Abstract
Objective:
The aim of the study was to assess the risk of asbestosis death based on the temporal pattern of exposure to asbestos.
Methods:
We followed up a cohort of asbestos textile workers, employed in 1946 to 1984, until November 2013. We measured the duration of the employment, the time since last employment (TSLE), the age, and the year of first employment. Hazard ratios (HR) were estimated through multivariable Cox regression models.
Results:
We observed 51 asbestosis deaths among 1823 workers. The HR of asbestosis death increased with exposure duration (HR 2.4 for ≥15 years compared with <5 years, P trend = 0.014) and declined with TSLE (HR 0.3 for ≥25 compared with <5 years, P = 0.004). The risk of asbestosis mortality strongly declined for exposure starting after 1968.
Conclusions:
The risk of asbestosis death strongly declines in the decades after cessation of the exposure.
Keywords: asbestos, asbestosis, cohort study, occupational exposure
Asbestosis is a diffuse interstitial lung disease that occurs after intense and prolonged exposure to asbestos.1 Because asbestosis may be clinically and histologically undistinguishable from other forms of pulmonary fibrosis, its diagnosis relies on the history of exposure to asbestos or detection of high level of asbestos bodies or asbestos fibers in the lung.2
In many European countries, asbestos has been intensively used during the twentieth century, until national regulations restricted or forbade its production, importation, or use. In Italy, the use of asbestos increased after World War II, peaked in the late 1970s, remained above 100,000 tons/yr until 1987, and almost ceased in 1992—after a national ban.3 Despite more than 20 years have elapsed since the ban, approximately 700 cases of asbestosis have been reported every year between 2011 and 2015 to the Italian National Institute for Insurance against Accidents at Work (INAIL).4 These figures suggest that the risk of asbestosis remains elevated for long periods after the end of the exposure. This observation is in line with the findings from an ecological study conducted in the United Kingdom using administrative data, which showed that mortality from asbestosis might continue to increase for at least 2 decades after cessation of occupational exposure.5 These authors explained this observation as a sign that the pathological process, once started, might continue even in the absence of exposure.5,6 However, the UK study was solely based on the analysis of mortality rates by birth cohort compared with the historical trends in the use of asbestos, without individual information on exposure.5
We conducted an analysis of a cohort of asbestos textile workers, thus providing novel individual-level evidence on the change in risk of death from asbestosis based on temporal patterns of exposure to asbestos.
MATERIALS AND METHODS
Study Population and Follow-Up
Detailed information on this cohort, as well as findings on mortality from asbestos-related cancers, was reported elsewhere.7–9 Briefly, this historical cohort includes all subjects (1083 women and 894 men) who had worked in an asbestos textile factory in Northern Italy between 1946 and 1984, when the factory was closed. Data on employment were obtained directly from the personnel records at the factory; information on specific job titles was not available. We linked our cohort to population registers maintained by the local authorities to ascertain vital status and causes of death (as officially coded for statistical purposes). We also actively collected any available information on the previous history of exposure to asbestos, and we identified 120 subjects (6.1%) who had been employed in a different asbestos factory before entering our cohort. For these workers we had evidence of relevant exposure to asbestos occurred before employment at the studies factory, but we could not access specific data on the characteristics or duration of exposure to asbestos. To maximize the validity of the results on the temporal pattern of exposure in determining the risk of asbestosis, we aimed at identifying a cohort as close as possible to an “inception cohort”—in our specific case, a cohort of workers without relevant exposure to asbestos before the baseline of the study. For this reason, we excluded the 120 workers with a former employment in an asbestos factory. We further excluded 34 deceased subjects with unknown cause of death.
We followed up the cohort from January 1, 1946 to November 30, 2013. The follow-up of each subject started at the beginning of the study period or at his first employment at the factory (whichever occurred later) and ended at the death, loss to follow-up, emigration, or end of the study, whichever occurred earlier. Also, we censored the follow-up at the age of 85 because of the possibility of frequent misclassification of the causes of death in the elderly.
This study was conducted according to the declaration of Helsinki for medical research involving human subjects.
