Supplemental Digital Content is available in the text
Keywords: AIDS, HIV, lung cancer, population health, public health, smoking
Abstract
Objective:
Lung cancer is the leading cause of non-AIDS-defining cancer deaths among HIV-infected individuals. Although lung cancer screening with low-dose computed tomography (LDCT) is endorsed by multiple national organizations, whether HIV-infected individuals would have similar benefit as uninfected individuals from lung cancer screening is unknown. Our objective was to determine the benefits and harms of lung cancer screening among HIV-infected individuals.
Design:
We modified an existing simulation model, the Lung Cancer Policy Model, for HIV-infected patients.
Data sources:
Veterans Aging Cohort Study, Kaiser Permanente Northern California HIV Cohort, and medical literature.
Target population
: HIV-infected current and former smokers.
Time horizon
: Lifetime.
Perspective
: Population.
Intervention:
Annual LDCT screening from ages 45, 50, or 55 until ages 72 or 77 years.
Main outcome measures:
Benefits assessed included lung cancer mortality reduction and life-years gained; harms assessed included numbers of LDCT examinations, false-positive results, and overdiagnosed cases.
Results of base-case analysis:
For HIV-infected patients with CD4+ cell count at least 500 cells/μl and 100% antiretroviral therapy adherence, screening using the Centers for Medicare & Medicaid Services criteria (age 55–77, 30 pack-years of smoking, current smoker or quit within 15 years of screening) would reduce lung cancer mortality by 18.9%, similar to the mortality reduction of uninfected individuals. Alternative screening strategies utilizing lower screening age and/or pack-years criteria increase mortality reduction, but require more LDCT examinations.
Limitations:
Strategies assumed 100% screening adherence.
Conclusion:
Lung cancer screening reduces mortality in HIV-infected patients with CD4+ cell count at least 500 cells/μl, with a number of efficient strategies for eligibility, including the current Centers for Medicare & Medicaid Services criteria.
Introduction
With the introduction of antiretroviral therapy (ART), the life expectancy of HIV-infected individuals has dramatically improved [1–3]. As HIV-infected individuals live longer, their risk of developing non-AIDS-defining cancers (NADCs) increases. Currently, lung cancer is the most common NADC and the leading cause of NADC mortality among HIV-infected individuals [4,5]. HIV-infected individuals are at an elevated risk of developing lung cancer because of a combination of higher smoking prevalence and an independent risk associated with HIV infection [4,6–15]. HIV-infected persons also develop lung cancer at a younger age than uninfected persons [14,15]. Most lung cancers are clinically diagnosed at an advanced stage and have 5-year survival rates less than 15% [16]. Therefore, an effective means of reducing the number of lung cancer deaths among the HIV-infected population is urgently needed.
The National Lung Screening Trial (NLST) demonstrated that screening with low-dose chest computed tomography (LDCT) led to a 20.0% reduction in lung cancer mortality [17]. Largely based on the results of NLST, the Centers for Medicare & Medicaid Services (CMS) initiated coverage of lung cancer screening in high-risk current and former smokers (age 55–77, 30 pack-years of smoking, current smoker or quit within 15 years) [18]. Similarly, multiple national organizations, such as the United States Preventive Service Task Force (USPSTF) and the National Comprehensive Cancer Network (NCCN), also recommended lung cancer screening for certain populations [19]. Although current guidelines recommend screening all high-risk current and former smokers, HIV-infected persons were not included in these prior studies or guideline recommendations. Therefore, considerable uncertainty exists regarding the appropriateness of lung cancer screening for HIV-infected persons.
The benefits and harms associated with LDCT-based lung cancer screening are unclear for HIV-infected smokers and may differ compared with uninfected persons. HIV-infected individuals may be good candidates for screening because of elevated risk and earlier age of diagnosis of lung cancer. However, despite improved life expectancy, HIV-infected persons can still potentially experience increased mortality compared with uninfected individuals because of complications of chronic HIV infection and elevated risks of comorbid illnesses, and, therefore, may not survive long enough to derive long-term benefits of screening [1,20,21]. Additionally, there are concerns that HIV infection may further exacerbate the harms associated with LDCT-based lung cancer screening by increasing the rate of false-positive screens and/or complications associated with invasive diagnostic testing resulting from positive tests [22].
Thus, effective lung cancer screening eligibility criteria among HIV-infected persons may differ from criteria for uninfected persons. We are the first to address whether lung cancer screening is associated with an overall mortality benefit for those with HIV and to quantify the benefits and harms of lung cancer screening in HIV-infected individuals with CD4+ cell count of 500 cells/μl and greater. As a randomized, clinical trial to address this relevant clinical question is very unlikely to be conducted, we adapted a computer-based mathematical model, which was used to inform the USPSTF recommendations on lung cancer screening in the general population, for HIV infection. We integrated currently available short-term trial data with observational data from large HIV cohorts to project long-term consequences. We then estimated the benefits and harms of screening strategies using CMS and alternative screening eligibility criteria to identify the optimal screening strategies for HIV-infected individuals.
