Supplemental Digital Content is available in the text
Keywords: epidemic potential, epidemiologic overlap, meta-analysis, sexually transmitted infection, systematic review
Abstract
Background:
Our objective was to assess the population-level association between herpes simplex virus 2 (HSV-2) and HIV prevalence.
Methods:
Reports of HSV-2 and HIV prevalence were systematically reviewed and synthesized following PRISMA guidelines. Spearman rank correlation ( ) was used to assess correlations. Risk ratios (RRHSV-2/HIV) and odds ratios (ORHSV-2/HIV) were used to assess HSV-2/HIV epidemiologic overlap. DerSimonian–Laird random-effects meta-analyses were conducted.
) was used to assess correlations. Risk ratios (RRHSV-2/HIV) and odds ratios (ORHSV-2/HIV) were used to assess HSV-2/HIV epidemiologic overlap. DerSimonian–Laird random-effects meta-analyses were conducted.
Results:
In total, 939 matched HSV-2/HIV prevalence measures were identified from 77 countries. HSV-2 prevalence was consistently higher than HIV prevalence. Strong HSV-2/HIV prevalence association was found for all data ( = 0.6, P < 0.001), all data excluding people who inject drugs (PWID) and children (
= 0.6, P < 0.001), all data excluding people who inject drugs (PWID) and children ( = 0.7, P < 0.001), female sex workers (
= 0.7, P < 0.001), female sex workers ( = 0.5, P < 0.001), and MSM (
= 0.5, P < 0.001), and MSM ( = 0.7, P < 0.001). No association was found for PWID (
= 0.7, P < 0.001). No association was found for PWID (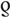 = 0.2, P = 0.222) and children (
= 0.2, P = 0.222) and children ( = 0.3, P = 0.082). A threshold effect was apparent where HIV prevalence was limited at HSV-2 prevalence less than 20%, but grew steadily with HSV-2 prevalence for HSV-2 prevalence greater than 20%. The overall pooled mean RRHSV-2/HIV was 5.0 (95% CI 4.7–5.3) and ORHSV-2/HIV was 9.0 (95% CI 8.4–9.7). The RRHSV-2/HIV and ORHSV-2/HIV showed similar patterns that conveyed inferences about HSV-2 and HIV epidemiology.
= 0.3, P = 0.082). A threshold effect was apparent where HIV prevalence was limited at HSV-2 prevalence less than 20%, but grew steadily with HSV-2 prevalence for HSV-2 prevalence greater than 20%. The overall pooled mean RRHSV-2/HIV was 5.0 (95% CI 4.7–5.3) and ORHSV-2/HIV was 9.0 (95% CI 8.4–9.7). The RRHSV-2/HIV and ORHSV-2/HIV showed similar patterns that conveyed inferences about HSV-2 and HIV epidemiology.
Conclusion:
HSV-2 and HIV prevalence are strongly associated. HSV-2 prevalence can be used as a proxy ‘biomarker’ of HIV epidemic potential, acting as a ‘temperature scale’ of the intensity of sexual risk behavior that drive HIV transmission. HSV-2 prevalence can be used to identify populations and/or sexual networks at high-risk of future HIV expansion, and help prioritization, optimization, and resource allocation of cost-effective prevention interventions.
Introduction
The ability to predict HIV epidemics and their scale is important to inform response [1]. This ability is of particular value in regions where HIV epidemics are emerging, such as in the Middle East and North Africa (MENA) [2], or in high-risk populations, where epidemics can emerge suddenly and rapidly, such as amongst MSM [3], or people who inject drugs (PWID) [4].
Self-reported sexual behavior data may provide one approach for understanding sexual networks and assessing HIV epidemic potential, but such data suffers from multiple limitations [5,6]. Self-reported data are subject to biases, and as they are egocentric, cannot capture the complexity of sexual-network structures [5,7]. Network structure, however, plays a critical role in determining the risk of exposure to HIV and other sexually transmitted infections (STIs) [6,8].
Mathematical modeling and empirical data have demonstrated recently that hepatitis C virus (HCV) prevalence can quantify the intensity of injecting risk behavior in PWID, and can predict HIV epidemic potential in this population [9–11]. This powerful concept of using the prevalence of one infection as an objective ‘biomarker’ to predict the prevalence of another infection, for infections that share the same mode of transmission, opens novel possibilities for understanding risk networks and the overlapping epidemiology of infectious diseases.
It was proposed and demonstrated recently, through in-silico simulations of HIV and herpes simplex virus type 2 (HSV-2) transmission in sexual networks, that HSV-2 prevalence could be used as a ‘summary collective measure’ of the intensity of sexual risk behavior in a sexual network, and can predict HIV epidemic potential [5,6].
Against this background, we investigated the empirical evidence for using HSV-2 prevalence as a ‘biomarker’ to predict HIV epidemic potential in a given population. We conducted a global systematic review of HSV-2 and HIV prevalence within the same population. We further conducted meta-analyses of HSV-2/HIV epidemiologic overlap, and assessed how this overlap varies by sex, region, and subpopulation. This is to our knowledge the first time in the literature that such an in-depth and systematic analysis of this ecological association for all world regions and for all different at-risk populations has been conducted and has quantified the association using primary data. We thus provided a comprehensive characterization of HSV-2/HIV overlapping epidemiology that yielded profound insights about HIV epidemiology and how sexual networks drive STI transmission.
