Abstract
Despite its anatomical location, the ovary is a site of pathogen exposure in the human female reproductive tract (FRT). However, the role of ovarian stromal fibroblasts in immune protection is unclear. We generated a population of ovarian stromal fibroblasts derived from normal human ovaries that expressed the pattern recognition receptors TLR3, TLR4, RIG-I, & MDA5. Poly (I:C) and LPS, respective mimics of viral and bacterial infections, selectively upregulated antiviral gene expression and secretion of chemokines and antimicrobials. Poly (I:C) exclusively stimulated the expression of interferon (IFN) β, IFNλ1, and the IFN-stimulated gene OAS2. Poly (I:C) also significantly increased secretion of elafin, CCL20, and RANTES, but had no effect on SDF-1α. In contrast, LPS had no effect on IFN or ISG expression but significantly increased secretion of RANTES and SDF-1α. Secretions from poly (I:C)-treated fibroblasts had both greater anti-HIV activity and induced higher levels of CD4+ T cell chemotaxis than those from LPS-treated cells. Our studies demonstrate a potential key role for ovarian fibroblasts in innate immune protection against incoming pathogens in the normal ovary.
Keywords: ovary, fibroblast, TLR, Poly (I:C), LPS, inflammation, HIV, chemotaxis
Introduction
The female reproductive tract (FRT) is a unique mucosal site whose immune system has evolved to optimize conditions for successful reproduction and immune protection [1]. The ovary represents a target site for sexually-transmitted infections (STIs). Movement from the vagina to the upper FRT (uterus, Fallopian tubes, and ovaries) occurs rapidly, potentially exposing a large mucosal surface to foreign pathogens [2, 3]. In recent studies Simian Immunodeficiency Virus (SIV) deposited in the vagina reached the ovaries and infected immune cells, thus demonstrating that viral pathogens can breach the surface epithelium to infect intra-ovarian CD4+ T cells [4, 5]. In studies with the human ovary, we found that CD4+ T cells and macrophages are present and readily infectible by HIV in vitro [6]. The ovary is also a target for bacterial STIs such as Neisseria gonorrhea and Chlamydia trachomatis, which can lead to Pelvic Inflammatory Disease (PID) [7]. PID has been proposed as a risk-factor for ovarian cancer [8], although other studies have shown no association [9]. Therefore, ovarian infections can potentially cause considerable pathology leading to increased morbidity and mortality in women.
The innate immune system is the first line of defense against incoming pathogens and is essential for reducing the possibility of a successful transmission event. Pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and Retinoic Acid Inducible Gene I (RIG)-like receptors (RLRs) recognize conserved moieties present on multiple classes of viral, bacterial, and fungal pathogens [10]. TLR3, RIG-I, and melanoma differentiation-associated protein 5 (MDA5) are three well-characterized PRRs that recognize dsRNA (poly (I:C)), a common viral marker [11]. In contrast, TLR4 recognizes lipopolysaccharide (LPS) present on the surface of Gram-negative bacteria [12]. Recognition by PRRs leads to the rapid induction of downstream signaling cascades that culminate in the expression of interferons (IFN), interferon-stimulated genes (ISG), antimicrobials, cytokines, and chemokines that increase immune protection, inhibit pathogen survival, and recruit immune cells to the site of exposure. For example, antimicrobials such as human beta-defensin 2 (HBD2), elafin, CCL20, RANTES, stromal-derived factor 1α (SDF-1α) can inhibit HIV infection [13–17], while functioning as chemokines for immune cells [18].
While the innate immune system has been extensively studied in the human FRT, most research has focused on epithelial cells and immune cells in the vagina, cervix, and endometrium. In contrast, the ovary has not been adequately investigated, and little is known about the role of normal ovarian fibroblasts in the innate immune response. In this study, we isolated and cultured purified populations of ovarian fibroblasts derived from normal human ovaries. The fibroblasts were exposed to poly (I:C), a viral ligand, and LPS, a bacterial ligand. We measured the expression of a panel of antiviral genes and secreted anti-HIV proteins in response to these treatments, as well as their effect of CD4+ chemotaxis and infection by HIV.
Methods and Materials
Source of Ovarian Tissue
Ovarian tissue was obtained from five women (average age 43.8yrs; range 34–50yrs) (Table 1) undergoing hysterectomy surgery at Dartmouth-Hitchcock Medical Center (Lebanon, NH). All tissues used were distal to the sites of pathology and determined to be unaffected by disease upon inspection by a pathologist. All investigations involving human subjects were conducted according to the principles expressed in the Declaration of Helsinki and carried out with the approval from the Committee for the Protection of Human Subjects (CPHS), Dartmouth Hitchcock Medical Center, and with written informed consent obtained from the patients before surgery.
Table 1.
