ABSTRACT
OBJECTIVE:
To examine the prevalence and characteristics of medical device–related pressure injuries (MDR PIs) in a large, generalizable database.
METHODS:
This study is a retrospective analysis of the 2016 International Pressure Ulcer Prevalence data. Data were limited to US and Canadian facilities. Facilities included acute care, long-term care, rehabilitation, long-term acute care hospitals, and hospice. Analysis included 102,865 adult patients; 99,876 had complete data and were the focus of the analysis and are reported in the results below.
RESULTS:
The overall PI prevalence was 7.2% (n = 7189), and the facility-acquired prevalence was 3.1% (n = 3113). The prevalence of MDR PIs was 0.60% (n = 601), which included both mucosal and nonmucosal MDR PIs. In this study, 75% of MDR PIs were facility acquired, whereas non-MDR PIs were most commonly present on admission. Facility-acquired MDR PIs formed 3 days faster than facility-acquired non-MDR PIs (12 vs 15 days; P < .05). By stage, most MDR PIs were superficial (58% were Stage 1 or 2), 15% were deep-tissue PIs, and 22% were full-thickness PIs (Stage 3 or 4 or unstageable). The most common anatomic locations for MDR PIs were the ears (29%) and the feet (12%). The most common devices associated with MDR PIs were nasal oxygen tubes, 26%; other, 19%; cast/splints, 12%; and continuous positive airway pressure/bilevel positive airway pressure masks, 9%.
CONCLUSIONS:
Because MDR PIs form faster than non-MDR PIs, timely proactive assessment and prevention measures are critical. Most MDR PIs occurred at the face and head region, and the ears specifically. The most common devices linked with MDR PIs were oxygen tubing and masks, making assessment and prevention efforts critical for patients who require those devices.
KEYWORDS: facility-acquired pressure injury, medical device–related pressure ulcer, medical device–related pressure injury, pressure injury prevalence, pressure ulcer prevalence, pressure injury incidence, pressure ulcer incidence
INTRODUCTION
Medical devices are an integral part of care of persons in all healthcare settings. Some medical devices such as endotracheal tubes are found primarily in acute care, whereas other devices such as oxygen masks, urinary catheters, cervical collars, tracheostomy tubes/ties, compression stockings, and nasogastric tubes, to name a few, are found across care settings. Pressure injuries (PIs) that form because of medical devices are a clinical phenomenon that deserves the attention of healthcare professionals.
The National Pressure Ulcer Advisory Panel (NPUAP) has been instrumental in raising awareness of the PIs that are caused by medical devices. In 2016, the NPUAP defined a medical device–related PI (MDR PI) as arising “from the use of devices designed and applied for diagnostic or therapeutic purposes. The resultant pressure injury generally conforms to the pattern or shape of the device.”1 The NPUAP also distinguished MDR PIs from PIs found on the mucosal membrane; these are PIs that are “found on mucous membranes with a history of a medical device in use at the location of the ulcer.”1,2 Both definitions indicate that the etiology of the PI is associated with a medical device,2 but the key difference is that mucosal PIs cannot be staged because, unlike skin, mucosa does not have keratinized epithelium.
As a call to action to prevent MDR PIs, the NPUAP has published 4 educational posters detailing MDR PI prevention best practices. These are available for free download from its website, www.npuap.org.3 Moreover, the NPUAP 2014 Clinical Practice Guideline4 included recommendations on identifying patients at risk of MDR PIs, how to assess those patients, and how to prevent MDR PIs. This was an advancement from the 2009 guideline that had only 1 recommendation on MDR PIs associated with repositioning techniques.5
The Wound, Ostomy and Continence Nurses Society included recommendations about medical devices in its 2017 position paper on unavoidable PIs; it proposed that research was needed to “investigate device-related pressure ulcers across the lifespan in all healthcare settings” and “conduct studies to describe device-related injuries and determine the risk factors as a basis for developing risk assessment tools, best practices, quality improvement initiatives, and safe material to prevent the injuries.”6 This study responds to this call for research by examining the prevalence and characteristics of MDR PIs. This includes investigating the likelihood of the PI to be present on admission or facility acquired and the distribution of MDR PIs by PI stage, anatomic location, and device type.
Literature Review
Prevalence and incidence data about PIs have been reported in the literature.7–15 VanGilder et al13 recently reported a decrease in both overall and facility-acquired PIs from an analysis of the International Pressure Ulcer Prevalence (IPUP) survey. The overall prevalence of PIs in all US healthcare settings decreased from 13.5% in 2006 to 9.3% in 2015, and in acute care, the overall PI prevalence was 13.3% in 2006, decreasing to 9.3% in 2015. The prevalence of facility-acquired PIs in all US healthcare settings decreased from 6.2% in 2006 (6.4% in acute care) to a range of 3.1% to 3.4% (2.9% in acute care in 2015) in the 2013 to 2015 period. A further analysis of these data demonstrated that while facility-acquired Stage 1 or 2 (or superficial) PIs have decreased in prevalence from 3.7% (2011) to 2.9% in 2015,13 full-thickness PIs (Stages 3 and 4, deep-tissue PI [DTPI], and unstageable) have remained constant, about 0.9% to 1.2% (2011 to 2016).16
Prevention of any PI, including those arising from medical devices, is desirable. To gauge the success or failure of PI prevention, facilities must assess their own incidence and prevalence rates and track changes in those numbers over time. Knowing the overall prevalence rates of MDR PIs allows facilities to benchmark and track their performance as it compares with other like facilities. However, finding information on MDR PIs for benchmarking purposes is difficult. The 2 NPUAP publications on PI prevalence and incidence do not have a dedicated chapter on MDR PIs, requiring researchers to look for information on MDR PIs throughout each chapter.10,11 Services such as the IPUP survey allow facilities to track their own prevalence over time and compare their numbers with similar facilities and unit types.