Methods
Data sources and search strategy
We systematically reviewed HSV-2/HIV prevalence data as informed by Cochrane Collaboration Handbook for Systematic Reviews[12] and reported findings following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13,14] (Text S1 of Supplementary Information. We searched PubMed, Embase, the abstract archives of the International AIDS Society (IAS) and International Society for Sexually Transmitted Diseases Research (ISSTDR) conferences, with no language, geographic region, population, or publication year restrictions. We searched PubMed and Embase using a strategy with both free text and MeSH/Emtree headings up to 12 October 2016 (Search criteria in Text S2 of Supplementary Information). Additional data were identified in several grey literature reports.
Study selection
Search results were imported into a reference manager, Endnote X7, where duplicates were identified and excluded. Titles and abstracts of remaining records were screened for relevance by one author (S.P.K.). Full texts of potentially relevant records were retrieved and assessed for eligibility; and reference lists of all relevant articles were screened for additional studies. Reports were included if they presented primary data with serological measurements of HSV-2 and HIV prevalence in the same population; and, used a type-specific assay (based on ELISA or western blot test) for HSV-2 diagnosis. Reports were excluded if they included self-reported prevalence; and, included only HSV-2/HIV-infected individuals, or included individuals based on their infection status - such as serodiscordant couples. Case studies, editorials, commentaries, letters to editor, and review papers were excluded, though bibliographies of reviews were screened for potentially relevant sources.
The term ‘report’ was used to refer to the documents (articles, conference abstracts, or country-level reports) presenting results of a study [12]. The term ‘datapoint’ or ‘study’ refers to a measure of matched HSV-2/HIV prevalence. Datapoints duplicated in more than one report were included only once (using the more detailed report). If two reports report the same study, the report with the largest sample size was used. Outcomes in more than one population/setting within a report were included as separate datapoints.
Data extraction
Datapoints were extracted by one author (S.P.K.) using a prepiloted data-extraction form and entered into a computerized database. To ensure consistency, a random sample (20%) of studies was checked by a second author (K.C.). Extracted information included publication type, study design, year(s) and location of data collection, study population, sampling method, sample size, proportion of men/women, and number of individuals tested for, and infected with, HSV-2/HIV. Any discrepancies were settled by consensus or by contacting authors. Data from abstracts were extracted from the English abstract directly if not published as an article or if the full-text article was not available. Data from articles in English and Spanish were extracted from the full-texts - data from other language publications were extracted from the English abstract.
Plan of analysis
Populations were categorized based on the perceived level of sexual risk behavior into: high-risk [female sex workers (FSWs), clients of FSWs, and MSM), intermediate risk (such as prisoners and STI-clinic attendees), and general population (such as pregnant women and healthy adults). A full list of population categories is included in Text S3 of Supplementary Information. For the regional analyses, datapoints were categorized into the six divides: North America, South/Latin America, Europe, MENA, Sub-Saharan Africa (SSA), and Asia.
Descriptive analyses
We examined the ecological population-level association between HSV-2/HIV prevalence using scatterplots for all data, and for specific population categories. Spearman rank correlation was used to assess the correlation (expressed as 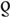 ) and P values, with significance set at P less than 0.05.
) and P values, with significance set at P less than 0.05.
Measures of the epidemiologic overlap
We explored both the risk ratio (RR) and odds ratio (OR) to quantify the epidemiological overlap between HSV-2/HIV, as has been done recently for HCV/HIV [10,11]. These two measures are closely related, but we used both because of subtle mathematical intricacies, and to provide description of the overlap using common measures in the literature [15].
The HSV-2/HIV RR was defined as the ratio of the prevalence (‘risk’) of HSV-2 to that of HIV in the same population:
 |
Similarly, the HSV-2/HIV OR was defined as the ratio of the odds of HSV-2 to that of HIV in the same population:
Histograms were used to assess the distribution of loge(RR) and loge(OR).
Meta-analyses
Meta-analyses for RRHSV-2/HIV/ORHSV-2/HIV were conducted by risk population, by subpopulation within the high-risk group, and for men and women separately. DerSimonian-Laird random-effects models were implemented using inverse-variance weighting [16]. This type of models assumes a normal distribution of true effect sizes across studies, and as such accounts for both, sampling variation and heterogeneity in effect size [17]. Meta-analyses excluded populations where the dominant mode of HIV transmission is perceived not to be sexual (PWID and children), studies with sample size less than 50, and studies with zero prevalence for both of HSV-2/HIV. Cochran's Q-test was used to assess evidence for heterogeneity in effect size - a P value less than 0.1 was considered significant [17,18]. I2 was computed to assess the proportion of between-study variation that is because of true differences in effect size [17]. Meta-analyses were conducted for all regions combined, and for all regions excluding SSA. SSA exclusion was because of the special nature of the HIV epidemics in this region.
For datapoints where HIV prevalence was zero, a 0.5 case number was added to allow for the computation of RRHSV-2/HIV/ORHSV-2/HIV. A sensitivity analysis was conducted by excluding all datapoints with zero HIV prevalence - results were invariable.
All analyses were carried out using STATA version 13.0 [19] (StataCorp LP, College Station, Texas, USA).
