Abstract
Purpose
Multiple myeloma (MM) remains an incurable malignancy of plasma cells despite considerable advances in treatment. The purpose of the study was to develop novel CARs for the treatment of MM and explore combinatorial therapy using CAR T cells and immunomodulatory drugs such as lenalidomide for increasing treatment efficacy.
Experimental design
We redirected central memory T cells to express second-generation CAR specific for CS1 and adoptively transferred them into MM tumor-bearing mice to test their anti-MM activity. CS1 CAR T cells were transduced and expanded in the presence of lenalidomide in vitro. The phenotype and effector function of CS1 CAR T cells treated with and without lenalidomide were compared. Finally, CS1 CAR T cells and lenalidomide were administered to treat MM bearing mice as combinatorial therapy.
Results
CS1 CAR T cells exhibited efficient antitumor activity when adoptively transferred into mice. Mechanistic studies indicated that the addition of lenalidomide during CS1 CAR T cell expansion in vitro enhanced the immune functions of CS1 CAR T cells, including cytotoxicity, memory maintenance, Th1 cytokine production, and immune synapse formation. Furthermore, lenalidomide enhanced the anti-tumor activity and persistence of adoptively transferred CS1 CAR T cells in vivo.
Conclusions
The study demonstrates that lenalidomide improves the anti-MM properties of CS1-directed CAR T cells and provides a basis for a planned clinical trial using the combination of lenalidomide with engineered T cells against CS1 in relapsed myeloma.
Keywords: lenalidomide, immunomodulation, CS1, CAR T cells, multiple myeloma
Introduction
Dramatic clinical responses have been reported recently using Chimeric Antigen Receptor (CAR) T cells specific for the B cell antigen CD19, in patients with chronic and acute lymphocytic leukemia (1, 2). Similar strategies have been explored in MM using CD19 as well as other targets such as BCMA (3, 4). In our study, we examine the target CS1 and demonstrate that combining immunomodulatory drugs (IMiDs) with adoptive cellular therapy (ACT) has the potential for synergistic effects via direct actions on the tumor cells together with IMiD stimulation of CAR T cells.
CS1 is a cell surface glycoprotein of the signaling lymphocyte activation molecule (SLAM) receptor family that is highly and selectively expressed on normal plasma cells and MM cells, with lower expression on natural killer (NK) cells and no expression on other normal tissue (5). CS1 is highly expressed in over 95% of cases of MM irrespective of cytogenetic abnormalities and throughout the disease process (5). The high expression of CS1 on MM cells coupled with the lack of or low expression in normal and essential tissues makes CS1 a reasonable target for CAR T cell therapy against MM.
IMiDs, e.g. lenalidomide, as a primary treatment modality for MM not only yields direct anti-MM effects but also co-stimulates T cells. Both in vitro and in vivo studies demonstrated that lenalidomide enhances tumor specific Th1 cytokines such as IL-2 and IFNγ (6, 7), preferentially stimulates CD8+ T cells (8), and inhibits IL-2-mediated generation of Tregs (9).
In the studies reported here, we investigated the effects of lenalidomide on CS1 CAR T cells in vitro and the anti-MM activity in a xenograft mouse model.
Materials and Methods
Antibodies and Flow Cytometry
Fluorochrome-conjugated isotype controls against CD3, CD4, CD8, PD1, Tim3, CD28, CD27, CD62L, CD127, CD107a, IFNγ, TNFα, and IL-2 and streptavidin-PE (SA-PE) were obtained from BD Biosciences. The antibody against CS1 was purchased from R&D Systems. Biotinylated Erbitux was generated from cetuximab purchased from the City of Hope pharmacy. All monoclonal antibodies were used according to the manufacturer's instructions. The percentage of cells in a region of flow cytometric analysis was calculated using FCS Express V4 (De Novo Software).
Lentivirus Vector Construction
The lentivirus CAR construct was modified from the previously described CD19-specific scFvFc:ζ chimeric immunoreceptor (10). The CS1 CAR contained a CS1-specific scFv (CS1R), a CD28z costimulatory domain, an IgG4-Fc spacer with a CH2 deletion to ensure enhanced potency and persistence (11), and a truncated human epidermal growth factor receptor (huEGFRt), which includes a cetuximab (Erbitux) binding domain. A T2A ribosome skip sequence links the codon-optimized CS1R:CD28:z sequence to the huEGFRt sequence, resulting in coordinate expression of both CS1R:CD28:z and EGFRt from a single transcript (CS1CARCD28EGFRt) (12). The CS1CARCD28EGFRt DNA sequence (optimized by GeneArt) was then cloned into a self-inactivating (SIN) lentiviral vector, pHIV7 (gift from Jiing-Kuan Yee, City of Hope).
Cell Lines
To generate LCL OKT3, allogeneic LCLs (EBV-transformed lymphoblastoid cell lines) were resuspended in nucleofection solution using the Amaxa Nucleofector kit T. OKT3-2A-Hygromycin_pEK plasmid was added to 5 mg/107 cells, the cells were electroporated using the Amaxa Nucleofector I, and the resulting cells were grown in RPMI-1640 with 10% fetal calf serum (FCS) containing 0.4 mg/mL hygromycin. MM.1s was cultured in RPMI-1640 with 10% FCS, and KG1a was cultured in IMDM with 10% FCS. To generate firefly luciferase+GFP+MM.1s (fflucGFPMM.1s), MM.1S purchased from ATCC were transduced with lentiviral vector encoding eGFP-ffluc. Initial transduction efficiency was 50%, so the GFP cells were sorted by FACS for >98% purity. U266B and MM.1R cells were purchased from ATCC. Banks of all cell lines were authenticated for the desired antigen/marker expression by flow cytometry prior to cryopreservation, and thawed cells were cultured for less than 6 months prior to use in assays. Bone marrow cells were obtained from patients with MM under protocols approved by the City of Hope Institutional Review Board.
Generation of MM Specific T Cells
Leukapheresis products were obtained from healthy donors under protocols approved by the City of Hope Institutional Review Board. Central memory T cells defined as CD45RO+CD62L+ were isolated on Miltenyi autoMACS (Miltenyi Biotec Inc). Briefly, PBMC were isolated by density gradient centrifugation over Ficoll-Paque (GE Healthcare) and incubated with anti-CD14 and anti-CD45RA microbeads (Miltenyi Biotec Inc) for 30 minutes at room temperature (RT). CD14+ and CD45RA+ cells were then immediately depleted using the autoMACS. The unlabeled negative fraction of cells was labeled with biotinylated-DREG56 mAb (COHNMC CBG) followed by incubation with anti-biotin microbeads (Miltenyi Biotec Inc). The CD62L+ TCM were purified with positive selection on auotMACS. Immediately after selection, the TCM were activated, transduced, and maintained as described (13). Cultures were supplemented with lenalidomide every 48 hours for 21 days before in vitro analysis.
Cytokine Production
5 × 105 CS1 CAR T cells were co-cultured overnight with 5 × 105 LCL OKT3, MM.1S or KG1a cells in 96-well tissue culture plates. The supernatant was collected, and cytokines were measured with Bioplex cytokine analysis (10-plex kit) (in triplicates) according to the manufacturer's instructions.
Intracellular Cytokine Staining
5 × 105 CS1 CAR T cells were activated overnight with 5 × 105 LCL OKT3, MM.1S, MM.1R, U266B or KG1a cells in 96-well tissue culture plates in the presence of brefeldin A (BD Biosciences). The cell mixture was then stained using anti-CD8, anti-CD4, and biotinylated Erbitux/streptavidin to analyze surface co-expression of CD8, CD4 and CAR, respectively. Cells were then fixed and permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences). After fixation, the T cells were stained with antibodies against INFγ, TNFα and IL-2. Cells were then analyzed using multicolor flow cytometry on MACSQuant (Miltenyi Biotec Inc.). In some experiments, co-cultures were set up in the presence of CS1 protein (BioVision).