There is limited information about MDR PIs.11,16–26 In 2009, VanGilder et al12 reported data from the 2009 IPUP survey of 86,932 US acute care patients who had 17,911 PIs; of those PIs, 9.1% (n = 1631) were device related. This study also found that 785 of the MDR PIs (48%) were facility acquired.12 The most common locations for these MDR PIs were the ear (20%), sacral/coccyx region (17%), heel (12%), and buttocks (10%).12 Apold and Rydrych20 analyzed 255 PIs (Stage 3, Stage 4, or unstageable) reported as adverse events from acute care hospitals in their 2012 study of the Minnesota SAFE Skin initiative.20 They reported that just under one-third (29%) of the serious PIs were device related.20 The types of devices that were associated with development of MDR PIs were cervical collars or braces (22%), other types of immobilizers (17%), oxygen tubing (13%), stockings or boots (12%), and nasogastric tubes (8%).20 They also examined the stage of the MDR PI when it was discovered and found that more than half (53%, n = 39) were unstageable, 5.4% (n = 4) were Stage 1, 20% (n = 15) were Stage 2, 20% (n = 15) were Stage 3, and 1.4% (n = 1) were Stage 4.20
The 2010 retrospective study by Black et al21 from a single medical center in the United States reported that 5.3% of patients (113 of 2079) in critical care, step-down, or medical-surgical units developed facility-acquired PIs. They found that 1.3% of the patients studied had at least 1 facility-acquired MDR PI.21 Of the facility-acquired MDR PIs, 5% were Stage 1, 32% were Stage 2, 24% were unstageable, 6% were DTPIs, and 3% were Stage 3.21 They did not observe any Stage 4 PIs.21
Coyer et al22 studied MDR PIs in a 2013 prospective study of 483 intensive care unit (ICU) patients conducted in Australia and the United States. The prevalence of MDR PIs was 3.1%, and most MDR PIs were associated with endotracheal or nasogastric tubes.22 A 2015 study by Schallom et al23 in Missouri found that the type of oxygen mask used made a difference in PI development. In fact, 20% of patients with a nasal-oral mask developed PIs, compared with only 2% of patients who used full-face masks.23
Amirah and colleagues24 studied 431 adult ICU patients in a large tertiary care acute care hospital in Saudi Arabia. Of these, 115 patients (26.7%) had at least 1 MDR PI. The total number of all PIs was 395; 128 of these were medical device related (32.4%). The devices associated with these MDR PIs were endotracheal tubes (37%), Foley catheters (37%), neck collars (12.5%), nasogastric tubes (9.4%), traction equipment (1.6%), and “other” devices. The types of devices reported in this study were similar to Coyer et al22; however, the prevalence in this study was quite a bit higher than both Black et al21 and Coyer et al.22
Arnold-Long et al25 reported a study of MDR PIs in 3 geographically diverse long-term acute care hospitals, which are specialty hospitals that commonly serve as a bridge for medically complex patients between ICU discharges and rehabilitation admissions. There were 142 MDR PIs that occurred over 1 year, which comprised 47% of the 304 hospital-acquired PIs in the study.25 Across the 3 locations, Stage 2 PIs represented most of the MDR PIs (51%); Stage 1 and DTPI both represented 18% of the sample; Stage 3, 7%; unstageable, 6%; and no Stage 4 MDR PIs were reported.25 The types of devices associated with MDR PIs were splints and braces (20%), oxygen tubing/continuous positive airway pressure (CPAP)/bilevel positive airway pressure (BiPAP) devices (15%), tubing associated with urine or fecal management (15%), heel relief devices (8%), percutaneous endoscopic gastrostomy tubing (6%), and “other” devices (35%).25
Medical device–related PIs can occur on mucosa or skin.1,2,26 However, the other articles in this review did not distinguish between MDR PIs on the mucosa versus skin, meaning there is even less information on the prevalence of these types of MDR PIs. The best indicator of what percentage of PIs are on the mucosa is from the Minimum Data Set (MDS 3.0), which is published by the Centers for Medicare & Medicaid Services (CMS).27,28 The CMS requires long-term care facilities to report PI data.27,28 Prevalence numbers for all PIs are recorded in MDS 3.0 in sections M0210-M0900 on skin conditions.28 The prevalence of all unhealed PIs in the first quarter of 2017 was 7.5%.28 However, the CMS does not separately report the prevalence of MDR PIs in sections M0210-M0900.28 The CMS does, however, report PIs on oral mucosa in a different section of the MDS (L0200C) and reported that PIs on oral mucosa had a prevalence of 0.2%.28 However, this prevalence included more than MDR PIs on the mucosa; it also includes oral masses and lesions such as those that form under dentures.28 Therefore, this number might provide an inflated estimate of MDR PIs on the mucosa, and the number of MDR PIs in long-term care as reported by CMS on either mucosa or skin is still unknown.
Medical device–related PIs are an important problem in the pediatric population.29–32 Fujii et al29 studied PI risk factors and rates in 7 neonatal ICUs in Japan. Of the 14 PIs that developed in 13 infants, 7 were located on the nose.29 Data by Boesch et al30 from a children’s hospital in the United States reported a reduction of MDR PIs in children with tracheostomies from 8.1% to 2.6% during implementation of a prevention care bundle and ultimately to a low of 0.3% after bundle implementation. A 2014 study of 741 neonatal ICU patients in a children’s hospital in the United States reported 28 patients developed PIs where 39 were caused by medical devices (79.6%).31 Infants who were born earlier (ie, fewer weeks of gestation) were more likely to have MDR PIs compared with infants with non-MDR PIs or conventional PIs.31-33
Given the small sample sizes of the few studies described previously, this study contributes to the literature by providing an in-depth analysis of MDR PIs using a large, robust sample of patients across healthcare settings with facility-acquired MDR PIs and MDR PIs present on admission. Because prevention and treatment guidelines are different for pediatric populations,32 this study focused on adult populations.
METHODS
International Pressure Ulcer Prevalence Survey
The IPUP survey has been conducted since 1989.7 It is an international, voluntary survey to enable facilities to benchmark and track their PI prevalence rates against other, similar facilities. Originally, only acute care hospitals participated, but current data include acute care hospitals, long-term acute care hospitals, long-term care, and rehabilitation facilities. The survey has grown over the years from 148 facilities and 34,987 patients participating in 1989 to approximately 1000 facilities surveying approximately 100,000 patients per year. In 2007, “medical device–related pressure ulcers” (now injuries) were added to the survey form. In 2016, a field to indicate the type of device that caused the MDR PI was added. The list of the devices that the survey responder had available to choose from included:
endotracheal tube
nasogastric tube
cast/splint
nasal oxygen (nose)
nasal oxygen (ears)
CPAP/BiPAP mask
halo
sequential compression device
cervical collar
tracheostomy neck plate
Methods and early results from IPUP surveys have been previously published.7,12–16 However, IPUP data on MDR PIs have not been published since 200812 and were therefore the focus of this study. Analyses were performed on the preexisting database and limited to adult patients/residents (>18 years old) who were hospitalized or resided in US or Canadian facilities. Analyses were limited to 2016 data, which allowed correlation between MDR PI and the type of device thought to be associated with the injury. To account for patients with multiple PIs, there were 2 types of analyses performed: (1) a patient-level analysis, and (2) a PI-level analysis. These analyses were further limited to patients for whom the survey responder completed data regarding the stage of the PI, the anatomic location of the PI, and whether the PI was present on admission or facility acquired. To analyze which devices were most frequently associated with MDR PIs, MDR PIs for which the survey responded did not list a device were coded as “other/unknown.”