Results
Search results
Figure 1 describes the study selection process, as per PRISMA guidelines [13]. Records were identified through PubMed (2403), Embase (3712), and conference abstract archives (2554). After multiple screening levels, 386 reports were eligible and contributed 849 datapoints on 756 829 participants from 77 countries, the majority being from Asia and SSA (Fig. 1 and Table 1).
Fig. 1.
Flow chart of included studies, adapted from Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.

Data from [13].
Table 1.
Meta-analyses of the pooled mean risk ratio (RRHSV-2/HIV) of herpes simplex virus type 2 infection relative to HIV infection, by region and sex.
| Total | Men | Women | ||||||||||
| Region | Studies total N | Countries total N | Samples total N | Pooled mean RR (95% CI) | Qb (P value) | I2c (%) | Pooled mean RR (95% CI) | Qb (P value) | I2c (%) | Pooled mean RR (95% CI) | Qb (P value) | I2c (%) |
| North America | 82 | 6 | 81005 | 12.1 (9.3–15.7) | 2326.7 (P < 0.001) | 97 | 5.1 (3.1–8.4) | 492.7 (P < 0.001) | 97 | 22.6 (14.5–35) | 667.8 (P < 0.001) | 96.4 |
| South/Latin America | 106 | 14 | 77267 | 8.4 (6.9–10.3) | 1722 (P < 0.001) | 95.8 | 4 (3.4–4.7) | 560.3 (P < 0.001) | 93 | 62 (32.7–117.4) | 150.7 (P < 0.001) | 91.4 |
| Europe | 35 | 11 | 16900 | 5.6 (4.1–7.6) | 346.8 (P < 0.001) | 91.3 | 2 (1.7–2.4) | 28.9 (P < 0.001) | 75.8 | 41.7 (15.9–109.4) | 3.7 (P = 0.817) | 0 |
| Asia | 297 | 13 | 225950 | 5.6 (5.1–6.2) | 7442.5 (P < 0.001) | 96.4 | 3 (2.6–3.6) | 747.9 (P < 0.001) | 91.7 | 6.2 (5.6–7.0) | 1976.5 (P < 0.001) | 93.7 |
| Middle East and North Africa | 16 | 6 | 18244 | 23 (8.8–59.6) | 60.2 (P = 0.001) | 81.7 | 19.8 (8.1–48.2) | 19.4 (P = 0.004) | 69 | 37.8 (3–484.4) | 28 (P < 0.001) | 85.7 |
| Sub-Saharan Africa | 305 | 27 | 337463 | 3.4 (3.1–3.6) | 13422.4 (P < 0.001) | 98 | 3 (2.4–3.6) | 2200.4 (P < 0.001) | 97.8 | 3.3 (3–3.6) | 4636.7 (P < 0.001) | 97.3 |
| Global | 849a | 77 | 756829 | 5 (4.7–5.3) | 26574.3 (P < 0.001) | 97.2 | 3.5 (3.1–3.8) | 4429.1 (P < 0.001) | 95.9 | 5.6 (5.1–6) | 10098.6 (P < 0.001) | 97 |
| Global excluding Sub-Saharan Africa | 544 | 50 | 419366 | 7 (6.4–7.6) | 12576.9 (P < 0.001) | 96.3 | 3.7 (3.3–4.1) | 2200.5 (P < 0.001) | 93.9 | 9.3 (8.3–10.5) | 3502.3 (P < 0.001) | 94.8 |
HSV-2, herpes simplex virus type 2; RR, risk ratio; CI, confidence interval.
aNumbers do not add up because of missing country variables for some of the datapoints. For sensitivity analyses, the analyses were repeated excluding these datapoints. Similar results were found.
bQ: the Cochran's Q-statistic, a measure assessing the existence of heterogeneity in effect size.
cI2: a measure assessing the magnitude of between-study variation that is because of differences in effect size across studies rather than chance.
Descriptive analysis
Scatterplots of the association between HSV-2/HIV prevalence are shown in Fig. 2. Strong and statistically significant correlation was found for all datapoints (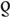 = 0.6, P < 0.001; Fig. 2a), FSWs (
= 0.6, P < 0.001; Fig. 2a), FSWs ( = 0.5, P < 0.001; Fig. 2b), MSM (
= 0.5, P < 0.001; Fig. 2b), MSM ( = 0.7, P < 0.001; Fig. 2c), and people who use but do not inject drugs (
= 0.7, P < 0.001; Fig. 2c), and people who use but do not inject drugs (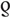 = 0.7, P < 0.001; Fig. 2d). However, the correlation was weak and not statistically significant for PWID (
= 0.7, P < 0.001; Fig. 2d). However, the correlation was weak and not statistically significant for PWID (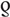 = 0.2, P = 0.222; Fig. 2e) and children including orphans of HIV+ mothers (
= 0.2, P = 0.222; Fig. 2e) and children including orphans of HIV+ mothers ( = 0.3, P = 0.082; Fig. 2f). By excluding all data points from nonsexual-transmission populations (PWID and children), the correlation increased in strength (
= 0.3, P = 0.082; Fig. 2f). By excluding all data points from nonsexual-transmission populations (PWID and children), the correlation increased in strength ( = 0.7, P < 0.001; Fig. 2g).
