Abstract
Background
Psoriasis is a chronic inflammatory disease associated with dyslipidemia, cardiovascular events and mortality. We aimed to assess and compare the effect of treatment of moderate-to-severe psoriasis with adalimumab or phototherapy on vascular inflammation (VI) and cardiovascular biomarkers.
Methods and Results
Randomized, double-blind, trial of adalimumab, phototherapy, and placebo (1:1:1) for 12 weeks, with cross-over to adalimumab for 52 weeks total. Outcomes included VI by 18F-FDG-PET/CT and biomarkers of inflammation, insulin resistance and lipoproteins. 97 patients were randomized, 92 completed the RCT portion; 81 entered the adalimumab extension with 61 completing 52 weeks of adalimumab.
There was no difference in change in VI at week-12 in the adalimumab group [change compared to placebo 0.64%, 95% CI −5.84% to 7.12%)] or the phototherapy group [−1.60%, (−6.78%, 3.59%)] or after 52-week adalimumab treatment (0.02% compared to initiation, 95% CI −2.85%, 2.90%). Both adalimumab and phototherapy decreased inflammation by serum CRP, IL-6. Only adalimumab reduced TNF and GlycA at 12 and 52 weeks. Neither had impact on metabolic markers (insulin, adiponectin, leptin). Only phototherapy increased HDL-p at 12 weeks. At 52-week of adalimumab cholesterol-efflux and HDL-p were reduced.
Conclusions
Adalimumab reduced key markers of inflammation including GlycA compared to phototherapy with no effect on glucose metabolism and VI, and potential adverse effects on HDL. GlycA improvement may partially explain the beneficial effects of adalimumab seen in observational studies. Larger studies with more detailed phenotyping of vascular disease should assess the comparative differences in the effects of adalimumab and phototherapy seen in our study.
SUBJECT TERMS: Nuclear Cardiology and PET, Vascular disease, Biomarkers
Keywords: Vascular inflammation, psoriasis, 18F-FDG PET/CT, cardiovascular disease, biomarkers, adalimumab, phototherapy
INTRODUCTION
Psoriasis is a common chronic Th1/Th17 inflammatory skin disease that affects over 125 million people worldwide1. Like other diseases of chronic inflammation, it is associated with an increased risk of impairments in lipoprotein metabolism (dyslipidemia and decreased cholesterol efflux capacity), insulin resistance and diabetes, and major adverse cardiovascular events2–5. The risk of cardiometabolic disease increases with increasing psoriasis severity, is independent of traditional risk factors, and culminates in a lifespan reduction of approximately 5 years5, 6.
The aberrant innate and adaptive immune pathways that drive the pathophysiology of psoriasis are known to also promote insulin resistance, atherosclerosis, and thrombosis7. Established and novel inflammatory markers such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6) and glycoprotein acetylation (GlycA) are increased in psoriasis8, 9, associate with skin disease severity8, 10 and are predictive of future cardiovascular risk in healthy individuals without psoriasis or any other chronic inflammatory condition11, 12. Consistent with the translational and epidemiological data linking psoriasis to cardiovascular disease, we and others have demonstrated that patients with psoriasis have increased vascular inflammation (VI), as measured by 18F-FDG-PET/CT, that is equivalent to approximately one decade of aging, and that increasing skin disease severity is associated with increasing VI independent of traditional cardiovascular risk factors10, 13. 18F-FDG-PET/CT is an attractive surrogate marker, as it predicts cardiovascular events14 and is highly sensitive to change with short-term (i.e., weeks-to-months) treatment with interventions known to lower cardiovascular risk, such as statins and therapeutic lifestyle changes 15. Psoriasis is a reliable disease to study cardiovascular effects of immune modulating therapy13 as it can be treated with a variety of targeted modalities7.
Adalimumab, a monoclonal antibody that blocks TNF-alpha, is a standard of care biologic therapy used to treat moderate to severe psoriasis16 and is suggested to be associated with a reduction in major cardiovascular events17. Narrow band ultraviolet B phototherapy (NB-UVB) is also highly effective in treating psoriasis, but is not associated with clinically significant alterations in systemic immune function7. A recently completed randomized trial demonstrated a beneficial effect of a systemic biologic anti-inflammatory therapy in reducing rate of myocardial infarction despite minimal change in LDL cholesterol18. Phototherapy provides a unique opportunity to compare treatment of psoriasis with a systemic immune-modulating biological therapy (adalimumab) to skin directed therapy (phototherapy). As such, we conducted a randomized controlled trial, in patients with moderate-to-severe psoriasis, of adalimumab, phototherapy, and placebo to determine the comparative impact of psoriasis treatment with adalimumab and phototherapy on VI measured by 18F-FDG-PET/CT and biomarkers of advanced lipoprotein characterization, glucose metabolism, and inflammation.
METHODS
The data will be made available to other researchers for purposes of reproducing the results, however, materials cannot be made available in view of the concerns regarding the patient privacy. The study was a multi-center randomized controlled trial designed to enroll 96 patients across 8 centers in the United States with 1:1:1 allocation to adalimumab subcutaneous injections or placebo injections every 2 weeks, or NB-UVB phototherapy at baseline (NCT01553058). At week 12, eligible patients entered an open label extension in which they were treated with adalimumab for 52 weeks (if initially assigned to placebo or phototherapy) or an additional 40 weeks if initially assigned to adalimumab, such that all patients received a total of 52 consecutive weeks of adalimumab (NCT01866592) (Figure 1). Primary outcome for our study was change in VI, estimated as a target-to-background ratio (TBR) of maximum target aortic activity to blood pool activity by 18F-FDG-PET/CT at week 12 compared to baseline in adalimumab and phototherapy groups compared to placebo. We also conducted a series of analyses of biomarkers of cholesterol, glucose metabolism, and inflammation which were selected based on their known association with psoriasis and/or cardiovascular disease at baseline, week 4, and week 12.
