Abstract
The number of epigenetic studies on brain functions and diseases are dramatically increasing, but little is known about the impact of post-mortem intervals and post-sampling effects on DNA modifications such as 5-methylcytosine (5mC) and 5-hydroxymethylcytosine (5hmC). Here, we examined post-mortem-induced changes in global brain 5mC and 5hmC levels at post-mortem intervals up to 540 min., and studied effects of tissue heat stabilization, using LUMA and ELISA. The global 5mC and 5hmC levels were generally higher in the cerebellum of adult rats than neonates. When measured by ELISA, the global 5mC content in adults, but not neonates, decreased with the post-mortem interval reaching a significantly lower level in cerebellum tissue at the post-mortem interval 540 min. (2.9 ± 0.7%; mean ± S.E.M.) compared to control (3.7 ± 0.6%). The global 5hmC levels increased with post-mortem interval reaching a significantly higher level at 540 min. (0.29 ± 0.06%) compared to control (0.19 ± 0.03%). This suggests that the post-mortem interval may confound 5mC and 5hmC analysis in human brain tissues as the post-mortem handling could vary substantially. The reactive oxygen species (ROS) level in cerebellum also increased over time, in particular in adults, and may be part of the mechanism that causes the observed post-mortem changes in 5mC and 5hmC. The global 5mC and 5hmC states were unaffected by heat stabilization, allowing analysis of tissues that are stabilized to preserve more labile analytes. Further studies in human samples are needed to confirm post-mortem effects on DNA methylation/hydroxymethylation and elucidate details of the underlying mechanisms.
The advances in epigenetics are revealing a molecular basis for how information other than the DNA sequence can influence gene function. Epigenetic mechanisms such as DNA methylation, histone modifications, chromatin remodelling and gene regulation by non-coding RNAs are crucial for the cellular development and function. It is clear that epigenetic states can be disrupted by environmental factors and the importance of epigenetic alterations in human diseases is becoming increasingly evident [1–3]. Newer classes of drugs are being developed to regulate epigenetic mechanisms and counteract disease states [4]. DNA methylation is a well-studied epigenetic modification that has been associated with several human diseases including cancer, cardiovascular disease, rheumatoid arthritis and brain disease [5–8]. While DNA methylation at position 5 of the cytosine pyrimidine ring (5mC) has been known for decades, other modifications of the DNA such as 5-hydroxymethylation (5hmC) are more recently highlighted as a biologically relevant feature [9–11]. Global 5hmC content varies between tissues, with highest levels in the brain, and appears to be correlated with tissue differentiation [12,13]. The functional significance of 5hmC is yet to be absolutely determined. It is suggested that this base modification is primarily involved in DNA demethylation, but functions may also include other regulatory processes important for diseases such as cancer and brain disorders [9,14–16].
When not using surrogate tissues such as peripheral blood and saliva, human DNA methylation studies are highly dependent upon the feasibility of analysing tissues from biobanks, where biological samples have been collected and stored for short periods or even up to decades [17]. Yet, surprisingly little is known about possible confounding effects of post-mortem interval and post-sampling handling on DNA modifications. Studies have demonstrated that DNA samples stored for several years at 80°C can be used for further applications, including DNA methylation analysis [18]. However, the post-mortem interval and pre-processing of biospecimens prior to storage are also critical for molecular studies. Death as well as the removal of a biological sample from its in vivo environment initiates a series of events as the cells trying to adapt to their new milieu [19–21]. Analyte levels such as RNA, lipids, metabolites, peptides, proteins and their modifications will rapidly start to change from their actual in vivo concentrations, making interpretation of analytical results difficult/inaccurate or even impossible [19–24]. Sample fixation by conductive heat inactivation of enzymes may reduce the heterogeneity as well as inhibit post-sampling effects on analyte levels due to tissue thawing and sample preparation [19,22,23,25]. Regarding DNA modifications, studies on placental tissue have indicated that DNA methylation is relatively stable [26,27] but, to our knowledge, no previous study has analysed 5hmC after storage delay. Such studies are important as standardized protocols for collection and processing of human tissues is often constrained by participant- or centre-related logistics, resulting in heterogeneous handling conditions, including delays of up to several hours before sample storage. This may be particularly true for biospecimens collected post-mortem, for example brain tissue [24].