= 0.7, P < 0.001; Fig. 2g).
Fig. 2.
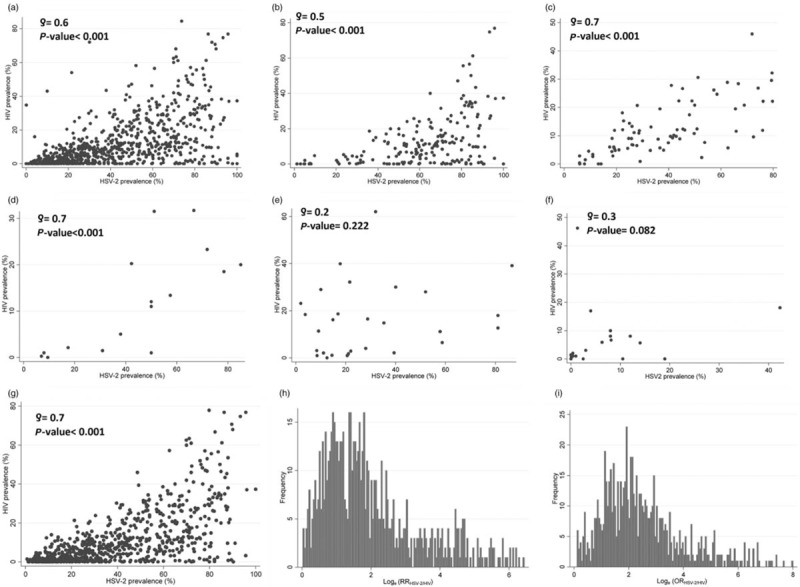
Descriptive analyses of the population-level association between herpes simplex virus type 2 (HSV-2) and HIV prevalence. Scatterplots of HIV versus HSV-2 prevalence in the following populations: (a) all datapoints; (b) female sex workers; (c) MSM; (d) people who use drugs but do not inject; (e) people who inject drugs; (f) children; and (g) all datapoints excluding populations where the dominant mode of HIV transmission was not sexual (people who inject drugs and children). The global distribution of loge(RR) and data (h) and loge(OR) data (i).
HSV-2 prevalence was consistently higher than HIV prevalence in all populations (Fig. 2a-d and g) apart from PWID and children (Fig. 2e and F). From visual inspection of Fig. 2, a threshold effect seems apparent where HIV prevalence was very low or vanishing at HSV-2 prevalence less than 20% or so, but growing steadily with HSV-2 prevalence for HSV-2 prevalence greater than 20% (Fig. 2g). This effect seemed most pronounced for FSWs (Fig. 2b).
Figure 2h and i present the loge(RR) and loge(OR) distributions including all data points excluding PWID and children. These figures suggest a roughly normal distribution with a positive skew and a median of 1.6 for loge(RR) (RRHSV-2/HIV = 5), and a median of 2.2 for loge(OR) (ORHSV-2/HIV = 9).
Measures of herpes simplex virus type 2/HIV epidemiologic overlap
Table 1 and Table S1 of Supplementary Information show the pooled mean RRHSV-2/HIV and ORHSV-2/HIV, respectively, by region and globally, for all data points excluding PWID and children.
Overall risk ratio and odds ratio
The overall RRHSV-2/HIV was 5 [95% confidence interval (CI) 4.7–5.3], increasing to 7 (95% CI 6.4–7.6) when SSA was excluded from the analysis (Table 1). The overall ORHSV-2/HIV was 9 (95% CI 8.4–9.7), increasing to 13.6 (95% CI 12.2–15.2) with SSA exclusion (Table S1). There was strong evidence for heterogeneity with a P value 0.001 or less. Most variation was because of heterogeneity in effect size (that is RRHSV-2/HIV or ORHSV-2/HIV) across studies rather than chance (I2 > 90%).
Risk ratio and odds ratio by region
The RRHSV-2/HIV was the lowest in SSA at 3.4 (95% CI 3.1–3.6) and the highest in MENA at 23 (95% CI 8.8–59.6). It was comparable in Asia and Europe at 5.6 (95% CI 5.1–6.2) and 5.6 (95% CI 4.1–7.6), respectively, and higher in South/Latin America and North America at 8.4 (95% CI 6.9–10.3) and 12.1 (95% CI 9.3–15.7), respectively.
The ORHSV-2/HIV (Table S1 of Supplementary Information) followed the same regional patterns as those of RRHSV-2/HIV. There was strong evidence for heterogeneity in both measures in all regions. Most variation was because of heterogeneity in effect size across studies.
Risk ratio and odds ratio by sex
The RRHSV-2/HIV for women was 5.6 (95% CI 5.1–6) versus only 3.5 (95% CI 3.1–3.8) for men (Table 1). After excluding SSA, the difference was even larger; 9.3 (95% CI 8.3–10.5) for women versus 3.7 (95% CI 3.3–4.1) for men. The RRHSV-2/HIV for women was consistently much higher than that for men across all regions except SSA – here the difference was minimal [3.3 (95% CI 3–3.6) for women versus 3 (95% CI 2.4–3.6) for men].
The ORHSV-2/HIV (Table S1 of Supplementary Information) followed the same sex patterns as those of RRHSV-2/HIV. There was generally strong evidence for heterogeneity in both measures. Most variation was because of heterogeneity in effect size across studies.