Degranulation
5 × 105 CS1 CAR T cells were co-cultured with 2.5 × 105 LCL OKT3, MM.1S, MM.1R, U266B or KG1a cells in RPMI T cell medium containing Golgi stop (BD Biosciences) and CD107a for six hours at 37°C. Degranulation was assessed using multicolor flow cytometry on MACSQuant. In some experiments, co-cultures were set up in the presence of CS1 protein (BioVision).
Cytotoxicity Assay
Four-hour luciferase based cytotoxicity assays (LCA) were performed as previously described (14) using CS1 CAR T cells as effector cells and CS1 expressing MM.1S as targets.
Side Population Assay (Hoechst Stain)
MM.1s cells were treated with different concentrations of lenalidomide (0, 1, and 10 μM) for 48 hours. Following treatment, cells were washed and resuspended in RPMI at a concentration of 10 × 106 cells/mL. Hoechst dye (Sigma) was added to suspension to achieve a final concentration of 5 μg/mL. Cells were incubated 2 hours with gentle vortexing every 30 minutes. Cells were then spun down at 2000 rpm for 2 minutes, and media were replaced with HBSS. 5 μg/mL propidium iodide was added, and cells were incubated 5 min at 4°C (15, 16) Analysis was conducted on MACSQuant.
Immune Synapse Staining
In an 8-well chamber, 60,000 MM.1S tumor cells were labeled with CellTracker Blue (Life Technologies) to visually distinguish from T cells and seeded in each well. They were co-cultured with the same amount of CS1 CAR T cells, which had been treated with different concentrations of lenalidomide. After 2 hour culture at 37°C and 10% CO2, the co-culture was washed with PBS three times and cells were fixed for 10 minutes with 4% paraformaldehyde (PFA) at 4°C, followed by air dry for 10 minutes, and another wash with PBS.
In order to detect CAR, the cells were blocked by 0.5% FCS PBS for 1 hour, then 1:150 diluted Fab antibody (goat anti mouse, Sigma, F4018) was added and incubated overnight at 4°C. After washing with 0.5% FCS PBS three times, the cells were stained with secondary antibody against goat (L+H) conjugated with Alexa488 for 1 hour at room temperature (RT). The F-Actin Visualization Kit (Biochem Kit™) was used according to the manufacturer’s instructions. Briefly, the cells were washed and permeabilized for 5 minutes at RT. After one wash with washing buffer, cells were stained with 200 μL working stock Rhodamine Phalloidin and incubated for 30 minutes at RT in the dark. After 3 washes, slides were covered by mounting medium. Each sample was duplicated and stained. For FACS based immune synapse formation analysis (17, 18), GFP+ MM.1S or GFP+KG1a cells were co-cultured with the same number of Erbitux- streptavidin-PE (SA PE) stained CS1 CAR T cells that had been treated with different concentrations of lenalidomide. After 30 min incubation, co-cultures were washed, and GFP and CAR double positive cells were quantified with flow cytometry.
Quantitative Image Analysis of Immune Synapse
Images were taken under microscope (Zeiss observe II, x40 oil). To quantify immune synapses, entire slides were scanned, and the area of F-actin and Fab conjugation were measured using software (Image propremier 9.2) by setting up the single staining F-actin, Fab, and CellTracker as background. The areas of cell conjugation (yellow-golden brown) were selected for measurement as polarization of synapse. The exported areas (pixel2) were further analyzed and converted to average pixel2 for entire slides.
Sequencing Library Preparation, Deep Sequencing Using Illumina HiSeq2500 and RNA-Seq Data Analysis
The CD8+CAR+ T cells treated with 0, 1, and 10 μM of lenalidomide were purified by FACS sorting after co-staining with antibodies against EGFR and CD8. The total RNA was isolated using RNeasy Plus Micro and Mini Kits according to protocol. RNA integrity was verified with the Agilent 2100 Bioanalyzer. The RNA sequencing libraries were prepared using SMARTer Stranded Total RNA-Seq Pico input Kit (Clontech). Briefly, 10 ng of DNA-free total RNA was reverse transcribed by SMARTScribe™ Reverse Transcriptase with random priming. The enzyme’s terminal transferase activity results in addition of a few non-templated nucleotides to the 3’ end of the cDNA, creating an extended template to enable the reverse transcriptase to continue replicating to the end of the oligonucleotide. The first round of PCR amplification added full-length Illumina adapters, including barcodes. The ribosomal cDNA (originating from rRNA) was then cleaved by ZapR in the presence of the mammalian-specific R-Probes. The rRNA-depleted cDNA were enriched via a second round of PCR amplification using primers universal to all libraries. The sequencing libraries were validated with running Agilent bioanalyzer DNA high sensitivity Chip, and quantified using Qubit and qPCR. The library templates were prepared for sequencing using cBot cluster generation system with HiSeq PE Cluster Kit V4 (Illumina). Sequencing runs were performed in the paired end mode of 101 cycles of read1, 7 cycles of index read and 101 cycles of read2 using HiSeq2500 platform with HiSeq SBS Kit V4 (Illumina). Real-time analysis (RTA) 2.2.38 software was used to process the image analysis and base calling.
qPCR analysis
The CD8+CAR+ T cells treated with 0, 1, or 10 μM of lenalidomide were purified by FACS sorting after co-staining with antibodies against EGFR and CD8. The cells from three different donors were purified and analyzed. The cDNA was used for Taqman qPCR (7500 FAST, Life Technology). The Taqman probes used in this study are listed in Supplemental Table 1. The gene expression levels are presented as 2^delta Ct.
Xenograph Models
All mouse experiments were approved by the City of Hope Institutional Animal Care and Use Committee. Six- to ten-week old NOD/Scid IL2RγCnull mice were injected intratibially on day 0 with 2 × 106 fflucGFP MM.1S cells. Five days later, mice were injected i.v. with dosed 1 × 106 CAR T cells or non-transduced mock cells. For experiments using lenalidomide, mice were administered 5-7.5 mg/kg of lenalidomide i.p. daily for 30 days. Anesthetized mice were imaged using a Xenogen IVIS 100 series system (Xenogen, Alameda, CA). Photons from ffLuc+ tumor xenografts were quantified using the software program Living Image (Xenogen), and the bioluminescence signal was measured as total photon flux normalized for exposure time and surface area and expressed in units of photons per second per cm2 per steradian. Human T-cell engraftment in peripheral blood, bone marrow, and spleen was determined by flow cytometry after staining with antibody against human CD45, CD8 and Erbitux for CAR detection.
Statistical Analysis
Analyses were performed using Prism (GraphPad Software Inc.). Log-rank (Mantel-Cox) test and Mann-Whitney t- test were used to ascertain the statistical significance of the in vivo data. The paired t-test (2-tailed) and two-way ANOVA were used for the analysis of in vitro data.
Results
CS1 is Highly Expressed on MM Cells and Primary MM Bone Marrow Cells
We conducted flow cytometry to characterize surface CS1 expression on MM cells. MM cell line MM.1S cells are highly (70-80%) CS1-positive. We also assessed antigen expression on bone marrow (BM) mononuclear cells from patients with newly diagnosed or relapsed MM. Consistently, primary MM cells across patients express high levels of CS1 (Figure 1A).
Figure 1. TCM-derived CS1 CAR T cells exhibit specific effector function and anti-MM activity in vivo.