The primary aim of this study was to investigate post-mortem-induced changes of global 5mC and 5hmC contents in brain tissue using a rat model to simulate post-mortem intervals up to 540 min. Age-dependent post-mortem effects were studied by analysing neonatal and adult brains. Oxidative stress levels were also assessed as the TET enzymes that convert 5mC to 5hmC may respond to oxidative stress [28]. A secondary aim was to examine the effects of tissue heat stabilization on DNA purity/quality and global 5mC/5hmC contents using neonatal brain tissue.
Material and Methods
Animal housing and sample collection
Pregnant Wistar rats were obtained from Taconic (Ejby, Denmark) and housed in Makrolon cages (59 × 38 × 20 cm) containing wood-chip bedding and nesting material. The animals were maintained on standard pellet food (R36 Labfor; Lantmännen, Kimstad, Sweden) and water ad libitum, and were housed in a temperature- and humidity-controlled environment with a 12-hr light/dark cycle (lights on at 6 a.m.). The animal experimental protocol was approved by the Uppsala Animal Ethical Committee and was consistent with the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Union Directive on the Protection of Animals Used for Scientific Purposes (2010/63/EU).
After birth, 37 neonatal female rats were killed on post-natal day 13 by decapitation [29] and the cerebellum was subsequently treated using six different protocols. To study the effect of post-mortem interval on global 5mC and 5hmC contents, the cerebellum from one group of animals (controls; n = 8) was immediately snap-frozen on dry ice, and the brains from groups two and three were kept at room temperature for 300 min. (n = 4) and 540 min (n = 7), respectively, to simulate differences in post-mortem intervals and allow post-mortem changes to appear before the dissection and storage. To determine the effect of tissue heat stabilization and examine possible influence of sample preparation on DNA purity/quality and global 5mC/5hmC states, the fourth group (n = 7) was instantly dissected after rapid enzyme inactivation by conductive heat stabilization with the Stabilizor™ system (Denator, Sweden) using the Auto Fresh mode on the intact brain prior to snap-freezing [30]. The brains from the fifth and sixth groups were also heat-stabilized but kept at room temperature for 300 min. (n = 4) and 540 min. (n = 7), respectively, before the dissection and snap-freezing in order to examine stability over time. To determine age-dependent post-mortem effects, the mothers, 12 adult female rats, were killed by decapitation and cerebellum snap-frozen immediately after dissection (n = 4) or kept at room temperature for 300 min. (n = 4) and 540 min. (n = 4), before snap-freezing, to simulate differences in post-mortem intervals. All samples were stored at −80°C until DNA extraction.
DNA extraction
DNA extraction was performed using the GeneElute™ Mammalian Genomic DNA miniprep kit (Sigma–Aldrich, St. Louis, MO, USA). In brief, brain samples were homogenized manually with a micropestle in 1.5-ml Eppendorf tubes together with lysis buffer containing Proteinase K. The samples were incubated at 55°C for 4 hr to allow for tissue digestion. RNase was added to allow for extraction of RNA-free genomic DNA. DNA isolation was performed using spin columns according to manufacturer’s protocol. The DNA was eluted in 100 μl of elution buffer. After centrifugation, the collected elution buffer was pipetted back on the binding column and the elution procedure was repeated once. The concentration and purity was measured on a Nanodrop (Thermo Scientific, Waltham, Massachusetts, USA). DNA quality was determined by running DNA on a 1% agarose gel to assess possible degradation.
Global DNA methylation
The luminometric methylation assay (LUMA) is a method that analyses global 5mC using restriction enzymes and detection with Pyrosequencing® [31]. The used enzymes HpaII and MspI are isoschizomer endonucleases that recognize the sequence CCGG and cleave between CG, but only MspI cleaves if the internal C is methylated. HpaII is sensitive to CpG methylation and will not cleave. This enzymatic treatment of the DNA creates 5′ overhang that can be quantified by a luminometric polymerase extension assay using Pyrosequencing®. The relative amount of DNA methylation is then expressed as an HpaII/MspI ratio, which gives the amount of CpG methylation in the context of CCGG. The used protocol is described in the publication by Luttropp et al. [32]. The amount of DNA used for each restriction cleavage reaction was 500 ng, and all samples were run in duplicates.