Primary analyses consisted of summary statistics (eg, counts, percentages, averages, and SDs). A t test was used to compare MDR PIs to non-MDR PIs based on days until facility-acquired PIs developed. χ2 tests were used to determine whether the distribution of MDR PIs versus non-MDR PIs was significantly different by care setting, whether the PI was present on admission or facility acquired, injury stage, and anatomic location. Significance was set to .05 for all analyses. This study was reviewed by the Schulman institutional review board (reference #201701754) and found to be exempt.
RESULTS
The 2016 survey included 117,988 patients in 1115 facilities. Limiting the data set to US and Canadian adults yielded 102,865 patients (Table 1). Of these, 10,179 patients had PIs, for an overall prevalence of 9.9%. Facility-acquired PI prevalence was 3.7% (n = 3763). The MDR PI prevalence for all subjects, regardless of whether or not they had complete data, was 0.65% (n = 668).
Table 1.
DATA SAMPLE
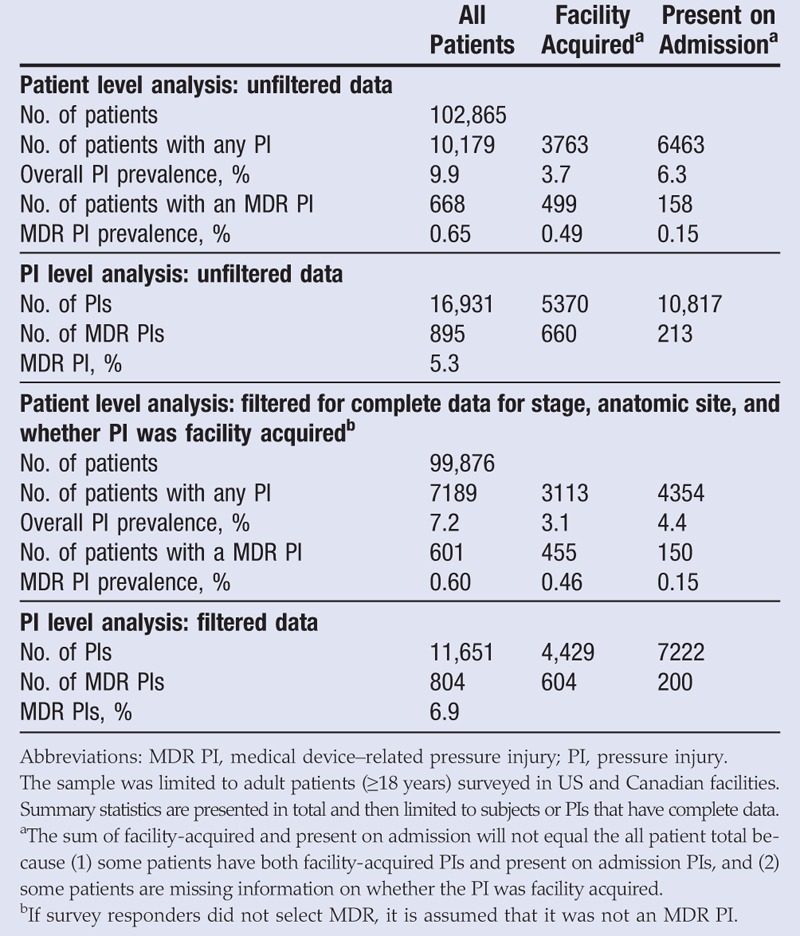
Because the surveys are self-reported, some of the data fields were not completed for some subjects. Therefore, to further analyze the data, subjects were limited to those who had complete data for PI stage, anatomic site, and whether the PI was facility acquired or present on admission. This yielded 99,876 patients; 7189 had a PI, and 601 patients had an MDR PI (0.60% prevalence).
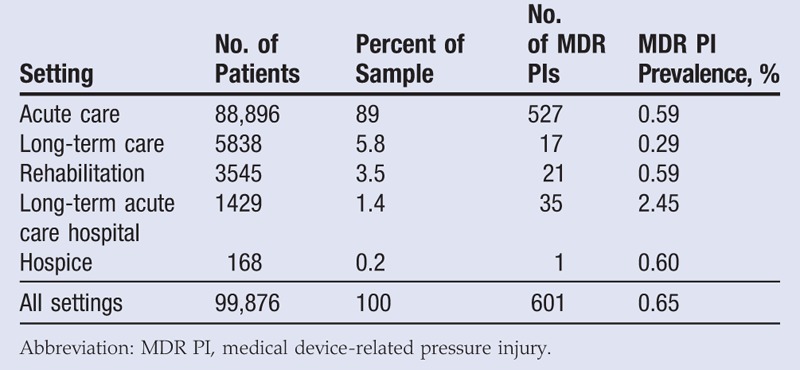
Data comprised patients from acute care units (89%), long-term care (5.8%), rehabilitation units (3.5%), long-term acute care hospitals (1.4%), and hospice units (0.2%). The MDR PI prevalence by care setting is reported in Table 2. A χ2 test found that the prevalence of MDR PIs was significantly different across care settings (χ2 = 91, P < .001). More specifically, a t test of proportions found that the prevalence of MDR PIs was significantly greater in long-term acute care hospitals than in acute care facilities (P < .001). However, there were very few MDR PIs in long-term care, rehabilitation, long-term acute care hospitals, and hospice care settings, and thus any analyses examining the differences in prevalence or characteristics of MDR PIs should be interpreted with caution. For this reason, all other characteristics are reported for all care settings and not separately.
Table 2.
ANALYSIS BY CARE SETTING
The PI-level analysis included 16,931 PIs in the total sample and 11,651 PIs that had complete data. Of those, 804 were identified as MDR PIs.