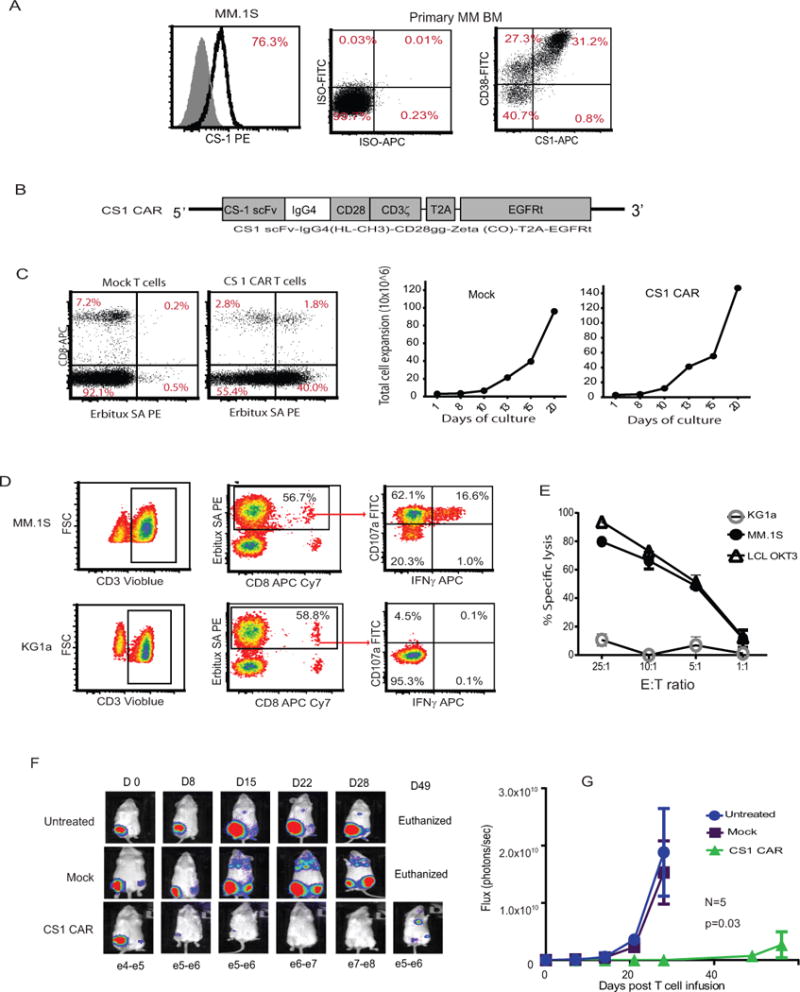
(A) MM cell line MM.1S and BM mononuclear cells from patients with MM were stained with fluorochrome-conjugated isotype controls and antibodies specific to CS1 and CD38. The percentages of positive cells are presented after exclusion of dead cells with DAPI. Representative data of ten MM patients’ BM are presented. (B) Schematics of CS1 and lentiviral CAR constructs, composed of an antigen-specific scFv, IgG4 hinge region, and a CD28 costimulatory domain. The IgG4 hinge region was shortened by deleting the CH2 portion. The CAR sequence is followed by a T2A ribosomal skip sequence and the coding sequence for the EGFRt tracking/suicide gene. (C) The selected central memory cells (TCM) were transduced with the second generation CS1 CAR following CD3/CD28 bead activation and expanded in the presence of rhIL-2 (50 U/mL) and IL-15 (0.5ng/mL) for 3 weeks. CAR expression was defined by Erbitux-biotin and streptavidin (SA)-PE staining. Percentages of CAR+ cells are indicated in each quadrant on the basis of gating of cells stained with SA-PE alone. Non-transduced TCM were used as control. Growth of total cell number was determined by Guava Viacount at different time points. (D) Expanded CS1 CAR T cells were co-cultured with MM.1S cells at a 1:1 ratio in medium containing Golgi plug and CD107a for six hours at 37°C. KG1a cells were used as negative stimulator. Degranulation was determined using multicolor flow cytometry. Percentages of CD107a+ and intracellular IFNγ+cells from gated Erbitux (CAR)+ T cells are presented. (E) CS1 T cells as effector cells were co-cultured with luciferase-expressing MM.1S as targets. After 4 hrs incubation, luminescence flux was read following addition of substrate luciferin. LCL OKT3 were used as a positive control and myeloid leukemic cells KG1a as a negative control. Representative data from six different donors are presented in C through E. (F) A total of 2 × 106 fflucGFP MM.1S cells were intratibially (i.t.) injected into NSG mice. Five days following tumor inoculation, mice were injected i.v. with dosed 1 × 106 CAR T cells or non-transduced mock cells. Tumor signals were monitored with Xenogen imaging once a week. (G) The bioluminescence signal was measured as total photon flux normalized for exposure time and surface area and expressed in units of photons (p) per second per cm2 per steradian (sr). Representative data from six separate in vivo experiments are presented (N=5 mice per group in each experiment).
Generation of TCM-derived CS1 CAR T Cells against MM
We isolated central memory T cells (TCM) from healthy donors, followed by activation and transduction with a lentivirus (13) encoding second-generation CAR specific for CS1, containing CD28 as costimulatory molecule and EGFRt as a potential molecule for detection, selection, and in vivo ablation (12) (Figure 1B). Engineered CS1 CAR T cells (Figure 1C) exhibited similar expansion in vitro (Figure 1C), comparable to the non-transduced mock T cells.
CS1 CAR T Cells Mediate Efficient Effector Function and Cytolytic Activity
To test the effector function of CS1 CAR T cells, we co-cultured expanded CAR T cells with MM.1S cells for 6 hours for degranulation assay and cytokine secretion determination. Gated CS1 CAR T cells express CD107a as an indicator of degranulation and IFNγ upon antigen stimulation (Figure 1D). We then co-cultured CAR T cells with luciferase-expressing MM.1S target cells for 4 hours; specific lysis was analyzed after adding the substrate luciferin (Figure 1E). CS1 CAR T cells exhibited specific and efficient killing of MM cells.
CS1 CAR T Cells Exhibit Efficient Antitumor Activity in vivo
A total dose of 1 × 106 CAR T cells specific for CS1 derived from TCM were intravenously transferred into MM.1S tumor-bearing mice. CS1 CAR T cells exhibited significantly higher antitumor activity as compared to mock T cells (P=0.03) (Figures 1F and G).
To further study whether tumor relapse is due to the low dose of CAR T cells, we increased the CS1 CAR T cell dose three-fold (3 × 106) with the same tumor burden. Tumor recurrence was not delayed (Figure S1), which suggested that other mechanisms were at play in regards to disease relapse.
Soluble CS1 Does Not Interfere with CS1 CAR Function
MM cells also secrete soluble forms of CS1 and BCMA (19, 20). Other investigators have previously shown that CAR-expressing T cells were not blocked from recognizing target cells by soluble proteins, including BCMA (21). CS1 concentrations of >10 times the median CS1 levels found in the serum of patients with MM did not block recognition of MM cells by CS1 CAR T cells, as indicated by equivalent CD107a degranulation and IFNγ production (Figure S2A and 2B). Furthermore, we analyzed cytolytic activity by adding graded concentrations of CS1 protein during the 4 hour luciferase based cytolytic assay (LCA). Again, soluble CS1 protein did not interfere with CS1 CAR-specific lysis (Figure S2C).
Lenalidomide Exerts a Costimulatory Effect on CS1 CAR T Cells in a Dose-dependent Fashion
Lenalidomide has known activity in the activation and expansion of normal T cells (22). We engineered and expanded CS1 CAR T cells in the presence of 0, 1 and 10 μM lenalidomide. We found that lenalidomide preferentially expanded the CD8+ CAR T cell subset in a dose-dependent manner (Figure 2A) along with increased CAR and total T cell expansion, but decreased the proportion of CD4+ T cells, irrespective of the different proportions of CD4+ and CD8+ T cells in the starting populations across different donors (Figures S3 and S4). The skew toward CD8+ cells became more dramatic when cultures were extended for >3 weeks (Figure 2A). CAR+ T cells, especially CD8+ CAR T cells, exhibited comparable levels of memory T cell signatures such as CD62L, CD127, CD27 and CD28 in the presence and absence of lenalidomide and maintained a relatively minor exhaustive phenotype as indicated by low expression of PD1 and Tim3 among both lenalidomide treated and untreated CAR T cells (Figure 2B). Th1 cytokines are critically important for the induction of antitumor cellular immunity in vivo (23), and lenalidomide has been shown to polarize toward the Th1 phenotype (24). We also demonstrated that lenalidomide-treated CS1 CAR T cells exhibited higher Th1 cytokine production, including IFNγ and TNFα, as measured in both the supernatant of co-cultures and cell-based intracellular cytokine analysis (Figures 2C, and D) in a dose-dependent manner. Interestingly, autocrine IL-2, the major determinant of CAR T cell potency and persistence, was greatly elevated in the CS1 CAR T cells treated with lenalidomide. However, the production of T cell-immune suppressive Th2 cytokines (e.g., IL-5, IL-10, and IL-4) was greatly reduced (Figure 2C). Lenalidomide exhibited a costimulatory function similarly on CD8+CAR+ and CD4+CAR+ T cells (Figures 2D). To prove these results are independent of MM cell lines, we tested two other MM cells, MM.1R and U266B, representing CS1 high and low expressers (Figures 3A). Upon stimulation with targets, CS1 CAR T cells express CD107a and secrete IFNγ and TNFα against all the MM cells tested. The effector functions are correlated with the CS1 expression levels on the targets (Figures 3B). Consistently, lenalidomide treated CS1 CAR T cells exhibited better effector function against different MM targets in a dose dependent manner (Figures 3C).