Patient characteristics.
| Patient | Age | Menopausal Status | Reason for Surgery |
|---|---|---|---|
| 1 | 34 | Pre-menopausal | Adenomyosis |
| 2 | 41 | Pre-menopausal | Adenomyosis |
| 3 | 46 | Post-menopausal | Fibroids |
| 4 | 48 | Post-menopausal | Prolapse |
| 5 | 50 | Post-menopausal | Prolapse |
Isolation of Ovarian Fibroblasts
Ovarian tissues were minced into 1 to 2mm fragments and subjected to digestion using an enzyme mixture containing final concentrations of 0.05% collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 0.01% DNAse (Worthington Biochemical, Lakewood, NJ). After enzymatic digestion for 1 hr at 37°C, cells were dispersed through a 250-μm mesh screen (Small Parts, Miami Lakes, FL), washed, and suspended in Hank’s Balanced Salt Solution (Thermo Fisher, Logan, UT) followed by further filtration through a 20-μm nylon mesh filter (Small Parts). Epithelial sheets and large debris were retained on the 20-μm filter, while the stromal fraction containing fibroblasts and immune cells passed through and were collected as part of the filtrate.
Ovarian Fibroblast Cell Culture and Treatment
To establish a purified in vitro cell culture system of human ovarian fibroblasts, the stromal filtrate was centrifuged (500 × g, 10 min), the pellet resuspended in complete media, and placed in a 75cm2 cell culture flask (Thermo Fisher). Medium was changed every 2 days. Complete medium consisted of DMEM/F12 supplemented with 20 mM HEPES (Invitrogen), 2 mM L-glutamine (Invitrogen), 50 mg/ml Primocin (Invivogen) and 10% heat-inactivated defined Fetal Bovine Serum (FBS) (Thermo Fisher). After reaching confluence, the cells were trypsinized and 1×106 cells added to a fresh 75cm2 flask. This was repeated at least once more before the cells were recovered and plated (1×106 cells/ml) in 24-well cell culture dishes (CytoOne, USA Scientific, Ocala, FL) in 500 μl of complete medium with 10% charcoal-dextran stripped FBS (Gemini, West Sacramento, CA) for at least 48 hrs prior to treatment. Cells were treated with HMW-poly (I:C) (Invivogen) at 2.5–25 μg/ml or Ultra-pure LPS-EK (derived from the Escherichia coli K12 strain) (Invivogen) at 0.1–1 μg/ml for up to 48 hrs.
TaqMan Real-Time RT-PCR
Total mRNA was isolated and purified using an RNeasy mini kit (Qiagen, Valencia, CA) with on-column DNase digestion using the RNase-Free DNase set (Qiagen) according to the manufacturer’s recommendations. 400ng of total RNA was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s recommendations. Relative mRNA expression levels of genes of interest were measured using the 5′ fluorogenic nuclease assay in real-time quantitative PCR using TaqMan chemistry on the ABI 7300 Prism real-time PCR instrument (Applied Biosystems, Carlsbad, CA). PCR was conducted using the following cycle parameters: 95°C, 12 min for 1 cycle (95°C, 20 s; 60°C, 1 min), for 40 cycles. Analysis was conducted using the sequence detection software supplied with the ABI 7300. Relative expression levels were expressed as a fold-increase in mRNA expression and calculated using the formula 2−ΔΔCt.
Flow Cytometry
Prior to treatment with poly (I:C) or LPS, cells were analyzed for surface expression of CD45-VioletFluor 450 (Tonbo Biosciences, San Diego, CA, USA), CD54-PE (Biolegend, San Diego, CA), CD73-PE (R&D Systems, Minneapolis, MN), CD90-APC (Thermo Fisher), CCR6-PE-Cy5.5 (Biolegend), PD-L1-PE-Cy7 (BD Biosciences, San Jose, CA), and EpCam-FITC (BD Bioscicences). Each marker was analyzed independently. Flow cytometry was performed using a MACSQuant flow cytometer (Miltenyi Biotec) using MASCQuantify software. Analysis was performed using FlowJo software (Tree Star Inc., Ashland, OR).
Cytokine Secretion
Elafin, Human Beta Defensin 2 (HBD2), CCL20, Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES), and Stromal-Derived Factor (SDF-1α) in cell culture supernatants were measured using a custom microsphere multiplex assay developed by our group [19].
CD4+ T cell chemotaxis
Chemotaxis assays were performed using Incucyte Clearview 96-well cell migration plates (Essen Biosciences, Ann Arbor, MI) and analyzed using an Incucyte Zoom (Essen Biosciences) as per the manufacturer’s instructions. Briefly, the apical chamber of the cell migration plates was coated with Protein G (Thermo Fisher) and ICAM-I (Thermo Fisher) prior to addition of activated CD4+ T cells (see below). Undiluted conditioned media from ovarian fibroblast cultures was placed in the basolateral chamber and chemotaxis of activated CD4+ T cells measured every 30 minutes for the following 24hrs using the Incucyte Zoom. Analysis of chemotaxis was performed using the Incucyte Zoom software module.
Virus
HIV-GFP-BaL: Replication-competent GFP-encoding infectious molecular clone (IMC) (Dr. Christina Ochsenbauer, University of Alabama at Birmingham), pNLENG1i-BaL.ecto [20], was derived from pNLENG1-ires [21] to express heterologous BaL env gene sequences in an isogenic backbone as described [22, 23]. Such reporter viruses, collectively referred to as Env-IMC-GFP, express GFP upon infection of HIV-1 susceptible cells [20, 24].
