Abstract
Sepsis is a major cause of hospital admissions and mortality. Nevertheless, there are significant gaps in our knowledge of the epidemiology of sepsis in obese people, who now represent more than one-third of the population in the United States. The objective of this study was to measure the association between obesity and mortality from presumed sepsis. A retrospective cohort study was used of 1,779 adult inpatients with presumed sepsis at a Tertiary Care Academic Institution from March 1, 2007 to June 30, 2011. Cases of sepsis were identified using a standardized algorithm for sepsis antibiotic treatment. Exposure (i.e., obesity) was defined as a body mass index ≥30 kg/m2. Multivariable logistic regression was used to assess the adjusted association between obesity and mortality. Patients with presumed sepsis were of a median age of 60.9 years (interquartile range 49.7–71) and 41.1 % were women. A total of 393 patients died, resulting in a 28-day in-hospital mortality of 22.1 %. In adjusted analysis, obesity was not significantly associated with increased mortality (odds ratio 1.11, 95 % CI 0.85–1.41, P = 0.47). There was also no difference in the in-hospital length of stay (P = 0.45) or maximum percent change in serum creatinine (P = 0.32) between obese and non-obese patients. Finally, there was no difference in the proportion of initial inadequate vancomycin levels (P = 0.1) after presumed sepsis. Obesity was not associated with increased mortality in patients with presumed sepsis. Further research is needed to determine how excess adiposity modulates inflammation from sepsis.
Keywords: Sepsis, Obesity, Hospital mortality, Renal insufficiency, Antibiotic levels, Outcome assessment (health care), Critical care
Intoduction
Sepsis is a leading cause of hospital admissions and mortality in the United States [1]. In 2007, hospital mortality from sepsis associated with organ dysfunction was 29 % [2]. Moreover, the incidence of sepsis has increased over the past 2 decades [3]. However, there is a poor understanding of the epidemiology of sepsis in highly prevalent chronic conditions such as obesity. Notably, the 2009–2010 age-adjusted prevalence of obesity (defined as a body mass index (BMI) ≥ 30 kg/m2) in the United States was 35.5 % in adult men and 35.8 % in adult women [4].
Several lines of evidence suggest that obesity may be a risk factor for adverse clinical outcomes from sepsis. First, in septic mouse models, excess adipose tissue up-regulates adhesion molecules and increases microvascular dysfunction [5]. Second, obese patients are less likely to receive recommended weight-based dosages for antibiotics in hospital infections [6, 7] and pharmacokinetic studies have demonstrated lower plasma concentrations of antibiotics in morbidly obese patients compared to patients with a normal BMI [8, 9]. Finally, in critical illness, several clinical studies suggest that obesity is associated with longer hospital stays, prolonged time on mechanical ventilation, and increased risk of hospital-acquired pneumonia [10–14].
We have found three published studies examining the association between obesity and mortality from sepsis. In 42 patients with intra-abdominal sepsis in Greece, obesity was not found to be a risk factor for hospital mortality (P = 0.89) [15]. Wurz-inger et al. [16] assessed 301 patients with septic shock in Austria and found a decreased odds of mortality in obesity (odds ratio (OR), 0.28, 95 % confidence interval (CI) 0.08–0.93, P = 0.04). A more recent study also performed in a cohort of patients with septic shock shows that obesity is associated with a lower mortality in crude analysis with the association becoming non-significant after adjusting for baseline characteristics [17]. However, these studies are limited by small sample size [15, 16], the absence of multivariable analysis [15], and missing data [17].
The objectives of this observational study were to define a novel association between obesity and mortality in sepsis, and to identify a potential high-risk population for future targeted interventions.
Methods
The University of Pennsylvania institutional review board approved this study.
Data source
The Pennsylvania Integrated Clinical and Administrative Research Database (PICARD) contains demographic, laboratory, and pharmacy information from two University of Pennsylvania Health System (UPHS) hospitals in Philadelphia: the Hospital of the University of Pennsylvania (HUP), a 725-bed academic tertiary care medical center and Pennsylvania Presbyterian Medical Center, a 344-bed urban community hospital. PICARD has been used effectively in prior studies of hospital infection [18–20].
Study cohort
We identified all adult inpatients with an episode of presumed sepsis between March 1, 2007 and June 30, 2011. We excluded patients who (a) were <18 years, (b) had a history of leg amputation or (c) were pregnant during the hospital admission of presumed sepsis. We applied the above exclusions because BMI is a poor correlate of adiposity in these populations.