Figure 2. Lenalidomide exerts a costimulatory effect on CS1 CAR T cells in a dose-dependent fashion.
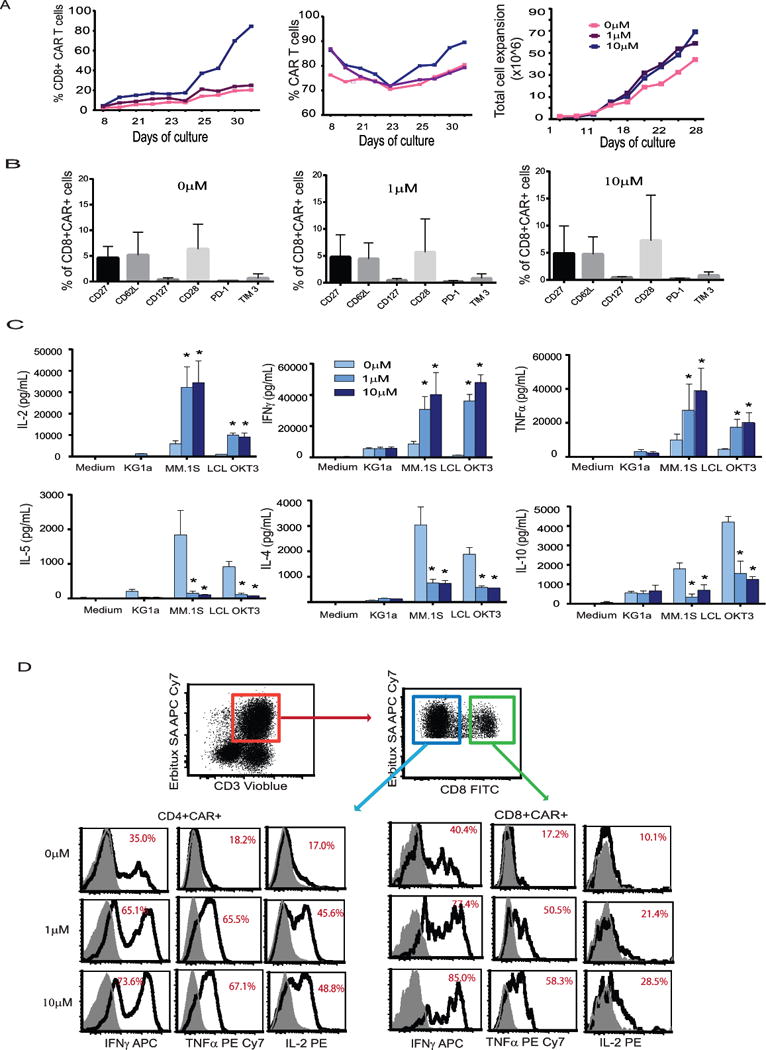
Central memory T cells were activated and transduced with lentivirus encoding CS1 CAR and were expanded in vitro in the presence of 0, 1, and 10 μM lenalidomide for 3-4 weeks. (A) Growth of total cell number was determined by Guava Viacount at different time points. Cells were stained with antibodies against CD8 and EGFRt, and percentages of positive cells at different time points are presented. (B) After 3 weeks of in vitro expansion, cells were labeled with indicated antibodies. Percentages of positive cells under CD8+CAR+ gating are presented. Means+SEM from three donors are presented. (C) 5 × 105 CS1 CAR T cells treated with different concentrations of lenalidomide were co-cultured overnight with 5 × 105 LCL OKT3, MM.1S or KG1a cells. The supernatant was collected, and cytokines were measured with Bioplex cytokine analysis from triplicates. Two-way ANOVA test was used. Representative data of two donors are presented. (D) 5 × 105 CS1 CAR T cells were activated overnight with 5 x105 MM.1S cells in the presence of brefeldin A. The cell mixture was then stained using Erbitux and streptavidin followed intracellular staining with antibody against INFγ, TNFα, and IL-2. Cytokine positive cells under indicated gating are presented. Representative data from three different donors are presented.
Figure 3. Lenalidomide exerts a costimulatory effect on CS1 CAR T cells against different MM cells.
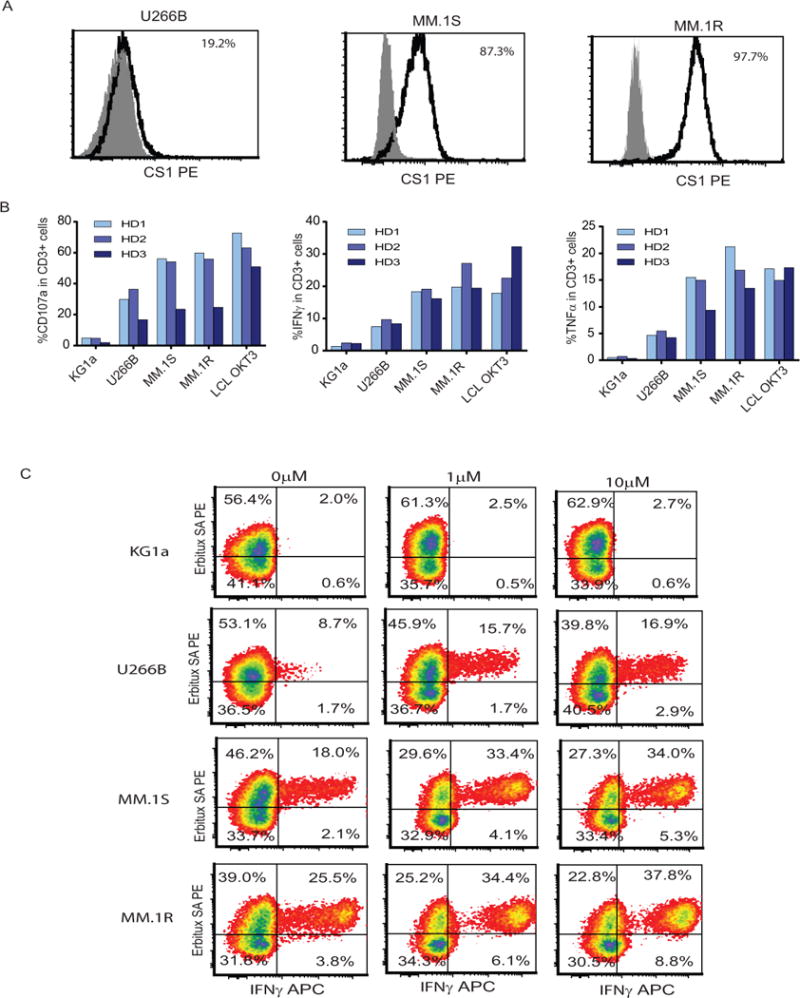
(A) CS1 expression on U266B, MM.1S and MM.1R. MM cells were analyzed by staining with fluorochrome-conjugated isotype controls and antibodies specific to CS1. The percentages of positive cells over isotype controls (filled grey) are presented after exclusion of dead cells with DAPI. (B) Expanded CS1 CAR T cells were co-cultured with U266B, MM.1S and MM.1R cells at a 1:1 ratio in medium containing Golgi plug and CD107a for six hours at 37°C. 5 × 105 CS1 CAR T cells were activated overnight with 5 x105 U266B, MM.1S and MM.1R cells in the presence of brefeldin A. The cell mixture was then stained using Erbitux and streptavidin followed intracellular staining with antibody against INFγ and TNFα. Percentages of CD107a positive and cytokine positive cells from the CD3+ population were determined using multicolor flow cytometry. KG1a cells were used as negative stimulator and LCL OKT3 cells as positive stimulator. Data from three different donors are presented. (C) Central memory T cells were activated and transduced with lentivirus encoding CS1 CAR and were expanded in vitro in the presence of 0, 1, and 10 μM lenalidomide for 3-4 weeks. 5 × 105 propagated CS1 CAR T cells were activated overnight with 5 x105 U266B, MM.1S and MM.1R cells in the presence of brefeldin A. The cell mixture was then stained using Erbitux and streptavidin followed intracellular staining with antibody against INFγ. Cytokine positive cells under CD3 gating are presented. Representative data from three different donors are presented.