Risk ratio and odds ratio by risk population and subpopulation
The RRHSV-2/HIV in the different risk populations, both including and excluding SSA, can be seen in Fig. 3a. The RRHSV-2/HIV was the highest among the general population at 5.8 (95% CI 5.4–6.3), but was comparable among high-risk and intermediate-risk populations at 4.7 (95% CI 4.4–5.2) and 4.2 (95% CI 3.7–4.7), respectively.
Fig. 3.
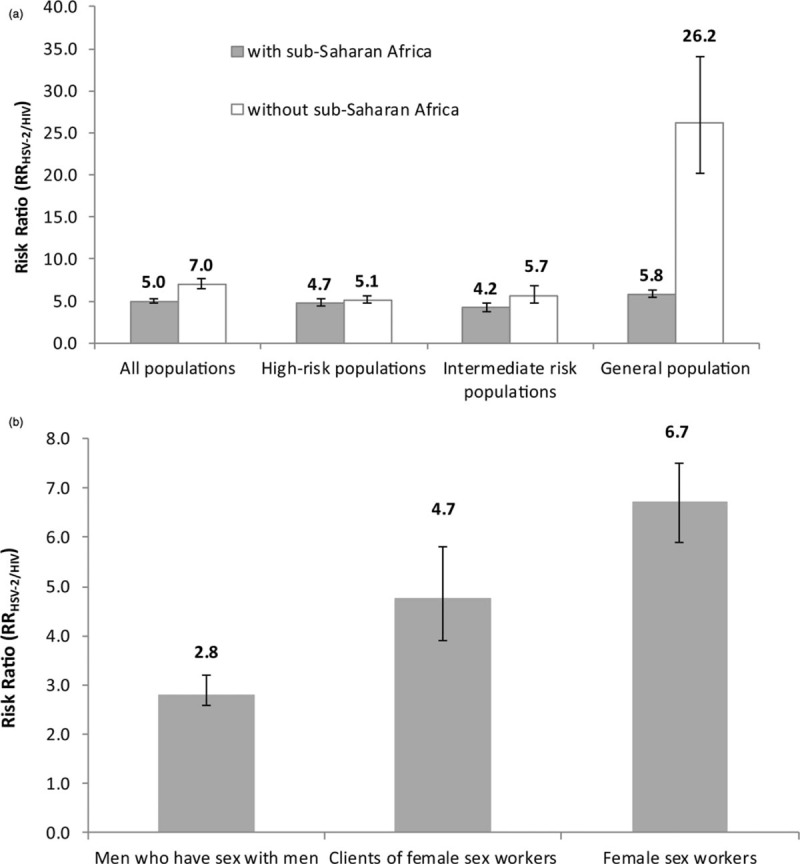
Meta-analyses of the pooled mean risk ratio (RRHSV-2/HIV) for herpes simplex virus type 2 (HSV-2) infection relative to HIV infection among (a) different population groups including and excluding data from Sub-Saharan Africa, and (b) different high-risk subpopulations.
After excluding SSA, the RRHSV-2/HIV was still the highest, but much higher, among the general population at 26.2 (95% CI 20.2–34.1), and remained comparable among high-risk and intermediate-risk populations at 5.1 (95% CI 4.7–5.6) and 5.7 (95% CI 4.7–6.8), respectively.
The RRHSV-2/HIV in the different subpopulations of the high-risk population can be seen in Fig. 3b. The highest RRHSV-2/HIV was among FSWs at 6.7 (95% CI 5.9–7.5), followed by clients of FSWs at 4.7 (95% CI 3.9–5.8), and MSM at 2.8 (95% CI 2.6–3.2).
The ORHSV-2/HIV (Fig. S1 of Supplementary Information) followed the same risk population and subpopulation patterns as those of RRHSV-2/HIV. The RRHSV-2/HIV/ORHSV-2/HIV for the general, intermediate-risk, and high-risk populations stratified by region are shown in Table S2 of Supplementary Information. There was generally strong evidence for heterogeneity in both measures. Most of the variation was because of heterogeneity in effect size across studies.
Discussion
Through a comprehensive global analysis, we found that there is a strong association between HSV-2 and HIV prevalence, with HSV-2 prevalence being consistently higher than HIV prevalence. This strong association was evident in all world regions and all populations where HIV is sexually transmitted. With such an association, HSV-2 prevalence can be used as a proxy of HIV prevalence, and could be used to predict future HIV prevalence in a population where HIV has not yet been introduced, or has not yet reached its epidemic potential.
These results are consistent with these two infections propagating in the same sexual networks and sharing closely linked epidemiology. Indeed, no association was found in populations where HIV's perceived mode of transmission was not sexual, such as for PWID and children. These findings, based on empirical data, validate mathematical modelling analyses that predicted similar findings based on HIV/HSV-2 transmission simulations [5,6].
These results imply also that HSV-2 prevalence is effectively a ‘summary collective measure’ of the intensity of sexual risk that drives STI transmission in a sexual network, and accordingly HSV-2 prevalence was strongly associated with HIV prevalence. A complementary explanation involves an HSV-2/HIV biological interaction, as suggested by earlier observational evidence [20–23]. This seems less likely, however, with the outcomes of clinical trials that have questioned this biological synergy [24–26], as well as a recent mathematical modeling analysis investigating the capacity of observational studies to assess such biological synergy [27]. The lack of an association we observed in PWID (Fig. 2E), despite the possibility of some residual sexual transmission among them, further sheds doubts about the existence or scale of an HSV-2/HIV biological synergy.