The pre-existing UPHS sepsis antibiotic algorithm was used as a marker for presumed sepsis. The algorithm was established to standardize empiric antibiotic therapy for presumed sepsis, and is initiated at the discretion of the treating physician when there is a clinical appearance of sepsis. It includes the antibiotics ‘vancomycin’ plus ‘ami-kacin or tobramycin or gentamicin’ plus ‘cefepime or levofloxacin or meropenem’. To be included in the study, a subject must have received one antibiotic from each of the three groups in a 24-h period. To preserve independence between observations, we included only the first episode for analysis for patients treated according to the algorithm during multiple admissions or multiple times during a single admission. Finally, in an unpublished pilot study on 73 patients, we found the above algorithm has a 94.5 % positive predictive value (95 % CI 86.6–98.5) for the 1992 ACCP/SCCM definition of sepsis. [21].
Exposures
We calculated BMI from height and weight recorded at the time of hospital admission using the following formula: weight in kilograms divided by height in meters squared. In the primary analysis, the exposure was obesity (BMI ≥ 30 kg/m2) and non-exposure was non-obesity (BMI ≥ 18.5 kg/m2 and <30 kg/m2). We excluded underweight patients (BMI < 18.5 kg/m2) from the primary analysis to avoid underestimation of the risk of obesity on mortality, given that low BMI has been linked with higher mortality in hospital infections [22].Wealso categorized BMI using World Health Organization criteria [23].
Outcomes
The cohort follow-up began at the time of the last antibiotic order in the initial sepsis antibiotic algorithm (t0). The primary outcome was 28-day in-hospital mortality, defined as death within the hospital from any cause occurring 28 days after t0. Secondary outcomes were (1) in-hospital length of stay (LOS), defined as total number of days in the hospital from t0 to discharge or death, (2) maximum percent change in serum creatinine in first 72 h after t0 and (3) initial plasma concentrations of vancomycin in the first 72 h after t0, defined as inadequate if <10 mg/L and toxic if >20 mg/L, based on guidelines for vancomycin therapeutic monitoring [24].
Control variables
PICARD provided demographic and clinical variables captured prior to presumed sepsis, including age, gender, race, year and hospital of admission, admission from the emergency department, in-hospital length of stay, inpatient use of antibiotics in the sepsis algorithm or immunosuppressives, and positive blood cultures. Sepsis severity was defined using ICU location at the time of presumed sepsis onset and the administration of a vasopressor in the 48 h prior to presumed sepsis. In addition, we defined the sepsis antibiotic algorithm as having adequate coverage if the antibiotics were ordered in the first 48 h after a culture sample was obtained and were ultimately shown to be active against the identified pathogen(s) based on in vitro susceptibility test results.
Baseline comorbidities were based on the International Classification of Disease, Ninth Revision (ICD-9) codes and included diagnostic codes from the hospital admission of presumed sepsis due to the high likelihood that they represented preexisting conditions [25, 26].
Statistical analysis
Baseline characteristics between non-obese and obese patients were compared using the Wilcoxon Rank Sum test for continuous variables [summarized as medians with interquartile ranges (IQR)] and the χ2 test for categorical variables (summarized as proportions). To test the linearity of continuous variables, we divided the variables into quartiles and plotted the predicted log odds of mortality against the midpoints of the variable quartiles [27]. In bivariable analysis, we determined the association between baseline variables and mortality. We also performed stratified analysis, as planned a priori for the following variables: gender, admitting hospital, and ICU location to determine statistical interaction. Interaction was assumed to be present when the test for homogeneity between the ORs for different strata yielded a significant result.
We used logistic regression for the multivariable analysis and purposeful forward selection to construct a final multivariable model. ICU location and administration of a vasopressor were included in the multivariable model a priori to adjust for sepsis severity. All variables with a P value <0.20 on bivariable analyses were considered for inclusion and maintained in the final model, if their inclusion resulted in a ≥15 % change in the odds ratio of the association between obesity and mortality from presumed sepsis [28].
In a secondary analysis, we used logistic regression to estimate ORs for each World Health Organization BMI category with the normal group as the reference. BMI was also modeled as a continuous variable. For our secondary outcomes, we assessed differences in LOS and maximum percent change in serum creatinine using the Wilcoxon Rank Sum test. Inadequate and toxic vancomycin concentrations were compared using logistic regression, adjusting for creatinine clearance calculated using the Cockcroft–Gault equation, time from vancomycin dose to vancomycin level, and initial vancomycin dose.
We performed a sensitivity analysis to determine the impact of unmeasured confounding from smoking status on the unadjusted OR of the association between obesity and mortality. We used estimates from published data to model smoking as a dichotomous variable in multivariable logistic regression using methods of Greenland [29, 30]. We assumed that the prevalence of smoking was lower in obese patients than non-obese patients. We also assumed that smoking was associated with a 1.5- to 3-fold increased odds of mortality.