Present on Admission Versus Facility Acquired
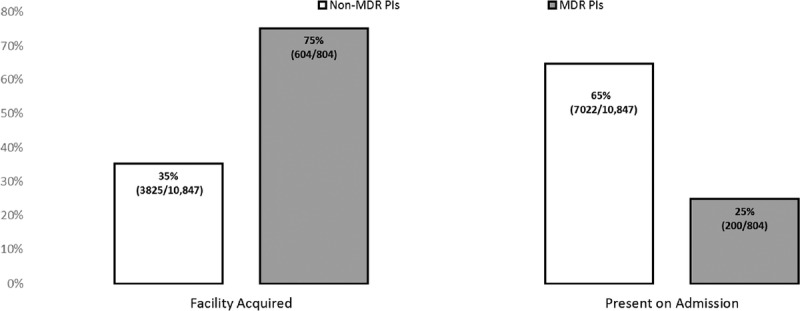
Of the 804 MDR PIs, 75% (n = 604) were facility acquired, and 25% (n = 200) were present on admission. Of the 10,847 non-MDR PIs, 35% (n = 3825) were facility acquired, and 65% (n = 7022) were present on admission (Table 1 and Figure 1). The MDR PIs were 2.1 times more likely to be facility acquired than non-MDR PIs. A χ2 test revealed that the distributions shown in Figure 1 are significantly different from each other (χ2 = 505, P < .001).
Figure 1.
PERCENTAGE OF PRESSURE INJURIES PRESENT ON ADMISSION VERSUS FACILITY ACQUIRED
Abbreviation: MDR PI, medical-device related pressure injury.
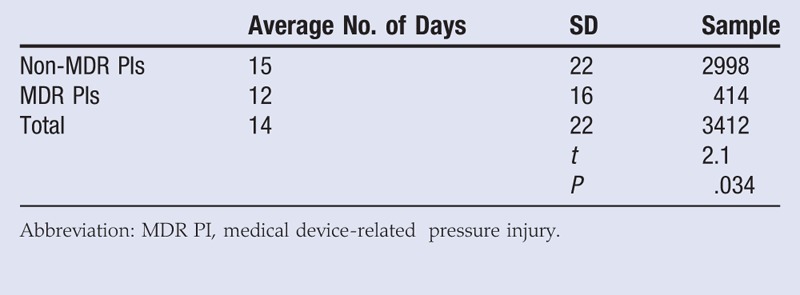
Survey responders were asked to report the number of days the patient was in the facility before the facility-acquired PI was discovered. A t test found that facility-acquired MDR PIs formed on average 3 days faster than non-MDR facility-acquired PIs (t = 2.1, P < .05; Table 3). The average number of days recorded before a non-MDR facility-acquired PI formed was 15 (SD, 22) days, but the average number of days recorded before a facility-acquired MDR PI formed was 12 (SD, 16) days (Table 3). The sample sizes for this analysis are reduced because the survey responders occasionally did not fill in the number of days before a facility-acquired PI was discovered.
Table 3.
T TEST EXAMINING TIME UNTIL FACILITY-ACQUIRED PRESSURE INJURY FORMED BY MEDICAL DEVICE-RELATED STATUS
Stage
The total number of PIs are broken out by stage in Table 4 and visualized in a bar graph in Figure 2. Of the 10,847 non-MDR PIs identified, the most frequent stage was Stage 2 (32%), followed by Stage 1 (20%), then unstageable PIs (15%), DTPIs (13%), Stage 3 (9.4%), Stage 4 (9.1%), and indeterminable PIs (those of unknown stage; 0.85%). Of the 804 MDR PIs identified, the most frequent stage was also Stage 2 (32%), followed closely by Stage 1 (28%), then DTPIs (15%), then unstageable PIs (13%), Stage 3 (7.5%), indeterminable PIs (3.7%), and Stage 4 (1.4%). Notably, MDR PIs are more likely to be classified as Stage 1 (28% vs 20%), but less likely to be classified as Stage 4 (1.4% vs 9.1%). A χ2 test reveals that the distributions across the stages differ significantly for MDR PIs as compared with all non-MDR PIs (χ2 = 146, P < .001). Importantly, as per the NPUAP, nonmucosal PIs cannot be staged, which might explain the relatively high percentage of indeterminable PIs in this analysis.
Table 4.
PRESSURE INJURIES BY STAGE
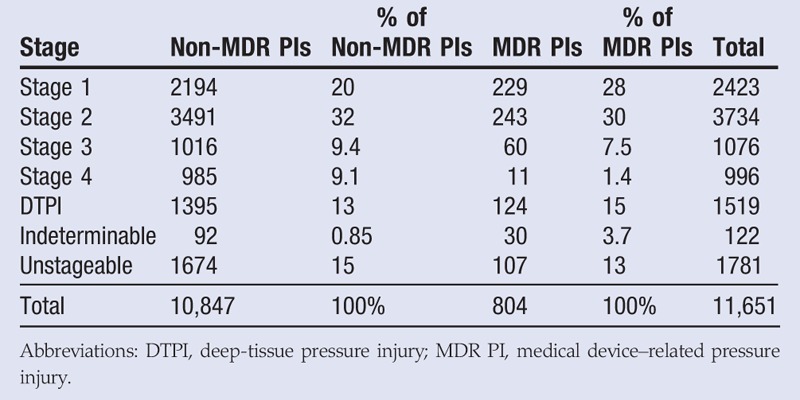
Figure 2.
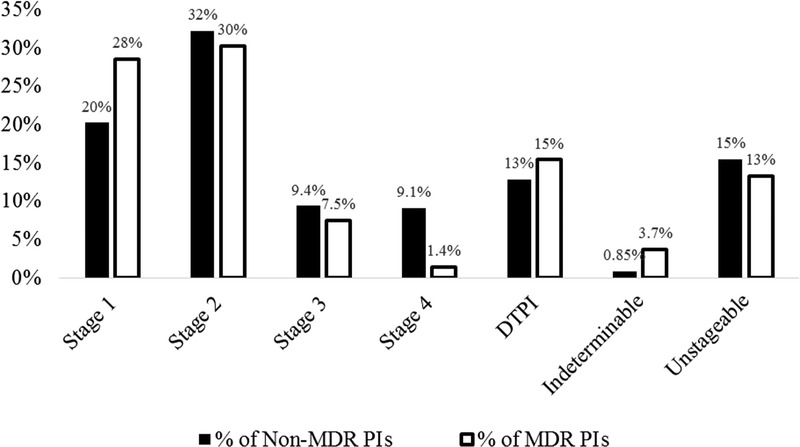
PERCENTAGE OF PRESSURE INJURIES BY STAGE
Abbreviations: DTPI, deep-tissue pressure injury; MDR PI, medical device-related pressure injury.
