Abstract
Background & objectives:
Bartonella henselae causes infections which closely resemble febrile illness and chronic diseases such as tuberculosis and haematological malignancies. There are not many studies on Bartonella infections from India. The present study was undertaken to diagnose B. henselae infection in diverse clinical conditions in a tertiary care hospital in north India.
Methods:
A total of 145 patients including those with fever and lymphadenopathy, infective endocarditis and neuroretinitis were enrolled in the study. Whole blood, serum and lymph node aspirate and valvular vegetations if available, were obtained. Samples were plated on chocolate agar and brain-heart infusion agar containing five per cent fresh rabbit blood and were incubated at 35°C for at least four weeks in five per cent CO2 with high humidity. Immunofluorescent antibody assay (IFA) was done for the detection of IgM antibodies in the serum using a commercial kit. Whole blood was used to perform polymerase chain reaction (PCR) for the citrate synthase gene (gltA).
Results:
IFA was positive in 11 of 140 (7.85%) patients and PCR was positive in 3 of 140 (2.14%) patients. Culture was negative in all the cases. A higher incidence of Bartonella infection was seen in patients with fever and lymphadenopathy (n=30), seven of whom were children. In ophthalmological conditions, four cases were IFA positive.
Interpretation & conclusions:
The present study shows that the threat of Bartonella infection is a reality in India. It is also an important treatable cause of fever and lymphadenopathy in children. Serology and PCR are useful tests for its diagnosis. Clinicians should consider Bartonella infection in the differential diagnosis of febrile illnesses and chronic diseases.
Keywords: Bartonella spp., citrate synthase gene, diagnostics methods, fever, lymphadenopathy, pyrexia of unknown origin
Bartonellosis is a zoonotic disease caused by bacterial genus Bartonella, which is virtually ubiquitous among mammals. There are 45 species described so far in the Bartonella genus, with Bartonella henselae, B. bacilliformis, B. quintana and B. elizabethae accounting for the majority of human cases1. Bartonella spp. infects closely related mammal species as its reservoir host, e.g. B. bacilliformis and B. quintana in humans, B. henselae in cats and dogs and B. elizabethae in rats. It establishes an intraerythrocytic and typically asymptomatic bacteraemia, although infections in incidental hosts may evoke discernible disease2,3. This long-lasting erythrocytic bacteraemia in due course leads to efficient transmission of the intraerythrocytic pathogen to other susceptible hosts, which is transmitted by blood-sucking arthropods such as body lice (B. quintana in body lice)4, fleas (B. henselae in cat fleas Ctenocephalides felis)4, sandflies (B. bacilliformis in Lutzomyia)4 and new potential vectors such as ixodid ticks for transmitting B. henselae5. Bartonella spp. are thought to multiply in the digestive tract of the vector and their transmission occurs by animal scratches or a bite/faeces of arthropods2.
B. henselae formerly known as Rochalimæa henselae was first discovered from the patient of cat scratch disease (CSD). Several genotypes have been identified so far, with two main genotypes designated Houston-1 (Type I) and Marseille (previously BATF) (Type II)6,7. Type 1 is a commonly isolated genotype in humans from Western Europe and Australia and it is considered to be more virulent7.
Infection with B. henselae shows myriad of clinical manifestation which is linked with immune status of the individuals. In immunocompetent individuals, it causes CSD which occurs mostly indirectly via contaminated flea faeces that are inoculated via a cat scratch. It affects all ages, but most commonly seen in <21 yr of age. It is a self-limiting infection characterized by lymphadenopathy, which may be accompanied by other unremarkable manifestations such as fever or fatigue. A few cases of meningoencephalitis, endocarditis and eye involvement (neuroretinitis), bacteraemia and neurological disorders have also been reported in immunocompetent individuals8. In immunocompromised individuals, B. henselae is known to be the agent of bacillary angiomatosis, peliosis hepatis, septicaemia, endocarditis, recurring fever and neurological disorders8.
Bartonella is Gram-negative fastidious bacteria having a peculiar and demanding nutritional requirement1. These can be grown on heart infusion, trypticase soy, Brucella agar and Columbia agar supplemented with five per cent rabbit blood or five per cent haemoglobin sheep and take 2-6 wk or longer period of incubation for visible colonies to appear in primary isolation. Due to difficulties with traditional culture methods for isolation, serologic testing for Bartonella infection, including immunofluorescent antibody assay (IFA) and ELISA, is the cornerstone for clinical diagnosis9.