Two-tailed P values of <0.05 were considered significant for all calculations. Statistical analyses were performed using STATA version 12.0 (StataCorp LP, College Station, TX).
Results
Study population
We identified 1,835 patients who received sepsis algorithm antibiotics during the study period. We excluded 38 patients with pre-existing leg amputation, 5 patients who were pregnant and 13 patients who were missing a weight or height. The remaining 1,779 patients were included in the final cohort for analysis. The distribution of BMI in the included cohort was as follows: 573 (32.2 %) were obese, 1,080 (60.6 %) were non-obese and 126 (7.1 %) were underweight. Baseline clinical and demographic characteristics of obese and non-obese patients are shown in Tables 1 and 2.
Table 1. Clinical and demographic characteristics of obese and non-obese patients with presumed sepsis.
| Characteristics | Obese (n = 573)a | Non-obese (n = 1,080)a | P valueb |
|---|---|---|---|
| Age, median years (IQR) | 60.6 (51.8–69) | 60.9 (49.2–71.9) | 0.65 |
| Female gender | 283 (49.4) | 391 (36.2) | <0.001 |
| Non-white racec | 173 (35.7) | 295 (31.2) | 0.08 |
| Admission to HUPd | 479 (83.6) | 918 (85) | 0.45 |
| Emergency department admissione | 197 (34.4) | 436 (40.4) | 0.02 |
| BMI, median (IQR) | 34.5 (31.9–39) | 24.5 (22–27) | <0.001 |
| Inpatient antibiotics prior to presumed sepsis | 74 (12.9) | 185 (17.1) | 0.02 |
| Inpatient immunosuppressives prior to presumed sepsisf | 51 (8.9) | 136 (12.6) | 0.02 |
| In-hospital length of stay prior to presumed sepsis, median days (IQR) | 3.83 (0.93–11.2) | 3.1 (0.83–11.7) | 0.45 |
| ICU location at time of presumed sepsis | 425 (74.2) | 749 (69.4) | 0.04 |
| Positive blood cultureg | 136 (23.7) | 226 (20.9) | 0.19 |
| Administration of a vasopressorh | 209 (36.5) | 302 (28) | <0.001 |
| Meropenemi | 63 (11) | 154 (14.3) | 0.14 |
| Amikacinj | 165 (28.8) | 300 (27.8) | 0.22 |
| Inadequate antibiotic coverage | 11 (1.92) | 23 (2.13) | 0.78 |
| 28 day in-hospital mortality | 140 (24.4) | 288 (21.1) | 0.12 |
| In-hospital length of stay after presumed sepsis, median days (IQR) | 9.53 (4–19.7) | 9.52 (3.96–19.9) | 0.45 |
| Maximum % change in serum creatinine, median (IQR) | +11.1 (-3.44, +36.3) | +9.74 (-4.17, +31) | 0.32 |
| Inadequate vancomycin level | 20 (19.2) | 55 (27.8) | 0.1 |
IQR interquartile range, HUP Hospital of the University of Pennsylvania, ICU Intensive Care Unit
Data are presented as No. (%) of subjects unless otherwise indicated
The χ2 test was used for categorical variables and the Wilcoxon Rank Sum test for continuous variables
Race was missing in 245 patients
As opposed to Pennsylvania Presbyterian Medical Center
As opposed to physician referral or transfer from another institution
Includes adalimubab, azathioprine, cyclosporine, etanercept, infliximab, interferon, methotrexate, mycophenolate, sirolimus, or tacrolimus
Blood culture ordered within 48 h prior to last antibiotic in the sepsis antibiotic algorithm
Includes dopamine, norepinephrine, epinephrine, vasopressin, or dobutamine
As opposed to cefepime or levofloxacin as part of the sepsis antibiotic algorithm
As opposed to gentamicin or tobramycin as part of the sepsis antibiotic algorithm
Table 2. Comorbidities of obese and non-obese patients with presumed sepsis.