Figure 1.
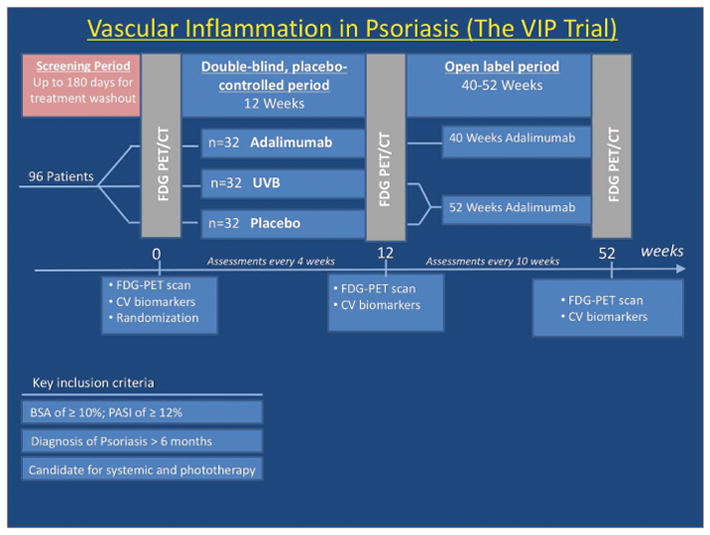
Study protocol for the Vascular Inflammation in Psoriasis (VIP) randomized, controlled trial
All participants had to be ≥18 years with a diagnosis of psoriasis for at least 6 months and that of moderate-to-severe psoriasis for at least 2 months, defined as body surface area ≥10% and psoriasis area severity index score ≥12 at baseline. Patients were excluded if they had any of the following treatments: UVB phototherapy within 14 days of baseline, psoralen-UVA phototherapy within 30 days of baseline, oral psoriasis treatments within 30 days of baseline, biologics within 90 days of baseline (or 180 days for ustekinumab); investigational agents within 30 days or 5 half-lives (whichever is longer) of baseline. Adalimumab (or corresponding placebo) therapy was administered in a double-blind manner as subcutaneous injection with an initial 80mg dose at week 0, followed by maintenance doses of 40mg every other week, starting from week 1 and then continued throughout the study. NB-UVB phototherapy dosing was based on estimated minimal erythema dose (MED) and Fitzpatrick skin type using a standardized protocol published by Zanolli and Feldman19. 18F-FDG PET/CT scans were analyzed to derive TBR values by previously published, and validated methods10. The extent of 18F-FDG uptake within the aorta was directly measured by using a dedicated image analysis software (OsiriX MD, Pixmeo SARL, Bernex, Switzerland) to measure VI calculated as TBR. The SUVmean from each of the superior vena cava slices were averaged to produce one venous value. To account for background blood activity, SUVmax values from each aortic slice were divided by the average venous SUVmean value yielding TBRmax values, the primary outcome measure. Patients underwent 18F-FDG PET/CT scans using the standard protocol20, 21 following overnight fast. Using a two-sided test with α=0.017 (with Bonferroni correction), we needed 32 patients in each arm to have 82% power in our study to detect a change in SUV of 0.1 with a presumed drop-out rate of 15%.
Primary analyses were the comparisons of the treatment effects of adalimumab, phototherapy and placebo, on the outcome measures using intention-to-treat approach. The changes in TBRmax and biomarker values were compared across groups using linear regressions, whereas additional multivariate linear regression models were fitted for TBRmax to assess sensitivity to potential imbalance of covariates, adjusting for clinical and demographic covariates using a backward model building approach. Natural log scale was used for all non-parametric variables. The secondary analyses were the changes in outcome measures from baseline to the end of open label extension period. The mean changes and proportions were calculated along with their respective 95% confidence intervals (CIs) and were reported as such. Sample size calculations were based on changes in SUV. Using a two-sided α=0.017 (applying a Bonferroni correction), we determined that 32 patients per arm would provide 82% power to detect the clinically significant change in SUV of 0.1 between groups assuming an anticipated standard deviation (SD) of the change in SUV of 0.11 and a drop-out rate of 15%.
Study approval was obtained from the Institutional Review Board (IRB) of the University of Pennsylvania or respective local IRB when indicated in accordance with the principles of Declaration of Helsinki. All guidelines for good clinical practice and those set forth by the Belmont Report (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research) were followed. All study participants provided written informed consent. The randomized placebo-controlled trial was overseen by an independent data monitoring committee. The sponsors (NHLBI and Abbvie) had no role in the analysis or reporting of the results. Abbvie reviewed the manuscript for compliance purposes. The detailed methods are elaborated in a separate Online only supplement.
RESULTS
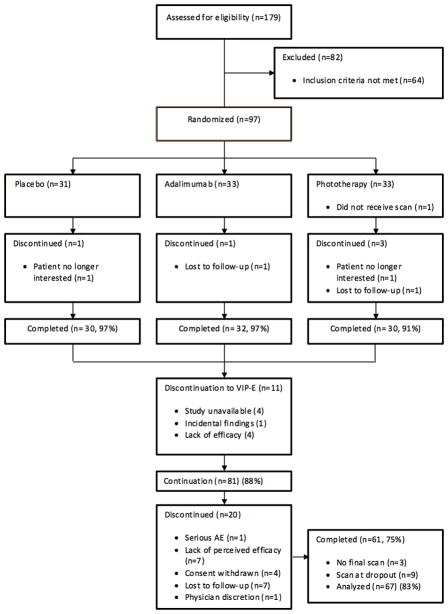
We screened 179 patients for eligibility and randomized 97 patients to placebo, adalimumab, or phototherapy treatment (1:1:1), with 92 (95%) patients completing the 12-week controlled portion of the study (Figure 2). 81 patients entered the open-label adalimumab extension portion of the study, of which 58 (72%) completed 52 weeks of adalimumab treatment and had an end of study scan. 9 patients (11%) had an early termination during the extension period and received an early termination scan, thus resulted in 67 (83%) patients being included in the analysis of the open-label extension portion of the study. Study subjects had an average age of 43; were predominantly male (69%); had an average duration of psoriasis of 17 years, and a mean PASI (a measure of psoriasis area and severity index) of 19. About 10% of patients had a history of psoriatic arthritis, and about 30% had previously been treated with systemic agents or phototherapy (Table 1). The study groups were well balanced for psoriasis characteristics and cardiovascular risk factors and were similar to populations typically seen in large phase III clinical trials of psoriasis therapeutic agents (Table 1).