Apart from serology, detection of specific DNA by conventional PCR, PCR-restriction fragment length polymorphism and real-time PCR with different gene targets have been described4,10. This study was undertaken to diagnose B. henselae infection in patients with diverse clinical conditions in a tertiary care centre in India.
Material & Methods
The present study was conducted during 2012-2016 in the department of Microbiology, All India Institute of Medical Sciences, New Delhi, India. A total of 145 patients were enrolled in the study. This included patients admitted in paediatrics and medicine wards with fever and lymphadenopathy, patients with culture-negative endocarditis and patients with clinical features suggestive of neuroretinitis. Samples were sent to the laboratory for cases where Bartonella infection was suspected.
Ethical approval for the study was obtained from the institute ethics committee. Written informed consent was taken from all patients. Patients with fever and lymphadenopathy due to tuberculosis, haematological malignancies and other pyogenic infections were excluded from the study. Similarly, patients with infective endocarditis whose blood culture yielded bacteria or fungi were also excluded. Whole blood samples (5 ml) were collected from all patients enrolled in the study. Screening tests for syphilis, toxoplasma, tuberculosis and other routine tests were performed on samples received from ophthalmology. Lymph node biopsy/aspirate samples were obtained from patients with fever and lymphadenopathy, and valvular vegetation was collected from patients with infective endocarditis whenever available.
Isolation of B. henselae was done by conventional culture from whole blood, lymph node biopsy/aspirate and valvular vegetation. Samples were plated on fresh chocolate agar and brain-heart infusion agar containing five per cent fresh rabbit blood and were incubated at 35°C for at least four weeks in five per cent CO2 with high humidity. B. henselae (ATCC 4982) strain served as positive control. Negative controls used were DNA extracted from ATCC strains of Klebsiella pneumoniae, Citrobacter sp. and Proteus vulgaris.
IFA was done for the detection of IgM antibodies from serum using a commercial kit (B. henselae IgM IFA kit, Vircell, Granada, Spain). For PCR, DNA was extracted from citrated blood, lymph node aspirates and valvular vegetations using QIAGEN DNA extraction kits (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. PCR for B. henselae citrate synthase gene (gltA) was done according to earlier published protocols using the following primers11:
Forward Primer: GGGGACCAGCTCATGGTGG
Reverse Primer: AATGCAAAAAGAACAGTAA ACA
PCR analysis was performed with 50 μl of a PCR mixture which contained 1 U of Taq DNA polymerase, 25 pmol of each primer and 100 ng of target DNA. Amplification was performed under standard conditions in a thermal cycler with 30 cycles each of one minute at 94°C, two minutes at 55°C and three minutes at 72°C, followed by a terminal extension step of 10 min at 72°C. Electrophoresis of the amplified PCR products was carried out on 1.5 per cent agarose gel containing ethidium bromide and examined under ultraviolet transilluminator. The PCR products for gltA gene showed an amplicon size of 379 base pairs.
Results
Of the 145 patients enrolled, 140 samples were processed. In the remaining five patients, three samples were inadequate and in another two the blood samples were coagulated. There were 73 males and 67 females. The age of the patients ranged from 0 - >60 years; of which 52 were children (0-12 yr), 24 were between 12-18 yr, 28 were in 19-39 yr age group, 15 were in 31-40 yr, 12 were in 41-50 yr, seven were in 51-60 yr age group and two were above 60 years. Of the 140 patients, 30 had fever and lymphadenopathy. The duration of fever ranged from 1-3 months. Cervical lymph nodes were most commonly enlarged. A history of maculopapular skin rash was elicited in eight patients. Twenty two patients presented with culture-negative endocarditis. The remaining 80 patients had ophthalmological symptoms, with papillitis being more common in 26 (37.14%), retrobulbar neuritis in 17 (24.28%) and optic neuritis in 15 (21.42%). IFAs for IgM antibodies were positive in 11 (7.85%, n=140) patients (Table). PCR for citrate synthase gene was positive in three (2.14%, n=140) patients (Figure). Culture was negative in all the cases. Of the 11 IFA-positive cases, seven presented with fever and lymphadenopathy. Five of these were diagnosed as having pyrexia of unknown origin (PUO), one with CSD and one with granulomatous disease. Among the four IFA-positive ophthalmology cases, one was a case of Parinaud's oculoglandular syndrome and the rest showed symptoms of neuroretinitis. Of the 11 IFA-positive cases, seven were children and four of them had PUO. Six of the IFA-positive patients gave a history of exposure to cats.