Anatomic Site
Tabulations of both non-MDR PIs and MDR PIs by anatomic location of the wound are presented in Table 5 and visualized in a pie chart in Figure 3. Of the 10,847 non-MDR PIs identified, the most frequent PI anatomic sites were the pelvic area (60%), followed by the lower extremities (33%), the back (2.5%), the upper extremities (1.8%), and the face/head (1.3%). The remaining 1.4% were recorded as “other.” Of the 804 MDR PIs identified, the most frequent wound locations were on the face and head (51%)—the ears (29%) and nose (10%) specifically. This was followed by the other location groups: the lower extremities (27%), the pelvic area (7.5%), the upper extremities (3.0%), and the back (1.5%). The remaining 10% were recorded as “other.”
Table 5.
PRESSURE INJURIES BY ANATOMIC LOCATION
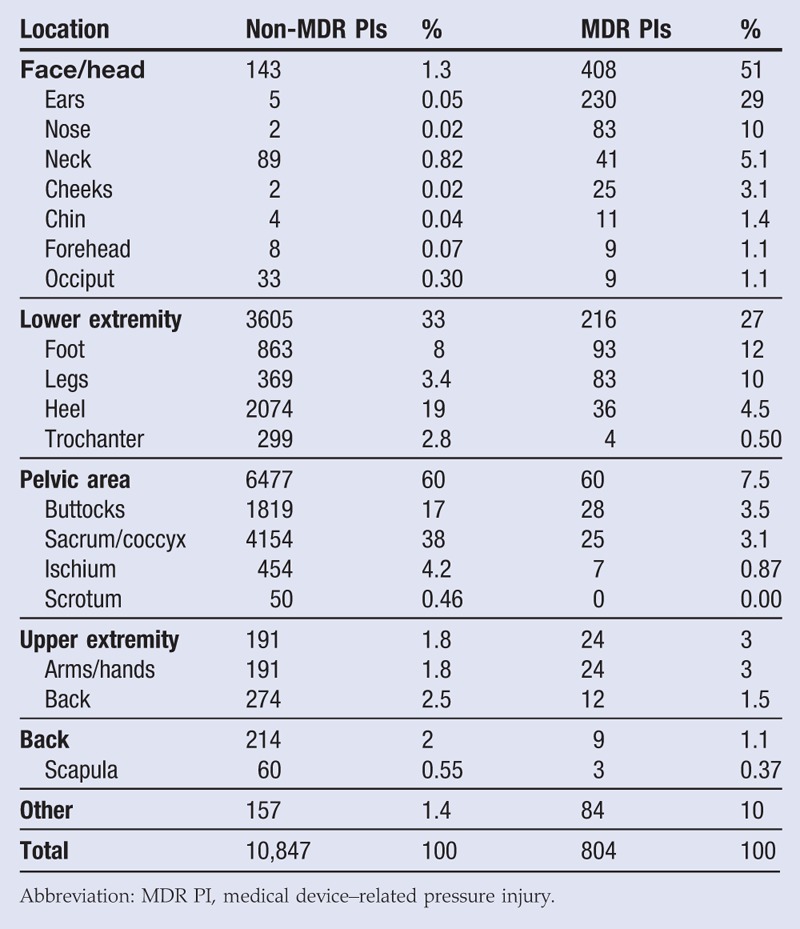
Figure 3.
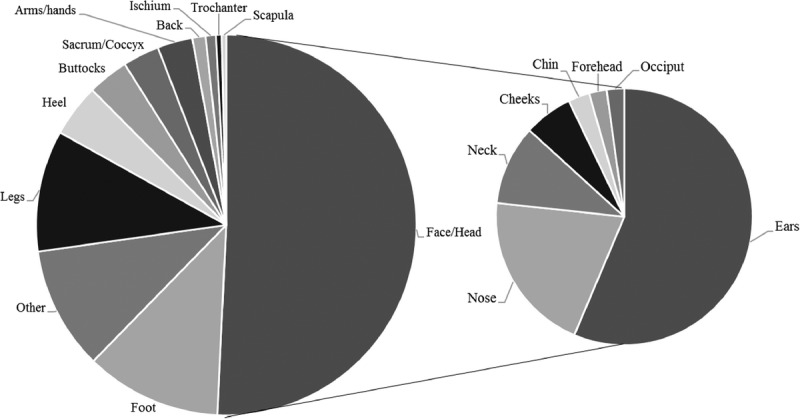
MEDICAL DEVICE–RELATED PRESSURE INJURIES BY ANATOMIC LOCATION
Notably, more than half (51%) of MDR PIs are located on the head or face, but only 1.3% of non-MDR PIs are in those areas. Most of the MDR PIs on the face or head were on the ears (29%, n = 230). A χ2 test reveals that the distributions across the locations do differ significantly for MDR PIs as compared with all non-MDR PIs (χ2 = 4800, P < .001).
Device Type
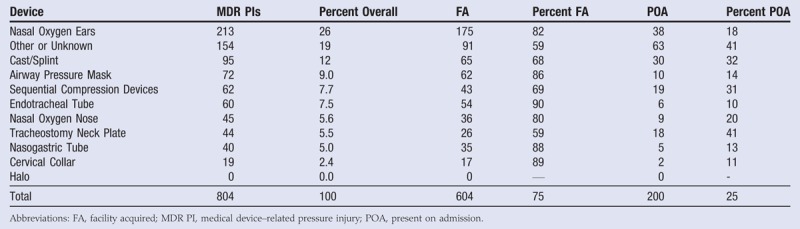
If survey responders identified a PI as device related, they were also instructed to identify what device was associated with the PI. In some cases, no device was chosen and these PIs were coded as “other or unknown” devices. Tabulations of MDR PIs by device type are listed in Table 6. The MDR PIs were most frequently associated with nasal oxygen devices (32%, n = 258), where 26% (n = 213) were associated with how the device interacted with the ears, and the other 5.6% (n = 45) were associated with how the device interacted with the nose. The next most frequent devices listed were casts and splints (12%), followed by CPAP or BiPAP masks (9.0%), then sequential compression devices (7.7%), endotracheal tubes (7.5%), tracheostomy neck plates (5.5%), nasogastric tubes (5.0%), and finally cervical collars (2.4%). No MDR PIs were associated with halo devices, and 19% of MDR PIs did not have a device filled out on the survey and so were coded as other or unknown.
