ABSTRACT
Background:
Mean values of hemodynamic variables are poorly effective in evaluating an actual recovery of the short-term autonomic mechanisms for blood pressure (BP) and heart rate (HR) regulation. The aim of this work is to analyze the response to therapy in the early phase of septic shock to verify possible associations between BP recovery and BP autonomic control.
Methods:
This is an ancillary study from the multicenter prospective observational trial Shockomics (NCT02141607). A total of 21 septic shock patients were studied at two time points during the acute phase of shock and were classified according to changes in SOFA score. Time series of BP components and HR were analyzed in time and frequency domain. Baroreflex sensitivity (BRS) was assessed, and a mathematical model for the decomposition of diastolic arterial pressure (DAP) oscillations was used to understand the different contributions of BRS and HR on peripheral vascular resistance control.
Results:
Only those patients, who significantly improved organ function (responders, R), showed an increase of mean value and low frequency (LF) power in BP time series. Fluid accumulation was higher in the non-responders (NR). BRS increased in NR and the model of DAP variability showed that the contribution of HR was highly reduced in NR.
Conclusions:
Although patients reached the mean BP target of 65 mmHg, our analyses highlighted important differences in terms of autonomic nervous system control. BP variability, HR variability and baroreflex trends can add information to individual vital sign measure such as mean BP, and can help in understanding the responsiveness to the combination of symphatomimetic drugs and fluid therapy.
Keywords: Baroreflex control, fluid balance, hemodynamics, septic shock, vasopressor
INTRODUCTION
It is well known from literature that early goal-directed therapy (EGDT) is not as effective as it initially advocated. Often, aggressive fluid resuscitation during the first hours may cause fluid overload that subsequently affects patients’ outcome. Persistent fluid overload during the first days increases the risk of cardiac, renal, and pulmonary dysfunction. Moreover, a positive fluid balance is associated with increased morbidity–mortality (1–4).
Indeed, since the first positive study of Rivers et al. (5), three recent large trials were not able to replicate these results and none of these studies demonstrated a benefit of EGDT protocol (6). The reasons for these inconsistencies in the medical literature are not fully clear: the complexity of the population under study and the lack of a pathophysiological approach to hemodynamic monitoring could be some possible explanations (7). In this regard, a high intersubject variability in response to treatment suggests the need for personalized approaches, i.e., patient-targeted therapy and not absolute thresholds.
The aim of EGDT is to restore blood pressure, i.e., mean arterial pressure (MAP), central venous pressure (CVP), and central venous oxygen saturation (ScvO2). However, the patient response to the initial hemodynamic optimization therapy cannot be evaluated solely by absolute values of vital signs, as they do not imply a restoration of the cardiovascular (CV) autonomic control. In other words, the restoration of the short-term physiological mechanisms for blood pressure and heart rate regulation cannot be inferred only from mean values of the classical hemodynamic variables.
In this study, the authors wanted to test the hypothesis that the changes in sympathetic autonomic nervous system (ANS) activity and in autonomic-mediated blood pressure (BP) control mechanisms may help to understand the actual effectiveness of fluid and vasopressor therapies and to correctly interpret the trend in mean arterial BP values (8–10). In particular, we analyzed patients’ response to therapy in early phase of septic shock and examined possible associations among BP recovery, BP autonomic control, and fluid accumulation.
PATIENTS AND METHODS
Study design and patients’ population
The present work is an ancillary study from the multicenter prospective observational trial Shockomics (see ClinicalTrials.gov Identifier NCT02141607). A complete description of the protocol can be found in (11). A subset of 21 septic shock patients enrolled at Genève University Hospital from October 2014 till December 2015 was included in this study, after approval of the Geneva regional research ethics committee (Commission cantonale d’éthique de la recherché, President: Prof. Bernhard Hirschel study number 14-041). All participants gave prior informed consent. Strict inclusion criteria were observed during the recruitment phase, as detailed reported in (11), to avoid a too high inhomogeneity within the population. The patients received initial therapy according to the standards (12) immediately after shock diagnosis (time T0). Patients were analyzed at two relevant time points: within 16 h from T0 when the inflammatory cascade has been just activated (T1), and within 48 h after T0 time point (T2).
SOFA score (Sequential Organ Failure Assessment score) was used to classify patients into two groups according to their responsiveness to early therapy: responder patients (R, n = 14) consisted in patients with a positive response to initial treatment. Those patients, who decreased their SOFA score of at least 5 points between T1 and T2 (ΔSOFAT2-T1 ≥5) or reached a SOFA score at T2 lower than 8 were classified as R. Non-responder patients (NR, n = 7) consisted in patients who still had a SOFA score at T2 higher than 8 or ΔSOFAT2-T1 <5.
Clinical and therapy data
Information on therapy and sedation administered to the patients were available and the overall dosage (μg/kg) of vasopressors and sedation drugs was calculated at T1 and T2. Vasopressors (VP) consisted in noradrenaline and adrenaline, whereas sedation drugs included dexmedetomidine, fentanyl, midazolam, and propofol. Fluid balance (FB, mL) was also retrieved at T1 and T2 for each patient.
Other clinical variables considered at T1 and T2 were as follows: lactate (mmol/L), C-reactive protein (CRP, mg/L), central venous oxygen saturation (ScvO2, %), cardiac output (CO, mL/min), stroke volume (SV, mL/beat), and ejection fraction (EF, %). Parameters related to specific organ systems were included: PaO2/FiO2 ratio (mmHg) and PEEP (mmHg) value for respiratory system together with the number of intubated patients, bilirubin (mg/dL) for the hepatic system, platelets count (103/mm3) for coagulation system, creatinine (mg/dL) together with urine output (mL/d), and the presence of acute kidney injury (AKI) for renal system, Glasgow Coma Scale (GCS) as an overall indication of the level of consciousness and sedation of the patients.
All patients except one had additional data provided by the hospital ICT system relating their entire intensive care unit (ICU) stay: minute-by-minute average measure of invasive systolic, diastolic, mean arterial pressure (SAP, DAP, MAP), and heart rate (HR), annotations of any change in the therapy (i.e., start, stop, or change of infusion rate for every administered drug). Information on daily fluid input, daily fluid output, and daily fluid balance of the entire ICU stay was also available for each patient. Mean and standard deviation were calculated every hour for SAP, DAP, MAP, and HR series; total cumulative amount of drugs administered was evaluated every 2 h. All the series were aligned with respect to time point T1. We focused on the first 10 ICU days as most of the patients in R group had an ICU length of stay less than 1 week before moving to another hospital ward.