Methods
Overview of the Lung Cancer Policy Model
The Lung Cancer Policy Model (LCPM) is a comprehensive Monte Carlo microsimulation model of lung cancer development, progression, detection, treatment, and survival [23,24]. The LCPM was created to explore different screening frequencies and smoking eligibility criteria in HIV-uninfected smokers as part of a joint analysis performed by the Cancer Intervention and Surveillance Modeling Network (CISNET) to inform the USPSTF's recommendations for lung cancer screening [25–27].
To modify the LCPM to reflect the unique conditions affecting lung cancer screening for HIV-infected individuals, we used a variety of data sources, including national surveys, the SEER registry linked to Medicare claims, the Kaiser Permanente Northern California (KPNC) HIV Cohort, and the Veterans Aging Cohort Study (VACS) [4,13,14,16,28–40]. The details of the original LCPM, parameters that were modified to tailor the simulation to HIV-infected persons, and the data sources used for those parameter modifications are described in Table 1 and the Appendix.
Table 1.
Key input parameters and calibration targets for developing a Lung Cancer Policy Model for HIV-infected patients.
| Observed data | Definition | Values | Sources |
| Smoking prevalence (95% CI), %a,c | Smoking prevalence in HIV-infected population | Current: 46.5 (43.3–49.8)Former: 17.8 (15.5–20.0)Never: 35.7 (32.5–39.0) | [28] |
| Cigarette smoked per day | Average packs of cigarettes smoked per day | 31% <0.5 pack41% between 0.5 and 1 pack17% between one and two packs11% more than two packs | [4] |
| Lung cancer treatment response | Percentage of treated lung cancer patients who responded to treatment | Complete response to front-line chemotherapy: 15%Partial response to front-line chemotherapy: 24% | [37] |
| Nonlung cancer mortality rateb | Mortality for causes of death other than lung cancer | Median survival for HIV-infected persons in 10-year age strata, CD4+ cell count | Primary data from cohorts described in [13,16,29,30,38] |
| Mortality rate by ART adherenceb | HIV-attributable mortality by ART adherence | See Appendix | [31,32] |
| Incidence rate ratioc | Incidence rate of lung cancer for HIV-infected compared with HIV-uninfected | 1.7 (1.5–2.1) | [14,38,40] |
| Hazard ratio (risk of death from lung cancer) c | Rate of death from lung cancer for HIV-infected compared with HIV-uninfected (hazard ratio for lung cancer mortality for HIV-infected compared with HIV-uninfected) | 1.3 (1.2–1.4) | [36] |
| Lung cancer stage at diagnosis d | Nonsmall cell lung cancer stage at diagnosis | Stage I: 21%Stage II: 5%Stage IIIA: 13%Stage IIIB: 21%Stage IV: 41% | [14,39] |
| Histological subtypec,d | Histological subtype of lung cancer diagnosis | Adenocarcinoma: 49%Squamous cell carcinoma: 32%Large cell carcinoma: 8%Other: 12% | [14,39] |
| Prevalence of suspicious nodules d | Percentage of patients who showed a positive result from screening LDCT | 25% | [33,34] |
ART, antiretroviral therapy; CI, confidence interval; LDCT. low-dose computed tomography.
aThe smoking prevalence between 40 and 49 years old.
bModel inputs are stratified by CD4+ cell count of 201–500 cells/μl and greater than 500 cells/μl.
cIndicates observed data that were used as calibration targets. See Appendix Figures 3–5 for the model fits.
dThe observed data are similar between HIV-infected and uninfected individuals.
Base case population in simulation runs
In the base case population, the simulated HIV-infected patients all received ART with 100% treatment adherence and had CD4+ cell count of 500 cells/μl and above, as patients with well controlled HIV disease are most likely to be considered as candidates to start cancer screening. The smoking prevalence of the HIV-infected population was informed by a study using nationally representative survey data [28]. For each simulation run, the LCPM followed one million men and one million women with HIV infection from age 40 to death. Our choice of simulation sample size was selected to ensure stable simulation results, not to approximate the prevalence of HIV infection in the United States.
Base case screening scenarios
For the base case analysis, we first used the LCPM to estimate the health outcomes of a reference scenario where no screening took place. In this no-screening scenario, lung cancers and benign pulmonary nodules could be detected following the development of clinical symptoms or by incidental imaging. Then, we simulated annual LDCT screening for HIV-infected individuals with 12 different screening scenarios based on varying age of screening initiation (45, 50, or 55 years), age of screening termination (72 or 77 years), and cigarette smoking history (20 or 30 pack-years). All scenarios screened former smokers for no more than 15 years since quitting. The CMS screening eligibility criteria was included within the 12 scenarios.