HIV Infection of CD4+ T cells
CD4+ T cells were isolated by negative bead selection (Miltenyi Biotec) as per the manufacturer’s instructions from the blood of a female donor. Following isolation, CD4+ T cells were activated by PHA and IL-2 for 48 hrs in X-Vivo-15 media containing phenol red (Lonza) supplemented with 10% charcoal-dextran stripped human serum. Prior to HIV infection, activated CD4+ T cells were transferred to round-bottom ultra-low attachment 96 well plates (Corning, NY, USA). To measure antiviral activity, conditioned media from ovarian stromal fibroblast cultures or control media were incubated for 1 hr with HIV-GFP-BaL (MOI=0.1), prior to addition to activated CD4+ T cells for a further 1 hr. Following this, CD4+ T cells were washed twice to remove any residual virus and conditioned media, and the cells maintained in vitro for 6 days. Media was changed every 2–3 days. Infection was determined by measuring GFP expression by flow cytometry. Only cells productively infected with HIV express GFP. Fresh media, and media containing poly (I:C) or LPS, mixed with HIV-GFP-BaL were used as controls for fibroblast conditioned media.
Statistical Analysis
Data analysis was performed using the GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). A two-sided P-value <0.05 was considered statistically significant. Comparison of two groups was performed with the non-parametric Wilcoxon paired test. Comparison of three or more groups was performed with the non-parametric paired Friedmann test followed by Dunn’s post-test. Data are presented as median +/− interquartile range (IQR).
Results
Characterization of Ovarian Fibroblasts
Primary human ovarian fibroblasts from pre- and post-menopausal women (Table 1) were recovered from enzymatically digested ovarian tissues and grown in vitro. Visually, confluent monolayers consisted of a collection of relatively uniform cells that had fibroblast “spread” morphology (Figure 1A) similar to results observed in other studies [25]. To further characterize the ovarian stromal fibroblasts, we measured expression of surface markers by flow cytometry. As seen in Figure 1B, 77% of the cells expressed PD-L1 (inhibitor of T-cell immunity), 44% expressed CD90 (adhesion molecule expressed on endometrial fibroblast populations), and less than 5% expressed CD70 (5′-ectonucleotidase) and CD54 (intracellular Adhesion Molecule 1). The lack of cells expressing the pan-immune cell marker CD45 (<5%), and the epithelial marker EpCam, is evidence that our cultures were virtually free of contaminating immune cells or epithelial cells.
Figure 1.

Ovarian stromal cells have a characteristic fibroblast phenotype in vitro and express pattern recognition receptors. Ovarian stromal fibroblasts derived from normal human ovaries were grown in vitro and analyzed by (A) light microscopy (20× magnification), (B) flow cytometry, and (C) real-time RT-PCR. Panel A shows ovarian fibroblasts grown in vitro from a representative individual sample. Panel B is the median in vitro expression of specific proteins on the surface of ovarian fibroblasts (n= 5 separate patients). Bars and horizontal lines represent the median +/− IQR. Panel C shows the median mRNA expression of TLR3, TLR4, RIG-I, and MDA5 in ovarian fibroblasts (n=5 separate patients). Bars represent median relative mRNA expression normalized to β-actin +/− IQR. **P<0.01. Statistical analysis was performed using the non-parametric Friedmann test with Dunn’s post-test correction for multiple comparisons.
Pattern recognition receptors (PRR) are essential for detecting and initiating responses to conserved moieties on a range of pathogens. We isolated mRNA from cultured ovarian fibroblasts and determined the expression levels of several PRR essential for responding to specific bacterial (TLR4) and viral (TLR3, RIG-I, & MDA5) pathogens. mRNA for all four TLRs was expressed by ovarian fibroblasts. As seen in Figure 1C, ovarian fibroblasts expressed significantly higher levels RIG-I mRNA in comparison to TLR3 (p<0.01). Similarly, there was a trend of higher TLR4 expression compared to TLR3. Together this suggests that ovarian fibroblasts can respond to both viral and bacterial ligands.
Poly (I:C) and LPS-Induced Responses by Ovarian Fibroblasts
Recognizing that fibroblasts in other regions of the FRT respond to pathogens, we investigated whether this trait is shared by ovarian fibroblasts. To test this, we exposed primary ovarian fibroblasts to the viral PRR ligand poly (I:C), or the bacterial ligand LPS for 24 hrs in vitro. mRNA expression was determined by RT-PCR, and protein secretion by a multiplex assay.
As seen in Figure 2, while there was variation between individuals, exposure of fibroblasts to poly (I:C) dose-dependently induced the expression of IFNβ by approximately 800-fold (Figure 2A), IFNλ1 by 1000-fold (Figure 2B) (p<0.01), and the interferon-stimulated gene (ISG) OAS2 by 100-fold (Figure 2C) (p<0.01). In contrast, LPS, irrespective of the dose used, had no effect on the expression of IFNβ, IFNλ1, or OAS2.
Figure 2.