Signal processing
The ICU is equipped with the Philips Intellivue MP70 monitor system. A laptop computer was connected to the monitor to synchronously download the waveforms by means of a dedicated software (iXtrend, ixellence GmbH, Germany). The arterial blood pressure (ABP) waveform was continuously recorded at 125 Hz at T1 and T2 during a stable condition of the patient, when no maneuvers or changes in the therapy occurred. The duration of ABP recordings spans from about 1 to 30 min (average time 13 min). We extracted beat-to-beat time series of systolic, diastolic, mean arterial pressure (SAP, DAP, and MAP, respectively), and heart period (HP), which is the time difference between consecutive onsets of ABP pulses, and it is considered as a surrogate of the RR-intervals time series. The beat-to-beat series were successively filtered according to standard procedures (13–15). Temporal relationships were maintained among the time series: given onset(i) as the onset of the current beat, HP(i) designated the difference between onset(i+1) and onset(i). SAP(i) follows onset(i) and is followed by DAP(i), which often coincides with onset(i+1). We subdivided each vital sign series into 2-min long windows and, to increase the number of windows, we adopted a 50% overlapping segmentation. Each window was resampled at 2 Hz by means of zero-order hold techniques, to obtain evenly spaced time series, and successively detrended using a 10th order polynomial function. Finally, we verified the stationary of the obtained time series by means of the Dickey–Fuller test.
Time and frequency indices were computed for each window and then averaged.
Hemodynamic analyses
We computed mean and standard deviation of each time series at T1 and at T2.
Spectral indices obtained from power spectra included low frequency (LF, 0.04–0.15 Hz) power; total power (TP), which represents the total area under the spectrum and it is a measure of the overall variability of the series; LF%, which represents the relative power in LF band, computed as LF/(TP−VLF)%, where VLF is the very low frequency band, 0 to 0.04 Hz.
The arterial baroreflex control mechanism was investigated through baroreflex sensitivity (BRS) analysis to assess the interrelationship between HP and SAP short-term oscillations. We adopted the bivariate model method, for further details see (16). Briefly, the two parameters of interest are the feedback gain, which represents the baroreflex mediated by the ANS, and the feedforward gain, which denotes the mechanical influence of RR intervals on systolic BP through the heart and vasculature, also named as runoff effect.
Finally, we implemented a model for the prediction of beat-to-beat variability of the diastolic component of peripheral blood pressure. Assuming that DAP variability is a surrogate measure of peripheral vascular resistance, we can say that the current DAP value is influenced both by the arterial baroreflex control mechanism and by the mechanical coupling between heart and circulatory system, the so-called runoff effect as previously introduced. A prolongation of the RR interval produces an immediate reduction in diastolic blood pressure (17) but, due to the increased time for cardiac filling, the subsequent stroke volume and pulse pressure increases via the Frank–Starling effect, the net result therefore is an increase in SAP in the next beat sensed by baroreceptor. This is followed subsequently by a more sustained decrease, reflecting the effect of a reduction in cardiac output produced by the lowered heart rate (18).
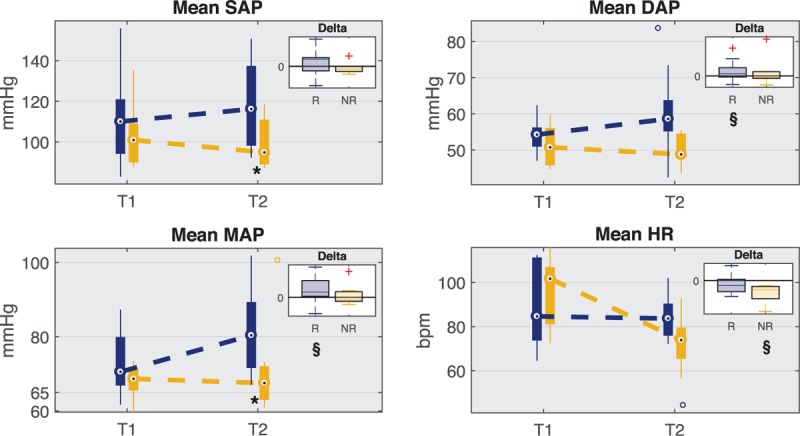
A black-box input–output model was used to disentangle these two mechanisms to highlight their different contribution on DAP variability at T1 and T2 in the two groups of patients. The model is represented by the following equation:
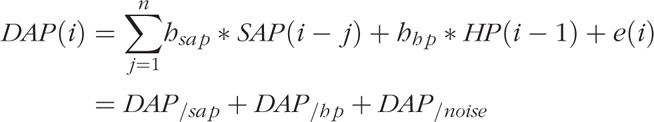 |
where the order n was fixed at 12 and the parameters were determined by least-squares minimization procedure. The DAP/sap component represents the black-box model for the arterial baroreflex-mediated sympathetic control of vasomotor tone, whereas the DAP/hp component is the mechanical effect of diastolic runoff.
The residual error (DAP/noise) includes all the remaining sources of variability not measured, such as the autoregulation-mediated control of peripheral resistance, artifacts, or noise.
To quantify the amount of DAP variability explained by the arterial baroreflex control or the mechanical runoff effect, a spectral decomposition was performed. In particular, we performed the spectral analysis of the model component series (DAP/sap, DAP/hp) and then we assessed the ratio between LF power of DAP/sap component over LF power of DAP (LF DAP/sap/LF DAP) and the ratio between LF power of DAP/hp component over LF power of DAP (LF DAP/hp/LF DAP).
Statistical analyses
We adopted the Mann–Whitney U test, also known as Wilcoxon rank-sum test, to verify significant differences in the indices values between the two groups (R and NR patients) separately at T1 and T2, whereas we used the Wilcoxon signed-rank test to assess significant changes from T1 to T2 within the same group of patients. The variations from T1 to T2 in the indices values, i.e., the deltas, were compared between the two groups by means of the Mann–Whitney U test.
For categorical parameters, i.e., data of incidence, we used the Fisher exact test.
Significance was considered with a P < 0.05.
RESULTS
Population and therapy
R and NR patients had similar clinical characteristics at admission, as reported in Table 1. NR stayed longer in the ICU and had higher mortality rate after 28 days from development of shock. The source of infection and the comorbidities were balanced between R and NR underlying that the different outcome of the two groups of patients was not due to preexisting pathologies, but it was highly dependent on patients’ response to therapy. Clinical variables and laboratory examinations values at time points T1 and T2 are shown in Table 2. According to inclusion criteria, all patients had a SOFA score higher than 8 at T1 and this was meant to ensure a similar severity of shock among patients at the enrollment. However, SOFA score at T1 was significantly higher in R than NR. This could be due to the limit number of patients and to a larger variance in the SOFA values in R group. For instance, in our clinical cohort two patients had a SOFA score of 16 at T1, but one improved (SOFAT2 = 10) and the other did not (SOFAT2 = 16). Moreover, CRP and lactate were comparable between the two groups at T1 confirming that the severity of inflammation was similar at the admission.