In addition, the 5mC DNA ELISA Kit™ from Zymo Research was used for the detection of 5mC. A DNA input of 100 ng per sample was used and the samples were run in duplicates according to the manufacturer’s protocol. The % 5mC in each sample is calculated using the y-intercept and the slope generated by logarithmically plotting the absorbance values of the six supplied DNA control samples with known 5mC amounts. The method gives the % 5mC content in the context of cytosine methylation regardless if the C is followed by a G, that is a CpG.
Global DNA hydroxymethylation
The Quest 5hmC DNA ELISA Kit™ from Zymo Research was used for the detection of 5hmC. A DNA input of 200 ng per sample was used and the samples were run in duplicates according to the manufacturer’s protocol. The % 5hmC in each sample is calculated using the y-intercept and the slope generated by plotting the absorbance values of the five supplied DNA control samples with known 5hmC amounts. The method gives the % 5hmC content in the context of cytosine hydroxylation regardless if the C is part of a CpG.
Oxidative stress
Adult and neonatal rats were killed by decapitation and cerebellum was immediately snap-frozen on dry ice (n = 8 per group). The samples were stored at −80°C until further analysis. Duplicates of all samples were gently homogenized in PBS (100 mg tissue/ml) kept on ice using a hand-driven glass homogenizer. The level of oxidative stress was measured using CM-H2DCFDA (Thermo Fischer, Waltham, Massachusetts, USA) as an indicator for reactive oxygen species (ROS). CM-H2DCFDA passively diffuses into cells and subsequent oxidation yields a fluorescent adduct that is trapped inside the cell. The samples were analysed for 360 min. at room temperature (21°C), by adding 10 μl of the probe (50 μM) to 100 μl of each sample, using a Polarstar Optima microplate reader (Bmg Labtech, Offenburg, Germany).
Statistical analysis
The data were analysed using analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (LSD) as post hoc test. Differences were considered statistically significant at p values <0.05. Statistica 12.0 software (StatSoft Inc. Tulsa, OK, USA) was used for the statistical analyses.
Results
The yield, purity or quality of the DNA extracted from cerebellum of neonatal or adult rats were not affected by the post mortem interval (data not shown). The average DNA yield from the heat-stabilized brain samples 814 ± 61 ng/μl (mean ± S.E.M.) was significantly increased (p < 0.05) compared to the snap-frozen non-stabilized tissue (617 ± 58 ng/μl), without any effects on purity (A260 nm/A280 nm and A260 nm/A230 nm; table 1) or quality as assessed by gel electrophoresis (data not shown).
Table 1.
Yield and purity of DNA (mean ± S.E.M.) extracted from neonatal non-stabilized snap-frozen (SF) samples and heat-stabilized samples (HS).
| Group | DNA yield (ng/μl) |
DNA purity1
|
Average2 5mC content (LUMA) | Average2 5hmC content (ELISA) | Average2 5hmC content (ELISA) | |
|---|---|---|---|---|---|---|
| 260/280 | 260/230 | |||||
| SF | 617 ± 58 | 1.87 ± 0.007 | 2.04 ± 0.04 | 69.9 ± 0.4% | 2.7 ± 0.1% | 0.13 ± 0.01% |
| HS | 814 ± 61* | 1.88 ± 0.005 | 2.05 ± 0.04 | 69.9 ± 0.3% | 2.7 ± 0.1% | 0.13 ± 0.01% |
Spectrophotometric analysis of DNA purity: A260/A280 assessment of protein contamination, A260/A230 assessment of contaminants such as guanidine thiocynate and carbohydrates.
The average global 5mC and 5hmC contents in all neonatal SF and HS samples as measured by LUMA and ELISA, respectively.
p < 0.05 compared with SF samples (Student’s t-test).