Ovarian fibroblasts selectively upregulate IFN and ISG in response to poly (I:C) and LPS. (A-C) Ovarian fibroblasts were stimulated with poly (I:C) (2.5 & 25μg/ml) or LPS (0.1 & 1μg/ml) for 24 hrs prior to mRNA recovery and analysis of expression levels for IFNβ (A), IFNλ1 (B), and OAS2 (C). n=5 separate patients. Bars represent the median fold increase in mRNA expression normalized to untreated control +/− IQR. *P<0.05. Statistical analysis was performed using the non-parametric Wilcoxon Signed Rank test.
The trend of specific responses by ovarian fibroblasts to either poly (I:C) or LPS was also seen with respect to protein secretion. We used a multiplex assay developed by us [19] to measure the secretion of a select panel of anti-HIV proteins (elafin, CCL20, RANTES, SDF-1α, and HBD2) by ovarian fibroblasts following stimulation with poly (I:C) or LPS. As seen in Figure 3, elafin, SDF-1α and RANTES were constitutively secreted by ovarian fibroblasts. Poly (I:C) significantly upregulated the secretion of elafin (p<0.01), CCL20 (p<0.01), and RANTES (p<0.05) but had no effect on the secretion of SDF-1α. LPS significantly upregulated the secretion of RANTES (p<0.01) and SDF-1α (p<0.01) and boosted RANTES secretion by approximately 100pg/ml more than poly (I:C). While there was a trend towards increased secretion of elafin and CCL20 following LPS exposure, this did not reach statistical significance. HBD2 was not detectable under the conditions present in our experiments.
Figure 3.

Ovarian fibroblasts selectively upregulate antimicrobial secretion in response to poly (I:C) and LPS. Ovarian fibroblasts were stimulated with poly (I:C) (25μg/ml) or LPS (1μg/ml) for 24 hrs prior to collection of secretions. Median concentration (pg/ml) of elafin (A), CCL20 (B), RANTES (C), and SDF-1α (D) was determined by an in-house multiplex assay. n=5 separate patients. Bars represent the median +/− IQR. *P<0.05, **P<0.01. Statistical analysis was performed using the non-parametric Friedmann test with Dunn’s post-test correction for multiple comparisons.
Ovarian Fibroblast Conditioned Media Increases CD4+ T cell Chemotaxis
Since the anti-HIV proteins present in ovarian fibroblast secretions also have chemotactic functions, we investigated whether their increased secretion following exposure to poly (I:C) or LPS leads to increased chemotaxis of activated CD4+ T cells.
As seen in Figure 4A, conditioned media from untreated fibroblasts had no effect on CD4+ T cell chemotaxis beyond that seen with control media alone. However, conditioned media from poly (I:C)-treated fibroblasts significantly increased (p<0.05) the chemotaxis of CD4+ T cells by up to 70% more compared to the conditioned media from untreated fibroblasts. In contrast, conditioned media from LPS-treated fibroblasts did not alter CD4+ T cell chemotaxis relative to untreated fibroblasts.
Figure 4.
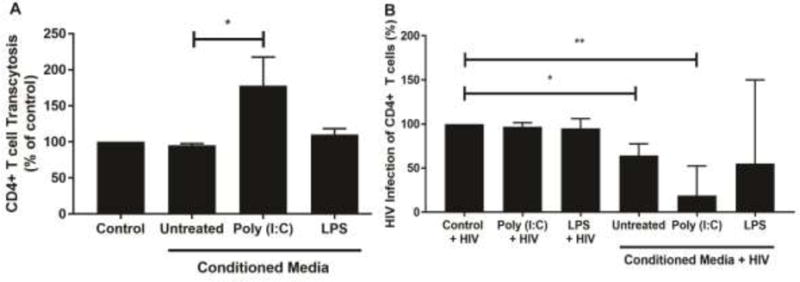
Conditioned media (CM) from poly (I:C)-treated fibroblasts induces chemotaxis and inhibits HIV infection of CD4+ T cells. (A) CM was recovered from untreated ovarian fibroblasts or fibroblasts treated with poly (I:C) (25μg/ml) or LPS (1μg/ml) for 24 hrs. Blood CD4+ T cells were activated with PHA/IL-2 for 48 hrs and then placed on the apical surface of a 96-well insert coated with Protein G and ICAM-I. Undiluted CM was placed in the basolateral compartment and CD4+ T cell chemotaxis measured using an IncuCyte Zoom. Data were normalized to % chemotaxis of control media. n=5 separate patients. Bars represent % median chemotaxis +/− IQR. *P<0.05. Statistical analysis was performed using the non-parametric Friedmann test with Dunn’s post-test correction for multiple comparisons. (B) CM from untreated fibroblasts or fibroblasts treated with poly (I:C) (25μg/ml) or LPS (1μg/ml) for 24 hrs was recovered, mixed with HIV (BaL-GFP) (MOI=0.1) for 1 hr, and subsequently added to activated CD4+ T cells for a further 1 hr followed by extensive washout of virus and CM. As controls, fresh media alone, poly (I:C) (25μg/ml) or LPS (1μg/ml) were mixed with HIV-BaL for 1 hr prior to incubation with activated CD4+ T cells for 1 hr and subsequent washout. Following washout, CD4+ T cells were incubated for a further 6 days with media being replenished every 48 hrs. After day 6, cells were analyzed by flow cytometry to determine expression levels of intracellular GFP, and thus HIV infection. Data were normalized to % HIV infection in control media. n=5 separate patients. *P<0.05, **P<0.01. Bars represent % median HIV infection +/− IQR. Statistical analysis was performed using the non-parametric Wilcoxon Signed Rank test.