Table.
Clinical presentation and laboratory findings in the 11 immunofluorescent antibody assay (IFA)-positive patients
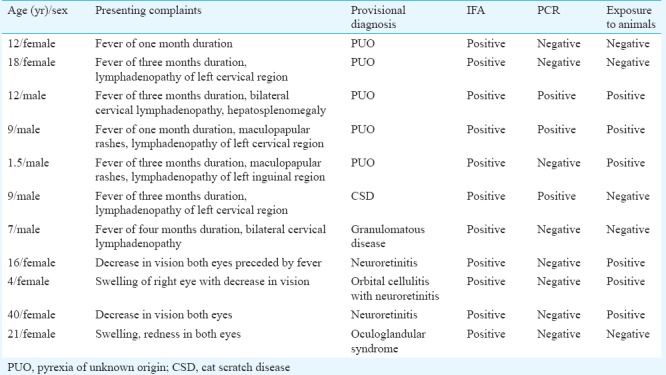
Figure.
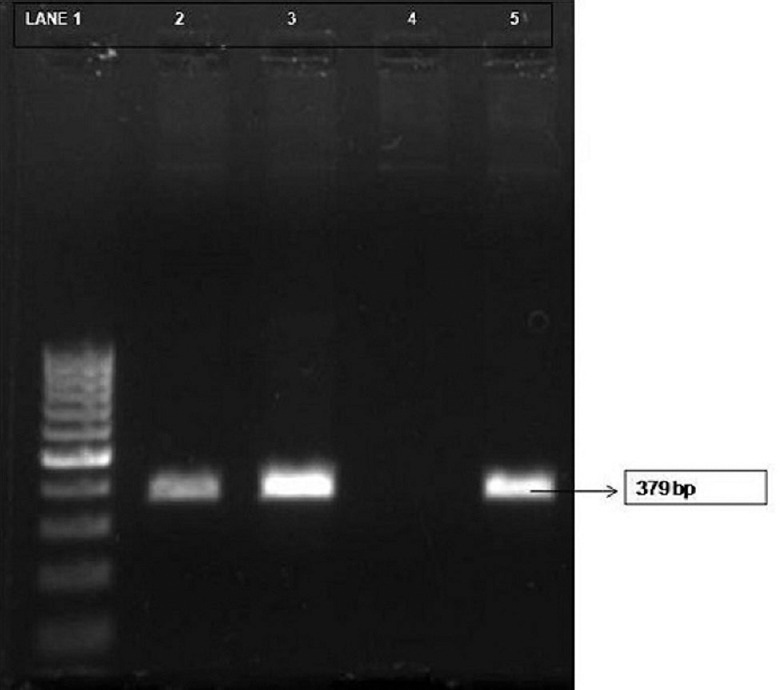
Polymerase chain reaction for Bartonella henselae citrate synthase gene (gltA). Lane 1, 100 bp DNA ladder; lane 2, clinical sample (laboratory no. 413) positive; lane 3, clinical sample (laboratory no. 738) positive; lane 4, negative control; lane 5, positive control (original image).
Discussion
The vector-borne pathogens Bartonella species are now considered as worldwide emerging diseases in humans. They present with the multitude of clinical manifestations which mimic other endemic diseases. Fever with lymphadenopathy and prolonged fever are common clinical presentations in patients with B. henselae infection3. As clinical features of B. henselae infection closely resembles symptoms associated with other febrile illnesses and chronic diseases, it is often left undiagnosed or underreported12. However, recognition of this infection is increasing worldwide and reports indicate its emergence even in developing and resource-limited countries. A study from Namibia by Noden et al12 showed the presence of B. henselae in 2.9 per cent of its study population by detecting IgG antibodies using IFA. The presence of IgG antibodies indicates epidemiological prevalence of Bartonella infection although it may not detect the recent infection.
Serological testing is at present the most commonly utilized means of clinically diagnosing infection with Bartonella spp., largely on account of accessibility and practicality. Of the 140 patients, 7.85 per cent showed serological evidence of B. henselae infection as confirmed by IFA. Among the various serological tests available for the diagnosis of B. henselae infection, IFA is the most commonly used method13. The duration of detection of IgM antibodies is less than three months; hence, a positive IgM indicates an acute disease3. However, the short duration and undetectable level of IgM antibodies and the absence of antibodies in bacteraemic phase make them infrequently discovered on serology. Hence, negative results do not necessarily exclude disease. Antigenic variability between the two B. henselae serotypes may also explain the poor antibody response13. Commercial kits use only B. henselae strain Houston-1 grown in cell culture as the antigen. Antibodies directed against the B. henselae strain Marseille may remain undetected13. The IFA developed by the Centers for Disease Control and Prevention, Atlanta, Georgia, reported sensitivities of 83-88 per cent and specificities of 94-98 per cent14.