Table 6.
MDR PIs POA VS FA BY DEVICE
Most MDR PIs are facility acquired; however, there are variations by device. For instance, of the 60 MDR PIs associated with endotracheal tubes, 90% (n = 54) were facility acquired. However, of the 44 MDR PIs associated with tracheostomy neck plates, only 59% (n = 26) were facility acquired. A stacked bar graph displays the differences (Figure 4).
Figure 4.
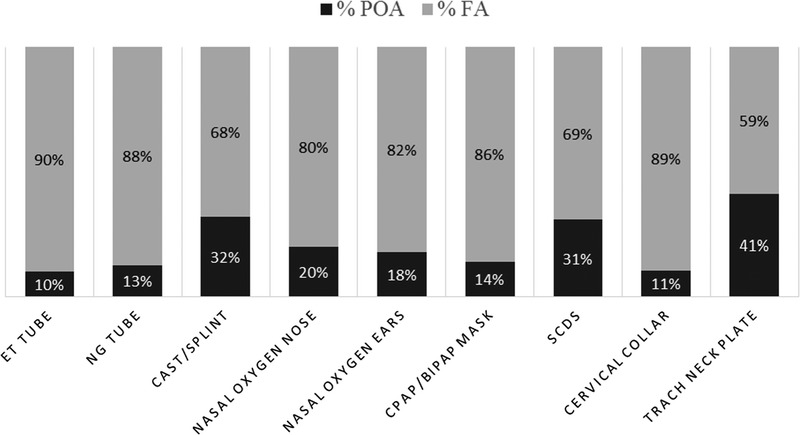
DEVICES RELATED TO MDR PIS BY POA VERSUS FA
Abbreviations: BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; ET, endotracheal; FA, facility acquired; MDR PI, medical device-related pressure injury; NG, nasogastric; POA, present on admission; SCD, sequential compression device; trach, tracheostomy.
DISCUSSION
Because this study’s sample represents many care settings (academic medical centers, community hospitals, long-term care facilities, long-term acute care hospitals, rehabilitation, and even hospice units), it is challenging to compare results with more narrowly defined settings. This broader representation might have resulted in a lower overall prevalence of MDR PIs of 0.60%. This was lower than the 1.4% reported by Black et al21 in an academic acute care medical center that was limited to patients without a PI present on admission. It was also lower than the 3.4% in ICU patients reported by Coyer et al22 and the 26.7% reported by Amirah et al.24 The lower prevalence might also reflect the positive impact of the NPUAP’s effort to raise awareness of MDR PIs and the impact of successful PI prevention efforts.
Study authors did find preliminary evidence of differences in prevalence of MDR PIs across care settings (Table 2). However, these results should be interpreted with caution because the sample sizes of MDR PIs in non–acute care settings were small. Similar to past studies of MDR PIs, these prevalence numbers do not distinguish between MDR PIs on the skin versus those on the mucosa. Future work should take this into account and report prevalence of both types of MDR PIs. The IPUP survey has been reworded such that this will be possible going forward.
There are different levels of analysis available to authors to determine the impact of a specific PI type. In a previous IPUP study,12 the relative proportion of all identified PIs (multiple PIs per patient are counted) that were MDR PIs was reported at 9.1% in acute care settings (2009 data), which was higher than the current study (6.9% of all documented PIs in a generalized care setting). In addition, 48% of MDR PIs were facility acquired in the previous analysis, as compared with 75% of MDR PIs in this study. These results may differ because of the addition of post–acute care settings. However, in the 2016 IPUP data, acute care settings made up 89% of the sample, so it is unlikely that the additional care settings explain the entire difference. Longitudinal studies are needed to know whether MDR PI prevalence has decreased over time and whether that decrease can be attributed to increased education and/or better guidelines from the NPUAP regarding MDR PIs.2 Moreover, the present study included data from Canada as well as the United States, and future research is needed to know whether the inclusion of a section on medical devices in the newly released best practice guideline by Wounds Canada will also have an impact on MDR PI prevalence in Canada.34
The present study found that MDR PIs were more likely to be facility acquired than non-MDR PIs (75% vs 25%). VanGilder et al12 found that 48% of the MDR PIs from the 2009 acute care IPUP data (785 of 1631) were facility acquired. Again, the present study included more than acute care settings, which might account for this difference. Study authors also found that facility-acquired MDR PIs developed faster during the course of admission than non-MDR facility-acquired PIs (12 vs 15 days, P < .05). This finding is new to the literature and highlights the importance of identification of patients at risk of MDR PIs and implementation of prevention strategies.
By stage, MDR PIs had higher percentages than non-MDR PIs in the following categories: Stage 1 (28% vs 20%), DTPIs (15% vs 13%), and indeterminable (3.7% vs 0.85%). The MDR PIs had lower percentages in the following categories: Stage 2 (30% vs 32%), Stage 3 (7.5% vs 9.4%), Stage 4 (1.4% vs 9.1%), and unstageable PIs (13% vs 15%). The percentage of MDR PIs reported as an “indeterminable” stage might reflect the fact that PIs on the mucosa cannot be staged. The 2016 survey data form did not include an option for mucosal PI. This is problematic because survey coordinators may have opted to mark mucosal PIs as indeterminable, or they might have left the stage field blank. If they left the field blank, they would have been dropped from this analysis for incomplete data. This revelation led to the decision to discontinue the indeterminable option on the data form and replace it with a field for PIs on the mucosal membrane for future IPUP surveys.
In their studies of facility-acquired MDR PIs, Black et al21 and Arnold-Long et al25 found that most MDR PIs were either Stage 1 or Stage 2 PIs. This corresponds with these results that most MDR PIs were Stage 1 (20%) or Stage 2 (32%). However, it is important to note that this study included present on admission and facility-acquired MDR PIs. Because of this study’s large sample, this research found 1.4% of MDR PIs were Stage 4, unlike Black et al21 and Arnold-Long et al25 who did not find any Stage 4 facility-acquired MDR PIs.