Figure 2.
Patient recruitment scheme for the study.
Table 1.
Baseline demographics and clinical characteristics
| Placebo | Adalimumab | Phototherapy | Total | |
|---|---|---|---|---|
|
| ||||
| N | 31 | 33 | 33 | 97 |
|
| ||||
| Age | ||||
| Mean (SD) | 44.32 (14.50) | 44.15 (13.97) | 41.97 (13.97) | 43.46 (14.03) |
|
| ||||
| Sex (%) | ||||
| Female | 11 (35.48) | 9 (27.27) | 10 (30.30) | 30 (30.93) |
| Male | 20 (64.52) | 24 (72.73) | 23 (69.70) | 67 (69.07) |
|
| ||||
| Pso Duration (Y) | ||||
| Median (IQR) | 20 (7–29) | 11 (2–22) | 12 (7–17) | 13 (6–25) |
|
| ||||
| PsA (%) | ||||
| 2 (6.45) | 4 (12.90) | 3 (9.68) | 9 (9.68) | |
|
| ||||
| BMI | ||||
| Mean (SD) | 31.95 (7.74) | 30.93 (7.42) | 32.61 (8.66) | 31.83 (7.91) |
| H/o CVD | 3 (9.68) | 2 (6.06) | 2 (6.06) | 7 (7.22) |
|
| ||||
| Diabetes | 1 (3.23) | 3 (9.09) | 0 | 4 (4.12) |
|
| ||||
| Hypertension | 7 (22.58) | 6 (18.18) | 5 (15.15) | 18 (18.56) |
|
| ||||
| Hyperlipidemia | 5 (16.13) | 5 (15.15) | 4 (12.12) | 14 (14.43) |
|
| ||||
| Statin use | 4 (12.90) | 1 (3.03) | 3 (9.09) | 8 (8.25) |
|
| ||||
| 10 year Framingham risk % | 4.9 (2.2–10.1) | 6.5 (2.5–12.0) | 3.7 (1.4–7.9) | 4.8 (1.9–10.7) |
| Median (IQR) | ||||
|
| ||||
| BSA % | ||||
| Median (IQR) | 21 (16–33) | 18 (15–25) | 19.5 (15–26) | 20 (15–29) |
|
| ||||
| PASI | ||||
| Median (IQR) | 15.0 (13.3–20.6) | 17.4 (15.4–22.0) | 16.8 (14.5–21.0) | 16.7 (13.9–21.6) |
|
| ||||
| H/o Phototherapy | ||||
| (%) | 11 (35.48) | 5 (16.13) | 13 (41.94) | 29 (31.18) |
|
| ||||
| H/o Oral Systemics | ||||
| (%) | 10 (32.26) | 10 (32.26) | 11 (35.48) | 31 (33.33) |
|
| ||||
| H/o Biologics | ||||
| (%) | 11 (35.48) | 10 (32.26) | 8 (25.81) | 29 (31.18) |
|
| ||||
| Baseline target-to-background ratio Mean (SD) | 1.620 (0.267) | 1.610 (0.334) | 1.636 (0.226) | 1.622 (0.278) |
Both adalimumab and phototherapy resulted in substantial improvements in psoriasis severity compared to placebo for physician reported measures (Supplemental Table 1). Patients evaluated during the open label adalimumab extension period achieved a high rate of skin clearance with 53% of patients being clear or almost clear of their skin disease (Supplemental Table 2). No statistically significant change was observed in physical activity (as measured using the IPAQ metabolic equivalent task (MET) minutes) between groups at week 12 or at end of extension period. On average, patients reported reduction in saturated and dietary cholesterol intake at week 12 and end of open label period compared to baseline, but there were no group-level differences.
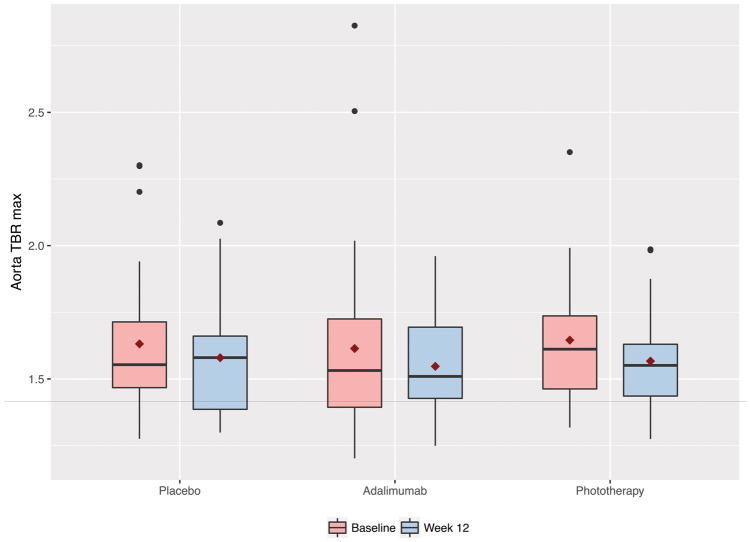
At baseline mean TBRmax values were 1.62, 1.61 and 1.64 in the placebo, adalimumab and phototherapy groups respectively (Table 1). Furthermore, the TBR and SUV values at subsequent visits for all three groups are noted under Supplementary Table 3. There was no difference in change in VI at week 12 in the adalimumab group (change compared to placebo 0.64%, 95% CI −5.84% – 7.12%) or the phototherapy group (−1.60%, 95% CI −6.78% – 3.59%) (Table 2 & Figure 3). Analysis within groups demonstrated a statistically significant reduction in VI by −4.09% (95% CI −7.78%, −0.39%) in the phototherapy treatment arm only. At the end of the extension period there was a −3.80% reduction in VI compared to the absolute study baseline (week 52 or 64 compared to week 0 for all) (95% −6.40%, −1.19%) (Table 3). However, there was no change in VI (0.02% difference, 95% CI −2.85%, 2.90%) during the adalimumab treatment only period comparing end of study assessment to adalimumab baseline (week 52 compared to week 0 for those initially assigned to adalimumab, and week 64 compared to week 12 for those who entered open-label extension but were initially assigned to placebo/phototherapy).