Table 1.
General characteristics of the two populations
| Responder | Non-responders | P | |
| Number of patients | 14 | 7 | |
| Age (yr) | 67.2 (62, 75) | 68.2 (61, 77.7) | NS |
| Weight (kg) | 85 (75, 90) | 75 (61.8, 78.8) | NS |
| Body mass index | 26.5 (24.1, 27.8) | 25.9 (20.4, 28.5) | NS |
| Males | 12 (85.7%) | 4 (57.1%) | NS |
| Total days in ICU | 4.5 (3, 6) | 10 (9.3, 19.8)* | 0.016 |
| Total days in hospital | 13.5 (11, 30) | 23 (17.5, 39) | NS |
| Mortality at 28 d | 2 (15.4%)n=13 | 5 (71.4%)§ | 0.022 |
| Mortality at 100 d | 2 (20%)n=10 | 5 (71.4%) | NS |
| Source of infection | |||
| Respiratory | 3 (21.4%) | 3 (42.9%) | NS |
| Abdominal | 3 (21.4%) | 3 (42.9%) | NS |
| Urinary tract | 5 (35.8%) | 1 (14.3%) | NS |
| Others | 3 (21.4%) | 0 (0%) | NS |
| Comorbidities | |||
| Chronic organ insufficiency | 13 (92.9%) | 6 (85.7%) | NS |
| Arterial hypertension | 5 (35.7%) | 3 (42.9%) | NS |
| Diabetes | 0 (0%) | 1 (14.2%) | NS |
| Coronary disease | 1 (7.1%) | 0 (0%) | NS |
| Systolic/diastolic disease | 0 (0%) | 1 (14.3%) | NS |
| Cerebrovascular disease | 1 (7.1%) | 1 (14.3%) | NS |
| Peripheral vascular disease | 1 (7.1%) | 0 (0%) | NS |
| Chronic lung disease | 2 (14.3%) | 2 (28.6%) | NS |
| Inflammatory bowel disease | 0 (0%) | 0 (0%) | NS |
| Liver disease | 1 (7.1%) | 1 (14.3%) | NS |
| Chronic kidney disease | 0 (0%) | 0 (0%) | NS |
| Acute heart failure | 8 (57.1%) | 4 (57.1%) | NS |
| Prolonged arrhythmias | 1 (7.1%) | 1 (14.3%) | NS |
Numerical data are reported as median (25th, 75th) percentile; categorical data are expressed as number of cases (percentage). Comparisons between R and NR: *P < 0.05 (Mann–Whitney U test). Comparisons between R and NR: §P < 0.05 (Fisher exact test)
Table 2.
Clinical data, laboratory data, total dose of vasopressor drugs VP (noradrenaline, adrenaline), and of sedation drugs (dexmedetomidine, fentanyl, midazolam and propofol) for responder and non-responder patients at time points T1 and T2
| Responder | Non-responder | |||||
| T1 | T2 | Delta | T1 | T2 | Delta | |
| SOFA score | 12.5 (11, 14) | 6 (6, 8) | −5 (−9, −4)§§§ | 16 (13.5, 16)* | 15 (13.5, 15.8)*** | 0 (−1.8, 0)°°° |
| Lactate (mmol/L) | 3.8 (2.8, 5.6) | 1.3 (1, 1.5) | −2.5 (−3.3, −1.4)§§§ | 4.7 (3.4, 7.8) | 2.7 (1.4, 3)* | −1.7 (−6.3, −0.4) |
| C-reactive protein (mg/L) | 271.5 (132.3, 378) | 212.8 (171.7, 257.7) | −34.6 (−104.2, 40.6) | 273.4 (96.8, 350.8) | 243.7 (154, 341.1) | 32.1 (−27.5, 103.2) |
| ScvO2 (%) | 76 (72, 78)n=10 | 71.5 (64.5, 72.5)n=8 | −6 (−10.3, −2.5) | 71 (61.8, 77.8)n=5 | 70 (61.5, 79.5)n=4 | −1.5 (−7, 1) |
| Cardiac output (L/min) | 5 (4.7, 6.9)n=11 | 5.7 (4.3, 6.1)n=13 | −0.4 (−1.1, 1.1) | 5.6 (3.7, 6.8) | 4.2 (3.5, 5.3)n=6 | −1.2 (−2.2, −0.3) |
| Stroke volume (mL/beat) | 64.9 (57.2, 74.9)n=11 | 58.6 (50.7, 75.2) | −0.18 (−2.4, 3.9) | 58.5 (45.5, 78.2)n=13 | 63.9 (54.8, 86.6)n=6 | 3.4 (−3.7, 7.8) |
| Ejection fraction | 55 (40, 60) | 47.5 (40, 60) | 0 (−5, 5) | 45 (22.5, 57.5) | 50 (35, 55)n=6 | 2.5 (−5, 5) |
| Fluid balance (mL) | 1466 (413, 2181) | −259 (−891, 557) | −1379 (−2400, −813)§ | 4075 (2552, 5039)** | 1488 (−133, 2837) | −3449 (−3888, −464)§ |
| VP dosage (mg) | 4.2 (0.6, 7.6) | 2.2 (1.7, 2.9)n=10 | −2.7 (−7.2, 1.4)n=10 | 11.9 (5.1, 16.5) | 15.7 (11.6, 17.9)*** | 3.8 (−0.7, 8.1)°° |
| Sedation dosage (mg) | 284.2 (160.6, 832)n=12 | 225 (1.4, 243 5.7)n=11 | −83.4 (−151, 126 4.4)n=10 | 203.9 (122.5, 749.9) | 125.5 (2.5, 242.2)n=6 | −212 (−606.4, 0.3)n=6 |
| Respiratory system | ||||||
| PaO2/FiO2 ratio (mmHg) | 183.7 (119, 265) | 272.4 (226.7, 316.7) | 72.8 (−7.9, 129.5)§ | 148.6 (132.1, 418.5) | 180 (171.9, 225.9) | 35.8 (−199.2, 47.2) |
| Tracheal intubation (#) | 13 (92.9%) | 8 (57.1%) | −5 (−35.7%)# | 6 (85.7%) | 6 (85.7%) | 0 (0%) |
| PEEP (mmHg) | 8 (7, 10)n=13 | 8.5 (7.5, 9.5)n=8 | −0.5 (−2.5, 0) | 10 (7, 12)n=6 | 8 (8, 10)n=6 | 0 (−2, 0) |
| Hepatic system | ||||||
| Bilirubin (mg/dL) | 1.3 (0.7, 1.9) | 0.65 (0.4, 1.5) | −0.4 (−0.9, −0.2)§§ | 1.3 (0.9, 14.1) | 1.4 (0.9, 14.2)* | 0 (0, 0.4)°° |
| Coagulation system | ||||||
| Platelets count (103/mm3) | 194 (101, 218) | 175 (77, 236) | −5 (−31, 20) | 112 (31.5, 138.3)* | 39 (17.5, 109.5)* | −8 (−41.8, 0) |
| Renal system | ||||||
| Creatinine (mg/dL) | 1.65 (1.1, 2) | 1.1 (0.6, 1.3) | −0.5 (−0.9, −0.2)§ | 1.7 (1.3, 2) | 1.3 (1.1, 2.4) | −0.1 (−0.5, 0.2) |
| Urine output (L/d) | 1.8 (0.8, 2.1) | 2.3 (1.4, 3.1) | 0.8 (−0.5, 1.3) | 0.8 (0.4, 1.5) | 0.6 (0.3, 1.7)** | −0.1 (−0.4, 0.2) |
| Acute kidney injury (#) | 3 (21.4%) | 2 (14.3%) | −1 (−7.1%) | 5 (71.4%) | 4 (57.1%) | −1 (−14.3%) |
| Nervous system | ||||||
| Glasgow Coma Score (GCS) | 3 (3, 4) | 11 (8, 14) | 6 (3, 11)§§ | 3 (3, 4.5) | 4 (3.3, 7.5)* | 1 (0, 2.5) |
Numerical data are reported as median (25th, 75th) percentile; categorical data are expressed as number of cases (percentage).