Global DNA methylation
The overall statistical comparison with ANOVA showed differences between the groups (F = 9.1, p < 0.001; F = 2.7, p < 0.05) and post hoc testing revealed an age-dependent increase in 5mC content when assessed with LUMA and ELISA, respectively (fig. 1A,B). The post hoc analysis of the ELISA data also demonstrated a significant (p < 0.05) decrease in 5mC levels for adult cerebellum tissue at the post-mortem interval 540 min. (2.9 ± 0.7%; mean ± S.E.M.) when compared to 0 min. (3.7 ± 0.6%). No statistically significant decrease in 5mC levels was observed between the post-mortem intervals in neonatal animals (fig. 1B). Heat stabilization of tissues did not affect global 5mC levels when measured by LUMA or ELISA. The average 5mC content of all the heat-stabilized samples combined was 69.9 ± 0.3% and 2.7 ± 0.1% compared to 69.9 ± 0.4% and 2.7 ± 0.1% for the snap-frozen non-stabilized neonatal samples, measured by LUMA and ELISA, respectively (table 1).
Fig. 1.
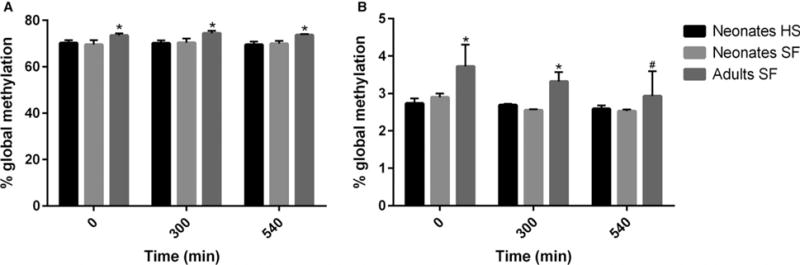
Effects of post-mortem interval and tissue heat stabilization (HS) on global DNA methylation in cerebellum measured by LUMA (A) and ELISA (B). To study the effect of post-mortem interval, the cerebellum from three groups of neonatal and adult animals were kept at room temperature for 0–540 min. before they were snap-frozen (SF) and stored at −80°C. To determine the effect of HS, the cerebellum from three groups of neonatal animals were instantly heat-stabilized and kept at room temperature for 0–540 min. before storage. Values represent mean ± S.E.M. *p < 0.05 compared with the neonatal SF group at respective time-points. #p < 0.05 compared with the adult 0-min. group. No differences were demonstrated between the neonatal SF and HS groups (ANOVA followed by Fisher’s LSD test).
Global DNA hydroxymethylation
The overall statistical comparison with ANOVA showed significant differences between the groups regarding global 5hmC measured by ELISA (F = 3.56; p < 0.01). The 5hmC content was higher for mothers at all post-mortem intervals compared to the neonates, but only reached statistical significance at 540 min (fig. 2). The average 5hmC level for all the neonatal samples – both heat-stabilized samples and the snap-frozen non-stabilized tissues – was 0.13 ± 0.01%, significantly lower than for the mothers (0.23 ± 0.03%; p < 0.001). The post hoc comparison also revealed a significant (p < 0.05) increase in 5hmC levels for adult cerebellum tissue at the post-mortem interval 540 min. (0.29 ± 0.06%) when compared to 0 min. (0.19 ± 0.03%). No statistically significant increase in 5hmC levels was observed between the post-mortem intervals in neonatal animals (fig. 2). Heat stabilization of tissues did not affect the global 5hmC levels. The average 5hmC content of all the heat-stabilized samples combined was 0.13 ± 0.01% compared to 0.13 ± 0.01% for the snap-frozen non-stabilized neonatal samples (table 1).
Fig. 2.