| Comorbidities | Obese (n = 573)a | Non-obese (n = 1,080)a | P valueb |
|---|---|---|---|
| Chronic pulmonary disease | 110 (19.2) | 161 (14.9) | 0.03 |
| Congestive heart failure | 118 (20.6) | 217 (20.1) | 0.81 |
| Diabetes mellitus | |||
| Without organ damage | 178 (31.1) | 209 (19.4) | <0.001 |
| With organ damagec | 77 (13.4) | 73 (6.8) | |
| Dialysis | 43 (7.5) | 78 (7.22) | 0.83 |
| Human immunodeficiency virus | 5 (0.87) | 11 (1.02) | 0.77 |
| Hypertension | |||
| Without chronic complications | 216 (37.7) | 349 (32.3) | <0.001 |
| With chronic complicationsd | 192 (33.5) | 289 (26.8) | |
| Leukemia/lymphoma | 114 (19.9) | 276 (25.6) | 0.01 |
| Liver disease | |||
| Mild | 60 (8.73) | 124 (11.5) | 0.1 |
| Moderate or severee | 47 (8.2) | 68 (6.3) | |
| Myocardial infarction | 82 (14.3) | 118 (10.9) | 0.05 |
| Solid tumor | |||
| Without metastasis | 40 (6.98) | 84 (7.78) | 0.17 |
| With metastasis | 50 (8.73) | 124 (11.5) | |
| Transplant (solid organ/hematopoietic stem cell) | 49 (8.55) | 153 (14.2) | <0.001 |
Data are presented as No. (%) of subjects
The χ2 test was used for categorical variables
Defined as neuropathy, retinopathy, or kidney disease
Defined as secondary causes of hypertension or associated kidney disease or congestive heart failure
Defined as liver disease with hepatic encephalopathy, ascites, esophageal varices, liver abscess or portal hypertension
Association between obesity and hospital mortality
The overall 28-day in-hospital mortality was 22.1 % (393 of 1,779 patients died). The mortality for obese patients was 24.4 % (140 of 573), while for non-obese patients the mortality was 21.1 % (228 of 1,080) (unadjusted OR 1.21, 95 % CI 0.95–1.54, P = 0.12). There was no effect modification by gender, admitting hospital, or ICU location on the odds ratio scale (test for homogeneity, P = 0.59, 0.54, and 0.29, respectively). The result of bivariable analysis between clinical and demographic variables and mortality is shown in Table 3. Following the a priori addition of ICU location and administration of a vasopressor, obesity did not have an increased odds of mortality (adjusted OR 1.11, 95 % CI 0.85–1.41, P = 0.47). No other variables significantly changed the OR and were therefore not retained in the final model. The OR also remained robust in the setting of unmeasured confounding from smoking (Table 4). A threefold difference in smoking prevalence between non-obese and obese patients was required to generate an OR outside the 95 % CI for the unadjusted association between obesity and mortality.
Table 3. Unadjusted risk factors for mortality from presumed sepsis.
| Variable | Survived (n = 1,285)a | Deceased (n = 368)a | P valueb | Odds ratio (95 % CI) |
|---|---|---|---|---|
| Age, median years (IQR) | 60.1 (48.9–70.4) | 62.6 (54.1–73.4) | <0.001 | – |
| Female gender | 524 (40.8) | 150 (40.8) | 0.99 | 1 (0.79–1.27) |
| Non-white race | 379 (33.7) | 89 (29.3) | 0.17 | 0.82 (0.62–1.08) |
| Admission to HUPc | 1,075 (83.7) | 322 (87.5) | 0.07 | 1.37 (0.97–1.93) |
| Emergency department admissiond | 498 (38.8) | 135 (36.7) | 0.47 | 0.97 (0.77–1.22) |
| Inpatient antibiotics prior to presumed sepsis | 192 (14.9) | 67 (18.2) | 0.13 | 1.27 (0.93–1.72) |
| Inpatient immunosuppressives prior to presumed sepsise | 165 (11.9) | 40 (10.8) | 0.34 | 0.85 (0.58–1.21) |
| In-hospital length of stay prior to presumed sepsis, median days (IQR) | 2.58 (0.81–10.3) | 5.9 (1.15–14.7) | <0.001 | – |
| ICU location at time of presumed sepsis | 837 (65.6) | 337 (91.6) | <0.001 | 5.82 (3.96–8.55) |
| Positive blood culturef | 276 (21.5) | 86 (23.4) | 0.44 | 1.11 (0.85–1.47) |
| Administration of a vasopressorg | 336 (26.2) | 175 (47.6) | <0.001 | 2.56 (2.02–3.25) |
| Meropenemh | 139 (10.8) | 78 (21.2) | <0.001 | 3.45 (2.38–5.01) |