FiO2, fraction of inspired oxygen; PaO2, arterial partial oxygen pressure; PEEP, positive end-expiratory pressure; ScvO2, central venous oxygen saturation; VP, vasopressors.
Comparisons between R and NR: *P < 0.05, **P < 0.01, ***P < 0.001 (Mann–Whitney U test).
Comparisons between T1 and T2: §P < 0.05, §§P < 0.01, §§§P < 0.001 (Wilcoxon signed-rank test).
Comparison between delta R and delta NR: °°P < 0.01, °°°P < 0.001 (Mann–Whitney U test).
Comparisons between R and NR: #P < 0.05 (Fisher exact test).
Table 2 describes the total dosage of vasopressor (noradrenaline, adrenaline) and sedation drugs (dexmedetomidine, fentanyl, midazolam, and propofol) administered. We observe that NR had a significantly higher dosage of vasopressors at T2 with respect to R and a large increase from T1 to T2, in contrast to R. We also report the total cumulative dose of vasopressors and sedation drugs, computed every 2 h, administered during the first 10 days of ICU stay (Fig. 1): note that R patients received on average a lower dosage of vasopressors after T1 with respect to NR patients, whereas both groups received a similar sedation therapy.
Fig. 1.
Trend of cumulative total dosage of vasopressors and sedation drugs administered during the first 10 days of ICU stay for responder (R) and non-responder (NR) groups.
Total dosage is computed as the sum of all the drugs administered every 2 h. The vertical black line marks T1 time point. The gray shaded area represents the standard error (SE). The two panels on the right show a detail of the trends from T1 and the next 2 days.
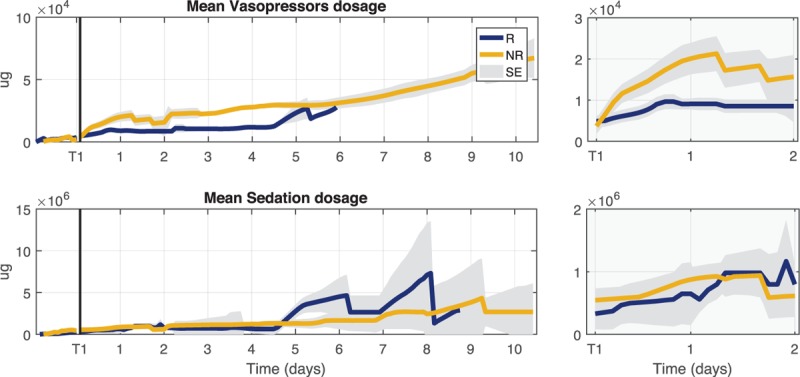
In addition, we analyzed the fluids administered as reported in Figure 2 where the daily trends of fluid input, fluid output, and fluid balance for the first 10 days of ICU stay are illustrated. NR received a similar initial fluid therapy at ICU admission (T0), but their fluid balance then became strongly positive at T1, in contrast to R patients, due to a higher dosage of fluid input and lower fluid output. The values of fluid balance at T1 and T2 and the delta between these two time points are also reported in Table 2.
Fig. 2.
Trends of daily measures of fluid input, fluid output, and fluid balance during the first 10 days of ICU stay for responder (R) and non-responder (NR) groups.
The gray shaded area represents the standard error (SE) of the mean estimation. T1 was used as time reference for all patients.
Hemodynamic variables and ANS indices
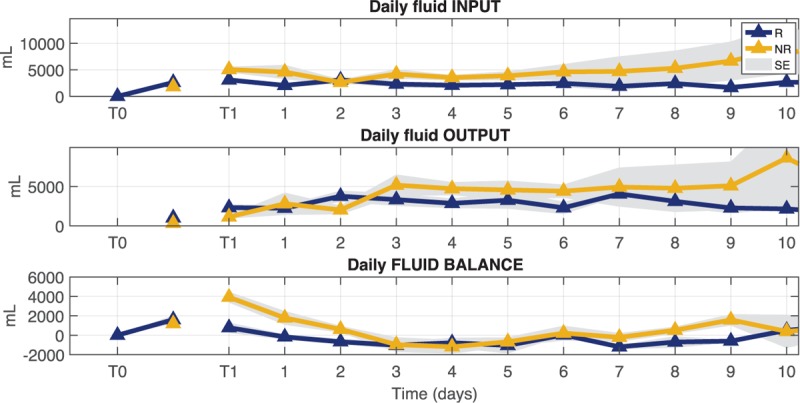
Figure 3 shows the distribution of mean values at each time point for SAP, DAP, MAP, and HR time series in both groups of patients. The upper right box displays the distribution of delta values computed as the difference between values at T2 and values at T1. The horizontal black line marks the zero value that means no change from T1 to T2.
Fig. 3.
Boxplot distribution of mean values for systolic (SAP), diastolic (DAP), mean (MAP) arterial pressure, and heart rate (HR) at time points T1 and T2 for responder (R) and non-responder (NR) patients.