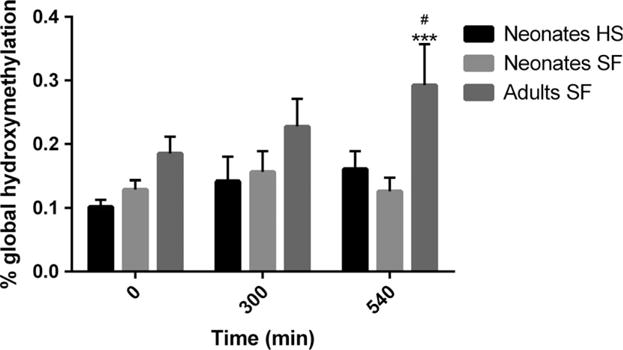
Effects of post-mortem interval and tissue heat stabilization (HS) on global 5hmC levels in cerebellum. To study the effect of post-mortem interval, the cerebellum from three groups of neonatal and adult animals were kept at room temperature for 0–540 min. before they were snap-frozen (SF) and stored at −80°C. To determine the effect of HS, the cerebellum from three groups of neonatal animals were instantly heat-stabilized and kept at room temperature for 0–540 min. before storage. Values represent mean ± S.E.M. ***p < 0.001, compared with the neonate SF group at respective time-points. #p < 0.05 compared with the adult 0-min. group. No differences were demonstrated between the neonatal SF and HS groups (ANOVA followed by Fisher’s LSD test).
Oxidative stress
The level of oxidative stress was measured using the CM-H2DCFDA probe as an indicator for ROS. The result demonstrates the post-mortem samples have an ongoing production of ROS. Higher production of ROS was demonstrated over time for adult cerebellum compared to the neonatal (fig. 3).
Fig. 3.
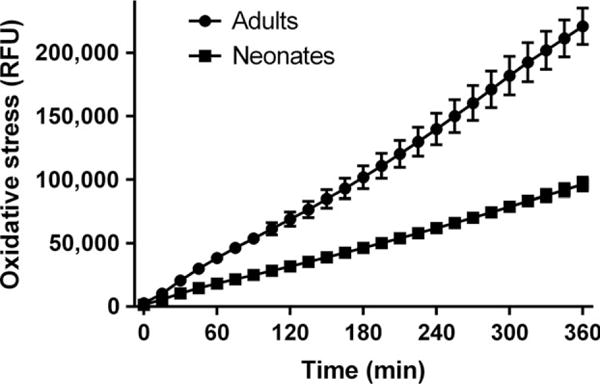
Oxidative stress was measured as relative fluorescence units (RFU) over time in cerebellum homogenates using the CM-H2DCFDA probe as an indicator for ROS. An ongoing production of oxidative stress was demonstrated in neonatal samples, and an even more distinct production in adults. Values represent mean ± S.E.M.
Discussion
One of the difficulties in studying human autopsy brain tissue is to distinguish between molecular changes related to the pathology and modifications occurring after death. Despite the dramatic increase in the number of epigenetic studies on brain functions and diseases, little is known about the impact of post-mortem intervals and post-sampling effects on DNA modifications such 5mC and 5hmC. Research has shown inconsistent and conflicting results further indicating the importance of considering methodological issues when analysing 5mC and 5hmC states of post-mortem brain tissues [15,33]. Better understanding of potential confounding effects of post-mortem handling, such as delay before freezing the samples, is therefore needed before any definite associations between the DNA methylation/hydroxymethylation state and certain diseases, phenotypes or environmental exposures are being made. Here, we report that the 5mC levels increased, while the 5hmC levels decreased, with post-mortem interval in the cerebellum of adult animals but not in neonates. The global 5mC and 5hmC contents were in general higher in adult animals compared to neonates.
The current study is based on a limited number of animals but suggests that DNA modifications may not be as static post-mortem as perhaps previously believed. Moreover, the level of ROS was revealed to increase over time in cerebellum. This indicates an ongoing post-mortem production of ROS in brain and shows that tissues can be affected by oxidative stress also during and after thawing of frozen samples. As the TET enzymes that convert 5mC to 5hmC may respond to oxidative stress, it was of interest to investigate whether the post-mortem interval could affect global 5hmC levels in cerebellum [28]. Interestingly, as above mentioned the 5mC levels seemed to increase and the 5hmC levels decrease, with postmortem time in the adult animals, but not in the neonatal animals. The reason for discrepancy between adult and neonatal cerebellum is not known but may be due to differences in regulation of the DNA methylation machinery or in the regulation/handling of ROS. Notably, higher production of ROS was demonstrated for adult cerebellum compared to the neonatal. Oxidative stress has been suggested to activate TET via increased production of alpha ketoglutarate, and lead to an increase in 5hmC levels [34]. Interestingly, oxidative stress has been reported to increase as well as decrease global and gene-specific DNA hydroxymethylation [28,35,36]. The oxidation reaction that converts 5mC to 5hmC can continue and generate other cytosine derivatives (e.g. 5caM or 5fM) or lead to demethylation of the cytosine. Hence, DNA methylation processes may, at least in part, be viewed as a cycle rather than a linear event. However, it is also possible that other yet undiscovered pathways that regulate DNA hydroxymethylation exist. Taken together, the results indicate that the post-mortem interval may lead to confounding effects when analysing global 5mC and 5hmC contents in human brain tissues, where the post-mortem handling of the brain samples may vary significantly. There should be no stability issues in conventional animal experiments as brain or other tissues usually are collected within seconds to minutes, generating global 5mC and 5hmC results unbiased by the post-mortem interval. Larger animal studies and more importantly further studies in human samples are needed to confirm post-mortem effects on DNA methylation/hydroxymethylation and elucidate details of the underlying mechanisms.