Base case outcomes
The model generated outputs for the total numbers of screening LDCT exams, false-positive cases, lung cancer cases and deaths, radiation-induced cancers, and life expectancy. All health outcomes are averaged using one million men and one million women with HIV infection from age 40 to death. Potential benefits and harms of each screening scenario were computed relative to the no-screening scenario. Benefits included lung cancer mortality reduction and life-years gained. Harms included number of false-positive results (including from surgery and biopsy), number of overdiagnosed lung cancer cases, and number of radiation-induced cancers. Overdiagnosed cases were estimated as the additional diagnoses in the screening scenarios compared with the no-screening scenario that would have likely had limited negative consequence [41]. Our method of estimating the number of radiation-induced cancers from screening is based on the Biological Effects of Ionizing Radiation (BEIR) VII report (see Appendix for details) [42]. We compared the trade-offs of harms and benefits of different scenarios using an ‘efficiency frontier.’ ‘Efficient’ strategies (collectively, the ‘frontier’) prevented the greatest number of lung cancer deaths for a given number of screening LDCT exams, the same method applied in our analysis with CISNET that informed USPSTF's lung cancer screening recommendations [26,27]. For the base case analysis, we assumed that all patients were receiving ART with 100% treatment adherence. However, the treatment adherence of some patients may eventually drop below 100%. The effect of ART adherence was tested by lowering from 100 to 80%. In the context of ART, 80% adherence means that the patient takes 80% of the prescribed medication. In the hypothetical ART sensitivity analysis, patients remain in a screening program even though their CD4+ cell counts fall below 500 cells/μl.
We also performed probabilistic sensitivity analysis to determine the likelihood of the optimal screening strategies remaining on the efficiency frontier as model parameters were varied according to their likely distributions. The uncertainties of the model parameters, including nonlung cancer mortality rate, are addressed in the probabilistic sensitivity analysis. The method and parameter distributions of the probabilistic sensitivity analysis are described in the Appendix.
Results
Comparison between HIV-infected and uninfected persons
We included simulations for uninfected persons using CMS screening criteria to compare with the HIV-infected population with CD4+ cell count of at least 500 cells/μl. The projected lung cancer mortality reductions because of screening with CMS guidelines, whenever averaged over the total population including both screened and unscreened simulated individuals, were 10.8 and 7.7% for uninfected and HIV-infected individuals, respectively. In the subgroup of patients who went through lung cancer screening, the estimations increased to 22.7 and 18.9% for uninfected and HIV-infected individuals, respectively. These results are comparable with the 20% mortality reduction observed in the NLST and are within the 95% confidence intervals reported in that study as shown in Appendix Figure 1. As different screening scenarios will screen different proportions of the total population, lung cancer mortality reductions among screened populations cannot be accurately compared. Therefore, lung cancer specific mortality reduction and life-years gained are shown for the total population.
Comparison of benefits and harms across scenarios
Table 2 summarizes the benefits of the 12 screening scenarios. Among all scenarios, screening HIV-infected individuals between ages 45 and 77 with a minimum 20 pack-years yielded the largest lung cancer mortality reduction (14.3%) and required screening the most individuals (34.3%). Eligibility criteria based on the CMS guidelines led to screening 19.4% of the total HIV-infected population and required 240 035 LDCT exams per 100 000 individuals. CMS-based criteria yielded 7.7% lung cancer mortality reduction among the total HIV-infected population and 1128 life-years gained per 100 000 individuals. Screening individuals between ages 55 and 72 with minimum of 30 pack-years required screening the fewest number of individuals (19.1%) and yielded the smallest lung cancer mortality reduction (6.9%). The lung cancer mortality reduction among the current and former HIV-infected smokers was estimated to range between 9.0–19.2% and 6.3–12.1%, respectively.
Table 2.
Benefits of the screening programs examined in this study with various eligibility criteria.