The upper right box displays the distribution of delta values computed as the difference between values at T2 and values at T1 for both groups. The horizontal black line marks the zero value that means no change from T1 to T2. ∗P < 0.05 Mann–Whitney U test between R and NR, §P < 0.05 Wilcoxon signed-rank test between T1 and T2.
Mean value of BP showed an increase between T1 and T2 in responders; moreover, at T2 R patients had significantly higher values than NR for MAP and SAP. All mean values of BP components were slightly decreasing in NR patients between the two time points. A significant decrease of HR mean value from T1 to T2 was observed in NR.
Figure 4 displays the trend of the average of minute-by-minute MAP and HR values during the first 10 ICU days. The trend of MAP during the initial days was very similar in the two groups and, in particular, at T1 and T2 both groups maintained the target MAP value of 65 mmHg. The trend of HR showed instead a decrease in NR group with respect to a more stable trend in R group.
Fig. 4.
Trends of mean arterial pressure (MAP) and heart rate (HR) series during the first 10 days of ICU stay for responder (R) and non-responder (NR) groups.
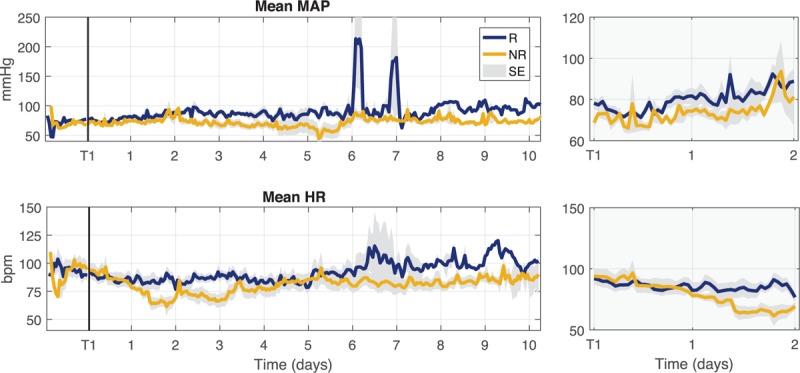
The reported values refer to hourly averaged values of the series. The vertical black line marks T1 time point. The gray shaded area represents the standard error (SE) of the mean estimation. T1 was used as time reference for all patients. The two panels on the right show a detail of the trends from T1 and the next 2 days.
Figure 5 shows the distribution of standard deviation (SD) and LF% values at each time point for SAP, DAP, MAP, and HR time series in both groups of patients. The overall variability in NR group was significantly lower with respect to R group: the standard deviation of SAP, DAP, and MAP was significantly lower at T2 in NR, and the standard deviation of HR was significantly lower at T1. In addition, at T2 the low-frequency component of DAP (LF% and LF absolute power, reported also in Table 3) was significantly lower in NR group.
Fig. 5.
Boxplot distribution of standard deviation (SD) and LF relative power (LF%) values for systolic (SAP), diastolic (DAP), mean arterial (MAP) pressure, and heart period (HP) at time points T1 and T2 for responder (R) and non-responder (NR) patients.
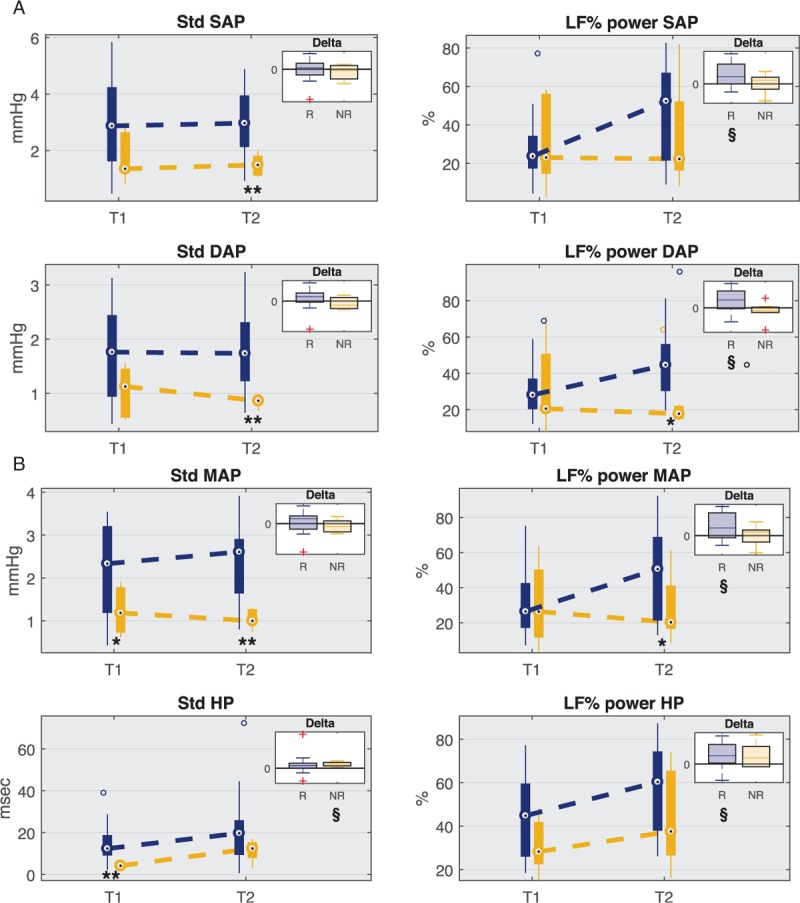
The upper right box displays the distribution of delta values computed as the difference between values at T2 and values at T1 for both groups. The horizontal black line marks the zero value that means no change from T1 to T2. ∗P < 0.05, ∗∗P < 0.01 Mann–Whitney U test between R and NR, §P < 0.05 Wilcoxon signed-rank test between T1 and T2, P < 0.05 Mann–Whitney U test between delta R and delta NR.
Table 3.