The first post-natal weeks in rats represent a critical developmental period for the neuronal system. There is a limited understanding of how DNA methylation processes change during this period of neurogenesis, synaptogenesis and neuronal circuit formation [37]. Even less is known about the role of 5hmC. In the present study, the global 5mC levels were found to be lower in cerebellum of neonates compared with adult animals. Studies of this developmental period in human beings have reported that DNA methylation levels increase rapidly and then stabilize by adulthood in both brain and blood [38]. Our results support this finding and indicate an important role also for 5hmC during brain development. The average 5hmC content for all neonatal samples combined was significantly lower than the adult average 5hmC content, and the neonatal 5hmC levels were found to be lower at all post-mortem intervals compared to adults. However, we cannot exclude that the differences in 5hmC states is due to the age-dependent postmortem effects discussed above as the only specific post-mortem interval that reached statistical significance was 540 min. Here we have assessed global 5mC and 5hmC levels in cerebellum but not whether any gene-specific changes were induced depending on post-mortem interval. The rationale for this design is that any effects due to post-mortem handling of the tissue should induce general effects on the DNA methylation machinery rather than specific. On the other hand, pathways such as ROS-regulated TET enzymes may be responsible for alterations of specific targets. The discrepancy between the 5mC results when measured by LUMA or ELISA may be due to important differences between the techniques. LUMA assesses global CpG methylation in a CCGG context using enzymatic digestion. The CCGG sequence accounts for only 8% of the CpG sites totally in the genome, but CpG-dense sites (CpG islands) are known to be located in promoter and other regulatory sites. In contrast, ELISA is an antibody-based technique here assessing methylated and hydroxymethylated C irrespective of sequence context. Moreover, we can conclude that both LUMA and ELISA are compatible with heat stabilization of samples as the global 5mC and 5hmC contents were unaffected compared with non-stabilized tissue. This is important because it allows analysis of DNA methylation/hydroxymethylation from tissues that have been heat-stabilized to preserve more labile analytes such as peptides and proteins during storage and sample preparation [19,22–25]. In addition, heat stabilization of the tissues increased the DNA yield compared to frozen tissues.
Conclusion
The global 5mC and 5hmC levels were generally higher in the adult than the neonatal cerebellum. When measured by ELISA, the global 5mC levels decreased, while the 5hmC levels increased with post-mortem time in the cerebellum of adult but not neonatal animals. This suggests that the post-mortem interval may lead to confounding effects when analysing DNA methylation/hydroxymethylation in human brain tissues as the post-mortem handling of the brain samples often varies substantially. To avoid biases, samples should be matched carefully for post-mortem interval and other potential confounding variables including age. Post-mortem tissue was demonstrated to produce ROS at higher rate for adults than neonates, which may be part of the mechanism that causes the observed 5mC and 5hmC alterations in adults. Further studies of post-mortem effects on the epigenome are warranted, especially in human brain tissues where post-mortem intervals are generally long.
Acknowledgments
Funding
Financial support was given by the Swedish Research Council Formas and Carl Tryggers Foundation.
Footnotes
Disclosure
The authors have nothing to disclose.