PCR-derived assays are helpful in rapid detection and identification of the bacteria directly from clinical samples. In our study, PCR for citrate synthase gene (gltA) picked up three positive cases. The gltA coding sequence in B. henselae is 1293 nucleotides in length and encodes a protein which has 431 amino acids. Citrate synthase gene (gltA) encodes for citrate synthase, which is the first enzyme of the tricarboxylic acid cycle and a key regulator of intracellular ATP production in both prokaryotic and eukaryotic cells. The nucleic acid sequence of the gltA gene has been determined for a number of bacteria, including B. henselae. The use of gltA comparisons to estimate divergence among closely related species was first reported by Regnery et al15. The PCR analysis was performed under stringent conditions, and earlier studies have proven that the protocol followed ensured no amplification of other members of alpha proteobacterium16. Failure to pick up more positive cases could be attributed to prior antibiotic use and the presence of inhibitors in the sample. False-negative results are also more likely to occur when samples contain less number of the bacteria17. It is well known that PCR for B. henselae has excellent specificity, but it lacks in sensitivity, which ranges from 43 to 76 per cent3.
B. henselae is a fastidious, slow-growing, intracellular organism. Its culture needs are characterized by complex nutritional requirements and prolonged incubation times17. Hence, culture of Bartonella species is difficult, requiring a 2-6 wk incubation for primary isolation3. In our study, all samples were culture negative. Isolation of the organisms from clinical samples is difficult because most of the patients had the history of prior antibiotic use. Moreover, Anderson and Neuman18 have reported that if patients lack systemic disease, isolation becomes unsuccessful. Isolation of bacteria from lymph node culture also offers poor yield, because lymphadenopathy is thought to be due to an aggravated immune response rather than the bacterial direct invasion and proliferation3. Novel culture media such as the alpha proteobacterium growth medium and techniques such as shell vial culture have improved the isolation rates of the organism19,20.
A higher incidence of Bartonella infection in our study was seen in patients with fever and lymphadenopathy. B. henselae infection is a common cause of lymphadenopathy in young patients3. Safont et al21 found that B. henselae and Mycobacterium avium infection occurs mostly in young patients, whereas patients with lymphadenopathy due to M. tuberculosis are significantly older. Asano et al22 also reported higher rates of B. henselae infection in children with cervical lymphadenopathy as compared to adults. Koehler et al23 reported evidence of Bartonella infection in 18 per cent of patients with prolonged fever. Of which, three per cent of either B. henselae or B. quintana was isolated from specimens of blood, tissue or both or had DNA detected in tissue. Mazur-Melewska et al24 identified B. henselae as the third leading infectious cause of PUO, after Epstein-Barr virus infection and osteomyelitis.
Among patients with ophthalmological findings, 4.9 per cent had B. henselae infection. One patient had Parinaud's oculoglandular syndrome and presented with eye redness, foreign body sensation in the eye, cervical lymphadenopathy and fever. B. henselae is the known causative agent for this syndrome and the route of infection is through direct conjunctival inoculation25. The other patients had a sudden painless loss of vision with swelling of the optic disc and stellate macular exudates characteristic of neuroretinitis. This is the most common posterior segment ocular complication of Bartonella infection. Chaudhry et al26 reported isolation of B. henselae in four of 29 patients suspected of optic neuritis. Apart from neuroretinitis, B. henselae has been reported from a 45 yr old patient diagnosed with acute endogenous endophthalmitis without any evidence of retinitis as well as in choroidal neovascularization, uveitis and optic disk oedema27,28,29,30. Hence, clinicians should think of infection with B. henselae not only in neuroretinitis but also in endophthalmitis and other ophthalmology conditions.
None of the 22 endocarditis patients were positive for B. henselae in our study. Negative results could be due to prior antibiotics intake or due to delayed collection of samples in the clinical course. In Bartonella endocarditis, amplification of Bartonella DNA from valvular tissue by PCR has been shown to have higher sensitivity and specificity, ranging from 72 to 98 per cent1. Okaro et al1 had suggested a positive titre of >16 for IgM and >64 for IgG, with a 4-fold rise in titre for IgG between acute-phase serum and convalescent-phase serum samples (collected at least two weeks apart) were preferred for definitive diagnosis.