Table 2.
Changes in TBRmax by treatment group during RCT period
| Placebo | Adalimumab | Phototherapy | |
|---|---|---|---|
|
| |||
| Global Change compared to baseline within group | |||
| Mean difference1 | −0.052 (0.112) | −0.067 (0.213) | −0.079 (0.020) |
| Mean % change (95% CI) 1 | −2.49% (−6.29%, 1.31%) (0.191) | −1.84% (−7.17%, 3.47%) (0.483) | −4.09% (−7.78%, −0.39%) (0.031) |
|
| |||
| Global Change compared to placebo | |||
| Difference of differences2 | NA | −0.015 (0.795) | −0.027 (0.647) |
| Difference of % change2 | NA | 0.64% (−5.84%, 7.12%) (0.844) | −1.60% (−6.78%, 3.59%) (0.540) |
One sample test (p-value)
Difference of differences (p-value)
(statistically significant findings bolded)
Figure 3.
Change in vascular inflammation for the randomized controlled trial period (baseline to week-12) stratified by the treatment group.
Table 3.
Changes in TBRmax open label extension
| Mean (p-value1) | |
|---|---|
|
| |
| Global change baseline compared to end of open label extension | |
| Difference | −0.08 (0.002) |
| % change (95% CI) | −3.80% (−6.40%, −1.19%) (0.005) |
|
| |
| Global change start of adalimumab compared to end of open label extension | |
| Difference | −0.02 (0.538) |
| % change | 0.02% (−2.85%, 2.90%) (0.987) |
One sample t-test
(statistically significant findings bolded)
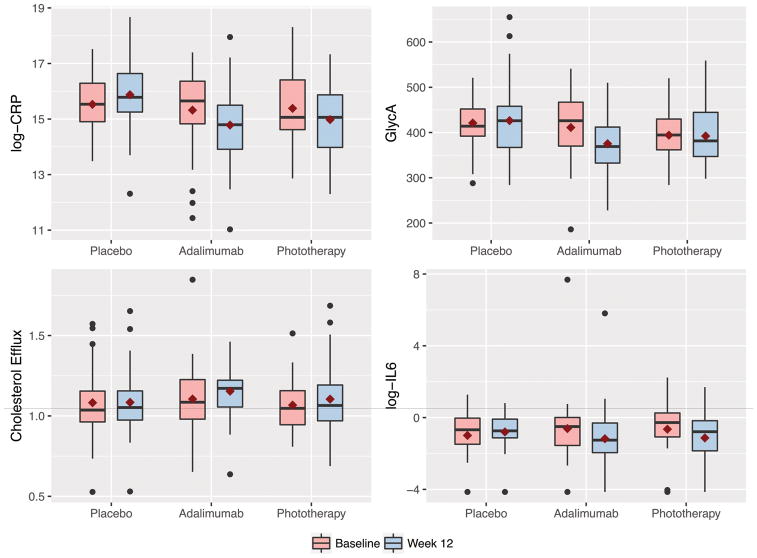
We evaluated biomarkers of advanced lipoprotein characterization, glucose metabolism, and inflammation (Figure 4). During the placebo-controlled period, a reduction in inflammation in the adalimumab group compared to placebo was observed for CRP, TNF-alpha, IL-6 and GlycA whereas only CRP and IL-6 were reduced in the phototherapy arm (Table 4). There was no change in lipoprotein characterization or glucose metabolism except for HDL-P which increased in the phototherapy group compared to placebo which was only modestly significant. At the end of the extension period, compared to absolute baseline (week 0 for all), there was no change in total cholesterol, LDL-P, or markers of glucose metabolism, but there was a reduction in efflux, and HDL-P. Furthermore, we also observed a reduction in markers of inflammation such as CRP, TNF-alpha, and GlycA, but an increase in the levels of IL-6 (Table 5). A similar pattern of biomarker change was observed during the adalimumab treatment only period comparing end of study assessment to adalimumab baseline (week 0 for those initially assigned to adalimumab or week 12 for those initially assigned to placebo or phototherapy) (Table 6).
Figure 4.
Change in key biomarkers for the randomized controlled trial period (baseline to week-12) stratified by the treatment group.
Table 4.