Baroreflex sensitivity and frequency indices for responder and non-responder patients at time points T1 and T2
| Responder | Non-responder | |||||
| T1 | T2 | Delta | T1 | T2 | Delta | |
| SAP | ||||||
| LF power (a.u.) | 121.8 (90, 173.4) | 246.3 (108.5, 278.4) | 51.5 (−2.2, 133) | 114.4 (60.6, 257.3) | 105.6 (81.3, 210) | 47.9 (−68.3, 62.7) |
| TP (a.u. ) | 525.7 (509, 626) | 534.6 (507.9, 549.9) | −0.9 (−72, 16) | 531.9 (520.5, 544) | 525 (498.7, 544.9) | −2.7 (−33.5, 31.6) |
| DAP | ||||||
| LF power (a.u. ) | 148.6 (94.7, 189) | 202 (156.1, 257.1) | 57.3 (4.3, 117.4)§ | 105.7 (80.5, 229.4) | 91.9 (74.8, 104.8)* | −13.8 (−91.5, 20.2)° |
| TP (a.u. ) | 521 (509.4, 549.5) | 526.1 (513.9, 546.8) | −1.5 (−24.1, 24.2) | 538.4 (516.2, 559.8) | 510.3 (446, 557.3) | −45.8 (−70.5, −3.3) |
| MAP | ||||||
| LF power (a.u. ) | 130.3 (79, 222.6) | 243.2 (106.5, 327.5) | 44.4 (−29.9, 245.3) | 119.9 (52.3, 225.1) | 99.3 (82, 223.2) | 44.3 (−86.5, 74.7) |
| TP (a.u. ) | 522.9 (502, 578.2) | 535.6 (525, 555.7) | 7 (−65.8, 25.4) | 546.9 (501.5, 557) | 533.2 (488, 558.5) | 11.5 (−67.3, 44.1) |
| HP | ||||||
| LF power (a.u. ) | 209 (127, 297.7) | 260.8 (185.6, 370.2) | 55.3 (21.9, 147.2) | 143.5 (113, 168.1) | 188 (131.3, 309.2) | 44.6 (6.7, 102.5) |
| TP (a.u. ) | 519.6 (511, 533.4) | 528.4 (514.7, 541.6) | 8.6 (−9, 36.2) | 529.6 (510.4, 546.9) | 536.6 (500.6, 545) | 24 (−60.8, 37.8) |
| BRS | ||||||
| Feedback (ms/mmHg) | 2.2 (1.4, 3.3) | 3.2 (2.1, 7.5) | 0.9 (−0, 3) | 1.3 (0.5, 1.7) | 5.2 (2.4, 8.1) | 3.5 (1.3, 6.1)§ |
| Feedforward (mmHg/ms) | 0.1 (0.05, 0.19) | 0.08 (0.04, 0.15) | −0.05 (−0.08, 0.05) | 0.15 (0.08, 0.3) | 0.06 (0.06, 0.1) | −0.04 (−0.29, 0.03) |
Values ad reported as median (25th, 75th) percentile.
BRS, baroreflex sensitivity; DAP, diastolic arterial pressure; HP, heart period; LF, low-frequency power; MAP, mean arterial pressure; SAP, systolic arterial pressure; TP, total power.
Comparisons between R and NR: *P < 0.05 (Mann–Whitney U test).
Comparisons between T1 and T2: §P < 0.05 (Wilcoxon signed-rank test).
Comparison between delta R and delta NR: °P < 0.05 (Mann–Whitney U test).
Figure 5 shows also a significant increase in LF% from T1 to T2 in responders for MAP, SAP, DAP, and HP, which was not reported in NR group.
With regard to the baroreflex sensitivity (Table 3), NR patients had significantly lower values of BRS feedback gain at T1 than R, but they showed a significant increase from T1 to T2 so that the gain became significantly higher in NR group at T2.
Diastolic blood pressure variability decomposition
Figure 6 illustrates the ratios distribution of DAP model components (LF DAP/sap/LF DAP and LF DAP/hp/LF DAP). In particular, in both groups the ratio computed between LF power of DAP/sap component over LF power of DAP, reflecting the portion of DAP variability mediated by the arterial baroreflex mechanism, was predominant with respect to the ratio between LF power of DAP/hp component over LF power of DAP, indicating the mechanical influence of the heart on the circulatory system (runoff effect). Moreover, we can notice that at T1 the component of DAP variability explained by the mechanical effect of the runoff was significantly lower in NR group. Finally, Table 4 reports the LF power values of DAP/sap and DAP/hp components, showing that the LF oscillations of the model component related to the HR were significantly lower in NR than in R group.
Fig. 6.
Ratio between LF absolute power of each predicted component and LF absolute power of diastolic blood pressure (DAP) at time points T1 and T2 for both groups of responder (R) and non-responder (NR) patients.
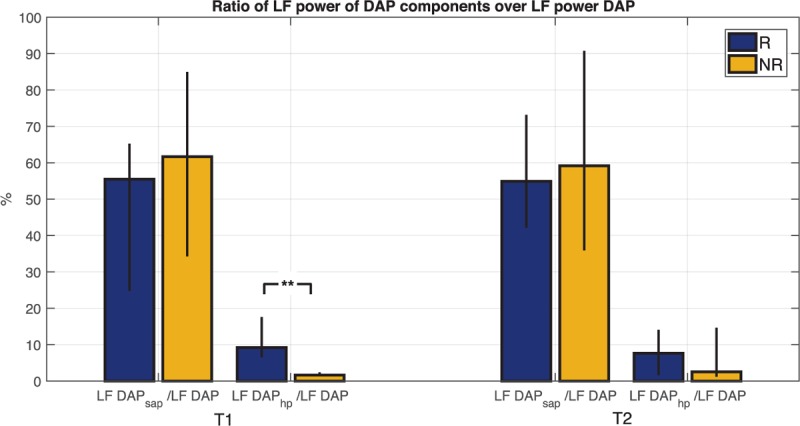
The height of the bar is the median value of the population; black bars indicate values of 25th and 75th percentile. ∗∗P < 0.01 Mann–Whitney U test between R and NR.
Table 4.
Low frequency (LF) power of DAP components variability for responder and non-responder patients at time points T1 and T2
| Responder | Non-responder | |||||
| T1 | T2 | Delta | T1 | T2 | Delta | |
| LF DAP/sap (a.u. ×10–3) | 5.82 (1.8, 11.6) | 8.38 (2.3, 17.3) | 1.47 (−2.7, 10.5) | 2.08 (0.4, 8.6) | 1.38 (0.6, 2)** | 0.16 (−7.5, 0.5) |
| LF DAP/hp (a.u. ×10–3) | 1.65 (0.6, 4.4) | 1.86 (0.2, 4.6) | −0.03 (−0.5, 1.2) | 0.06 (0, 0.1)** | 0.06 (0, 0.4)* | 0.04 (−0, 0.2) |
Values ad reported as median (25th, 75th) percentile.
Comparisons between R and NR: *P < 0.05, **P < 0.01 (Mann–Whitney U test)
DISCUSSION
In the present study, the early response to standard therapy in 21 septic shock patients was investigated by means of several hemodynamic indices. Our major finding is that common mean targets routinely used to guide hemodynamic optimization and fluid therapy in acute shock patients, such as mean arterial blood pressure, are not sufficient alone to explain the evolution of patient's organ dysfunction.