Anti-HIV Activity of Ovarian Fibroblast Conditioned Media
The ovary contains a population of CD4+ T cells that are susceptible to HIV infection [6]. Previous studies have shown that fibroblasts from elsewhere in the FRT can potentially affect the outcome of HIV transmission [26]. Recognizing that ovarian fibroblasts secrete anti-HIV proteins (Figure 3), both constitutively and in response to poly (I:C) and LPS, we investigated whether ovarian fibroblast secretions can inhibit the infection of blood CD4+ T cells by HIV. Since the majority of sexually-transmitted infections are driven by CCR5-tropic HIV [27], we used GFP-BaL, the lab-adapted CCR5-tropic strain of HIV, which we used previously to infect ovarian CD4+ T cells [6]. Our rationale was to incubate the conditioned media with BaL for 1hr prior to infection of blood CD4+ T cells for 1hr. This would allow any antiviral factors present in the conditioned media to interact with HIV before infection of the CD4+ T cells. This would also reduce the exposure time of the CD4+ T cells to the conditioned media. As expected, given their short exposure time (1hr) to CD4+ T cells, the controls consisting of HIV mixed with fresh media alone, or fresh media containing either poly (I:C) or LPS, had no effect on HIV infection of CD4+ T cells (Figure 4B). Conditioned media from untreated ovarian fibroblasts significantly decreased HIV-GFP-BaL infection of CD4+ T cells by approximately 40% (p<0.05) compared to virus alone. Conditioned media from fibroblasts exposed to poly (I:C) had greater anti-HIV activity and significantly reduced HIV infection by approximately 70% (p<0.01) compared to virus alone, while conditioned media from LPS-treated fibroblasts had no significant effect on HIV infection.
Conclusions
Human primary ovarian fibroblasts grown in vitro respond to poly (I:C) and LPS. Each ligand induces a unique response by ovarian fibroblasts characterized by the specific induction of genes and secretion of anti-HIV proteins. Furthermore, fibroblast secretions possess innate anti-HIV activity that is enhanced following exposure to poly (I:C). Poly (I:C) also increased the chemotaxis of activated CD4+ T cells by fibroblast secretions. In contrast, LPS increased secretion of SDF-1α and RANTES, but had no effect on HIV infection, chemotaxis of CD4+ T cells, or fibroblast IFN and ISG expression. Together these studies suggest fibroblasts are an integral part of the innate immune response to incoming pathogens in the ovary and can initiate protective responses following pathogen exposure.
The upper FRT is not a sterile environment and is routinely exposed to contents from the lower FRT. For example, labelled macrospheres and dyes deposited in the lower FRT reach the upper FRT within minutes to hours [2]. Similarly, the ovary is accessible to incoming viral and bacterial pathogens [4, 5]. The presence of functional PRRs allows for the rapid recognition of pathogens and the initiation of innate and adaptive immune responses. PRRs are expressed throughout the FRT in epithelial cells, stromal fibroblasts, and immune cells [28–33]. Our study extends these findings by demonstrating that TLR3, TLR4, RIG-I, and MDA5 are present in human ovarian fibroblasts. Previous studies using human, murine, or bovine ovaries have shown that multiple cell types express PRRs and respond to pathogenic stimulation. For example, granulosa cells express functional TLRs 1–9, RIG-I, and MDA5, and respond to poly (I:C) [34, 35], ovarian surface epithelia express functional TLRs 2–5 [34], and cumulus cells express TLRs 2 4, 8, and 9 [36, 37]. We further demonstrate that normal human ovarian fibroblasts respond to poly (I:C), a dsRNA ligand for TLR3, RIG-I, and MDA5, as well as LPS (TLR4). Together this suggests that ovarian fibroblasts are functionally capable of initiating immune responses against viral and bacterial pathogens that gain access to the ovaries via the FRT or the peritoneum.
It is increasingly evident that stromal fibroblasts have functions beyond their structural role and are likely essential mediators of immune protection within the tissue environment [38]. Our results suggest that ovarian fibroblasts can initiate unique immune responses against specific viral and bacterial pathogens that breach or bypass the ovarian surface epithelium. This occurs elsewhere in the FRT, where endometrial [30, 39] and oviductal [40] fibroblasts induce an inflammatory response following exposure to viral and/or bacterial PRR ligands. For example, endometrial stromal fibroblasts upregulate Hepatocyte Growth Factor (HGF) in response to poly (I:C) but not other PRR ligands [30], while endometrial ECC-1 epithelial cells increase their expression of MCP-1 in response to LPS but not poly (I:C) [32].