Lenalidomide Eradicates MM-initiating Cells without Downregulating CS1 Expression on MM Cells
We assayed the sensitivity of MM.1S cells to lenalidomide as well as the maintenance of surface CS1 during lenalidomide treatment. The overall growth of MM.1S cells was greatly inhibited by the addition of lenalidomide every other day in the culture, indicating that MM.1S cells are sensitive to lenalidomide in vitro (Figure S5A). We performed flow cytometry-based Hoechst staining to evaluate the existence of a MM tumor-initiating population with stem-like features known as side population cells (SP). Forty-eight hours after treatment of CS1 CAR T cells, a dramatic decrease of SP was observed in the treated MM.1 S cells (Figure S5B). Meanwhile, lenalidomide did not downregulate CS1 expression on the MM.1S cells, assuring intact antigen for CAR T cell targeting (Figure S5C).
Lenalidomide Improves Immune Synapse Formation between CAR T Cells and Tumor Cells
The term immunological synapse (IS) refers to the organization of membrane proteins that occurs at the interface between the T cell and the target cells when contact is made during the effector phase (25). The enhancement of immune function by lenalidomide on CAR T cells may be related to increased immune synapse formation. We co-cultured CAR T cells that had been treated with lenalidomide with target cells for 2 hours. The areas of cell conjugation and polarization between Fab positive CAR T cells and CellTracker labeled tumor cells were defined as immune synapses and were quantified using average pixel2. We also directly measured the numbers of conjugates as a result of immunological synapse formation between CS1 CAR T cells and MM.1S target cells using flow cytometry (18). Consistently, immune synapse formation was found to be significantly increased with the lenalidomide-treated CS1 CAR T cells as compared with untreated CS1 CAR T cells (Figures 4A, B, C and Figure S6A). No immune synapse formation was observed when CS1 CAR T cells were co-cultured with KG1a or mock T cells were co-cultured with MM.1S cells (Figure 4D and Figure S6B and S6C). In concert with these findings, cytolytic activity was increased with lenalidomide-treated CAR T cells in a dose-dependent fashion, which became especially apparent when a low E:T ratio was used (Figures 4E, and F). These data support that enhanced immune synapse formation is associated with the improved cytolytic activity of CAR T cells.
Figure 4. Lenalidomide improves immune synapse formation between CAR T cells and tumor cells.
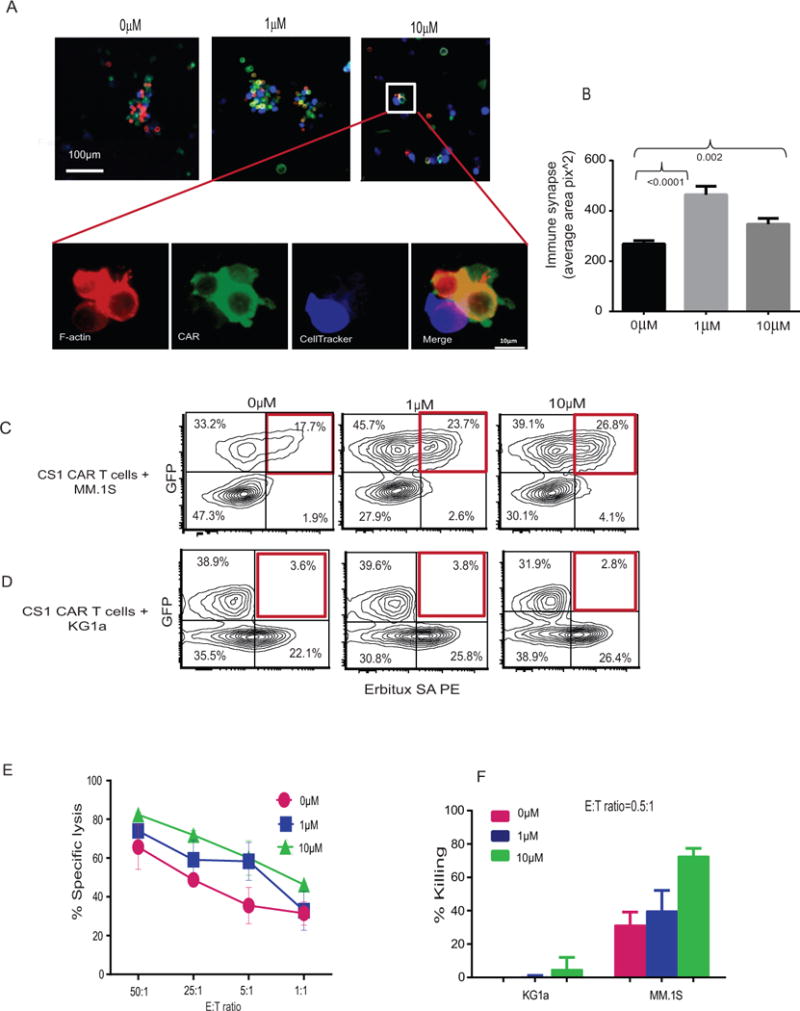
MM tumor cells labeled with CellTracker Blue were co-cultured with the same number of CS1 CAR T cells treated with different concentration of lenalidomide. After 2 hours incubation, co-cultures were washed and fixed with 4% paraformaldehyde (PFA). In order to detect CAR, the cells were stained with Fab antibody followed by secondary antibody conjugated with Alexa 488. The F-actin was labeled in red. (A) The images were taken under microscope. (B) To quantify immune synapses, entire slides were scanned and the area of F-actin and Fab conjugation were measured using Image propremier 9.2 software by setting up single staining F-actin, Fab, and CellTracker as background. The areas (yellow-golden brown) of cell conjugation and polarization for synapse were selected for measurement. Statistical analysis was performed with 2-tailed paired-t test. P values are indicated. (C and D) In FACS based synapse analysis, GFP+ MM.1S cells or GFP+KG1a were co-cultured with the same number of Erbitux SA-PE stained CS1 CAR T cells that had been treated with different concentrations of lenalidomide. After 30 min incubation, co-cultures were washed, and GFP and CAR double positive cells were quantified with flow cytometry. (E) CS1 CAR T cells treated with different concentrations of lenalidomide were co-cultured with luciferase-expressing MM.1S for 4 hours. Specific lysis was analyzed after adding substrate luciferin. Average from three replicates are depicted. (F) CS1 CAR T cells treated with different concentrations of lenalidomide were co-cultured with MM.1S at a E:T ratio (Effector:Target) of 0.5 to 1 for 24 hours. Percent killing was analyzed and calculated on the basis of flow cytometric analysis of the tumor cells remaining in the culture. Average killing from two separate experiments of two donors are presented.