| Strategy (age-start_stop_pack-years) | Cohort screened (%) | # Screening LDCT per 100ka | Screening detected Lung cancer cases per 100ka | Lung cancer mortality reduction among total population (%) | Lung cancer mortality reduction among current smokers | Lung cancer mortality reduction among former smokers | Life-years gained per 100ka |
| Age_45_72_PY20 | 34.3 | 552 102 | 813 | 13.3 | 18.1 | 10.9 | 2246 |
| Age_45_72_PY30 | 24.1 | 388 513 | 727 | 10.5 | 14.6 | 8.0 | 1752 |
| Age_45_77_PY20 | 34.3 | 585 621 | 959 | 14.3 | 19.2 | 12.1 | 2359 |
| Age_45_77_PY30 | 24.4 | 412 719 | 860 | 11.3 | 15.6 | 8.9 | 1841 |
| Age_50_72_PY20 | 30.9 | 420 056 | 705 | 11.3 | 15.0 | 9.6 | 1806 |
| Age_50_72_PY30 | 22.0 | 306 173 | 635 | 9.0 | 12.2 | 7.4 | 1414 |
| Age_50_77_PY20 | 30.9 | 453 543 | 850 | 12.3 | 16.2 | 10.9 | 1919 |
| Age_50_77_PY30 | 22.3 | 330 343 | 768 | 9.8 | 13.1 | 8.2 | 1498 |
| Age_55_72_PY20 | 26.5 | 298 133 | 575 | 8.8 | 11.4 | 8.1 | 1361 |
| Age_55_72_PY30 | 19.1 | 215 902 | 520 | 6.9 | 9.0 | 6.3 | 1042 |
| Age_55_77_PY20 | 26.5 | 331 597 | 718 | 9.9 | 12.5 | 9.3 | 1474 |
| Age_55_77_PY30 (CMS) | 19.4 | 240 035 | 652 | 7.7 | 9.8 | 7.2 | 1128 |
LDCT. low-dose computed tomography.
aNumbers are per 100 000 individuals (100k) at age 40 followed to death.
Table 3 shows the harms associated with each scenario. Screening individuals between ages 55 and 72 years with minimum of 30 pack-years resulted in the fewest overdiagnosed cases (30.6 cases per 100 000 individuals) corresponding to 18.2 and 5.9% overdiagnosed cases among screening-detected and all lung cancers, respectively. The CMS-based eligibility criteria resulted in 34.3 overdiagnosed cases per 100 000 individuals. The most effective mortality reduction strategy (14.3%), screening between ages 45 and 77 years with minimum of 20 pack-years, resulted in the most number of scans (585 621 LDCT exams per 100 000 individuals) and overdiagnosed cases (50.8 cases per 100 000 individuals). The radiation-induced lung and breast cancers were estimated to range to between 6.5–21.1 and 2.6–4.8 per 100 000 individuals, respectively.
Table 3.
Harms of the screening programs examined in this study with various eligibility criteria.
| Strategy (age-start_stop_pack-years) | Average screening examinations per person screened | Average false-positive results per person screened | Overdiagnosed cases per 100ka | Overdiagnosis, percentage of screening-detected cases | Overdiagnosis, percentage of all cases | Radiation-induced lung cancers per 100ka | Radiation-induced breast cancers per 100ka,b |
| Age_45_72_PY20 | 16.1 | 5.9 | 46.9 | 20.0 | 5.8 | 21.1 | 4.8 |
| Age_45_72_PY30 | 16.1 | 5.8 | 37.6 | 20.1 | 5.2 | 15.7 | 3.7 |
| Age_45_77_PY20 | 17.1 | 5.8 | 50.8 | 20.4 | 5.3 | 21.1 | 4.8 |
| Age_45_77_PY30 | 16.9 | 5.7 | 40.8 | 20.5 | 4.7 | 15.9 | 3.7 |
| Age_50_72_PY20 | 13.6 | 5.7 | 42.1 | 18.6 | 6.0 | 13.7 | 3.7 |
| Age_50_72_PY30 | 13.9 | 5.7 | 34.2 | 18.9 | 5.4 | 10.5 | 3.1 |
| Age_50_77_PY20 | 14.7 | 5.7 | 46.3 | 19.3 | 5.5 | 13.7 | 3.7 |
| Age_50_77_PY30 | 14.8 | 5.6 | 37.8 | 19.6 | 4.9 | 10.7 | 3.1 |
| Age_55_72_PY20 | 11.3 | 5.5 | 36.8 | 17.6 | 6.4 | 8.4 | 3.0 |
| Age_55_72_PY30 | 11.3 | 5.5 | 30.6 | 18.2 | 5.9 | 6.5 | 2.6 |
| Age_55_77_PY20 | 12.5 | 5.5 | 41.3 | 18.4 | 5.8 | 8.5 | 3.0 |
| Age_55_77_PY30 (CMS) | 12.4 | 5.6 | 34.3 | 19.0 | 5.3 | 6.6 | 2.7 |
aNumbers are per 100 000 individuals (100k) at age 40 followed to death.
bThe number of radiation-induced breast cancers in female patients.
Evaluation of screening strategies using the efficiency frontier
To determine the optimal screening scenario(s), we used lung cancer mortality reduction and number of screening LDCT exams as the benefit and harm, respectively, to construct the efficiency frontier. This method of determining the optimal screening scenario(s) was used in prior analyses informing the USPSTF's lung cancer screening recommendations [26,27]. Figure 1 shows the scenarios on the efficiency frontier, including the CMS screening criteria. The scenarios that include screening from age 50 or 55 until age 77 with a minimum of 20 pack-years were also on the frontier; both were projected to yield superior reduction in lung cancer mortality, but with a higher number of LDCT exams.
Fig. 1.