This study found that most MDR PIs (51%) were located on the face, head, or neck, with 29% on the ears specifically. Apold and Rydrych20 found that 70% of MDR PIs were located on the face. That study included only MDR PIs that were categorized as Stage 3, Stage 4, or unstageable, whereas the present study included all stages, which might have accounted for the difference in the locations. Future research is needed to explore the relationship between location and severity of the PI. Black et al21 found that the ears were the most common location (35%), which is similar to the result in the present study that ears were the most common location (230 of the 804 MDR PIs, or 29%).
The devices most commonly associated with MDR PIs were nasal oxygen tubes, which affected both the ears and nose for a total of 32%. The results of the present study differ from ICU studies reported by both Coyer et al22 and Amirah et al,24 which found endotracheal or nasogastric tubes to be the most common devices related to PIs. In the present study, endotracheal and nasogastric tubes comprised 7.5% and 5.0% of the MDR PIs, respectively, across all care settings. Of the MDR PIs captured in this study, 28% were recorded in ICU settings, as opposed to the aforementioned studies,22,24 which exclusively studied ICU settings. It is likely that a generalized population uses different devices than the ICU population.
The current study results also differed from Apold and Rydrych,20 who reported that the most common devices associated with serious PIs in acute care were cervical collars or braces (22%). The present study found that cervical collars were the second least frequent device related to an MDR PI (2.4%). This difference is possibly linked with the differences in stages of PIs included in the analysis. Apold and Rydrych20 analyzed only serious PIs (Stage 3, Stage 4, or unstageable). In the present study, Stage 3, Stage 4, and unstageable MDR PIs made up only 22% of the 804 MDR PIs in the sample.
Future research is needed to explore the relationship between type of device and the depth of tissue involved of the MDR PI; certain devices might be associated with higher PI numerical stages. Unfortunately, the IPUP survey did not collect data on the use of medical devices for patients who did not have an MDR PI. Therefore, it was not possible to examine the percentage of patients with a given device who developed an MDR PI. As the relative proportion of those patients using nasal oxygen tubing in the studied care settings to those who developed MDR PIs is unknown, it cannot be known whether they are more of a causative device than other device types.
This study has some important clinical implications because the devices that have been identified as associated with MDR PIs are very common in clinical practice. This study found that in a generalized, large database of patients, the most common anatomic PI sites were the ears, and the most commonly associated devices were nasal oxygen tubes. However, previous studies have shown that the devices most commonly associated with MDR PIs vary substantially with care setting. To enable standardization, future work should capture the number of patients with each type of device and how many of them later developed an associated MDR PI. This percentage would standardize results across care settings by accounting for how frequently (or infrequently) certain devices are used in different care settings.
This study also found that it is particularly critical to be proactive in assessing MDR PIs, because facility-acquired MDR PIs form faster than facility-acquired non-MDR PIs. Providers must select devices that protect the skin whenever possible, apply the right device and apply it appropriately, assess the skin/tissue under the device frequently, and mitigate any early signs of PI.
Limitations
There are several important limitations to this work. First, the survey teams had limited options regarding the device field for MDR PIs. The survey should have included an “other” or “unknown” device option, which would have allowed researchers to determine whether the survey responder did not know the type of device or if they left the question blank for some other reason.
Second, choices for anatomic sites did not include “lips” or “mouth” to yield more information about the location of MDR PIs, particularly of those that were likely to be a mucosal PI.
Third, survey coordinators could choose “indeterminable” as a PI stage. This category might have included mucosal PIs; however, interpretation is limited because the intent of the survey coordinator was not captured in the form. Relatedly, mucosal PI was not listed as a staging category to allow the nonstaged mucosal PIs to be correctly recorded. Mucosal PIs have been added in the 2017 survey.
Fourth, only adults were included in this analysis, and further research is warranted in the pediatric population.
Fifth, the IPUP survey focuses primarily on acute care settings, and therefore, these sample sizes from each setting were quite small, making it infeasible to examine differences in characteristics of MDR PIs across care settings. Studies using large samples from non–acute care settings are needed to know whether these results can be generalized beyond acute care.
Finally, the current analysis was limited to self-reported data from facilities that choose to participate in the IPUP survey. There might be differences in mandated reporting versus voluntary reporting of PIs.
CONCLUSIONS
Medical devices are common in all clinical environments, and their use is associated with the formation of MDR PIs.1,2 This study found that the prevalence of MDR PIs was 0.6%, which was lower than previously reported. The lower prevalence might reflect the positive impact of the NPUAP’s effort to raise awareness of MDR PIs and the impact of successful PI prevention efforts. Most MDR PIs were facility acquired (75%) and were commonly located on the ears (29%) and the feet (12%). The devices most commonly associated with MDR PIs were nasal oxygen tubes (32%), which affect both the ears and the nose. Finally, this study reinforces existing guidelines on the early assessment and treatment of MDR PIs4 by finding that facility-acquired MDR PIs form on average 3 days faster than facility-acquired non-MDR PIs.
REFERENCES
- 1.National Pressure Ulcer Advisory Panel. National Pressure Ulcer Advisory Panel announces a change in terminology from pressure ulcer to pressure injury and updates the stages of pressure injury. 2016. www.npuap.org/national-pressure-ulcer-advisory-panel-npuap-announces-a-change-in-terminology-from-pressure-ulcer-to-pressure-injury-and-updates-the-stages-of-pressure-injury. Last accessed March 19, 2018.
- 2.National Pressure Ulcer Advisory Panel. Mucosal Pressure Ulcers: An NPUAP Position Statement August 2008. 2008. www.npuap.org/wp-content/uploads/2012/01/Mucosal_Pressure_Ulcer_Position_Statement_final.pdf. Last accessed March 19, 2018.
- 3.National Pressure Ulcer Advisory Panel. Best Practices for Prevention of Medical Device-Related Pressure Injuries: Posters. www.npuap.org/resources/educational-and-clinical-resources/best-practices-for-prevention-of-medical-device-related-pressure-injuries. Last accessed March 19, 2018.