References
- 1.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–40. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson O, Baccarelli AA. Environmental health and long non-coding RNAs. Curr Environ Health Rep. 2016;31:178–87. doi: 10.1007/s40572-016-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasolino M, Liu S, Wang Y, Zhou Z. Distinct cellular and molecular environments support aging-related DNA methylation changes in the substantia nigra. Epigenomics. 2017;9:21–31. doi: 10.2217/epi-2016-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefanska B, MacEwan DJ. Epigenetics and pharmacology. Br J Pharmacol. 2015;172:2701–4. doi: 10.1111/bph.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A, Taran-tini L, et al. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–28. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Souza RA, Islam SA, McEwen LM, Mathelier A, Hill A, Mah SM, et al. DNA methylation profiling in human Huntington’s disease brain. Hum Mol Genet. 2016;25:2013–30. doi: 10.1093/hmg/ddw076. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Cabrero D, Almgren M, Sjoholm LK, Hensvold AH, Ringh MV, Tryggvadottir R, et al. High-specificity bioinformatics framework for epigenomic profiling of discordant twins reveals specific and shared markers for ACPA and ACPA-positive rheumatoid arthritis. Genome Med. 2016;8:124. doi: 10.1186/s13073-016-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng N, Wang Y, Zheng M, Yu X, Lin H, Ma RN, et al. Genome-wide analysis of DNA methylation and their associations with long noncoding RNA/mRNA expression in non-small-cell lung cancer. Epigenomics. 2017 Jan 23; doi: 10.2217/epi-2016-0120. https://doi.org/10.2217/epi-2016-0120. [Epub ahead of print] [DOI] [PubMed]
- 9.Rodger EJ, Chatterjee A, Morison IM. 5-hydroxymethylcytosine: a potential therapeutic target in cancer. Epigenomics. 2014;6:503–14. doi: 10.2217/epi.14.39. [DOI] [PubMed] [Google Scholar]
- 10.Weichenhan D, Plass C. The evolving epigenome. Hum Mol Genet. 2013;22:R1–6. doi: 10.1093/hmg/ddt348. [DOI] [PubMed] [Google Scholar]
- 11.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethyl-cytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–30. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Globisch D, Munzel M, Muller M, Michalakis S, Wagner M, Koch S, et al. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One. 2010;5:e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valinluck V, Tsai HH, Rogstad DK, Burdzy A, Bird A, Sowers LC. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–8. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppieters N, Dieriks BV, Lill C, Faull RL, Curtis MA, Dragunow M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol Aging. 2014;35:1334–44. doi: 10.1016/j.neurobiolaging.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Szyf M. The elusive role of 5′-hydroxymethylcytosine. Epigenomics. 2016;8:1539–51. doi: 10.2217/epi-2016-0076. [DOI] [PubMed] [Google Scholar]
- 17.Langie SA, Moisse M, Declerck K, Koppen G, Godderis L, Van-den Berghe W, et al. Salivary DNA methylation profiling: aspects to consider for biomarker identification. Basic Clin Pharmacol Toxicol. 2017;121(Suppl 3):93–101. doi: 10.1111/bcpt.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer I, Martinez A, Boluda S, Parchi P, Barrachina M. Brain banks: benefits, limitations and cautions concerning the use of post-mortem brain tissue for molecular studies. Cell Tissue Banking. 2008;9:181–94. doi: 10.1007/s10561-008-9077-0. [DOI] [PubMed] [Google Scholar]
- 19.Skold K, Alm H, Scholz B. The impact of biosampling procedures on molecular data interpretation. Mol Cell Proteomics. 2013;12:1489–501. doi: 10.1074/mcp.R112.024869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandana R, Mythri RB, Mahadevan A, Shankar SK, Srinivas Bharath MM. Biochemical analysis of protein stability in human brain collected at different post-mortem intervals. Indian J Med Res. 2009;129:189–99. [PubMed] [Google Scholar]