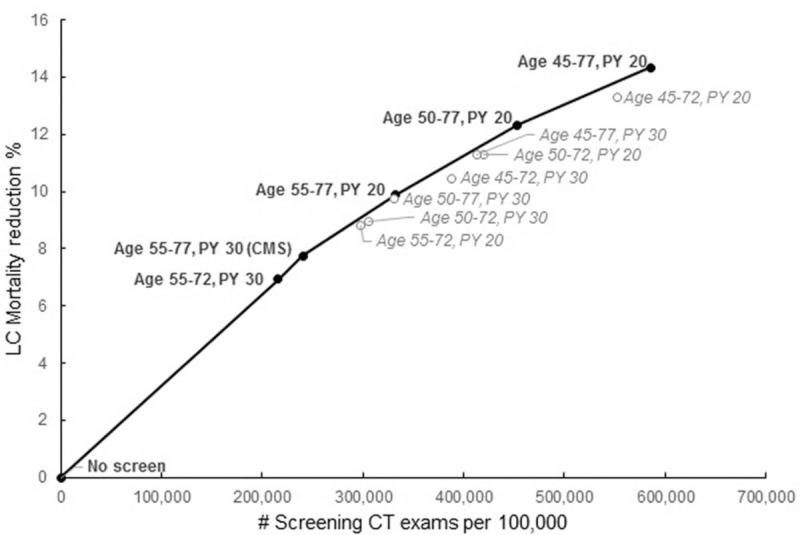
Lung cancer mortality reduction and total screens efficiency frontier for HIV-infected individuals for different screening strategies. Estimated lung cancer mortality reduction from annual LDCT screening of a HIV-infected cohort, for programs with different screening eligibility criteria, including the scenario using the CMS guideline. The black solid circles are the strategies on the frontier. The gray open circles are the strategies below the frontier. CMS, Centers for Medicare & Medicaid Services.
Sensitivity analysis
In our model, ART adherence influenced HIV viral suppression. Therefore, we chose our base case to be highly ART adherent and thus virally suppressed, again representative of the clinical population most likely to be considered for screening [43]. To examine the impact of ART adherence, we included model projections of the benefits and harms of lung cancer screening if the ART adherence fell below 100% (80% ART adherence in Appendix Tables 2 and 3). The percentages of cohort screened, numbers of screening LDCT exams, and mortality reduction were similar between the 80 and 100% ART adherence groups. Differences existed in the screening-detected lung cancer cases and life-years gained as HIV-infected patients with a lower ART adherence experienced higher mortality from competing causes of death. Thus, fewer of these patients lived long enough to develop lung cancer. Life-years gained were also diminished because patients with lower ART adherence did not live long enough to accumulate the downstream benefits of early lung cancer detection. Average screening LDCT exams per person screened and overdiagnosis were both lower for patients with 80% adherence rate compared with 100%. However, average false-positive results per person screened and the screening strategies considered efficient were similar between the two groups.
From our probabilistic sensitivity analysis, the probabilities of the strategies being on the frontier are shown in Table 4. Appendix Figure 6 shows the corresponding scatterplot. Four strategies, including CMS's recommendations, have a 100% probability of being on the efficiency frontier, meaning they were efficient in each of the sensitivity analyses. Screening from age 55 to 72 with a minimum of 30 pack-years, which generated a lower mortality reduction and required fewer screening LDCT exams, had a 93.2% probability of being on the efficiency frontier.
Table 4.
The probability of each strategy being on the frontier.
| Strategy (age-start_stop_pack-years) | Probability of being on the efficiency frontier |
| Age_45_72_PY20 | 0.0% |
| Age_45_72_PY30 | 0.0% |
| Age_45_77_PY20 | 100.0% |
| Age_45_77_PY30 | 0.0% |
| Age_50_72_PY20 | 0.0% |
| Age_50_72_PY30 | 0.0% |
| Age_50_77_PY20 | 100.0% |
| Age_50_77_PY30 | 0.0% |
| Age_55_72_PY20 | 0.0% |
| Age_55_72_PY30 | 93.2% |
| Age_55_77_PY20 | 100.0% |
| Age_55_77_PY30 (CMS) | 100.0% |
Discussion
In this study, we are the first to address the clinically relevant question of whether HIV-infected patients with CD4+ cell count of 500 cells/μl and above derive overall benefit from lung cancer screening, and whether different eligibility criteria might be more efficient in those with HIV. We tailored the LCPM to HIV-infected individuals using data from the literature and several large, representative HIV cohorts. We incorporated long-term relationships between ART adherence and HIV mortality and used the modified LCPM to estimate the impact of LDCT screening on lung cancer mortality reduction, life-years gained, and other health outcomes in HIV-infected individuals. We found that LDCT screening can provide a similar mortality reduction benefit to HIV-infected and HIV-uninfected patients. Application of CMS criteria for screening was on the efficiency frontier and resulted in reduced mortality and increased life-years gained. Screening from age 45 to 77 with a minimum of 20 pack-years of smoking was also on the frontier and resulted in the greatest life-years gained, yet required the highest number of LDCT scans performed and yielded the highest rate of overdiagnosis. As HIV-infected patients have shorter life expectancy even in the late ART-era, our results indicated that lowering the screening termination age from 77 to 72 might be an alternative way to screen these patients for lung cancer, as this strategy was also on the frontier and produced a similar mortality reduction with fewer LDCT scans obtained. Our findings highlight the importance of considering both benefits and harms of screening in considering optimal screening strategies.