This is the first demonstration that secretions from both untreated ovarian fibroblasts and those exposed to poly (I:C) can inhibit HIV infection of CD4+ T cells. Previous studies have shown that the ovary is a site for SIV transmission in macaques [4, 5], and that in humans, there is a population of HIV-infectible ovarian CD4+ T cells [6]. To better mimic viral transmission in vivo, we used BaL, a CCR5-tropic strain (R5) of HIV, recognizing that CCR5-tropic HIV is predominantly responsible for the sexual transmission of HIV [27]. Our demonstration of constitutive anti-HIV activity present in secretions from untreated fibroblasts suggest the presence of a baseline level of protection against incoming virus that is not dependent upon viral recognition. Other FRT cellular secretions have constitutive anti-HIV activity. For example, endometrial epithelial cell secretions inhibit the infection of reporter cells [41]. The identity of the secreted proteins responsible for the anti-HIV activity have not been determined, but likely include additional proteins, beyond those measured by us (see below), acting additively or synergistically. Our findings suggest that ovarian fibroblasts are an essential line of defense in the early hours following HIV exposure.
Intriguingly, exposure to poly (I:C) increased the anti-HIV activity of ovarian fibroblast secretions. Increased anti-HIV activity could be due to increased secretion of elafin, CCL20, and RANTES elicited in response to poly (I:C). However, each of the antimicrobials we measured was below the reported minimums for in vitro HIV inhibitory concentration (elafin: 0.01–100 ng/ml, CCL20: 2–200 ng/ml, RANTES: 3 ng/ml, SDF-1α: 200 ng/ml) [14, 16, 42–45], suggesting that no one antimicrobial is responsible for the observed anti-HIV activity and that they act together to exert their antiviral effect. It is also likely that poly (I:C) increases the secretion of a wide range of antimicrobials and cytokines beyond those measured by us. Other known anti-HIV proteins such as SLPI and cathelicidin are expressed in the ovary [46, 47] and restrict HIV infection at other sites [48–50]. Therefore, the increased anti-HIV response could also be due to additional secreted antimicrobials and cytokines. That fibroblast anti-HIV responses can be upregulated following poly (I:C) exposure suggests that therapeutics incorporating specific TLR agonists may be able to boost anti-HIV immune responses.
In contrast to secretions from poly (I:C)-treated cells, conditioned media from LPS-treated fibroblasts, despite increased secretion of SDF-1α and RANTES, showed no corresponding increase in anti-HIV activity. The reasons for this are unclear. One explanation is that while LPS upregulates secretion of specific anti-HIV proteins, their complement is distinct from those induced by poly (I:C), leading to different antiviral effects, and possibly the creation of a more permissive environment for BaL survival. For example, SDF-1α can block infection by CXCR4-tropic HIV by virtue of its being a ligand for the CXCR4 co-receptor we would not expect it to have an effect on infection by BaL (CCR5-tropic HIV) of CD4+ T cells despite LPS increasing its secretion by fibroblasts.
Secretions from ovarian fibroblasts exposed to poly (I:C) increased the chemotaxis of CD4+ T cells. This suggests that fibroblasts can link the innate and adaptive immune systems, by being directly involved in the initial response to viral pathogens while also summoning immune cells to areas of viral exposure. While the identity of the chemotactic agent(s) remains to be determined, CCL20 and RANTES, which are both upregulated by poly (I:C), have been implicated in the trafficking of T cell subsets in other systems [51–53]. However, like our anti-HIV effects, it is likely that poly (I:C) induces the secretion of a wide spectrum of chemokines which may also be involved in CD4+ T cell chemotaxis. The ovary contains a resident population of immune cells, including T cells and macrophages, whose numbers and location within the ovary varies across the ovarian cycle [54–57]. It is likely that in addition to CD4+ T cells, secretions from ovarian fibroblasts are also able to induce chemotaxis of other immune cell subsets. For example, RANTES and SDF-1α can induce the chemotaxis of neutrophils and monocytes, respectively [58, 59].
In conclusion, we demonstrate that ovarian fibroblasts are poised to respond to viral and bacterial pathogens that reach the ovary. In particular, stimulation with poly (I:C) induces the expression of antimicrobials that inhibit HIV infection and chemokines that induce chemotaxis of immune cells. Ovarian fibroblasts are thus capable of inhibiting the transmission of HIV. Recognizing that infections in the ovary can cause significant complications, including infertility, cancer, and death, future studies are needed to integrate ovarian fibroblasts into models of innate and adaptive immune protection at this site. Furthermore, fibroblast senescence that occurs with increased aging may affect the immune response to incoming pathogens in elderly women. By defining the immune response of normal ovarian fibroblasts, these studies provide a foundation for understanding how they may be dysregulated in ovarian cancer, where cancer-associated fibroblasts have been implicated in the progression of cancer.
HIGHLIGHTS.
Normal human ovarian fibroblasts induce distinct inflammatory responses to viral and bacterial TLR ligands.
Secretions from normal human ovarian fibroblasts inhibit HIV infection of CD4+ T cells, with antiviral activity increased by exposure to poly (I:C).
Secretions from normal human ovarian fibroblasts exposed to viral TLR ligands increase the chemotaxis of CD4+ T cells.