| Amikacini | 327 (25.5) | 138 (37.5) | 0.006 | 1.47 (1.12–1.93) |
| Inadequate antibiotic coverage | 28 (2.18) | 6 (1.63) | 0.68 | 0.74 (0.31–1.81) |
| Congestive heart failure | 270 (21) | 65 (17.7) | 0.16 | 0.81 (0.6–1.09) |
| Chronic pulmonary disease | 213 (16.6) | 58 (15.8) | 0.71 | 0.94 (0.69–1.29) |
| Diabetes mellitus | ||||
| Without organ damage | 308 (24) | 79 (21.5) | 0.24 | 0.84 (0.64–1.21) |
| With organ damagej | 121 (9.42) | 29 (7.88) | 0.28 | 0.79 (0.51–1.21) |
| Myocardial infarction | 148 (11.5) | 52 (14.1) | 0.18 | 1.26 (0.9–1.78) |
| HTN | ||||
| Without chronic complications | 439 (34.2) | 126 (34.2) | 0.48 | 0.91 (0.69–1.19) |
| With chronic complicationsk | 385 (30) | 96 (26.1) | 0.11 | 0.79 (0.59–1.05) |
| HIV | 15 (1.17) | 1 (0.27) | 0.12 | 0.23 (0.03–1.75) |
| Dialysis | 102 (7.94) | 19 (5.16) | 0.07 | 0.63 (0.38–1.05) |
| Liver disease | ||||
| Mild | 133 (10.4) | 41 (11.1) | 0.31 | 1.22 (0.84–1.77) |
| Moderate or severel | 64 (4.98) | 51 (13.9) | <0.001 | 3.14 (2.12–4.64) |
| Leukemia/lymphoma | 295 (23) | 85 (25.8) | 0.26 | 1.17 (0.89–1.52) |
| Transplant (solid organ/hematopoietic stem cell) | 154 (12) | 48 (13) | 0.58 | 1.10 (0.78–1.56) |
| Solid tumor | ||||
| Without metastasis | 97 (7.55) | 27 (7.34) | 0.37 | 1.1 (0.68–1.65) |
| With metastasis | 115 (8.95) | 59 (16) | <0.001 | 1.95 (1.39–2.74) |
CI confidence interval, HUP Hospital of the University of Pennsylvania, ICU Intensive Care Unit, HTN hypertension, HIV human immunodeficiency virus
Data are presented as No. (%) of subjects unless otherwise indicated
The χ2 was used for categorical variables and the Wilcoxon Rank Sum test for continuous variables
As opposed to Pennsylvania Presbyterian Medical Center
As opposed to physician referral or transfer from another institution
Includes adalimubab, azathioprine, cyclosporine, etanercept, infliximab, interferon, methotrexate, mycophenolate, sirolimus, or tacrolimus
Blood culture ordered within 48 h prior to last antibiotic in the sepsis antibiotic algorithm
Includes dopamine, norepinephrine, epinephrine, vasopressin, or dobutamine
As opposed to cefepime or levofloxacin as part of the sepsis antibiotic algorithm
As opposed to gentamicin or tobramycin as part of the sepsis antibiotic algorithm
Defined as neuropathy, retinopathy, or kidney disease
Defined as secondary causes of hypertension or associated kidney disease or congestive heart failure
Defined as liver disease with hepatic encephalopathy, ascites, esophageal varices, liver abscess or portal hypertension
Table 4. Sensitivity analysis for unmeasured confounding from smoking.
| Prevalence of smoking in non-obese patients (%) | Prevalence of smoking in obese patients (%) | True OR if the OR for the association between smoking and mortality = 1.25 | True OR if the OR for the association between smoking and mortality = 1.5 | True OR if the OR for the association between smoking and mortality = 2 |
|---|---|---|---|---|
| 40 | 30 | 1.24 | 1.26 | 1.30 |
| 40 | 20 | 1.27 | 1.32 | 1.41 |
| 40 | 10 | 1.30 | 1.38 | 1.54a |
| 30 | 20 | 1.24 | 1.26 | 1.31 |
| 30 | 10 | 1.27 | 1.32 | 1.43 |
| 20 | 10 | 1.24 | 1.27 | 1.32 |
OR odds ratio
Greater than the upper bound of the 95 % confidence interval for the unadjusted association between obesity and mortality
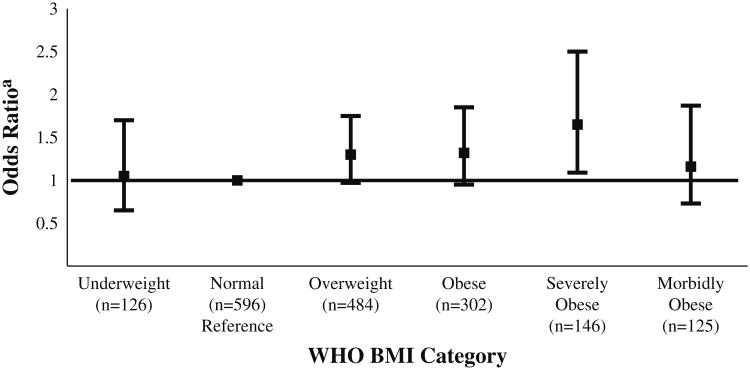
In a secondary analysis, we evaluated the relationship between increasing values of BMI and mortality. There was no linear association between BMI and mortality (P = 0.14). There was also no significant difference in mortality rates across World Health Organization categories (P = 0.18) (see Fig. 1), although severely obese patients had a borderline significantly higher odds of mortality after adjustment for sepsis severity compared to the normal group (OR 1.53, 95 % CI 0.96–2.36, P = 0.05).