Outcome Definition
The main outcome measure was the hazard ratio (HR) of death due to asbestosis. The underlying cause of death, classified according to the International Classification of Diseases, 9th Edition (ICD-9), was extracted from the official death records. Deaths due to asbestosis were identified by the ICD-9 code 501. We also took note of death from other chronic lung diseases, such as ICD-9 500, coal workers’ pneumoconiosis; 502, pneumoconiosis due to other silica or silicates; 503, pneumoconiosis due to other inorganic dust; 504, pneumopathy due to inhalation of other dust; 505, pneumoconiosis, unspecified.
We also identified all deaths attributed to established asbestos-related cancers, including peritoneal cancer (ICD-9 158), laryngeal cancer (161), lung cancer (162), pleural cancer (163), and ovarian cancer (183).10
As a secondary outcome, we calculated the mortality rate (MR) from asbestosis per 100,000 person-years across strata of selected exposure variables.
Exposure Characteristics and Exposure Variables
Various types of asbestos were used in the factory, including crocidolite. Environmental data on exposure to asbestos between 1968 and 1977 were published elsewhere.7,8 In the late 1960s, the recorded exposure levels were extremely high (100 ff/mL) in the opening and carding areas, but elevated levels were observed also in the spinning department (10 to 15 ff/mL). During the 1970s, an important decrease in the environmental concentration of asbestos was documented, and the average level of exposure measured in 1977 was at most 2 ff/mL in all the departments of the factory.7,8 Although these environmental monitoring data were available to us, we could not reconstruct individual exposure levels of the cohort. Thus, we quantified the occupational exposure to asbestos of each subject based on duration of employment at the factory; we also calculated time since last employment (TSLE), defined according to the cessation of the employment or the shutdown of the factory. Duration of employment, TSLE and age were calculated as time-varying variables. Considering the documented change in the environmental levels of asbestos and the possible role of age at first exposure,5,7,8 we also identified for each individual the period and age at first employment, classified according to the date of hiring. Finally, we explored the role of sex in determining the risk of asbestosis in our cohort.
Statistical Analysis
Summary statistics were expressed as number and percentages; we also reported the MR and their 95% confidence intervals (CIs) across strata of selected variables. We plotted the cumulative hazard function for death from asbestosis by means of the Nelson-Aalen estimator.11
We fitted Cox proportional hazard regression models to study the association between the exposure variables and the risk of asbestosis mortality. As the incidence of asbestosis is strongly age related,5 in all the regression models we specified age as the main temporal axis. At first, we fitted univariate regression models including a binary (sex) or ordinal (age and year of first exposure, duration of employment, and TSLE—cutoffs were selected a priori) indicator for the variable under study. We then fitted five separate multivariable regression models, one for each potential predictor. We chose a priori the covariates to be included in these models, to avoid collinearity between temporal variables; sex and period of first employment were always included among the covariates, whereas the inclusion of age at first exposure, duration of exposure, or TSLE depended on the variable under study. Indeed, the simultaneous inclusion of age at first exposure, duration of exposure, and TSLE in multivariable Cox models with age specified as the main temporal axis induced multiple collinearity; hence, we prioritized covariates as follows: (1) duration of exposure, (2) TSLE, and (3) age at first exposure. Aside collinearity, multivariable models also presented the risk of over-parameterization due to the relatively limited number of events (N = 51). For this reason, we introduced in each model only the main predictor as an ordinal variable, whereas the other covariates, included as potential confounders, were modeled as continuous variables. After assessing the dose–response variable through the analysis of the HR across strata of ordinal variables, we further adapted a cubic spline regression model selected according to the procedure proposed by Royston and Sauerbrei.12 This iterative procedure allows performing a backward selection of covariates (at P < 0.1) to be included in the final model and selecting the parameterization of continuous variables that minimize the deviance of the model; we applied the strategy starting from a full model including duration of exposure, TSLE, year of first exposure, and sex.
We conducted two sensitivity analyses. In the first set, we extended the case definition to all chronic lung diseases identified by ICD-9 codes 500 to 505. The second set of sensitivity analyses was aimed at assessing the role of competing mortality causes in determining the observed pattern of death due to asbestosis. Hence, we estimated subhazard ratio (SHR) of asbestosis by fitting Fine and Gray competing risk regression models, where potentially asbestos-related cancers were treated as competing events.13
All the analyses were performed using Stata 14.1 SE (Stata Corp, College Station, TX). All tests were two-sided, and a P < 0.05 was used to define significance.