Lenalidomide Enhances Transcriptional Signatures of Immune Synapse and T Cell Function
We sought to compare the transcriptome of the CAR T cells treated with and without lenalidomide. RNAseq gene expression analysis was conducted with purified CD8+CAR+ T cells (>90%) (Figure 5A). More than 600 genes were differentially expressed (fold change > 2) among the lenalidomide-treated and untreated CD8+CAR+ T cells (Figure 5B) in a dose-dependent manner (389 progressively upregulated and 276 downregulated). (Full gene names are listed in Supplemental Tables 2, 3). Among these genes, immune synapse-related genes such as cell junction and biological assembly-related genes are significantly increased with lenalidomide treatment (P = 4.55 × 10−4 - 2.08 × 10−7) (Figure 5C, Supplemental Table 4). Moreover, lenalidomide causes global changes in gene transcripts that are characteristic of improved stem memory, homing and effector function of CAR T cells. Genes associated with stem memory or less differentiated phenotypes, such as IL-7, ACTIN1, KIT, WNT5B, IL-9, and MYB (26, 27), are up-regulated together with genes associated with enhanced effector function, such as IRF4 (28) and IFI44 (29). Lenalidomide-treated CS1 CAR T cells have elevated gene signatures that are related to better homing capacity such as integrins ITGA4 and ITGB3 (30), which all responded in a dose-dependent manner (Figure 5D). In contrast, exposure to lenalidomide suppresses genes in CAR T cells that are associated with exhaustion such as PDCD1, CD160 (31, 32), terminal differentiation genes such as GZMK and GZMH, EOMES (33, 34) and immune suppression signals such as TGFBR3 and IL-10 (35) (Figure 5E). Collectively, these findings align with the changes noted above supporting that lenalidomide exerts a costimulatory effect on CS1 CAR T cells. To rule out donor-dependent factors, we purified CD8+CAR+ cells from three different donors and analyzed the expression of representative genes. Consistently, dose-dependent effects of lenalidomide on CAR T cells were observed (Figures 5F-H).
Figure 5. Lenalidomide enhances transcriptional signatures of immune synapse and T cell function.
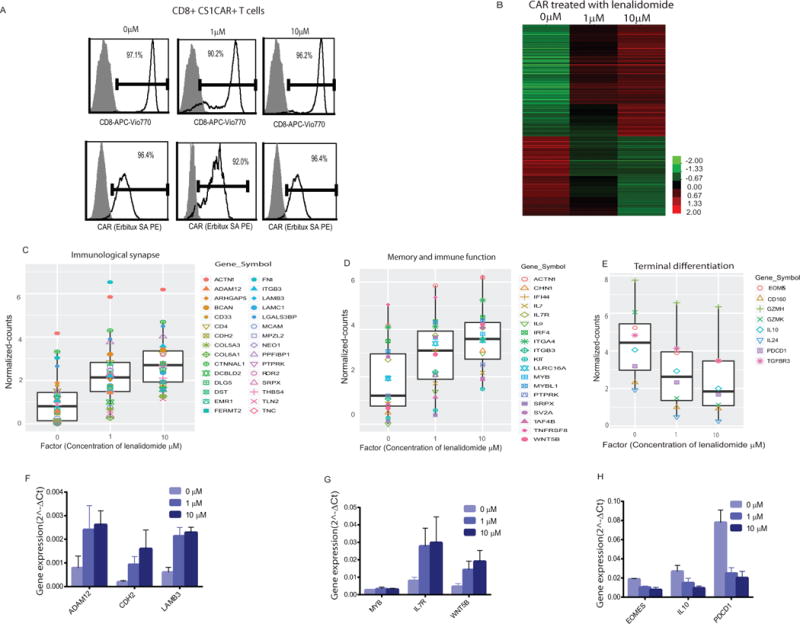
(A) CD8+CS1CAR+ T cells in expanded CS1 CAR T cell population treated with different concentrations of lenalidomide were purified. Percentages of CD8+ and CAR+ cells are depicted. (B) Differentially expressed genes that have RPKM fold change ≥ 2 between compared groups of indicated concentrations of lenalidomide are presented. Normalized counts for (C) immune synapse, (D) memory and immune function, and (E) terminal differentiation genes in CAR T cells are presented. Reprehensive genes in purified CD8+CAR+ T cells derived from three donors were analyzed using RT-qPCR. Means+SEMs of 2^delta Ct of (F) immune synapse, (G) memory and immune function, and (H) terminal differentiation genes are presented.
Lenalidomide Improves CS1-targeted anti-MM Activity and Prolongs Functional CAR T Cell Persistence in vivo
We investigated the synergistic effects of CS1 CAR T cells and lenalidomide against MM in our established xenograft mouse model (Figure 6A). Although 5-7.5 mg/kg lenalidomide daily injection alone and mock T cells plus daily injection of lenalidomide did not induce tumor regression, a single CS1 CAR T cell infusion in conjunction with lenalidomide induced complete tumor remission during lenalidomide treatment (30 days) (Figure S7) and significantly prolonged survival (P=0.008 and P=0.001) (Figure 6B) as compared to the group treated with CS1 CAR T cells alone. Fifty days post CAR T cell infusion, CAR T cells were detected only in the group of mice administered both CS1 and lenalidomide, but not in mice given CS1 CAR T cells alone, suggesting improved CAR T cell persistence with lenalidomide (Figure 6C). Upon further evaluation, we found that persisting CAR T cells in the group receiving lenalidomide and CS1 CAR combinatorial treatment were predominantly CD8+, in contrast to the input CAR T cells that were CD4+ dominant, which is consistent with the in vitro data showing enhanced CD8+CAR T cell expansion (Figure 6D).
Figure 6. Lenalidomide improves the anti-MM activity of CS1 CAR T cells and prolongs functional CAR T cell persistence in vivo.
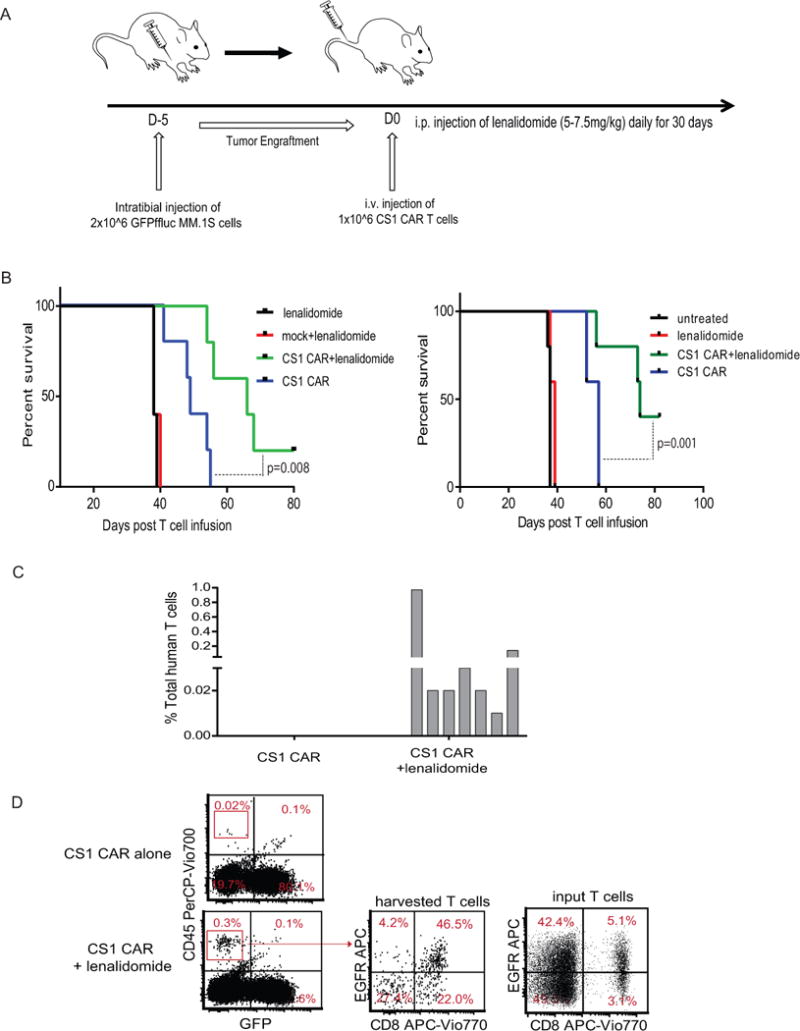
(A) A total of 2 × 106 fflucGFP MM.1S cells were intratibially (i.t.) injected into NSG mice. Five days following tumor inoculation, mice received a single injection (i.v) of dosed 1 × 106 CAR T cells and daily i.p injections of 5-7.5 mg/kg lenalidomide for 30 days. Non-transduced mock cells were infused into control mice. (B) Kaplan-Meier survival curves are presented. N=5 mice per group. Representative data from four separate experiments are presented. (C) Fifty days post CAR T cell infusion, human T cells harvested from peripheral blood, bone marrow, and spleen (three mice per group) were analyzed with flow cytometry after staining with antibody against human CD45. Human CD45+GFP- cells are depicted. (D) The harvested bone marrow cells were labeled with antibodies against CD45, CD8 and EGFR. Percent of positive cells in gated CD45+ population are depicted. Representative data of four in vivo experiments are presented.
