibility impairment induced by acute ethanol in the Barnes maze task in rats. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:1059–1071. doi: 10.1007/s00210-016-1269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Ann N Y Acad Sci. 2000;920:321–327. doi: 10.1111/j.1749-6632.2000.tb06942.x. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and Apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Guadagnoli T, Caltana L, Vacotto M, Gironacci MM, Brusco A. Direct effects of ethanol on neuronal differentiation: An in vitro analysis of viability and morphology. Brain Res Bull. 2016;127:177–186. doi: 10.1016/j.brainresbull.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Othmer E, Halikas JA, Freemon F. Loss of short term memory as a predictor of the alcoholic “Blackout”. Nature. 1970;227:201–202. doi: 10.1038/227201a0. [DOI] [PubMed] [Google Scholar]
- Han BN, D’Costa A, Back SA, Parsadanian M, Patel S, Shah Ar, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Shaw G. Ethanol effects on neonatal rat cortex: comparative analyses of neurotrophic factors, apoptosis-related proteins, and oxidative processes during vulnerable and resistant periods. Brain Res Dev Brain Res. 2003;145:249–62. doi: 10.1016/j.devbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicotinamide Inhibits Ethanol-Induced Caspase-3 and PARP-1 Over-activation and Subsequent Neurodegeneration in the Developing Mouse Cerebellum. Cerebellum. 2018 doi: 10.1007/s12311-017-0916-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kim DH, Zhao X. BDNF protects neurons following injury by modulation of caspase activity. Neurocrit Care. 2005;3:71–6. doi: 10.1385/NCC:3:1:071. [DOI] [PubMed] [Google Scholar]
- Krenz M, Korthuis RL. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol. 2012;52:93–104. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, He Z, Shen J, Huang Q, Li W, Liu X. Apoptotic caspases regulate induction of iPSCs from human fibroblasts. Cell Stem Cell. 2010;7:508–20. doi: 10.1016/j.stem.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie SE, James M, West R. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:149–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC. Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci. 2010;67:73–88. doi: 10.1007/s00018-009-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Teppen TL, Miller KM, Sexton HG, Pandey SC, Swartzwelder HS. Donepezil reverses dendritic spine morphology adaptations and Fmr1 epigenetic modifications in hippocampus of adult rats after adolescent alcohol exposure. Alcohol Clin Exp Res. 2018;42:706–717. doi: 10.1111/acer.13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan SK, Pachunka JM, Mott JL. Role of microRNAs in Alcohol-Induced Multi-Organ Injury. Biomolecules. 2015;5:3309–38. doi: 10.3390/biom5043309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res. 2011;20:263–269. doi: 10.1007/s12640-011-9239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. National and state costs of excessive alcohol consumption. Am J Prev Med. 2010;49:73–9. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol Aging. 2004;25:1127–39. doi: 10.1016/j.neurobiolaging.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Schachter AS, Davis KL. Guidelines for the appropriate use of cholinesterase inhibitors in patients with Alzheimer’s disease. CNS Drugs. 1999;11:281–288. [Google Scholar]
- Shen H, Kihara T, Hongo H, Wu X, Kem W, Shimohama S. Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of α7 nicotinic receptors and internalization of NMDA receptors. British Journal of Pharmacology. 2010;161:127–139. doi: 10.1111/j.1476-5381.2010.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Rabins PV, Barry PP, Buckhotz NS, DeKosky ST, Ferris SH, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–71. [PubMed] [Google Scholar]
- Su F, Guo AC, Li WW, Zhao YL, Qu ZY, Wang YJ, et al. Low-dose ethanol preconditioning protects against oxygen-glucose deprivation/reoxygenation-induced neuronal injury by activating large conductance, Ca2+-activated K+ channels in vitro. Neurosci Bull. 2017;33:28–40. doi: 10.1007/s12264-016-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Chen L, Zheng W, Wei X, Wu W, Duysen EG, Jiang W. Study of acetylcholinesterase activity and apoptosis in SH-SY5Y cells and mice exposed to ethanol. Toxicology. 2017;384:33–39. doi: 10.1016/j.tox.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Tharp VK, Rundell OH, Lester BK, Williams HL. Alcohol and information processing. Psychopharmacologia. 1974;40:33–52. doi: 10.1007/BF00429446. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;28(281):1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotox Res. 2005;7:319–322. doi: 10.1007/BF03033888. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Qualls Z, Brown DO, Chin Y, Hurley LL, Taylor RE. Neuroprotective effects of low alcohol concentration against LPS-induced toxicity in cutured cells. Alc Clin Exp Res. 2012;36(6 Suppl):21A. [Google Scholar]
- Tizabi Y, Getachew B, Ferguson CL, Csoka AB, Thompson KM, Gomez-Paz A, Ruda-Kucerova J, Taylor RE. Low Vs. High Alcohol: Central Benefits Vs. Detriments. Neurotox Res. 2018 doi: 10.1007/s12640-017-9859-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. State-dependent effects of alcohol on explicit memory: the role of semantic associations. Psychopharmacology (Berl) 2000;149:98–106. doi: 10.1007/s002139900349. [DOI] [PubMed] [Google Scholar]
- Xicoy H, Wieringa B, Martens GJ. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener. 2017;24(12(1)):10. doi: 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Shioda N, Han F, Moriguchi S, Fukunaga K. Donepezil-induced neuroprotection of acetylcholinergic neurons in olfactory bulbectomized mice. Yakugaku Zasshi. 2010;130:717–21. doi: 10.1248/yakushi.130.717. [DOI] [PubMed] [Google Scholar]