Specific blood pressure targets have been recommended for septic shock patients, for instance, sepsis guidelines recommend vasopressors administration to achieve and maintain a MAP of at least 65 mmHg in patients not responding to initial fluid resuscitation. In this regard, the actual blood pressure targets and their implementation are the main determinants of the patient's exposure to fluids and vasopressors (19). Interestingly, the cumulative vasopressor administration is independently associated with morbidity and mortality, whereas reduction of exposure to vasopressors has the potential of improving outcomes. For these reasons, the key point is to understand if new hemodynamic markers and targets can be defined for a more effective therapy with a real benefit on organ recovery and outcome.
In this study, patients were stratified as R or NR to therapy according to change in SOFA score between T1 and T2. All patients had similar severity of shock at the enrollment and they received the same initial therapy, but the R group improved their organs condition within the first 2 days after ICU admission (i.e., between T1 and T2), whereas the NR patients did not improve or even worsened.
NR patients displayed a higher fluid balance during the first days after shock development due to a larger fluid infusion and a reduction in urine output (Fig. 2). There are various possible explanations for this phenomenon, for instance, a higher capillary leak in these patients, or a prolonged vasoplegic state, which required a prolonged infusion of vasopressors with concomitant fluid infusions. Moreover, we must remind that vasopressors are intravenously administered and a prescription of a higher vasopressor dosage usually translates into a higher infusion rate with a consequently additional fluid infusion. The additional administration of fluids to maintain hydration should take into account this portion, but in the acute phase the decision on fluid therapy is not straightforward and the recovery from hypotension is of primary importance.
Interestingly, fluid accumulation is known to affect ANS response to sympathetic stimuli. In fact, previous studies reported that the large increase in the power of LF oscillations in RR series observed during active or passive standing in healthy subjects was not observed during other sympathetic activation activities in which central volume did not change, like the prolonged handgrip (20). Moreover, the magnitude of LF oscillations observed during exercise was reported to be influenced by body position, i.e., standing or supine positions—both of which are characterized by sympathetic activation (21). Another study reported that the LF variability component of RR intervals and muscle sympathetic nerve activity (MSNA) decreased in heart failure patients despite an overall increased sympathetic activation; one can interpret this result as being due to the increased central volume present in this pathological condition (22). Finally, a recent study compared the changes in cardiac sympathetic nerve activity (CSNA) during volume loading in two groups of normal and heart failure sheep, showing that the decrease in CSNA due to the fluid infusion in the normal sheep was not present in the heart failure group (9).
Given these premises, we hypothesized that the fluid overload in NR could have been one of the possible mechanisms that prevented the sympathetic outflow to the heart and to the peripheral resistance in these patients. In fact, a different trend in the overall variability and low frequency oscillations was observed: R patients increased LF% values of SAP, DAP, MAP series (Fig. 5), whereas NR patients did not despite a higher dosage of vasopressors (Table 2), which mainly act on the sympathetic outflow to the periphery. It is widely accepted that changes in LF oscillations of blood pressure can be related to the changes in the outflow of sympathetic nervous system and spectral analysis of blood pressure has been proved to be a powerful tool for the identification of the different cardiovascular control mechanisms that regulate BP (23, 24). From this perspective, the absence of LF increase in NR patients can be read as a sign of non-responsiveness to the vasopressor therapy or let think to a mechanism that prevented sympathetic nervous system outflow. Moreover, the large decrease in mean value of HR between T1 and T2 in the non-responders further supports this hypothesis: although an increasing trend in LF power of HR oscillations in NR patients, this did not translate into an increase in cardiac frequency. This could be a sign of a possible activation of vagal outflow to the heart mediated by the cardiopulmonary (CP) baroreflex. CP baroreceptors are low-pressure receptors located in the walls of the pulmonary artery and the cardiac chambers that sense the changes in central volume pressure and regulate ANS outflow to the heart and vessels.
The interaction between cardiopulmonary and arterial baroreflex has been widely demonstrated in literature: reflex forearm vascular resistance in response to carotid neck suction/pressure was augmented when the cardiopulmonary baroreceptors were unloaded (25); the reflex control of total peripheral resistance mediated by cardiopulmonary baroreflex almost doubled in seven conscious dogs after chronic arterial baroreceptor denervation (26).
In our study the feedback gain of the baroreflex mechanism, which quantifies the relationship between SAP and HR mediated by the nervous system, increased significantly at T2 in NR. If cardiopulmonary afferent activation indirectly affects the arterial baroreflex control of heart and peripheral resistance, then the elevated loading (or stretching) of CP baroreceptors, due to large central volume pressures, could be a possible explanation of the different trend in BRS feedback gain observed in NR patients with respect to R patients whose fluid balance significantly decreased at T2.
Commonly, the rising of BRS feedback gain is interpreted as a sign of recovery in various pathological conditions (27, 28). However, in NR patients the increase in BRS gain is accompanied by a fall in HR and by a slight decrease in mean BP from T1 to T2. The model of DAP variability decomposition can help in understanding this paradox: the portion of LF DAP modulated by the HR (LF DAP/hp) was highly reduced in NR compared to R (Table 4), meaning that the regulatory mechanism of DAP based on the mechanical effects of the runoff was suppressed in NR patients. These results hint that the rising of BRS feedback gain observed in NR is not a sign of recovery, but a compensatory mechanism to a condition of diminished HR and reduced sympathetic control of heart and vessels’ resistance.
We want to highlight that the decrease in HR and increase of BRS in NR patients could be interpreted as a prevalence of vagal tone. In fact, many studies demonstrate that the increase in baroreflex sensitivity control of HR is, in part, mediated by the enhancement of the vagal nerve (28).
All patients were able to maintain their MAP over the targeted threshold of 65 mmHg (Fig. 3), even if the trend between T1 and T2 in NR patients was slightly decreasing and they displayed lower values with respect to R patients both at T1 and T2. Looking at the international guidelines for acute shock resuscitation all these patients seemed to be responsive to the therapy.
However, the hemodynamic analyses allowed to discriminate those patients who actively responded restoring the autonomic nervous system regulation of BP and HR, and those who recovered from hypotension without a marked improvement in the cardiovascular autonomic control.
Finally, it is not unusual for septic shock patients to receive excess fluids even after they are weaned from vasopressors (29). In our opinion, one of the reasons to explain this observation is that indices relating to CV autonomic control are not commonly used to guide fluid therapy and our work is meant to contribute in this direction: this type of indices offer noninvasive options to guide fluid therapy and permit to assess the likely hemodynamic response of patients to the therapy.
Limitations
The main limitation of this study consists in the limited size of the population, collected in a single center. The small sample size did not allow to take into account all possible confounding factors, such as comorbidities or previous history of vasoactive therapy. Further studies should be conducted on a larger population and by taking into account the response to changes in vasopressor dosage or fluid administration in a tighter way so to better infer patient conditions.