Fig. 1.
Mortality in World Health Organization Body Mass Index Categories. WHO World Health Organization, BMI Body Mass Index. aOdds ratio presented with 95 % CI
Different mortality time-points
We repeated the primary analysis for mortality at 3 and 7 days after presumed sepsis onset, adjusting for sepsis severity. Obese patients had non-significantly higher odds of mortality at 3 days (OR 1.36, 95 % CI 0.98–1.89, P = 0.06) and at 7 days (OR 1.22, 95 % CI 0.91–1.62, P = 0.19).
Obesity and secondary clinical outcomes
The median LOS after presumed sepsis was comparable between obese patients (9.53 days, IQR 4.01–19.7) and non-obese patients (9.52 days, IQR 3.96–19.9, P = 0.45). There was also no difference in the maximum percent change in serum creatinine in the first 72 h after presumed sepsis between obese and non-obese patients (+11.1 %, IQR −3.44, +36.3 versus +9.74 %, IQR −4.17, +31, P = 0.32, respectively).
The majority of patients (81.7 %) did not have a vancomycin level in the 72 h after presumed sepsis. Septic patients with versus without a vancomycin level did not differ in terms of ICU location, administration of a vasopressor and unadjusted mortality. Obese patients had non-significantly higher odds of having an adequate vancomycin level (OR 1.59, 95 % CI 0.85–2.99, P = 0.15) as well as a toxic vancomycin level (OR 1.11, 95 % CI 0.63–1.96, P = 0.71) compared to non-obese patients.
Comment
The findings of our study suggest that there is no association between obesity and increased mortality in adult patients with presumed sepsis. Prior data on obesity and mortality have been limited. Kalfarentzos et al. [15] show that obesity is not a risk factor for mortality in 42 patients with intra-abdominal sepsis (P = 0.89). Nevertheless, their study did not use multivariable analysis to adjust for potential confounding and defined obesity from ideal body weight, rather than BMI, which may misclassify obesity in relation to actual adiposity. A 2010 study using 301 patients from an Austrian referral hospital finds, in a secondary analysis, that obese patients have a significantly lower odds of ICU mortality after adjustment for baseline characteristics and disease severity (OR 0.28, 95 % CI 0.08–0.93, P = 0.04) compared to patients with a normal BMI [16]. Nonetheless, only four obese patients died while in the ICU. In logistic regression, small numbers in strata can result in significant bias away from the null [27]. Finally, Arabi et al. [17] published a more recent study of 2,882 patients admitted to the ICU for septic shock. In an unadjusted analysis, obese patients have a lower hospital mortality (51.3 %) compared to patients with a normal BMI (54.4 %, P = 0.003). After adjustment for baseline characteristics, the association became non-significant. Of note, only 33.2 % of their septic shock cohort had available BMI data.
Compared to the previous studies, our study includes patients with a range of sepsis severities. We also reported a validation of our cases of sepsis, according to definitions developed in 1992. These definitions have been used in several prior studies on sepsis [31–33]. In 2001, the definition of sepsis was revisited. However, the current evidence did not support a change to the 1992 definition of sepsis for use in research [34]. Even so, the mortality of our cohort was similar to a cohort of severe sepsis and septic shock patients previously reported on from our institute that used the 2001 International Sepsis Definitions criteria [35]. We did not use diagnostic codes to define cases of sepsis because significant heterogeneity has been described between different database abstraction methods based on codes [36]. Nonetheless, the positive predictive value of our approach, from unpublished pilot data, was comparableto previously validated algorithms that used diagnostic codes for defining sepsis in retrospective databases [37]. Our method also defines a temporal relationship between sepsis and clinical outcomes, which is difficult with codes that are defined after discharge or death, and eliminates the risk of controlling for variables that occur after the onset of sepsis and are in the causal pathway between obesity and mortality.
In a secondary analysis, there was a trend toward increased odds of mortality in the severely obese group compared to the normal group. However, our study was not powered to detect differences in subgroups and further evaluation of excess mortality for higher categories of BMI may be warranted. In another secondary analysis, we found a decrease in the adjusted OR between obesity and mortality at longer time-points from presumed sepsis onset, with the largest association at 3 days. There may be several biological mechanisms that contribute to the association between obesity and mortality over the course of sepsis. First, adipose tissue can produce pro-inflammatory cytokines in response to inflammation, potentially contributing to increased early mortality [5]. Second, leptin, a hormone that regulates adipose tissue mass, is known to be elevated both in sepsis and in obesity [38, 39]. Leptin enhances the phagocytic activity of macrophages and higher levels have been associated with survival in septic patients [40–42]. Late survival in obese patients may also be related to improved nutritional reserves compared to leaner patients.