As HIV-infected individuals experience an elevated lung cancer risk, one would expect that lung cancer screening would be beneficial to these patients. However, HIV-infected individuals experience increased risk of competing causes of death compared with uninfected persons, even with improved HIV management. Therefore, for lung cancer screening to be effective among HIV-infected individuals, patients with lung cancer diagnoses need to survive these competing causes of death long enough to experience the benefits of early lung cancer detection. The estimates from our simulation model suggest competing effects of higher lung cancer incidence and lower life expectancy. There are other factors that can also influence the benefits and harms, or overall effectiveness, of screening, such as lung cancer stage, histological subtype at diagnosis, and prevalence of suspicious nodules; these rates are similar between HIV-infected and uninfected individuals [14,33,34,39]. Our estimated overdiagnosis rate from lung cancer screening for HIV-infected patients is close to the reported overdiagnosis rate of 18.5% for uninfected individuals [41]. Our analyses support that lung cancer screening in HIV-infected individuals provides similar lung cancer mortality reduction as does screening in uninfected individuals, representing a notable benefit to this population.
Our estimate for radiation-induced cancers from screening is lower than the estimate of Rampinelli et al.[44] because they determined the life-time attributable risk of radiation-induced cancers using Table 12D-1 of the BEIR VII report [42]. However, Table 12D-1 should be used only for individuals with average risk of dying from all causes. HIV-infected smokers have a higher mortality rate and, thus, a higher probability of dying before developing radiation-induced cancers, resulting in fewer radiation-induced cancers. Overall, the number of screening-detected lung cancers far exceeds the number of radiation-induced cancers from LDCT screening.
Limited clinical trial data evaluating lung cancer screening in HIV-infected smokers exist, and it is unlikely that a randomized controlled trial of screening will be conducted with enough power to determine the lung cancer mortality reduction among the HIV-infected population. A recent trial conducted in France reported the results of a single round of LDCT-based lung cancer screening in 442 HIV-infected individuals [45] and found that screening in HIV-infected smokers was feasible and well tolerated, but the study design did not allow for estimation of the long-term benefits and harms of screening. The only other published lung cancer screening trial in HIV-infected persons included 224 participants and showed a lower prevalence of both positive screens and lung cancers diagnosed compared with the NLST. This trial enrolled younger patients (median age 47) with less smoking exposure than did the NLST – a possible explanation for their findings [46]. Advanced simulation modeling techniques, such as those used in the LCPM, are the most feasible way to inform clinical management and guide policy. Our findings show that lung cancer screening is beneficial to HIV-infected patients and that several potential strategies are efficient. Diverging from the CMS screening criteria results in greater mortality reduction and life-years gained, but at the cost of administering more LDCT scans and increased risk of misdiagnosis.
Our study benefitted from the use of a well validated lung cancer natural history model modified using data from several large, modern HIV cohorts. Our base case is representative of a large proportion of the US HIV-infected population who would be eligible for lung cancer screening. Recent data show greater than 75% US HIV-infected patients in care have viral suppression [43]. However, there are several limitations that warrant specific consideration. First, our estimated benefits and harms are based on a 100% screening adherence rate, as was done in the CISNET analyses that informed the USPSTF recommendations [26,27]. Lung cancer screening at the national level is in an early stage of implementation. Although adherence to LDCT screening was approximately 95% in the NLST, there are currently limited data to inform the screening adherence rate in the real-world setting. A national survey found that adherence rates were 54.6, 69.3, 85.8, and 46.4% in 2010 for colorectal, breast, cervical, and prostate cancer screening for the general population, respectively, and it seems unlikely that lung cancer screening will exceed these rates [47]. Our results, therefore, should be viewed as the best-case scenario for lung cancer screening, as is the case with other modeling work for the HIV-uninfected population. Although a large-scale screening trial among the HIV-infected population may not be feasible, performing smaller trials to gather adherence data, simultaneously for both screening and ART, would provide the necessary estimates to improve the prediction of future simulation studies. Second, we did not exhaustively examine all possible screening eligibility criteria. We simulated 12 different screening scenarios that we believe to provide sufficient clinical information to determine the optimal screening protocol(s) for HIV-infected smokers. Third, competing mortality rates were stratified into current, former, and never smokers, but not stratified by pack-years. Smoking history varies widely within the categories of current and former. To our knowledge, there are no US HIV cohorts that contain smoking quantity data with adequate numbers of participants and follow-ups to create stratified estimates suitable to inform our model. We strongly believe that the data sources and methods by which we obtained our nonlung cancer mortality estimates were adequate for the study purposes, and we have explored the potential uncertainty around those estimates using probabilistic sensitivity analyses. Fourth, our model considered cigarette smoking as the main risk factor for developing lung cancer. We did not account for other lung cancer risk factors, such as cannabis, asbestos, and radon exposure, in the model. We also did not modify lung cancer risk according to CD4+ cell count, as there is conflicting data regarding the impact of that exposure (see Appendix). Lastly, there are not sufficient data in the literature to inform the impacts of screening stratified by race and HIV risk profile (e.g. MSM and injection drug use). Thus, we did not include these factors in the current analysis.