We observed that mice treated with combining CAR T cells and daily injection of lenalidomide remain tumor free. However, relapse occurs once ceasing lenalidomide treatment for two weeks (Figure S7), suggesting that lenalidomide is required for durable tumor regression as it is used in the clinic as a long-term maintenance therapy. We further investigated whether the presence of lenalidomide is needed for sustained and enhanced CAR T cell function in vivo. CS1 CAR T cells treated ex vivo with and without lenalidomide were injected into MM-bearing mice without giving exogenous lenalidomide administration. Consistently, no improved antitumor activity was observed when mice were treated with the lenalidomide pre-treated CAR T cell products as compared with untreated CS1 CAR T cells (Figure S8). To further support this scenario, we performed an experiment where we treated the CS1 CAR T cells with lenalidomide for two weeks. We then split the culture into two, one culture underwent continued lenalidomide treatment every other day for an additional two weeks and the other culture no longer treated with lenalidomide. Consistently, in the culture with continued lenalidomide, we observed increased levels of CD107a and percentages of CD8+ T cells at the end of the experiment, compared with values of untreated cultures. We also noted increased intracellular IL-2, which is a hallmark of T cell persistence and function. However, discontinuation of lenalidomide resulted in decreased IL-2 production and a lower percentage of CD8+ T cells, while CD107a levels remained the same. In addition, CAR T cells expressed higher levels of PD1 upon discontinuation of lenalidomide (Figure S9). Overall, our data indicated that the costimulatory effects of CAR T cells mediated by lenalidomide rely on the continuing activation of the T cell signaling pathway. The role of lenalidomide in this context is therefore not an enhancement during manufacturing but rather a combinatorial agent that improves the function of these CAR T cells.
Discussion
In this study, we demonstrate that CS1 CAR T cells exhibit efficient anti-MM activity in vivo. Secondly, we show that lenalidomide, in addition to its known anti-myeloma effects, can also augment T cell function and number. This in turns leads to a synergistic increase in the anti-myeloma activity of CAR T cells through increased cytotoxicity and improved persistence of the engineered cells.
To validate the potential use of CS1 CAR T cells for MM, we examined the effector function of CS1 CAR T cells against different MM cells, varying in the levels of CS1 expression. Data demonstrated that CS1 CAR T cells exhibited antigen dependent effector function as indicated by degranulation and cytokine production, supporting a selective targeting for MM cells which express high CS1 on the surface. More interestingly, CS1 CAR T cells exhibited efficient effector function against MM.1R, which are resistant to dexamethasone because of mutant glucocorticoid receptor(36, 37). These data may indicate the effectiveness of CS1 CAR T cells on dexamethasone-resistant patients with MM.
Although significant tumor remission was observed in the CS1 CAR T cell-treated mice, tumor relapse eventually occurred. We first speculated that tumor antigen escape was the cause. Unexpectedly, recurrent tumors remained CS1-positive regardless of CS1 CAR T cell treatment. We further evaluated CAR T cell persistence in the tumor-bearing mice, as lack of CAR T cell persistence is another contributor to failure of T cell therapy, and greater T cell persistence and survival are associated with high rates of complete remission. To our surprise, CAR T cells were detected in CS1 CAR T cell-treated mice. Although these persisting CAR T cells had low expression of exhaustion markers such as PD1 and Tim3, the levels were too low to prevent tumor relapse (data not shown). These data highlight the need for a strategy that can improve CAR T cell function and expansion/persistence to achieve durable tumor remissions.
Lenalidomide has been shown to promote immunologic memory in the T cell populations of patients with MM by resulting in an excess of memory T cells and decrease of terminally differentiated T cells (38). In agreement with others’ observations (38), our data showed that lenalidomide preferentially expanded CD8+CAR T cells, resulting in increased total cell expansion. The expanded CD8+CAR T cells exhibited less differentiated phenotypes but maintained minimal exhaustive features as reported (39) and terminal differentiation genes, suggesting that lenalidomide improves memory CAR T cell maintenance.
Lenalidomide exerts a costimulatory effect on T cell responses that includes increased production of IL-2 and IFN-γ and inhibits production of anti-inflammatory cytokines (40, 41). Consistently, in our studies, CAR T cells treated with lenalidomide exhibited a strong capacity to produce Th1 cytokines upon stimulation with different CS1+ tumors such as U266B, MM.1S and MM.1R; these cytokines are required for durable antitumor activity of T cells (42). By contrast, Th2 cytokines that hamper the induction of effective T cell responses (42, 43) were downregulated, demonstrating that lenalidomide can favorably moderate the immune milieu. Furthermore, lenalidomide-treated CAR T cells exhibited increased cytolysis of MM cells. These effects may be attributed to the enhanced immune synapse formation between the lenalidomide-treated CAR T cells and tumors as is supported by gene signatures enriching for immune synapse formation and is in accordance with previous studies (44, 45).
In the murine model we chose a relatively low dose of lenalidomide to allow assessment of potential synergistic effects when combined with CAR T cell treatment. In spite of the suboptimal dose of lenalidomide in the NSG model as compared with the doses used by others (46, 47), combination therapy completely eradicated tumor. Therefore, the combinatorial effects mainly reflect the impact of lenalidomide on CAR T cells. Patients treated with lenalidomide have shown faster CD8+ T cell reconstitution compared with CD4+ cells (48). Consistently, CD8+CAR T cells became dominant within the harvested human T cells from mice, which markedly differs from the CD4-dominant composition of input CAR T cells, further confirming our in vitro data showing CD8+CAR enrichment in the presence of lenalidomide. Pre-treated CS1 CAR T cells exhibited similar antitumor activity to untreated CS1 CAR T cells in vivo, indicating the requirement of sustained lenalidomide to maintain the T-cell co-stimulatory pathway and obtain synergistic effects with CAR T cells.
We also noticed that recurrent tumors in mice that had received combination therapy had a different distribution pattern than tumors in mice treated with CS1 CAR T cells alone. Specifically, CS1 CAR T cell-treated tumors were located mainly in the BM; in contrast, mice with recurrent tumors from combination treatment had to be euthanized because of large extramedullary subcutaneous tumors where there were no CAR T cells detected (data not shown). Lenalidomide disrupts the microenvironmental interactions between MM cells and BM stromal cells (49), resulting in inhibition of tumor growth. Our data suggest that lenalidomide may impair BM microenvironmental support for MM growth, directing the relapsed tumor to seed preferentially in extramedullary tissues. Evidently, CS1 CAR T cells did not penetrate into the metastasized extramedullary tumors and faced the same barriers presented by other types of solid tumors (50).
In summary, this study provides novel mechanistic insights into CAR T cell functional augmentation by lenalidomide in terms of in vivo antitumor activity and persistence. The synergistic effects could be the consequence of directly inhibiting tumor-initiating cells (SP) while enhancing the antitumor activity of CAR T cells. This study provides the basis for a planned clinical trial using the combination of lenalidomide with engineered T cells against CS1 in relapsed myeloma.