One manifestation of how HSV-2 prevalence provides a measure of the intensity of sexual risk behavior is the apparent threshold effect (Fig. 2b and g). In populations, where HSV-2 prevalence was less than 20%, HIV prevalence seemed limited or vanishing; risk-behavior intensity was not sufficient to sustain HIV transmission, though it could sustain (the more infectious) HSV-2 infection. In populations where HSV-2 prevalence was greater than 20%, HIV prevalence increased steadily with HSV-2 prevalence, as risk-behavior intensity was sufficient to sustain the propagation of both infections, with higher prevalence for both with higher risk-behavior intensity. These findings support the modeling analyses that predicted the existence of this threshold effect at HSV-2 prevalence of about 20% [5,6]. Further quantitative analyses are needed to investigate this apparent threshold effect, estimate its magnitude for the different at-risk populations, examine potential confounding factors affecting it, and link it to the theoretical mathematical modeling predictions [5,6].
For the global data, the RRHSV-2/HIV (that is ratio of HSV-2 to HIV prevalence) had a value of 5 indicating that, in average, HSV-2 prevalence was five times higher than HIV prevalence. This ratio, however, differed by sex, risk population/subpopulation, and region. It was much higher for women than men (Table 1). This could be in part explained by women being more biologically susceptible to HSV-2 infection, and thus have higher prevalence [28], whereas HIV is biologically equally transmissible for both sexes [29]. The RRHSV-2/HIV for MSM was very low (Fig. 3b), probably a consequence of anal-sex HIV transmission probability being 10-fold higher than that through vaginal sex [30,31], leading to a relatively higher HIV prevalence than expected for a heterosexual population.
Our results provide suggestive evidence that the RRHSV-2/HIV (or ORHSV-2/HIV) may have value as ‘epidemic diagnostics’ of the phase, sustainability, and type of HIV epidemics circulating in a population. The RRHSV-2/HIV was low for both SSA and for men in all regions but MENA (Table 1). This may be explained by the established phase of the HIV epidemics (reaching their epidemic potential) in SSA and among MSM in most regions, as supported by our knowledge of the global epidemiology of HIV [32,33]. Meanwhile, the RRHSV-2/HIV in MENA was very high, consistent with the evidence indicating recently emerging HIV epidemics that have not yet reached their epidemic potential [3,4,34].
The RRHSV-2/HIV in the general population was much lower in SSA than that in other regions (Fig. 3a). This may be explained by the heterosexual HIV epidemics in the general population in SSA, whereas the general-population component of the HIV epidemic has been weak and nonsustainable in the other regions [1,32,33]. The potential use of the RRHSV-2/HIV (or ORHSV-2/HIV) as ‘epidemic diagnostics’ merits further investigation not only because of the practical epidemiologic relevance, but also because of theoretical implications about how STIs propagate in sexual networks [6].
Our study has several limitations. The availability of data varied by region, country, sex, and risk population/subpopulation, thereby constraining the scope of the analyses. Although we identified strong HSV-2/HIV correlation, HSV-2 alone cannot explain the variation in HIV prevalence. It has been recently suggested that there are distinctions in how sexual networks affect each infection transmission in a population [6]. Biological cofactors (such as male circumcision [35,36]) can also affect each infection differentially [37], thereby complicating the association. Interventions, whether they affect both infections (such as condom use), or one of them (such as antiretroviral treatment) can further complicate the association. There was heterogeneity in the RRHSV-2/HIV (and ORHSV-2/HIV) across studies. Further work is needed to better understand this heterogeneity and implementation of these measures as epidemic diagnostics.
A main challenge to this approach remains that, overall, HIV data are more abundant than HSV-2 data, as there are no specific testing or screening programs for HSV-2. However, there are different situations where HSV-2 data are available and hence can be used to make predictions about HIV future spread. This approach is especially useful in situations where HIV prevalence is low and where there might be lower incentive for continuous HIV surveillance, especially in more resource-limited settings with competing health priorities – say different countries in Asia, most notably in India with ample HSV-2 data [38]. In such cases, HSV-2 prevalence, if available, can be used as a guide to whether HIV prevalence might increase and, therefore, whether HIV monitoring and surveillance should be prioritized. Of notice that whereas repeated HIV surveillance surveys are needed to track the HIV epidemic, a single HSV-2 survey may be sufficient to assess the levels and intensity of sexual risk behavior and HIV epidemic potential in a population – thus helping in the prioritization of limited surveillance resources.
One example to this end is in Pakistan where surveillance studies have been conducted among key populations at higher risk of infection, some of which had measured the prevalence of both HIV and HSV-2. Although in earlier studies HIV prevalence was very limited among different types of male sex workers, HSV-2 prevalence was exceptionally high among transgender sex workers – also called hijras [39], suggesting considerable HIV epidemic potential among this male sex workers subgroup. Indeed, repeated rounds of surveillance confirmed this HSV-2-based prediction where HIV prevalence increased among hijras from 0.8% in 2005 [40] to 1.8% in 2006 [41], and to 6.4 [42] and 5.2% [43] in 2008 and 2011, respectively, whereas it remained at about 1% among the other male sex worker subgroups who had relatively low HSV-2 prevalence [40–43].