In conclusion, lung cancer screening with LDCT among HIV-infected smokers appears to provide similar mortality reduction as in the general population and HIV-infected persons with CD4+ cell count of at least 500 cells/μl should be considered candidates for lung cancer screening as programs are implemented. Our study demonstrates that an accepted general population screening regimen, the CMS screening criteria, was an efficient strategy. Screening individuals from ages 45 to 77 with 20 pack-years of smoking history was also efficient and resulted in the greatest life-years gained, but led to the highest number of LDCT scans and cases overdiagnosed. In addition to the current CMS criteria, there are opportunities to decrease mortality and increase life-years gained by moving to other strategies on the efficiency frontier. Future studies should evaluate the cost-effectiveness of these screening strategies, and evaluate the inclusion of validated prognostic indices such as the VACS index [48] to determine the appropriateness of lung cancer screening in HIV-infected persons with comorbid illnesses.
Acknowledgments
Author contribution: All authors for this study meet the following four criteria specified by the ICMJE:
-
(1)
substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work;
-
(2)
drafting the work or revising it critically for important intellectual content;
-
(3)
final approval of the version to be published; and
-
(4)
agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Source of funding: This report is based on research conducted through support from the National Cancer Institute (R01CA173754 to C.Y.K., D.F.S., J.P.W., K.C., R.S.B. and K07CA180782 to K.S.). The Program Official contact at the National Cancer Institute is Susan M. Scott (E-Mail: scotts2@mail.nih.gov). The funding source had no role in the design, conduct, or analysis of the study or in the decision to submit the manuscript for publication.
Conflicts of interest
C.I.H. is an inventor of patents and pending patents owned by Cornell Research Foundation. As of April 2009, she has divested herself of all royalties and other interests arising from these. J.W. is a member of the research board of EHE International and has received consulting honorarium from Quintiles, Merck, and AstraZeneca, and research grants from Sanofi and Quorum. M.J.S. reports grants from Pfizer and Merck outside the submitted work.
Excluding the aforementioned disclosure and the funding acknowledgements, all authors declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Supplementary Material
References
- 1.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP, Jr, Klein DB, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis 2013; 26:17–25. [DOI] [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol 2006; 24:1383–1388. [DOI] [PubMed] [Google Scholar]
- 5.Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis 2010; 51:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadranel J, Garfield D, Lavole A, Wislez M, Milleron B, Mayaud C. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax 2006; 61:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS 2007; 21:207–213. [DOI] [PubMed] [Google Scholar]
- 8.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Swiss HIV Cohort. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005; 97:425–432. [DOI] [PubMed] [Google Scholar]
- 9.Hakimian R, Fang H, Thomas L, Edelman MJ. Lung cancer in HIV-infected patients in the era of highly active antiretroviral therapy. J Thorac Oncol 2007; 2:268–272. [DOI] [PubMed] [Google Scholar]
- 10.Hessol NA, Seaberg EC, Preston-Martin S, Massad LS, Sacks HS, Silver S, et al. WIHS Collaborative Study Group. Cancer risk among participants in the women's interagency HIV study. J Acquir Immune Defic Syndr 2004; 36:978–985. [DOI] [PubMed] [Google Scholar]
- 11.Kirk GD, Merlo C, O’ Driscoll P, Mehta SH, Galai N, Vlahov D, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 2007; 45:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr 2010; 55:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011; 183:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012; 26:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst 2015; 107:pii: dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus JL, Chao C, Leyden WA, Xu L, Yu J, Horberg MA, et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev 2015; 24:1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). 2015. [Google Scholar]
- 19.National Comprehensive Cancer Network. Lung cancer screening version 1. 2017. [Accessed 7 July, 2017] [Google Scholar]
- 20.Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, et al. Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO8 APROCO-COPILOTE Study Group; Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO3 AQUITAINE Study Group. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr 2007; 46:72–77. [DOI] [PubMed] [Google Scholar]
- 21.Gueler A, Moser A, Calmy A, Gunthard HF, Bernasconi E, Furrer H, et al. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS 2017; 31:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigel K, Pitts R, Crothers K. Lung malignancies in HIV infection. Semin Respir Crit Care Med 2016; 37:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon PM, Kong CY, Johnson BE, Weinstein MC, Weeks JC, Kuntz KM, et al. Estimating long-term effectiveness of lung cancer screening in the Mayo CT screening study. Radiology 2008; 248:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon PM, Kong CY, Johnson BE, Weinstein MC, Weeks JC, Tramontano AC, et al. Chapter 9: the MGH-HMS lung cancer policy model: tobacco control versus screening. Risk Anal 2012; 32 Suppl 1:S117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyer VA. Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338. [DOI] [PubMed] [Google Scholar]