- 21.ElHajj Z, Cachot A, Muller T, Riederer IM, Riederer BM. Effects of postmortem delays on protein composition and oxidation. Brain Res Bull. 2016;121:98–104. doi: 10.1016/j.brainresbull.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Stingl C, Soderquist M, Karlsson O, Boren M, Luider TM. Uncovering effects of ex vivo protease activity during proteomics and peptidomics sample extraction in rat brain tissue by oxygen-18 labeling. J Proteome Res. 2014;13:2807–17. doi: 10.1021/pr401232e. [DOI] [PubMed] [Google Scholar]
- 23.Jernerén F, Söderquist M, Karlsson O. Post-sampling release of free fatty acids – effects of heat stabilization and methods of euthanasia. J Pharmacol Toxicol Methods. 2015;71:13–20. doi: 10.1016/j.vascn.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Buesa C, Maes T, Subirada F, Barrachina M, Ferrer I. DNA chip technology in brain banks: confronting a degrading world. J Neuropathol Exp Neurol. 2004;63:1003–14. doi: 10.1093/jnen/63.10.1003. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson O, Segerström L, Sjöback R, Nylander I, Borén M. qPCR based mRNA quality score show intact mRNA after heat stabilization. Biomol Detect Quantif. 2016;7:21–6. doi: 10.1016/j.bdq.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilahur N, Baccarelli AA, Bustamante M, Agramunt S, Byun HM, Fernandez MF, et al. Storage conditions and stability of global DNA methylation in placental tissue. Epigenomics. 2013;5:341–8. doi: 10.2217/epi.13.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avila L, Yuen RK, Diego-Alvarez D, Penaherrera MS, Jiang R, Robinson WP. Evaluating DNA methylation and gene expression variability in the human term placenta. Placenta. 2010;31:1070–7. doi: 10.1016/j.placenta.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Delatte B, Jeschke J, Defrance M, Bachman M, Creppe C, Calonne E, et al. Genome-wide hydroxymethylcytosine pattern changes in response to oxidative stress. Sci Rep. 2015;5:12714. doi: 10.1038/srep12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierozan P, Jerneren F, Ransome Y, Karlsson O. The choice of euthanasia method affects metabolic serum biomarkers. Basic Clin Pharmacol Toxicol. 2017;121:113–8. doi: 10.1111/bcpt.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson M, Boren M, Skold K, Falth M, Sjogren B, Andersson M, et al. Heat stabilization of the tissue proteome: a new technology for improved proteomics. J Proteome Res. 2009;8:974–81. doi: 10.1021/pr8006446. [DOI] [PubMed] [Google Scholar]
- 31.Karimi M, Johansson S, Stach D, Corcoran M, Grander D, Schalling M, et al. LUMA (LUminometric Methylation Assay)–a high throughput method to the analysis of genomic DNA methylation. Exp Cell Res. 2006;312:1989–95. doi: 10.1016/j.yexcr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Luttropp K, Sjöholm LK, Ekström TJ. Global Analysis of DNA 5-Methylcytosine Using the Luminometric Methylation Assay, LUMA. Methods Mol Biol. 2015;1315:209–19. doi: 10.1007/978-1-4939-2715-9_16. [DOI] [PubMed] [Google Scholar]
- 33.Chouliaras L, Mastroeni D, Delvaux E, Grover A, Kenis G, Hof PR, et al. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol Aging. 2013;34:2091–9. doi: 10.1016/j.neurobiolaging.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chia N, Wang L, Lu X, Senut M-C, Brenner CA, Ruden DM. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics. 2011;6:853–6. doi: 10.4161/epi.6.7.16461. [DOI] [PubMed] [Google Scholar]
- 35.Coulter JB, O’Driscoll CM, Bressler JP. Hydroquinone increases 5-hydroxymethylcytosine formation through ten eleven translocation 1 (TET1) 5-methylcytosine dioxygenase. J Biol Chem. 2013;288:28792–800. doi: 10.1074/jbc.M113.491365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Yang Y, Wang X, Chong Z, Yin R, Song SH, et al. Redox-active quinones induces genome-wide DNA methylation changes by an iron-mediated and Tet-dependent mechanism. Nucleic Acids Res. 2014;42:1593–605. doi: 10.1093/nar/gkt1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons RK, Stringfellow SA, Glover ME, Wagle AA, Clinton SM. DNA methylation markers in the postnatal developing rat brain. Brain Res. 2013;1533:26–36. doi: 10.1016/j.brainres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell. 2015;14:924–32. doi: 10.1111/acel.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]