Acknowledgments
Study supported by NIH grants AI102838, AI071761, and AI117739 (CRW). We thank all study participants, Pathologists, Obstetrics, and Gynecology surgeons, operating room nurses and support personnel at Dartmouth-Hitchcock Medical Center. Flow Cytometry studies were carried out in DartLab, the Immune Monitoring and Flow Cytometry Shared Resource. The shared resource is supported in part by a Norris Cotton Cancer Center Support Grant (P30CA023108-36) and an Immunology COBRE Grant (P30GM103415-14) from the National Institute of General Medical Sciences.
Supported by NIH AI102838, AI071761, AI117739 (Charles R. Wira)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nature Reviews Immunology. 2015;15(4):217–30. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zervomanolakis I, et al. Physiology of upward transport in the human female genital tract. Annals of the New York Academy of Sciences. 2007;1101:1–20. doi: 10.1196/annals.1389.032. [DOI] [PubMed] [Google Scholar]
- 3.Kunz G, et al. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Human Reproduction. 1996;11:627–632. doi: 10.1093/humrep/11.3.627. [DOI] [PubMed] [Google Scholar]
- 4.Barouch DH, et al. Rapid Inflammasome Activation following Mucosal SIV Infection of Rhesus Monkeys. Cell. 2016;165(3):656–67. doi: 10.1016/j.cell.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stieh DJ, et al. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell Host Microbe. 2016;19(4):529–40. doi: 10.1016/j.chom.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Z, et al. Characterization of immune cells and infection by HIV in human ovarian tissues. American Journal of Reproductive Immunology. 2017;78(1):e12687–n/a. doi: 10.1111/aji.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunham RC, Gottlieb SL, Paavonen J. Pelvic Inflammatory Disease. New England Journal of Medicine. 2015;372(21):2039–2048. doi: 10.1056/NEJMra1411426. [DOI] [PubMed] [Google Scholar]
- 8.Lin HW, et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;12(9):900–4. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen CB, et al. Pelvic inflammatory disease and risk of invasive ovarian cancer and ovarian borderline tumors. Cancer Causes & Control. 2013;24(7):1459–1464. doi: 10.1007/s10552-013-0216-y. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker SW, et al. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annual Review of Immunology. 2015;33(1):257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato H, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. The Journal of Experimental Medicine. 2008;205(7):1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow JC, et al. Toll-like Receptor-4 Mediates Lipopolysaccharide-induced Signal Transduction. Journal of Biological Chemistry. 1999;274(16):10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 13.Sun L, et al. Human β-Defensins Suppress Human Immunodeficiency Virus Infection: Potential Role in Mucosal Protection. Journal of Virology. 2005;79(22):14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh M, et al. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2010;129(2):207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocchi F, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh M, et al. CCL20/MIP3α is a Novel Anti-HIV-1 Molecule of the Human Female Reproductive Tract. American Journal of Reproductive Immunology. 2009;62(1):60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberlin E, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 18.Jin T, Xu X, Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine. 2008;44(1):1–8. doi: 10.1016/j.cyto.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boesch AW, et al. A multiplexed assay to detect antimicrobial peptides in biological fluids and cell secretions. J Immunol Methods. 2013;397(1–2):71–6. doi: 10.1016/j.jim.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochiel DO, et al. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PLoS ONE. 2010;5(12):e14306. doi: 10.1371/journal.pone.0014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelderblom HC, et al. Viral complementation allows HIV-1 replication without integration. Retrovirology. 2008;5(1):60. doi: 10.1186/1742-4690-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochsenbauer C, et al. Generation of Transmitted/Founder HIV-1 Infectious Molecular Clones and Characterization of Their Replication Capacity in CD4 T Lymphocytes and Monocyte-Derived Macrophages. Journal of Virology. 2012;86(5):2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmonds TG, et al. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408(1):1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Garcia M, et al. Estradiol Reduces Susceptibility of CD4+ T Cells and Macrophages to HIV-Infection. PLoS ONE. 2013;8(4):e62069. doi: 10.1371/journal.pone.0062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quiros RM, et al. Ovarian normal and tumor-associated fibroblasts retain in vivo stromal characteristics in a 3-D matrix-dependent manner. Gynecol Oncol. 2008;110(1):99–109. doi: 10.1016/j.ygyno.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidleman JA, et al. Mucosal stromal fibroblasts markedly enhance HIV infection of CD4+ T cells. PLoS Pathog. 2017;13(2):e1006163. doi: 10.1371/journal.ppat.1006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences. 2008;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart KM, et al. Functional expression of pattern recognition receptors in tissues of the human female reproductive tract. Journal of Reproductive Immunology. 2009;80(1–2):33–40. doi: 10.1016/j.jri.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh M, et al. Pathogen Recognition in the Human Female Reproductive Tract: Expression of Intracellular Cytosolic Sensors NOD1, NOD2, RIG-1, and MDA5 and response to HIV-1 and Neisseria gonorrhea. American Journal of Reproductive Immunology. 2013;69(1):41–51. doi: 10.1111/aji.