Conclusion
We identified and quantified a strong HSV-2/HIV prevalence association through a systematic global analysis. We also identified an apparent threshold effect whereby HIV prevalence was limited below a specific HSV-2 prevalence level. We used two measures of the HSV-2/HIV epidemiologic overlap, and found that these measures can potentially be used as ‘epidemic diagnostics’ of the phase, sustainability, and type of HIV epidemics. These findings open new approaches to understand the link between sexual risk behavior and STI transmission, and how data on one STI can inform the epidemiology of another STI. Further research is needed to investigate similar associations between any two pairs of STIs, and all of them collectively, to elucidate their overlapping epidemiology in different population groups and settings.
Our findings demonstrate that HSV-2 prevalence can be used as a proxy ‘biomarker’ of HIV epidemic potential, acting as a ‘temperature scale’ of the intensity of sexual risk behavior that drives HIV transmission. HSV-2 prevalence can be used to identify populations and/or sexual networks at high-risk of future HIV expansion, and as a criterion in the prioritization, optimization, and resource allocation of cost-effective HIV/STI-specific prevention interventions. These findings are of special importance in countries (such as in MENA) with poor surveillance systems, where the status of the HIV epidemic remains poorly known, and where high-risk populations are hidden and stigmatized. They also highlight the importance and value of including HSV-2 prevalence in integrated biobehavioral surveillance surveys that may find vanishing HIV prevalence, as a zero HIV prevalence does not necessarily imply no potential for future HIV spread.
Acknowledgements
Funding: This publication was made possible by NPRP grant number 5-752-3-177 from the Qatar National Research Fund (a member of Qatar Foundation). The findings achieved herein are solely the responsibility of the authors. The authors are also grateful for infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine-Qatar. R.O. also acknowledges the support of JSPS Grant-in-Aid for Young Scientists (B) 15K19217 of Japan Society for the Promotion of Science.
Authors’ contributions: S.P.K. conducted the systematic review of the literature, data retrieval, extraction, analysis, and synthesis. M.H. with S.P.K. wrote the first draft of the article. K.C. contributed to the data extraction process. G.M. contributed to study design and methodology development. R.O. contributed to data analysis. P.V. contributed to study conception and data analysis. L.J.A. conceived and led the design of the study, analyses, and drafting of the article. All authors contributed to the conduct of the research, interpretation of the results, and drafting of the article, and approved the final manuscript.
Conflict of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Abu-Raddad LJ, Akala FA, Semini I, Riedner G, Wilson D, Tawil O. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa: time for strategic action. Middle East and North Africa HIV/AIDS Epidemiology Synthesis Project. World Bank/UNAIDS/WHO Publication. Washington DC: The World Bank Press; 2010. [Google Scholar]
- 2.UNAIDS. Prevention Gap Report. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland, July 2016. Available at: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf [Accessed October 2016] [Google Scholar]
- 3.Mumtaz G, Hilmi N, McFarland W, Kaplan RL, Akala FA, Semini I, et al. Are HIV epidemics among men who have sex with men emerging in the Middle East and North Africa?: a systematic review and data synthesis. PLoS Med 2011; 8:e1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mumtaz GR, Weiss HA, Thomas SL, Riome S, Setayesh H, Riedner G, et al. HIV among people who inject drugs in the Middle East and North Africa: Systematic review and data synthesis. PLoS Med 2014; 11:e1001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Schiffer JT, Ashley R, Mumtaz G, Alsallaq RA, Akala FA, et al. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics 2010; 2:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omori R, Abu-Raddad LJ. Sexual network drivers of HIV and herpes simplex virus type 2 transmission. AIDS 2017; 31:1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RM, Renzetti CM. The problems of researching sensitive topics. Am Behav Scientist 1990; 33:510–528. [Google Scholar]
- 8.Kretzschmar M, Morris M. Measures of concurrency in networks and the spread of infectious disease. Math Biosci 1996; 133:165–195. [DOI] [PubMed] [Google Scholar]
- 9.Vickerman P, Hickman M, May M, Kretzschmar M, Wiessing L. Can hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis. Addiction 2010; 105:311–318. [DOI] [PubMed] [Google Scholar]
- 10.Mumtaz GR, Weiss HA, Vickerman P, Larke N, Abu-Raddad LJ. Using hepatitis C prevalence to estimate HIV epidemic potential among people who inject drugs in the Middle East and North Africa. AIDS 2015; 29:1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbarzadeh V, Mumtaz GR, Awad SF, Weiss HA, Abu-Raddad LJ. HCV prevalence can predict HIV epidemic potential among people who inject drugs: mathematical modeling analysis. BMC Public Health 2016; 16:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiley-Blackwell, The Cochrane collaboration. Cochrane Handbook for Systematic Reviews of Interventions. 2008. [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. PA, USA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 16.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Front matter. Introduction to meta-analysis. New Jersey, USA: John Wiley & Sons, Ltd; 2009. i–xxix. [Google Scholar]