Change in advanced lipoprotein characterization, glucose metabolism, and inflammation between baseline and Week 12, by treatment group during RCT period
| Adalimumab vs Placebo1 | Phototherapy vs Placebo1 | F-test p2 | |
|---|---|---|---|
| TC | 6.481 (0.386) | 8.154 (0.280) | 0.514 |
| Efflux | 0.046 (0.357) | 0.035 (0.496) | 0.629 |
| LDL-P | 9.193 (0.897) | 52.320 (0.467) | 0.743 |
| HDL-P | 2.558 (0.089) | 3.319 (0.030) | 0.071 |
| Log Insulin | 0.014 (0.934) | 0.071 (0.679) | 0.909 |
| Log Adiponectin | −0.142 (0.672) | −0.151 (0.655) | 0.879 |
| Log Leptin | −0.086 (0.504) | 0.050 (0.695) | 0.568 |
| Log CRP | −0.883 (0.002) | −0.752 (0.009) | 0.004 |
| Log TNF-alpha | −0.411 (<0.001) | −0.177 (0.065) | <0.001 |
| Log IL6 | −0.764 (0.007) | −0.683 (0.019) | 0.014 |
| GlycA | −41.165 (0.006) | −7.199 (0.628) | 0.015 |
Difference of differences (statistically significant findings bolded)
Global difference (statistically significant findings bolded)
Table 5.
Change in advanced lipoprotein characterization, glucose metabolism, and inflammation between baseline and end of open label extension
| Baseline (SD) | End of Study (SD) | Diff (SE) | p-value | |
|---|---|---|---|---|
| TC | 171.000 (37.159) | 174.164 (34.347) | 3.164 (4.216) | 0.456 |
| Efflux | 1.088 (0.233) | 0.871 (0.163) | −0.217 (0.032) | <0.001 |
| LDL-P | 1256.701 (395.937) | 1279.239 (400.703) | 22.537 (44.283) | 0.613 |
| HDL-P | 33.299 (7.543) | 30.315 (6.721) | −2.984 (0.786) | <0.001 |
| Log Insulin | 6.284 (0.911) | 6.402 (0.795) | 0.118 (0.136) | 0.388 |
| Log Adiponectin | 2.240 (0.738) | 2.166 (0.571) | −0.074 (0.068) | 0.277 |
| Log Leptin | 9.110 (1.342) | 9.158 (1.233) | 0.048 (0.195) | 0.808 |
| Log CRP | 15.412 (1.425) | 14.597 (1.383) | −0.815 (0.192) | <0.001 |
| Log TNF-alpha | 0.688 (0.554) | 0.413 (0.873) | −0.275 (0.131) | 0.040 |
| Log IL6 | −0.820 (1.809) | 0.234 (1.160) | 1.054 (0.243) | <0.001 |
| GlycA | 399.742 (64.876) | 370.184 (65.916) | −29.559 (7.749) | <0.001 |
(statistically significant findings bolded)
Table 6.
Change in advanced lipoprotein characterization, glucose metabolism, and inflammation between start of adalimumab and end of open label extension
| Start of adalimumab (SD) | End of Study (SD) | Diff (SE) | p-value | |
|---|---|---|---|---|
| TC | 170.955 (37.236) | 174.164 (34.347) | 3.209 (4.004) | 0.426 |
| Efflux | 1.102 (0.235) | 0.871 (0.163) | −0.230 (0.032) | <0.001 |
| LDL-P | 1261.358 (364.259) | 1279.239 (400.703) | 17.881 (40.416) | 0.660 |
| HDL-P | 32.896 (7.220) | 30.315 (6.721) | −2.581 (0.824) | 0.003 |
| Log Insulin | 6.150 (0.772) | 6.402 (0.795) | 0.252 (0.128) | 0.053 |
| Log Adiponectin | 2.259 (0.792) | 2.174 (0.571) | −0.085 (0.071) | 0.235 |
| Log Leptin | 9.171 (1.334) | 9.158 (1.233) | −0.013 (0.197) | 0.947 |
| Log CRP | 15.522 (1.441) | 14.597 (1.383) | −0.925 (0.184) | <0.001 |
| Log TNF-alpha | 0.725 (0.571) | 0.409 (0.867) | −0.315 (0.126) | 0.015 |
| Log IL6 | −0.842 (1.734) | 0.233 (1.152) | 1.075 (0.235) | <0.001 |
| GlycA | 407.848 (80.882) | 370.184 (65.916) | −37.665 (9.022) | <0.001 |
(statistically significant findings bolded)
DISCUSSION
We conducted a randomized, controlled trial to determine the impact of systemic anti-TNF immune targeted (i.e., adalimumab) treatment and skin directed treatment (i.e., ultraviolet B phototherapy) on key markers of vascular disease risk compared to placebo in patients with psoriasis, an inflammatory disease well established to be associated with increased VI10, metabolic dysfunction, and an increased risk of cardiovascular disease and mortality3. TNF inhibitors are used in hundreds of thousands of patients with inflammatory bowel, joint, skin and eye disease. Thus, our findings establish provocative information about their cardiometabolic effects under rigorous experimental conditions in humans. Foremost, the study results strongly demonstrate that use of TNF inhibition has no impact, be it adverse, or ameliorative, on VI. Indeed, the 95% confidence interval of our estimate in the open label extension suggests we had adequate statistical power to exclude clinically significant alterations in VI from adalimumab considering statin effects as reference15.
Our results are in line with a study that demonstrated no beneficial effects of TNF inhibition in patients with myocardial infarction22, however, they are in strong contrast to a non-controlled study in rheumatoid arthritis of anti-TNF treatment which observed a reduction in VI by 18F-FDG-PET/CT following 8 weeks of therapy23. This study was open label and assessed a reduction in the “hottest plaque” (most diseased aortic segment). We have also demonstrated improvements in VI in an observational study of psoriasis patients being treated systemically8, 24. Similarly, we observed a reduction in VI in the overall clinical trial population (comparing baseline to end of study), but this was due to improvements seen in the period in which patients received placebo or phototherapy. Our patient reported outcomes suggest dietary improvements consistent with lifestyle changes advised by the American Heart Association25 in the overall study population, which may in part explain the improvements in VI we observed that do not differentiate from placebo. These results emphasize the importance of randomization and placebo control in studies evaluating these highly sensitive outcomes.