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman KD, et al. Modulation of Hepatocyte Growth Factor Secretion in Human Female Reproductive Tract Stromal Fibroblasts by Poly (I:C) and Estradiol. American Journal of Reproductive Immunology. 2012;67(1):44–53. doi: 10.1111/j.1600-0897.2011.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasu K, Narahara H. Pattern Recognition via the Toll-Like Receptor System in the Human Female Genital Tract. Mediators of Inflammation. 2010;2010 doi: 10.1155/2010/976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer TM, et al. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112(3):428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh M, et al. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C) Fertility and Sterility. 2008;89(5, Supplement 1):1497–1506. doi: 10.1016/j.fertnstert.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M, et al. Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunol Immunother. 2009;58(9):1375–85. doi: 10.1007/s00262-008-0650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan K, et al. Toll-like receptor 3 and RIG-I-like receptor activation induces innate antiviral responses in mouse ovarian granulosa cells. Molecular and Cellular Endocrinology. 2013;372(1–2):73–85. doi: 10.1016/j.mce.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Shimada M, et al. Induced expression of pattern recognition receptors in cumulus oocyte complexes: novel evidence for innate immune-like functions during ovulation. Mol Endocrinol. 2006;20(12):3228–39. doi: 10.1210/me.2006-0194. [DOI] [PubMed] [Google Scholar]
- 37.Shimada M, et al. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135(11):2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- 38.Owens BM. Inflammation, Innate Immunity, and the Intestinal Stromal Cell Niche: Opportunities and Challenges. Front Immunol. 2015;6:319. doi: 10.3389/fimmu.2015.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman KD, et al. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertility and Sterility. 2009;92(3):1107–1109. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh H, et al. Human oviductal stromal fibroblasts, but not oviductal epithelial cells, express Toll-like receptor 4: the site-specific mucosal immunity of the human fallopian tube against bacterial infection. American Journal of Reproductive Immunology. 2006;56(2):91–101. doi: 10.1111/j.1600-0897.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 41.Wira CR, et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunology. 2011;4(3):335–42. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocchi F, et al. Identification of RANTES, MIP-1α, and MIP-1β as the Major HIV-Suppressive Factors Produced by CD8+ T Cells. Science. 1995;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 43.Maréchal V, et al. Opposite Effects of SDF-1 on Human Immunodeficiency Virus Type 1 Replication. Journal of Virology. 1999;73(5):3608–3615. doi: 10.1128/jvi.73.5.3608-3615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valenzuela-Fernández An, et al. Optimal Inhibition of X4 HIV Isolates by the CXC Chemokine Stromal Cell-derived Factor 1α Requires Interaction with Cell Surface Heparan Sulfate Proteoglycans. Journal of Biological Chemistry. 2001;276(28):26550–26558. doi: 10.1074/jbc.M100411200. [DOI] [PubMed] [Google Scholar]
- 45.Drannik AG, et al. Anti-HIV-1 Activity of Elafin Is More Potent than Its Precursor’s, Trappin-2, in Genital Epithelial Cells. Journal of Virology. 2012;86(8):4599–4610. doi: 10.1128/JVI.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JJ, et al. Induction of secretory leukocyte protease inhibitor (SLPI) in estradiol valerate (EV) induced polycystic ovary. Arch Pharm Res. 2011;34(8):1389–97. doi: 10.1007/s12272-011-0820-x. [DOI] [PubMed] [Google Scholar]
- 47.Coffelt SB, et al. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. International Journal of Cancer. 2008;122(5):1030–1039. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 48.Kedzierska K, et al. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Reviews in Medical Virology. 2003;13(1):39–56. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 49.Wahl SM, et al. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 1997;3(Suppl 1):S64–9. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 50.Bergman P, et al. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5(4):410–5. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki T, et al. CCR6 Regulates the Migration of Inflammatory and Regulatory T Cells. Journal of immunology (Baltimore, Md : 1950) 2008;181(12):8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schall TJ, et al. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347(6294):669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 53.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160(2):550–4. [PubMed] [Google Scholar]
- 54.Best CL, et al. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11(4):790–7. doi: 10.1093/oxfordjournals.humrep.a019256. [DOI] [PubMed] [Google Scholar]
- 55.Castro A, et al. Luteal leukocytes are modulators of the steroidogenic process of human mid-luteal cells. Hum Reprod. 1998;13(6):1584–9. doi: 10.1093/humrep/13.6.1584. [DOI] [PubMed] [Google Scholar]
- 56.Brannstrom M, et al. Localization of leukocyte subsets in the follicle wall and in the corpus luteum throughout the human menstrual cycle. Fertil Steril. 1994;61(3):488–95. [PubMed] [Google Scholar]
- 57.Gaytan F, et al. Macrophages, cell proliferation, and cell death in the human menstrual corpus luteum. Biol Reprod. 1998;59(2):417–25. doi: 10.1095/biolreprod59.2.417. [DOI] [PubMed] [Google Scholar]
- 58.Pan ZZ, et al. Inducible lung-specific expression of RANTES: preferential recruitment of neutrophils. Am J Physiol Lung Cell Mol Physiol. 2000;279(4):L658–66. doi: 10.1152/ajplung.2000.279.4.L658. [DOI] [PubMed] [Google Scholar]
- 59.Chatterjee M, et al. Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4-CXCR7. Cell Death Dis. 2015;19(6):233. doi: 10.1038/cddis.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]