- 26.de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014; 160:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon PM, Meza R, Plevritis SK, Black WC, Tammemagi CM, Erdogan A, et al. Comparing benefits from many possible computed tomography lung cancer screening programs: extrapolating from the National Lung Screening Trial using comparative modeling. PloS One 2014; 9:e99978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–344. [DOI] [PubMed] [Google Scholar]
- 29.Crothers K, Goulet JL, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Goetz MB, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS education and prevention: official publication of the International Society for AIDS Education 2009; 21 (3 Suppl):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a ‘virtual’ cohort using the National VA Health Information System. Med Care 2006; 44 (8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 31.Braithwaite RS, Fiellin DA, Nucifora K, Bryant K, Roberts M, Kim N, et al. Evaluating interventions to improve antiretroviral adherence: how much of an effect is required for favorable value?. Value Health 2010; 13:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khademi A, Braithwaite RS, Saure D, Schaefer AJ, Nucifora K, Roberts MS. Should expectations about the rate of new antiretroviral drug development impact the timing of HIV treatment initiation and expectations about treatment benefits?. PloS One 2014; 9:e98354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigel K, Wisnivesky J, Shahrir S, Brown ST, Justice A, Kim J, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS 2014; 28:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthew T, Keith S, Alison MM, Alycia P, Juan PW, Chung YK, et al. The association between emphysema and non-calcified lung nodules in HIV-infected and uninfected individuals and implications for lung cancer screening. In: C25 HIV-associated lung infections/disorders from basic to clinical. American Thoracic Society; 2016. p. A4719. [Google Scholar]
- 35.Sigel K, Wisnivesky JP, Kong CY, Braithwaite RS, Park LS, Dubrow R, et al. Frequency of complications after lung biopsy in HIV-infected compared to HIV-uninfected patients: implications for lung cancer screening. In: B61 Managing lung cancer screening and its downstream findings. American Thoracic Society; 2015. pp. A3586–A3586. [Google Scholar]
- 36.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol 2015; 33:2376–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Jaen GA, Pantanowitz L, Bower M, Buskin S, Neil N, Greco EM, et al. Human immunodeficiency virus-associated primary lung cancer in the era of highly active antiretroviral therapy: a multiinstitutional collaboration. Clin Lung Cancer 2010; 11:396–404. [DOI] [PubMed] [Google Scholar]
- 38.Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009; 23:2337–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigel K, Crothers K, Dubrow R, Krauskopf K, Jao J, Sigel C, et al. Prognosis in HIV-infected patients with nonsmall cell lung cancer. Br J Cancer 2013; 109:1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanik EL, Katki HA, Engels EA. Cancer risk among the HIV-infected elderly in the United States. AIDS 2016; 30:1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patz EF, Jr, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemagi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014; 174:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NRC (National Research Council). Health risks from exposure to low levels of ionizing radiation. BEIR VII Phase 2. Washington, DC: National Academy Press; 2005. [PubMed] [Google Scholar]
- 43.Bradley H, Mattson CL, Beer L, Huang P, Shouse RL. Medical Monitoring Project. Increased antiretroviral therapy prescription and HIV viral suppression among persons receiving clinical care for HIV infection. AIDS 2016; 30:2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casiraghi M, Veronesi G, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017; 356:j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makinson A, Eymard-Duvernay S, Raffi F, Abgrall S, Bommart S, Zucman D, et al. ANRS EP48 HIV CHEST study Team. Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. AIDS 2016; 30:573–582. [DOI] [PubMed] [Google Scholar]
- 46.Hulbert A, Hooker CM, Keruly JC, Brown T, Horton K, Fishman E, et al. Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol 2014; 9:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke TC, Soler-Vila H, Fleming LE, Christ SL, Lee DJ, Arheart KL. Trends in adherence to recommended cancer screening: the US population and working cancer survivors. Front Oncol 2012; 2:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bebu I, Tate J, Rimland D, Mesner O, Macalino GE, Ganesan A, et al. Infectious Disease Clinical Research Program HIV Working Group. The VACS index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 65:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