We propose several theories for the discrepancy between our experimental results evaluating VI, a surrogate marker of future cardiovascular events, and observational studies of actual cardiovascular events17, 26. It is possible that TNF associated benefits on cardiovascular events are mediated by pathways beyond VI such as thrombosis27. Moreover, large molecules (i.e., antibodies) may not be directly active in large vessels such as the aorta due to impaired tissue penetration, which may explain why small molecules such as statins do have a strong anti-inflammatory effect on VI as early as after 4 weeks of treatment15. Psoriasis increasingly is recognized to be a Th17 driven disease7 and thus more targeted treatments may be necessary to alter VI in this specific patient population. We observed adverse impacts of adalimumab on key mediators of lipid metabolism such as cholesterol efflux capacity, which is a validated surrogate marker for the ability of HDL to perform reverse cholesterol transport, and HDL-P with no evidence of change in LDL-particle number. In contrast, we demonstrated strong and consistent improvements in skin inflammation (i.e., clearance of psoriasis) and strong reductions in systemic inflammation as measured by serum TNF-alpha, CRP, and GlycA with short and longer-term adalimumab treatment, but an increase in IL-6. It is therefore possible that anti-inflammatory benefits of adalimumab may be counteracted by adverse impacts on advanced measures of HDL lipoprotein structure and function resulting in a neutral effect on VI.
While CRP, IL-6, TNF-alpha are established markers of cardiovascular disease12, GlycA is an emerging novel biomarker of systemic inflammation and cardiovascular disease derived from nuclear magnetic resonance, with value in psoriasis8, 11. It may be possible that the improvement in inflammatory biomarkers including GlycA seen in our study over a short-term follow-up may partially explain the observational evidence that has demonstrated better cardiovascular outcomes in psoriasis patients treated with anti-TNF therapy17. We observed a disconnect between changes in serum inflammatory markers and VI which was reported in a previous study28. Indeed, anti-TNF therapy has been associated with a decrease in systemic inflammatory markers28 and future CV events in claims database studies17. However, in the short term, anti-TNF therapy has been shown to lead to an “apolipoprotein B” shift whereby LDL and triglycerides increase following initiation of therapy. Furthermore, IL-6 levels increased following anti-TNF treatment. Therefore, it may be possible that potential beneficial effects of anti-TNF therapy on VI were offset by the pro-atherogenic shift in lipoproteins and IL-6. It may also be possible that this younger group of patients included in our study had changes in biomarkers with less profound impact compared to statin studies which included older adults with more advanced atherosclerosis. Furthermore, other vascular beds including the coronary arteries may have changed coincident to biomarker changes but were not assessed in this study. Additionally, while there is early data for the use of FDG to evaluate treatment change15, 29, untoward effects of various kinds of treatment on FDG quantification require exploration. Finally, following adalimumab or phototherapy, we did not observe change in glucose levels or insulin resistance by HOMA-IR nor in adiponectin or leptin. It is entirely possible that HOMA-IR, which detects peripheral skeletal muscle insulin resistance, but not hepatic insulin resistance did not change because insulin resistance was not affected in the skeletal muscle. Future research should consider assessment of clamp studies to understand whether hepatic insulin resistance improves.
Our results are similar to a contemporaneous placebo controlled trial of adalimumab on VI in psoriasis30 which showed no evidence of change in VI in the aorta assessed by 18F-FDG-PET/CT, albeit the specified trial showed an increase in VI in the carotids. Furthermore, our inclusion of a phototherapy arm provides us with a unique opportunity to analyze the effect of a biological treatment in relation to an established skin directed treatment modality on cardiovascular and inflammatory markers, compared to placebo. Additionally, while the previous study only explored the impact of anti-TNF therapy on VI and assessed the baseline association between a few biomarkers and VI as well as psoriasis severity, we provide a more comprehensive assessment of indices of subclinical and clinical atherosclerosis by demonstrating modulation of contemporary inflammatory, cardiometabolic and lipoprotein biomarkers compared to more standard markers assessed in the prior study.
Despite being a randomized and placebo-controlled, our study has important limitations that warrant mention. First, though we had more than 80% power per our sample size calculations based on primary outcome, our sample size was still relatively small, and our follow-up duration relatively short for cardiovascular disease study. As such, our results should be interpreted with certain caution, and future trials should consider employment of longer-duration follow-ups with expanded vascular outcomes, such as coronary CT angiography, to further test our findings. Our use of surrogate outcomes instead of hard cardiovascular endpoints limits our ability to derive definite conclusions. Furthermore, since phototherapy arm cannot be blinded, our comparisons between adalimumab and phototherapy arms in relation to placebo arm are subject to participant bias and as such our results should be interpreted with caution. The subset of 61 patients who participated in the open-label extension period provided critical one-year data suggesting no significant benefit of anti-TNF in reducing VI over one-year despite changes in biomarkers. However, one-fourth of the patients did not make it to one-year of ADA treatment, and therefore these results should be interpreted with caution. The main reason for drop out was failure of treatment response in the skin and therefore, our findings in the extension study may over estimate benefits of adalimumab. Finally, we did not evaluate VI in carotid arteries, which in some studies was suggested to be more sensitive to change29, however, a large body of literature has focused on VI in the aorta as a surrogate for atherosclerosis and overall cardiovascular disease risk14, 31.
In summary, our study demonstrates that anti-TNF therapy has strong and consistent anti-inflammatory effects in the skin and blood of patients with psoriasis in contrast to phototherapy, while both anti-TNF therapy and phototherapy had no impact on VI as assessed by 18F-FDG-PET/CT compared to placebo. Furthermore, anti-TNF therapy had no impact on glucose metabolism with potentially adverse effects on reverse cholesterol transport (a function of HDL) and HDL-particle size. These findings have important implications for our understanding of the relationship between targeted inhibition of TNF in a rigorous experimental trial of humans and its effect on cardiometabolic biomarkers and biomarkers of systemic and VI. Future experimental work is ongoing to determine the impact of biologics that target IL12/23 and IL-17 as well as a small molecule (apremilast) that targets PDE-4 on pathways interrogated in the current study. Each of these therapies carries with it a highly specific potential effect on both systemic inflammation and cardiometabolic diseases, thereby providing critically needed human data to understand other pathways at play in atherogenesis that result in premature morbidity and mortality in hundreds of millions of people worldwide.