Supplementary Material
Translational Reference.
Chimeric Antigen Receptor (CAR)-directed T cell therapy is a promising approach for hematological malignancies, including multiple myeloma (MM); however, uncertainties surround the optimal target as well as enhancing the persistence and expansion of engineered cells in vivo. This study demonstrated that CS1-specific CAR T cells mediated efficient effector function in vitro and anti-MM activity in vivo. Furthermore, we explored further improving the function and persistence of these engineered CS1-specific T cells using a combinatorial therapy of CAR T cells with immunomodulatory drugs such as lenalidomide. We found that lenalidomide plays a co-stimulatory role in modulating CAR T cells and strengthens the anti-tumor activity and persistence of adoptively transferred CS1 CAR T cells against MM in vivo. The rational combination of these immunotherapeutic regimens is an effective strategy for increasing efficacy against MM and provides a generalizable approach for combining lenalidomide and CAR T cells.
Acknowledgments
Grant Support: This study was supported by City of Hope Lymphoma SPORE (NCI P50 CA107399) (to S.J Forman), the City of Hope Cancer Center Support Grant (NCI P30 CA33572) (to S.J Forman), and Multiple Myeloma Research Foundation (MMRF) (to S.J Forman and X Wang).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare they have no competing interests
Authorship Contributions
X.W, S.J.F, M.H, and A.K designed, interpreted data, and co-wrote the manuscript. R.U, M.W, L. W, L. L, C.H,C. W.W, and W.C performed research and collected data. S.T, C.E.B, F.P, L.Y, and J.F.S analyzed data, and participated in the manuscript review.
References
- 1.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373:1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187:1885–92. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dredge K, Marriott JB, Todryk SM, Muller GW, Chen R, Stirling DI, et al. Protective antitumor immunity induced by a costimulatory thalidomide analog in conjunction with whole tumor cell vaccination is mediated by increased Th1-type immunity. J Immunol. 2002;168:4914–9. doi: 10.4049/jimmunol.168.10.4914. [DOI] [PubMed] [Google Scholar]
- 8.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–6. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 9.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 11.Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther. 2015;23:757–68. doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–63. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Naranjo A, Brown CE, Bautista C, Wong CW, Chang WC, et al. Phenotypic and Functional Attributes of Lentivirus-modified CD19-specific Human CD8+ Central Memory T Cells Manufactured at Clinical Scale. J Immunother. 2012;35:689–701. doi: 10.1097/CJI.0b013e318270dec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CE, Wright CL, Naranjo A, Vishwanath RP, Chang WC, Olivares S, et al. Biophotonic cytotoxicity assay for high-throughput screening of cytolytic killing. J Immunol Methods. 2005;297:39–52. doi: 10.1016/j.jim.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petriz J. Flow cytometry of the side population (SP) In: Paul Robinson J, et al., editors. Current protocols in cytometry/editorial board. 2007. Chapter 9: Unit9 23. [DOI] [PubMed] [Google Scholar]
- 17.Markey KA, Gartlan KH, Kuns RD, MacDonald KP, Hill GR. Imaging the immunological synapse between dendritic cells and T cells. J Immunol Methods. 2015;423:40–4. doi: 10.1016/j.jim.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Na B, Jun C. Assessment of immunological synapse formation by flow cytometry. Bio-protocol. 2016;6:e1758. [Google Scholar]
- 19.Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158:727–38. doi: 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- 20.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–37. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–60. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, Kufe T, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62:39–49. doi: 10.1007/s00262-012-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl):S52–61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- 24.De Keersmaecker B, Fostier K, Corthals J, Wilgenhof S, Heirman C, Aerts JL, et al. Immunomodulatory drugs improve the immune environment for dendritic cell-based immunotherapy in multiple myeloma patients after autologous stem cell transplantation. Cancer Immunol Immunother. 2014;63:1023–36. doi: 10.1007/s00262-014-1571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. Pillars article: The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]; J Immunol. 2015;194:4066–72. [PubMed] [Google Scholar]
- 26.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A Distinct Subset of Self-Renewing Human Memory CD8(+) T Cells Survives Cytotoxic Chemotherapy. Immunity. 2009;31:834–44. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao S, Buzo BF, Pham D, Jiang L, Taparowsky EJ, Kaplan MH, et al. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013;39:833–45. doi: 10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyogoku C, Smiljanovic B, Grun JR, Biesen R, Schulte-Wrede U, Haupl T, et al. Cell-specific type I IFN signatures in autoimmunity and viral infection: what makes the difference? PLoS One. 2013;8:e83776. doi: 10.1371/journal.pone.0083776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J Cell Sci. 2003;116:4695–705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 31.Vigano S, Banga R, Bellanger F, Pellaton C, Farina A, Comte D, et al. CD160-associated CD8 T-cell functional impairment is independent of PD-1 expression. PLoS pathogens. 2014;10:e1004380. doi: 10.1371/journal.ppat.1004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–3. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 34.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305. doi: 10.1158/0008-5472.CAN-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma S, Lichtenstein A. Dexamethasone-induced apoptotic mechanisms in myeloma cells investigated by analysis of mutant glucocorticoid receptors. Blood. 2008;112:1338–45. doi: 10.1182/blood-2007-11-124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moalli PA, Pillay S, Weiner D, Leikin R, Rosen ST. A mechanism of resistance to glucocorticoids in multiple myeloma: transient expression of a truncated glucocorticoid receptor mRNA. Blood. 1992;79:213–22. [PubMed] [Google Scholar]
- 38.Simone R, Marsilio S, Patten PEM, Ferrer G, Chen S-S, Yan XJ, et al. Lenalidomide Promotes The Expansion Of CD8 T Cells With An Effector Memory Phenotype In a Murine Xenograft Model Of Chronic Lymphocytic Leukemia. Blood. 2013;122 abstract 119. [Google Scholar]
- 39.Gorgun G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, et al. Lenalidomide Enhances Immune Checkpoint Blockade-Induced Immune Response in Multiple Myeloma. Clin Cancer Res. 2015;21:4607–18. doi: 10.1158/1078-0432.CCR-15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BN, Gao H, Cohen EN, Badoux X, Wierda WG, Estrov Z, et al. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117:3999–4008. doi: 10.1002/cncr.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–6. [PubMed] [Google Scholar]
- 42.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3:3856–93. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–8. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 44.Kuramitsu S, Ohno M, Ohka F, Shiina S, Yamamichi A, Kato A, et al. Lenalidomide enhances the function of chimeric antigen receptor T cells against the epidermal growth factor receptor variant III by enhancing immune synapses. Cancer Gene Ther. 2015;22:487–95. doi: 10.1038/cgt.2015.47. [DOI] [PubMed] [Google Scholar]
- 45.Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica. 2009;94:1198–202. doi: 10.3324/haematol.2009.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Bi E, Hong S, Qian J, Zheng C, Wang M, et al. CD4(+) T cells play a crucial role for lenalidomide in vivo anti-tumor activity in murine multiple myeloma. Oncotarget. 2015;6:36032–40. doi: 10.18632/oncotarget.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otahal P, Prukova D, Kral V, Fabry M, Vockova P, Lateckova L, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2016;5:e1115940. doi: 10.1080/2162402X.2015.1115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clave E, Douay C, Coman T, Busson M, Bompoint C, Moins-Teisserenc H, et al. Lenalidomide consolidation and maintenance therapy after autologous stem cell transplant for multiple myeloma induces persistent changes in T-cell homeostasis. Leuk Lymphoma. 2014;55:1788–95. doi: 10.3109/10428194.2013.865182. [DOI] [PubMed] [Google Scholar]
- 49.Gorgun G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–37. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newick K, O’Brien S, Sun J, Kapoor V, Maceyko S, Lo A, et al. Augmentation of CAR T-cell Trafficking and Antitumor Efficacy by Blocking Protein Kinase A Localization. Cancer immunology research. 2016;4:541–51. doi: 10.1158/2326-6066.CIR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