Lastly, we did not find that obese patients were more likely to have an initial inadequate vancomycin level compared to non-obese patients as originally hypothesized, in part because they received higher doses of vancomycin (1 mg/kg up to 70 kg and 1.5 mg/kg above 75 kg) and had comparable increases in serum creatinine over the first 72 h after presumed sepsis onset. A more detailed pharmacokinetic analysis was limited by the number of patients without vancomycin levels. Antibiotic pharmacokinetics during sepsis is complex and mediated by several factors that fluctuate over the course of infection, including kidney function and volume of distribution. Future studies should focus on determining how obesity interacts with other clinical variables to influence plasma antibiotic concentrations.
In assessing levels across the cohort, we found that 24.6 % of all initial vancomycin levels were inadequate and 27.7 % were toxic. Inadequate levels may increase the risk of treatment failure and the emergence of antimicrobial resistance and toxic levels may exacerbate renal dysfunction in an already susceptible population. The role of therapeutic drug monitoring of antibiotics in sepsis needs to be better defined. More levels should be drawn in septic patients to monitor toxicity and gauge adequacy. This should be coupled with research that identifies appropriate dosing schedules to hopefully improve the excessive morbidity and mortality from sepsis.
There are several potential limitations of the present study. There may have been error in the measurements of body height and weight resulting in misclassification of BMI. This error is likely to bias towards the null since measurements were recorded prior to knowledge of the study outcome. While BMI is a commonly used proxy for adiposity in clinical research, there may be more accurate correlates that needed to be further explored. Selection bias was minimized using a standardized antibiotic algorithm to identify cases of sepsis in a retrospective database. The prevalence of obesity in our cohort (32.2 %) is also comparable to a large population of ICU patients at another academic institution, suggesting that sepsis algorithm antibiotics were administered independent of patient BMI [43]. Nonetheless, the results of our study may not be generalizable to patients who do not receive broad empiric antibiotics for presumed sepsis.
Confounding from unmeasured variables is a limitation of all observational research. Specifically, we did not have data on infection source, which can result in different rates of mortality and can be differential between obese and non-obese patients. However, we were able to measure severity of illness variables such as the presence of positive blood cultures, use of vasopressors, ICU location that would influence the effect of infection source on mortality. We were also able to control for a number of comorbidities such as diabetes. Our estimates also remained robust in the setting of confounding from smoking status based on a sensitivity analysis. Finally, the present study was conducted in a single healthcare system, and these results may not be generalizable to other institutions.
Conclusions
We find that obesity is not associated with increased mortality in presumed sepsis. Further studies should focus on understanding how excess adiposity modulates inflammation from sepsis, and the role of antibiotic therapeutic drug monitoring in improving clinical outcomes.
Acknowledgments
A research grant from the Infectious Disease Society of America (IDSA) Medical Scholars Program supported this study.
Footnotes
Conflict of interest: None.
Contributor Information
Timothy Glen Gaulton✉, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women's Hospital, CWN-L 1, Rm L111, 75 Francis Street, Boston, MA 02115, USA.
Mark Gordon Weiner, Division of General Internal Medicine of the Department of Medicine, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA.
Knashawn Hodge Morales, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA; Department of Biostatistics and Epidemiology, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA.
David Foster Gaieski, Department of Emergency Medicine, Center for Resuscitation Science, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA.
Jimish Mehta, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA; Department of Pharmacy, Hospital of the University of Pennsylvania, Philadelphia, PA, USA.
Ebbing Lautenbach, Department of Biostatistics and Epidemiology, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA; Division of Infectious Diseases of the Department of Medicine, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA, USA.