As no pathognomonic clinical features is attributed to Bartonella infections, laboratory testing is required for a definitive diagnosis of Bartonella infection. In this study, serology was found to be a useful test for diagnosing B. henselae infection in patients with suggestive clinical features. PCR was able to confirm the same. However, neither clinical symptoms nor tests such as serology nor PCR alone are satisfactory for diagnosis of B. henselae infection.
Since IgG IFA could not be performed due to financial constraints in our study, we might have missed some more positive cases which would be a possible limitation of the present study along with the low sample size.
In conclusion, our study depicts the presence of Bartonella infection in 7.85 per cent patients with diverse clinical conditions studied by IFA. These might be just the tip of the iceberg. Our study underscores the need for further screening of the susceptible human population. Moreover, screening procedures should also include likely reservoirs of infection such as cats as well as unlikely reservoirs such as birds.
Acknowledgment
Authors acknowledge Dr Rakesh Yadav for providing endocarditis samples.
Footnotes
Financial support & sponsorship: Authors thank the All India Institute of Medical Sciences, New Delhi, India, for providing intramural grant for conducting this study
Conflicts of Interest: None.
References
- 1.Okaro U, Addisu A, Casanas B, Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev. 2017;30:709–46. doi: 10.1128/CMR.00013-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulliainen AT, Dehio C. Persistence of Bartonella spp.stealth pathogens: From subclinical infections to vasoproliferative tumor formation. FEMS Microbiol Rev. 2012;36:563–99. doi: 10.1111/j.1574-6976.2012.00324.x. [DOI] [PubMed] [Google Scholar]
- 3.Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: Widening spectrum of Bartonella henselae infection. Pediatrics. 2008;121:e1413–25. doi: 10.1542/peds.2007-1897. [DOI] [PubMed] [Google Scholar]
- 4.Breitschwerdt EB. Bartonellosis: One health perspectives for an emerging infectious disease. ILAR J. 2014;55:46–58. doi: 10.1093/ilar/ilu015. [DOI] [PubMed] [Google Scholar]
- 5.Cotté V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis HJ, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. 2008;14:1074–80. doi: 10.3201/eid1407.071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomel BB, Boulouis HJ, Breitschwerdt EB. Cat scratch disease and other zoonotic Bartonella infections. J Am Vet Med Assoc. 2004;224:1270–9. doi: 10.2460/javma.2004.224.1270. [DOI] [PubMed] [Google Scholar]
- 7.Boulouis HJ, Chang CC, Henn JB, Kasten RW, Chomel BB. Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet Res. 2005;36:383–410. doi: 10.1051/vetres:2005009. [DOI] [PubMed] [Google Scholar]
- 8.Klotz SA, Ianas V, Elliott SP. Cat-scratch disease. Am Fam Physician. 2011;83:152–5. [PubMed] [Google Scholar]
- 9.Hoey JG, Valois-Cruz F, Goldenberg H, Voskoboynik Y, Pfiffner J, Tilton RC, et al. Development of an immunoglobulin M capture-based enzyme-linked immunosorbent assay for diagnosis of acute infections with Bartonella henselae. Clin Vaccine Immunol. 2009;16:282–4. doi: 10.1128/CVI.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litwin CM, Anderson B, Tsolis R, Rasley A. The Bartonellaceae, Brucellaceae, and Francisellaceae. In: Detrick B, Schmitz JL, Hamilton RG, editors. Manual of Molecular and Clinical Laboratory Immunology. 8th ed. Washington: ASM Press; 2016. pp. 473–81. [Google Scholar]
- 11.Avidor B, Kletter Y, Abulafia S, Golan Y, Ephros M, Giladi M, et al. Molecular diagnosis of cat scratch disease: A two-step approach. J Clin Microbiol. 1997;35:1924–30. doi: 10.1128/jcm.35.8.1924-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noden BH, Tshavuka FI, van der Colf BE, Chipare I, Wilkinson R. Exposure and risk factors to coxiella burnetii, spotted fever group and typhus group Rickettsiae, and Bartonella henselae among volunteer blood donors in Namibia. PLoS One. 2014;9:e108674. doi: 10.1371/journal.pone.0108674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurin M, Rolain JM, Raoult D. Comparison of in-house and commercial slides for detection by immunofluorescence of immunoglobulins G and M against Bartonella henselae and Bartonella quintana. Clin Diagn Lab Immunol. 