- 4.Haesler E, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Cambridge, United Kingdom: Cambridge Media; 2014. [Google Scholar]
- 5.European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Washington, DC: National Pressure Ulcer Advisory Panel; 2009. [Google Scholar]
- 6.Wound, Ostomy, and Continence Nurses Society. WOCN Society Position Paper: Avoidable Versus Unavoidable Pressure Ulcers (Injuries). Mt Laurel, NJ: WOCN; 2017. [DOI] [PubMed] [Google Scholar]
- 7.Amlung SR, Miller WL, Bosley LM. The 1999 National Pressure Ulcer Prevalence Survey: a benchmarking approach. Adv Skin Wound Care 2001;14:297-301. [DOI] [PubMed] [Google Scholar]
- 8.Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud 2013;50(7):974-1003. [DOI] [PubMed] [Google Scholar]
- 9.Meehan M. Multi site prevalence ulcer prevalence study. Adv Skin Wound Care 1990;3:14-9. [Google Scholar]
- 10.Cuddigan J, Berlowitz DR, Ayello EA. Pressure ulcers in America: prevalence, incidence, and implications for the future. Adv Skin Wound Care 2001;14:208. [DOI] [PubMed] [Google Scholar]
- 11.Pieper B, National Pressure Ulcer Advisory Panel, eds. Pressure Ulcers: Prevalence, Incidence, and Implications for the Future. Washington, DC: National Pressure Ulcer Advisory Panel; 2012. [Google Scholar]
- 12.VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008-2009 International Pressure Ulcer Prevalence Survey and a 3-year, acute care, unit-specific analysis. Ostomy Wound Manage 2009;55(11):39-45. [PubMed] [Google Scholar]
- 13.VanGilder C, Lachenbruch C, Algrim-Boyle C, Meyer S. The International Pressure Ulcer Prevalence Survey 2006-2015. J Wound Ostomy Cont Nurs 2017;44(1):20-;8. [DOI] [PubMed] [Google Scholar]
- 14.VanGilder C, MacFarlane GD, Harrison P, Lachenbruch C, Meyer S. The demographics of suspected deep tissue injury in the United States: an analysis of the International Pressure Ulcer Prevalence Survey 2006-2009. Adv Skin Wound Care 2010;23:254-61. [DOI] [PubMed] [Google Scholar]
- 15.VanGilder C, MacFarlane G, Meyer S, Lachenbruch C. Body mass index, weight, and pressure ulcer prevalence: an analysis of the 2006-2007 International Pressure Ulcer Prevalence Surveys. J Nurs Care Qual 2008;24(2):127-35. [DOI] [PubMed] [Google Scholar]
- 16.Kayser S, VanGilder C, Lachenbruch C. Predictors of hospital-acquired pressure injuries: a multivariate regression analysis of International Pressure Ulcer Prevalence Survey data (2011-2016). Poster presented at the 49th WOCN Annual Conference; Salt Lake City, Utah; May 19-23, 2017.
- 17.Watts D, Abrahams E, MacMillan C, et al. Insult after injury: pressure ulcers in trauma patients. Orthop Nurs 1998;17(4):84-91. [PubMed] [Google Scholar]
- 18.Powers J, Daniels D, McGuire C, Hilbish C. The incidence of skin breakdown associated with use of cervical collars. J Trauma Nurs 2006; 13(4):198-200. [DOI] [PubMed] [Google Scholar]
- 19.Ackland HM, Cooper DJ, Malham GM, Kossman T. Factors predicting cervical collar-related decubitus ulceration in major trauma patients. Spine 2007;32(4):423-8. [DOI] [PubMed] [Google Scholar]
- 20.Apold A, Rydrych D. Preventing device-related pressure ulcers: using data to guide statewide change. J Nurs Care Qual 2012;27(1):28-34. [DOI] [PubMed] [Google Scholar]
- 21.Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Medical device related pressure ulcers in hospitalised patients. Int Wound J 2010;7:358-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coyer FM, Stotts NA, Blackman VS. A prospective window into medical device–related pressure ulcers in intensive care. Int Wound J 2013;11:656-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schallom M, Cracchiolo L, Falker A, et al. Pressure ulcer incidence in patients wearing nasal-oral versus full-face noninvasive ventilation masks. Am J Crit Care 2015;24(4):349-57. [DOI] [PubMed] [Google Scholar]
- 24.Amirah MF, Rasheed AM, PJ P, Nu’man OS, Muteb MA. A cross-sectional study on medical device–related pressure injuries among critically ill patients in Riyadh, Kingdom of Saudi Arabia. WCET J 2017;37(1):8-11. [Google Scholar]
- 25.Arnold-Long M, Ayer M, Borchert K. Medical device–related pressure injuries in long-term acute care hospital setting. J Wound Ostomy Cont Nurs 2017;44(4):325-30. [DOI] [PubMed] [Google Scholar]
- 26.Delmore BA, Ayello EA. Pressure injuries caused by medical devices and other objects a clinical update. Am J Nurs 2017;117(12):36-45. [DOI] [PubMed] [Google Scholar]
- 27.Ayello EA. CMS MDS 3.0 Section M skin conditions in long-term care: pressure ulcers, skin tears, and moisture-associated skin damage update. Adv Skin Wound Care 2017;30:415-29. [DOI] [PubMed] [Google Scholar]
- 28.MDS frequency report. www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/Minimum-Data-Set-3-0-Public-Reports/Minimum-Data-Set-3-0-Frequency-Report.html. Last accessed March 19, 2018.
- 29.Fujii K, Sugama J, Okuwa M, Sanada H, Mizokami Y. Incidence and risk factors of pressure ulcers in seven neonatal intensive care units in Japan: a multisite prospective cohort study. Int Wound J 2010;7(5):323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boesch RP, Myers C, Garrett T, et al. Prevention of tracheostomy-related pressure ulcers in children. Pediatrics 2012;129(3):e792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visscher M, Taylor T. Pressure ulcers in the hospitalized neonate: rates and risk factors. Scientific Reports 2014;1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baharestani MM. Pressure ulcers in pediatric populations. In: Pieper B, National Pressure Ulcer Advisory Panel, eds. Pressure Ulcers: Prevalence, Incidence, and implications for the Future. Washington, DC: National Pressure Ulcer Advisory Panel; 2012. [Google Scholar]
- 33.Fischer C, Bertelle V, Hohlfeld J, Forcada-Guex M, Stadelmann-Diaw C, Tolsa JF. Nasal trauma due to continuous positive airway pressure in neonates. Arch Dis Child Fetal Neonatal Ed 2010;95(6):F447-51. [DOI] [PubMed] [Google Scholar]
- 34.Norton L, Parslow N, Johnston D, et al. Best practice recommendations for the prevention and management of pressure injuries. 2017. Wounds Canada. www.woundscanada.ca/docman/public/health-care-professional/bpr-workshop/172-bpr-prevention-and-management-of-pressure-injuries-2/file. Last accessed March 19, 2018.