- 18.Higgin J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analysis. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stata/se 13.1. StataCorp. Stata: Release 13. Statistical Software. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 20.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM, Jr, Self SG, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008; 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanton C, Abu-Raddad LJ, Weiss HA. Time to refocus on HSV interventions for HIV prevention?. J Infect Dis 2011; 204:1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Looker KJ, Elmes JAR, Gottlieb SL, Schiffer JT, Vickerman P, Turner KME, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis 2017; 17:1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010; 375:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, et al. HPTN 039 Protocol Team. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, et al. HSV trial team; Steering and Data Monitoring Committees. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med 2008; 358:1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omori R, Nagelkerke N, Abu-Raddad LJ. HIV and herpes simplex virus type 2 epidemiological synergy: misguided observational evidence? A modelling study. Sex Transm Infect 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 2002; 186 Suppl 1:S3–S28. [DOI] [PubMed] [Google Scholar]
- 29.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005; 191:1403–1409. [DOI] [PubMed] [Google Scholar]
- 30.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol 2010; 39:1048–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS 2014; 28:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Griensven F, de Lind van Wijngaarden JW, Baral S, Grulich A. The global epidemic of HIV infection among men who have sex with men. Curr Opin HIV AIDS 2009; 4:300–307. [DOI] [PubMed] [Google Scholar]
- 33.Awad SF, Abu-Raddad LJ. Could there have been substantial declines in sexual risk behavior across sub-Saharan Africa in the mid-1990s?. Epidemics 2014; 8:9–17. [DOI] [PubMed] [Google Scholar]
- 34.Mumtaz GR, Riedner G, Abu-Raddad LJ. The emerging face of the HIV epidemic in the Middle East and North Africa. Curr Opin HIV AIDS 2014; 9:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss HA, Halperin D, Bailey RC, Hayes RJ, Schmid G, Hankins CA. Male circumcision for HIV prevention: from evidence to action?. AIDS 2008; 22:567–574. [DOI] [PubMed] [Google Scholar]
- 36.Weiss HA, Thomas SL, Munabi SK, Hayes RJ. Male circumcision and risk of syphilis, chancroid, and genital herpes: a systematic review and meta-analysis. Sex Transm Infect 2006; 82:101–109. discussion 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Raddad LJ, Barnabas RV, Janes H, Weiss HA, Kublin JG, Longini IM, Jr, et al. Have the explosive HIV epidemics in sub-Saharan Africa been driven by higher community viral load?. AIDS 2013; 27:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Summary Report – India (July 2011), Integrated Behavioural and Biological Assessment (IBBA), Round 2 (2009-2010). New Delhi: Indian Council of Medical Research and FHI 360. [Google Scholar]
- 39.Hawkes S, Collumbien M, Platt L, Lalji N, Rizvi N, Andreasen A, et al. HIV and other sexually transmitted infections among men, transgenders and women selling sex in two cities in Pakistan: a cross-sectional prevalence survey. Sex Transm Infect 2009; 85 Suppl 2:ii8–ii16. [DOI] [PubMed] [Google Scholar]
- 40.Pakistan National AIDS Control Program. HIV Second Generation Surveillance in Pakistan. National Report Round I. Canada-Pakistan HIV/AIDS Surveillance Project. National Aids Control Program, Ministry of Health, Pakistan, 2005. Available at: http://www.nacp.gov.pk/library/reports/Surveillance%20&%20Research/HIV-AIDS%20Surveillance%20Project-HASP/HIV%20Second%20Generation%20Surveillance%20in%20Pakistan%20-%20Round%201%20Report%20-%202005.pdf [Accessed October 2016] [Google Scholar]
- 41.Pakistan National AIDS Control Program. HIV Second Generation Surveillance in Pakistan. National Report Round II. Canada-Pakistan HIV/AIDS Surveillance Project. National Aids Control Program, Ministry of Health, Pakistan, 2006-07. Available at: http://www.nacp.gov.pk/library/reports/Surveillance%20&%20Research/HIV-AIDS%20Surveillance%20Project-HASP/HIV%20Second%20Generation%20Surveillance%20in%20Pakistan%20-%20Round%202%20Report%202006–07.pdf [Accessed October 2016] [Google Scholar]
- 42.Pakistan National AIDS Control Program. HIV Second Generation Surveillance in Pakistan. National Report Round III. Canada-Pakistan HIV/AIDS Surveillance Project. National Aids Control Program, Ministry of Health, Pakistan, 2008. Available at: http://www.nacp.gov.pk/library/reports/Surveillance%20&%20Research/HIV-AIDS%20Surveillance%20Project-HASP/HIV%20Second%20Generation%20Surveillance%20in%20Pakistan%20-%20National%20report%20Round%20III%202008.pdf, [Accessed October 2016] [Google Scholar]
- 43.Pakistan National AIDS Control Program. HIV Second Generation Surveillance in Pakistan. National Report Round IV. Canada-Pakistan HIV/AIDS Surveillance Project. National Aids Control Program, Ministry of Health, Pakistan, 2011. Available at http://www.nacp.gov.pk/library/reports/Surveillance%20&%20Research/HIV-AIDS%20Surveillance%20Project-HASP/HIV%20Second%20Generation%20Surveillance%20in%20Pakistan%20-%20National%20report%20Round%20IV%202011.pdf [Accessed October 2016] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