Supplementary Material
Acknowledgments
The investigators are deeply grateful to our patient volunteers who participated in this clinical trial.
FUNDING: This study was supported by grants (NHLBI R01-HL111293, K24-AR-064310) and by an unrestricted grant from AbbVie to the Trustees of the University of Pennsylvania). Nehal N. Mehta is supported by National Institutes of Health Intramural Research Program (Z01 HL-06193). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ABBREVIATIONS
- 18F-FDG PET/CT
18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography
- PASI
Psoriasis Area and Severity Index
- HOMA-IR
Homeostasis model assessment of insulin resistance
DISCLOSURES
Dr. Mehta is a full-time US Government Employee and receives research grants to the NHLBI from AbbVie, Janssen, Celgene and Novartis.
Dr. Gelfand in the past 12 months has served as a consultant for Coherus (DSMB), Dermira, Janssen Biologics, Merck (DSMB), Novartis Corp, Regeneron, Dr. Reddy’s labs, Sanofi and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Regeneron, Sanofi, Celgene, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly and Abbvie. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma.
Dr. Takeshita receives a research grant from Pfizer Inc. (to the Trustees of the University of Pennsylvania) and has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly.
Dr. Troxel is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma.
Dr. Tyring conducts clinical studies sponsored by the following companies: Abbvie/BI; Celgene; Coherus; Dermira; Eli Lilly; Janssen; Leo; Merck; Novartis; Pfizer; Regeneron/Sanofi and Valeant. He is a speaker for Abbvie, Eli Lilly, Janssen, Leo, Novartis, Pfizer, Regeneron/Sanofi and Valeant.
Dr. Armstrong has received research grants and/or honorarium from AbbVie, Celgene, Janssen, Novartis, Eli Lilly, Regeneron, Sanofi, and Valeant and has participated in continuing medical education work related to psoriasis that was indirectly supported by Eli Lilly and AbbVie.
Dr. Duffin has received grant/research/clinical trial support from Amgen, Abbvie, Celgene, Eli Lilly, Janssen, Bristol-Myers Squibb, Stiefel, Novartis, and Pfizer over the last 24 months. Additionally, Dr. Duffin has served as a consultant/on the advisory boards for Amgen, Abbvie, Celgene, Eli Lilly, Janssen, Bristol-Myers Squibb, Stiefel, Novartis, and Pfizer.
Dr. Chiesa Fuxench has no conflicts of interest. However, she was being funded, at the time, by a research grant from the National Psoriasis Foundation and a training grant from the National Institutes of Health.
Dr. Hubbard receives grant funding from the National Institutes of Health and Patient-Centered Outcomes Research Institute.
Dr. Rader is the co-founder of Vascular Strategies and holds equity in the company.
Dr. Kalb has received grants/research funding with AbbVie, Amgen, Boehringer Ingelheim, Janssen-Ortho Inc., Merck & Co., Inc., and Novartis Pharmaceuticals Corp. over the last 24 months. During this time frame, he has also served as a consultant honoraria for Dermira, Janssen-Ortho Inc. Sun Pharmaceutical Industries Ltd. and a DSMB member honoraria for Eli Lilly and Co.
Dr. Simpson has served as a consultant for AbbVie, Anacor, Celgene, Dermira, Genentech, Leo, Glaxo Smith Kline, Pfizer, Regeneron, Sanofi-Genzyme, Menlo, and Eli Lilly in the last 24 months. During this time frame, he has also acted as the primary investigator for the following sponsored trials: Anacor, Celgene, Chugai, Dermira, Eli Lilly, Genentech, MedImmune, Merck, Novartis, Regeneron, Roivant, Tioga, and Vanda.
Dr. Torigian is the co-founder of Quantitative Radiology Solutions LLC.
Dr. Van Voorhees has served on the advisory board of Celgene, Dermira, Allergan, Merck, Pfizer, Aqua, Astra Zeneca, Jannsen, Amgen, Leo, Allergan, and Lilly. For Novartis and AbbVie, Dr. Van Voorhees acts as a consultant as well as serves on the board. Dr. Van Voorhees has received a portion of ex-spouse pension from Merck.
Dr. Menter in the last 24 months has served on the advisory board for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli-Lilly, Janssen Biotech, Inc., and LEO Pharma. He has also worked as a consultant for AbbVie, Allergan, Amgen, Eli-Lilly, Galderma, Janssen Biotech, Inc., LEO Pharma, Novartis, Pfizer, Vitae, and Xenoport. Additionally, he has acted as an investigator for AbbVie, Allergan, Amgen, Anacor, Boehringer Ingelheim, Celgene, Dermira, Eli-Lilly, Janssen Biotech, Inc., LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Symbio/Maruho, and Xenoport. He also serves as a speaker for AbbVie, Amgen, Janssen Biotech, Inc., and LEO Pharma. He has received compensation in the form of grants from AbbVie, Allergan, Amgen, Anacor, Boehringer Ingelheim, Celgene, Dermira, Janssen Biotech, Inc., LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Symbio/Maruho, and Xenoport. He has also received honoraria from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli-Lilly, Galderma, Janssen Biotech, Inc., LEO Pharma, Novartis, Pfizer, Vitae, and Xenoport.
All other authors have no conflict of interest.