References
- 1.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 2.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the united states from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Singer G, Stokes KY, Terao S, Granger DN. Sepsis-induced intestinal microvascular and inflammatory responses in obese mice. Shock. 2009;31(3):275–279. doi: 10.1097/SHK.0b013e3181834ab3. [DOI] [PubMed] [Google Scholar]
- 6.Garey KW, Pai MP, Suda KJ, et al. Inadequacy of fluconazole dosing in patients with candidemia based on Infectious Diseases Society of America (IDSA) guidelines. Pharmacoepidemiol Drug Saf. 2007;16(8):919–927. doi: 10.1002/pds.1365. [DOI] [PubMed] [Google Scholar]
- 7.Hall RG, Payne KD, Bain AM, et al. Multicenter evaluation of vancomycin dosing: emphasis on obesity. Am J Med. 2008;121(6):515–518. doi: 10.1016/j.amjmed.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS., Jr Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob Agents Chemother. 2006;50(4):1222–1227. doi: 10.1128/AAC.50.4.1222-1227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman D, Scheetz MH, Adeyemi OA, et al. Serum piperacillin/tazobactam pharmacokinetics in a morbidly obese individual. Ann Pharmacother. 2007;41(10):1734–1739. doi: 10.1345/aph.1K256. [DOI] [PubMed] [Google Scholar]
- 10.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134(5):974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 13.El-Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest. 2001;120(6):1989–1997. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 14.Bochicchio GV, Joshi M, Bochicchio K, Nehman S, Tracy JK, Scalea TM. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006;203(4):533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Kalfarentzos FE, Dougenis DV, Cristopoulos DC, Spiliotis JD, Williams M, Androulakis J. Prognostic criteria in intra-abdominal sepsis. Int Surg. 1987;72(3):185–187. [PubMed] [Google Scholar]
- 16.Wurzinger B, Dunser MW, Wohlmuth C, et al. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wien Klin Wochenschr. 2010;122(1–2):31–36. doi: 10.1007/s00508-009-1241-4. [DOI] [PubMed] [Google Scholar]
- 17.Arabi YM, Dara SI, Tamim HM, et al. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit Care. 2013;17(2):R72. doi: 10.1186/cc12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lautenbach E, Synnestvedt M, Weiner MG, et al. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control Hosp Epidemiol. 2009;30(12):1186–1192. doi: 10.1086/648450. [DOI] [PubMed] [Google Scholar]
- 19.Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol. 2006;27(9):893–900. doi: 10.1086/507274. [DOI] [PubMed] [Google Scholar]
- 20.Rattanaumpawan P, Morales KH, Binkley S, et al. Impact of antimicrobial stewardship programme changes on unnecessary double anaerobic coverage therapy. J Antimicrob Chemother. 2011;66(11):2655–2658. doi: 10.1093/jac/dkr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest Physicians/Society of critical care medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Falagas ME, Athanasoulia AP, Peppas G, Karageorgopoulos DE. Effect of body mass index on the outcome of infections: a systematic review. Obes Rev. 2009;10(3):280–289. doi: 10.1111/j.1467-789X.2008.00546.x. [DOI] [PubMed] [Google Scholar]
- 23.WHO Expert Committee. WHO Technical Report Series No 854. WHO; Geneva: 1995. Physical status: the use and interpretation of anthropometry. [PubMed] [Google Scholar]
- 24.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Pharmacotherapy. 2009;29(11):1275–1279. doi: 10.1592/phco.29.11.1275. [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 26.Naessens JM, Campbell CR, Berg B, Williams AR, Culbertson R. Impact of diagnosis-timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources. Med Care. 2007;45(8):781–788. doi: 10.1097/MLR.0b013e3180618b7f. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Model building strategies and methods for logistic regression. In: Hosmer DW, Lemeshow S, editors. Applied logistic regression. 2nd. John Wiley & Sons; New York: 2000. [Google Scholar]
- 28.Flegal KM. The effects of changes in smoking prevalence on obesity prevalence in the United States. Am J Public Health. 2007;97(8):1510–1514. doi: 10.2105/AJPH.2005.084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huttunen R, Heikkinen T, Syrjanen J. Smoking and the outcome of infection. J Intern Med. 2011;269(3):258–269. doi: 10.1111/j.1365-2796.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996;25(6):1107–1116. [PubMed] [Google Scholar]
- 31.Annane D, Cariou A, Maxime V, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341–348. doi: 10.1001/jama.2010.2. [DOI] [PubMed] [Google Scholar]
- 32.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 33.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 34.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 35.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 36.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 37.Carnahan RM, Herman RA, Moores KG. A systematic review of validated methods for identifying transfusion-related sepsis using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):222–229. doi: 10.1002/pds.2322. [DOI] [PubMed] [Google Scholar]
- 38.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 39.Yousef AA, Amr YM, Suliman GA. The diagnostic value of serum leptin monitoring and its correlation with tumor necrosis factor-alpha in critically ill patients: a prospective observational study. Crit Care. 2010;14(2):R33. doi: 10.1186/cc8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA. 1996;93(25):14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bornstein SR, Licinio J, Tauchnitz R, et al. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83(1):280–283. doi: 10.1210/jcem.83.1.4610. [DOI] [PubMed] [Google Scholar]
- 42.Arnalich F, Lopez J, Codoceo R, Jimnez M, Madero R, Montiel C. Relationship of plasma leptin to plasma cytokines and human survival in sepsis and septic shock. J Infect Dis. 1999;180(3):908–911. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- 43.Finkielman JD, Gajic O, Afessa B. Underweight is independently associated with mortality in post-operative and non-operative patients admitted to the intensive care unit: a retrospective study. BMC Emerg Med. 2004;4(1):3. doi: 10.1186/1471-227X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]