CONCLUSIONS
In conclusion, BP variability, HR variability, and baroreflex trends can add information to individual vital signs such as MAP, and can help understanding the responsiveness to the combination of sympathomimetic drugs and fluid therapy.
The results of the present study suggest that mean values of vital signs only give an approximate overview of hemodynamic status of shocked patients. Indeed, static values of blood pressure do not carry information about the short-term cardiovascular regulatory mechanisms of heart rate and blood pressure, which are at the basis of the recovery of organs dysfunction in shocked patients.
Footnotes
MC and BBP are first co-authors.
KB and MF equally contributed to the work.
This study is supported by the EU FP7 Health Programme, ShockOmics project, Grant #602706.
The authors report no conflicts of interest.
REFERENCES
- 1.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, et al. ARISE Investigators, ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506. [DOI] [PubMed] [Google Scholar]
- 2.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372:1301–1311. [DOI] [PubMed] [Google Scholar]
- 3.The ProCESS investigators: a randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015; 43:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377. [DOI] [PubMed] [Google Scholar]
- 6.Lief L, Arbo J, Berlin DA. The physiology of early goal-directed therapy for sepsis. J Intensive Care Med 2016; 32:1–7. [DOI] [PubMed] [Google Scholar]
- 7.Kalil AC, Kellum JA. Is early goal-directed therapy harmful to patients with sepsis and high disease severity? Crit Care Med 2017; 45:1265–1267. [DOI] [PubMed] [Google Scholar]
- 8.Ligtenberg G, Blankestijn PJ, Koomans HA. Hemodynamic response during lower body negative pressure: role of volume status. J Am Soc Nephrol 1998; 9:105–113. [DOI] [PubMed] [Google Scholar]
- 9.Ramchandra R, Hood SG, Watson AMD, May CN. Responses of cardiac sympathetic nerve activity to changes in circulating volume differ in normal and heart failure sheep. Am J Physiol Regul Integr Comp Physiol 2008; 295:R719–R726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrario M, Moissl U, Garzotto F, Cruz DN, Tetta C, Signorini MG, Ronco C, Grassmann A, Cerutti S, Guzzetti S. The forgotten role of central volume in low frequency oscillations of heart rate variability. PLoS One 2015; 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletti F, Conti C, Ferrario M, Ribas V, Bollen Pinto B, Herpain A, Post E, Romay Medina E, Barlassina C, De Oliveira E, et al. ShockOmics: multiscale approach to the identification of molecular biomarkers in acute heart failure induced by shock. Scand J Trauma Resusc Emerg Med 2016; 24:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellinger R, Levy M, Rhodes A. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Intensive care Med 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 13.Zong W, Heldt T, Moody GB, Mark RG. An open-source algorithm to detect onset of arterial blood pressure pulses. Comput Cardiol 2003; 259–262. [Google Scholar]
- 14.Sun JX, Reisner AT, Mark RG. A signal abnormality index for arterial blood pressure waveforms. Comput Cardiol 2006; 33:13–16. [Google Scholar]
- 15.Wessel N, Voss A, Malberg H, Ziehmann C, Voss HU, Schirdewan A, Meyerfeldt U, Kurths J. Nonlinear analysis of complex phenomena in cardiological data. Herzschr Elektrophys 2000; 11:159–173. [Google Scholar]
- 16.Wyller VB, Barbieri R, Saul JP. Blood pressure variability and closed-loop baroreflex assessment in adolescent chronic fatigue syndrome during supine rest and orthostatic stress. Eur J Appl Physiol 2011; 111:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baselli G, Cerutti S, Civardi S, Malliani A, Pagani M. Cardiovascular variability signals: towards the identification of a closed-loop model of the neural control mechanisms. IEEE Trans Biomed Eng 1988; 35:1033–1046. [DOI] [PubMed] [Google Scholar]
- 18.Khoo MCK. Modeling of autonomic control in sleep-disordered breathing. Cardiovasc Eng 2008; 8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takala J. Should we target blood pressure in sepsis? Crit Care Med 2010; 38:S613–S619. [DOI] [PubMed] [Google Scholar]
- 20.Kiviniemi A, Frances M, Tiinanen S, Craen R, Rachinsky M, Petrella RJ, Seppänen T, Huikuri HV, Tulppo MP, Shoemaker J. α-Adrenergic effects on low-frequency oscillations in blood pressure and R–R intervals during sympathetic activation. Exp Physiol 2011; 96:718–735. [DOI] [PubMed] [Google Scholar]
- 21.Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol 2003; 90:317–325. [DOI] [PubMed] [Google Scholar]
- 22.Van de Borne P, Montano N, Pagani M, Oren R, Somers V. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation 1997; 95:1449–1454. [DOI] [PubMed] [Google Scholar]
- 23.Stauss HM, Anderson EA, Haynes WG, Kregel KC. Frequency response characteristics of sympathetically mediated vasomotor waves in humans. Am J Physiol 1998; 274:H1277–H1283. [DOI] [PubMed] [Google Scholar]
- 24.Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 2007; 34:362–368. [DOI] [PubMed] [Google Scholar]
- 25.Cooper VL, Hainsworth R. Effects of head-up tilting on baroreceptor control in subjects with different tolerances to orthostatic stress. Clin Sci 2002; 103:221–226. [DOI] [PubMed] [Google Scholar]
- 26.Mukkamala R, Kim JK, Li Y, Sala-Mercado J, Hammond RL, Scislo TJ, O’Leary DS. Estimation of arterial and cardiopulmonary total peripheral resistance baroreflex gain values: validation by chronic arterial baroreceptor denervation. Am J Physiol Heart Circ Physiol 2006; 290:H1830–H1836. [DOI] [PubMed] [Google Scholar]
- 27.Nardocci G, Martin A, Abarzúa S, Rodríguez J, Simon F, Reyes EP, Acuña-Castillo C, Navarro C, Cortes PP, Fernández R. Sepsis progression to multiple organ dysfunction in carotid chemo/baro-denervated rats treated with lipopolysaccharide. J Neuroimmunol 2015; 278:44–52. [DOI] [PubMed] [Google Scholar]
- 28.Laterza MC, De Matos LDNJ, Trombetta IC, Braga AMW, Roveda F, Alves MJNN, Krieger EM, Negrão CE, Rondon MUPB. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension 2007; 49:1298–1306. [DOI] [PubMed] [Google Scholar]
- 29.Cunha ARL, Lobo SMA. What happens to the fluid balance during and after recovering from septic shock? Rev Bras Ter Intensiva 2015; 27:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