Footnotes
Clinical Trial Registration Information
URL: https://clinicaltrials.gov/ct2/show/NCT01866592?term=vascular+inflammation+in+psoriasis&rank=1
UID: NCT01866592, NCT01553058
References
- 1.Goff KL, Karimkhani C, Boyers LN, Weinstock MA, Lott JP, Hay RJ, Coffeng LE, Norton SA, Naldi L, Dunnick C, Armstrong AW, Dellavalle RP. The global burden of psoriatic skin disease. The British journal of dermatology. 2015;172:1665–1668. doi: 10.1111/bjd.13715. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 3.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. European heart journal. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, Raper A, Wilcox M, Baer A, DerOhannesian S, Wolfe M, Reilly MP, Rader DJ, VanVoorhees A, Gelfand JM. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–221. doi: 10.1016/j.atherosclerosis.2012.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, Shin DB, Attor R, Troxel AB, Gelfand JM. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA dermatology. 2013;149:1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, Margolis DJ, Strom BL. The risk of mortality in patients with psoriasis: results from a population-based study. Archives of dermatology. 2007;143:1493–1499. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 7.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY, Gottlieb AB. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 8.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, Krishnamoorthy P, Ng Q, Aridi TZ, Salahuddin T, Natarajan B, Lockshin BN, Ahlman MA, Chen MY, Rader DJ, Reilly MP, Remaley AT, Bluemke DA, Playford MP, Gelfand JM, Mehta NN. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circulation research. 2016;119:1242–1253. doi: 10.1161/CIRCRESAHA.116.309637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta NN, Li K, Szapary P, Krueger J, Brodmerkel C. Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. Journal of translational medicine. 2013;11:194. doi: 10.1186/1479-5876-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, Ng Q, Joshi AA, Krishnamoorthy P, Dave J, Rose SM, Doveikis J, Playford MP, Prussick RB, Ehrlich A, Kaplan MJ, Lockshin BN, Gelfand JM, Mehta NN. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connelly MA, Otvos JD, Shalaurova I, Playford MP, Mehta NN. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. Journal of translational medicine. 2017;15:219. doi: 10.1186/s12967-017-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 13.Harrington CL, Dey AK, Yunus R, Joshi AA, Mehta NN. Psoriasis as a human model of disease to study inflammatory atherogenesis. American journal of physiology Heart and circulatory physiology. 2017;312:H867–H873. doi: 10.1152/ajpheart.00774.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U, Tawakol A. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovascular imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. Journal of the American College of Cardiology. 2013;62:909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb AB, Kalb RE, Langley RG, Krueger GG, de Jong EM, Guenther L, Goyal K, Fakharzadeh S, Chevrier M, Calabro S, Langholff W, Menter A. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. Journal of drugs in dermatology : JDD. 2014;13:1441–1448. [PubMed] [Google Scholar]
- 17.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Archives of dermatology. 2012;148:1244–1250. doi: 10.1001/archdermatol.2012.2502. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 19.Zanolli MD, Feldman SR. Phototherapy treatment protocols: for psoriasis and other phototherapy responsive dermatoses. New York: Taylor & Francis; 2004. [Google Scholar]
- 20.Chen W, Bural GG, Torigian DA, Rader DJ, Alavi A. Emerging role of FDG-PET/CT in assessing atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2009;36:144–151. doi: 10.1007/s00259-008-0947-2. [DOI] [PubMed] [Google Scholar]
- 21.Bural GG, Torigian DA, Chamroonrat W, Houseni M, Chen W, Basu S, Kumar R, Alavi A. FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2008;35:562–569. doi: 10.1007/s00259-007-0528-9. [DOI] [PubMed] [Google Scholar]
- 22.Padfield GJ, Din JN, Koushiappi E, Mills NL, Robinson SD, Cruden Nle M, Lucking AJ, Chia S, Harding SA, Newby DE. Cardiovascular effects of tumour necrosis factor alpha antagonism in patients with acute myocardial infarction: a first in human study. Heart. 2013;99:1330–1335. doi: 10.1136/heartjnl-2013-303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC, Rudd JH, Wilkinson IB. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126:2473–2480. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 24.Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, Teague HL, Harrington CL, Rivers JP, Chung JH, Kabbany MT, Natarajan B, Silverman JI, Ng Q, Sanda GE, Sorokin AV, Baumer Y, Gerson E, Prussick RB, Ehrlich A, Green LJ, Lockshin BN, Ahlman MA, Playford MP, Gelfand JM, Mehta NN. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol. 2017;2:1013–1018. doi: 10.1001/jamacardio.2017.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ American College of Cardiology/American Heart Association Task Force on Practice G. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2960–2984. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pircher J, Merkle M, Wornle M, Ribeiro A, Czermak T, Stampnik Y, Mannell H, Niemeyer M, Vielhauer V, Krotz F. Prothrombotic effects of tumor necrosis factor alpha in vivo are amplified by the absence of TNF-alpha receptor subtype 1 and require TNF-alpha receptor subtype 2. Arthritis research & therapy. 2012;14:R225. doi: 10.1186/ar4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duivenvoorden R, Mani V, Woodward M, Kallend D, Suchankova G, Fuster V, Rudd JH, Tawakol A, Farkouh ME, Fayad ZA. Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study. JACC Cardiovascular imaging. 2013;6:1087–1094. doi: 10.1016/j.jcmg.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A dal PI. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, Maari C, Delorme I, Lynde CW, Tardif JC. TNF-alpha Antagonist and Vascular Inflammation in Patients with Psoriasis Vulgaris: A Randomized Placebo-Controlled Study. The Journal of investigative dermatology. 2017;137:1638–1645. doi: 10.1016/j.jid.2017.02.977. [DOI] [PubMed] [Google Scholar]
- 31.Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, Agostini D, Ubleis C, Gimelli A, Hacker M Cardiovascular Committee of the European Association of Nuclear M. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. European journal of nuclear medicine and molecular imaging. 2016;43:780–792. doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.