2002;9:1004–9. doi: 10.1128/CDLI.9.5.1004-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opavsky MA. Cat scratch disease: The story continues. Can J Infect Dis. 1997;8:43–9. doi: 10.1155/1997/982908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–89. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diddi K, Chaudhry R, Sharma N, Dhawan B. Strategy for identification & characterization of Bartonella henselae with conventional & molecular methods. Indian J Med Res. 2013;137:380–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Qian X, Jin L, Hayden RT, Macon WR, Lloyd RV. Diagnosis of cat scratch disease with Bartonella henselae infection in formalin-fixed paraffin-embedded tissues by two different PCR assays. Diagn Mol Pathol. 2005;14:146–51. doi: 10.1097/01.pas.0000176771.64165.fb. [DOI] [PubMed] [Google Scholar]
- 18.Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–19. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cadenas MB, Maggi RG, Diniz PP, Breitschwerdt KT, Sontakke S, Breithschwerdt EB, et al. Identification of bacteria from clinical samples using Bartonella alpha-proteobacteria growth medium. J Microbiol Methods. 2007;71:147–55. doi: 10.1016/j.mimet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Angelakis E, Richet H, Rolain JM, La Scola B, Raoult D. Comparison of real-time quantitative PCR and culture for the diagnosis of emerging Rickettsioses. PLoS Negl Trop Dis. 2012;6:e1540. doi: 10.1371/journal.pntd.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safont M, Angelakis E, Richet H, Lepidi H, Fournier PE, Drancourt M, et al. Bacterial lymphadenitis at a major referral hospital in France from 2008 to 2012. J Clin Microbiol. 2014;52:1161–7. doi: 10.1128/JCM.03491-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano T, Ichiki K, Koizumi S, Kaizu K, Hatori T, Fujino O, et al. High prevalence of antibodies against Bartonella henselae with cervical lymphadenopathy in children. Pediatr Int. 2010;52:533–5. doi: 10.1111/j.1442-200X.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 23.Koehler JE, Sanchez MA, Tye S, Garrido-Rowland CS, Chen FM, Maurer T, et al. Prevalence of Bartonella infection among human immunodeficiency virus-infected patients with fever. Clin Infect Dis. 2003;37:559–66. doi: 10.1086/375586. [DOI] [PubMed] [Google Scholar]
- 24.Mazur-Melewska K, Mania A, Kemnitz P, Figlerowicz M, Służewski W. Cat-scratch disease: A wide spectrum of clinical pictures. Postepy Dermatol Alergol. 2015;32:216–20. doi: 10.5114/pdia.2014.44014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biancardi AL, Curi AL. Cat-scratch disease. Ocul Immunol Inflamm. 2014;22:148–54. doi: 10.3109/09273948.2013.833631. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry R, Mukherjee A, Menon V. Isolation & characterization of Bartonella sp. from optic neuritis patients. Indian J Med Res. 2012;136:985–90. [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein DA, Mouritsen L, Friedlander S, Tessler HH, Edward DP. Acute endogenous endophthalmitis due to Bartonella henselae. Clin Infect Dis. 2001;33:718–21. doi: 10.1086/321884. [DOI] [PubMed] [Google Scholar]
- 28.Latanza L, Viscogliosi F, Solimeo A, Calabrò F, De Angelis V, De Rosa P, et al. Choroidal neovascularisation as an unusual ophthalmic manifestation of cat-scratch disease in an 8-year-old girl. Int Ophthalmol. 2015;35:709–16. doi: 10.1007/s10792-015-0093-3. [DOI] [PubMed] [Google Scholar]
- 29.Drancourt M, Bodaghi B, Lepidi H, Le Hoang P, Raoult D. Intraocular detection of Bartonella henselae in a patient with HLA-B27 uveitis. J Clin Microbiol. 2004;42:1822–5. doi: 10.1128/JCM.42.4.1822-1825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wade NK, Levi L, Jones MR, Bhisitkul R, Fine L, Cunningham ET, Jr, et al. Optic disk edema associated with peripapillary serous retinal detachment: An early sign of systemic Bartonella henselae infection. Am J Ophthalmol. 2000;130:327–34. doi: 10.1016/s0002-9394(00)00599-7. [DOI] [PubMed] [Google Scholar]