RESULTS
The cohort included 1977 workers employed between 1946 and 1984. After the exclusion of 120 subjects who had been previously occupationally exposed to asbestos and of 34 with unknown cause of death, 1823 were included in the main analysis (Fig. 1). At the end of the follow-up, 768 (42.1%) subjects were alive and aged less than 85, 104 (5.7%) were right-censored at the age of 85, 19 (1.0%) had emigrated, 26 (1.4%) were lost to follow-up, and 906 (49.7%) died before the age of 85 (51 due to asbestosis). Selected characteristics of the cohort are presented in Table 1. Asbestosis was reported as the underlying cause of deaths in 5.6% (51 out of 906) of workers deceased before the age of 85 years. The overall crude MR for asbestosis was 74 cases per 100,000 person-year at risk (95% CI, 56 to 97), but we observed important variation depending on the temporal pattern of exposure. In particular, the highest MRs were observed among workers employed first before 1961 (MR = 118) and at the age of 35 years or more (MR = 202). Also, asbestosis mortality steadily increased with duration of employment (from 24 per 100,000 for <5 years to 422 per 100,000 person-year at risk for ≥15 years of employment). Regarding TSLE, the MR increased up to 112 cases per 100,000 person-year at risk in the first 25 years, and then it declined to 62 cases per 100,000 person-year at risk.
FIGURE 1.
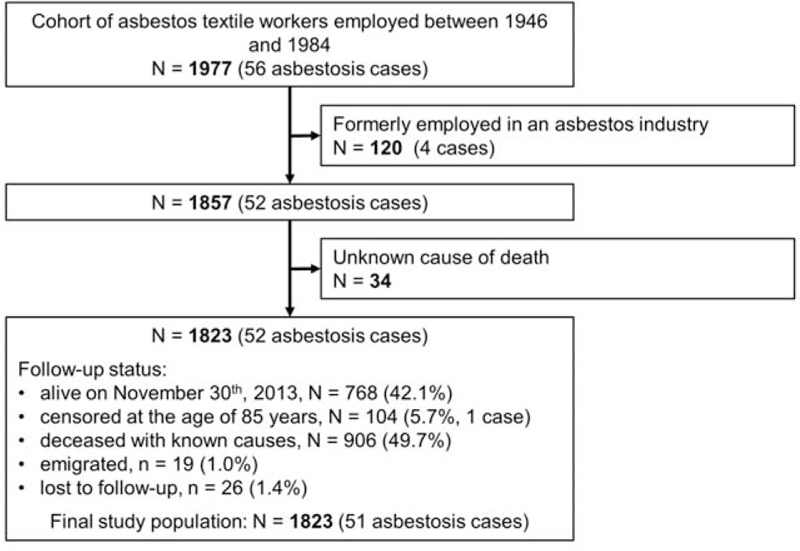
Flow diagram of the study population.
TABLE 1.
Characteristics of the Study Population and Mortality From Absestosis
| Asbestosis (ICD9 501) | |||||||
| No (N = 1772) | Yes (N = 51) | Mortality | |||||
| Characteristic | Median Value* | N | (%) | N | (%) | MRR† | (95% CI)† |
| Sex | |||||||
| Female | NA | 1002 | (98.0) | 20 | (2.0) | 46 | (29–71) |
| Male | NA | 770 | (96.1) | 31 | (3.9) | 121 | (85–172) |
| Age at first employment | |||||||
| <25 yrs | 19 | 761 | (99.0) | 8 | (1.0) | 23 | (11–45) |
| 25–34 yrs | 30 | 440 | (97.8) | 10 | (2.2) | 56 | (30–105) |
| ≥35 yrs | 44 | 571 | (94.5) | 33 | (5.5) | 202 | (144–285) |
| Year of first employment | |||||||
| ≤1960 | 1951 | 529 | (95.3) | 26 | (4.7) | 118 | (80–173) |
| 1961–1968 | 1964 | 675 | (97.2) | 20 | (2.9) | 74 | (48–115) |
| ≥1969 | 1970 | 568 | (99.1) | 5 | (0.9) | 25 | (10–59) |
| Duration of employment | |||||||
| <5 yrs | 0.9 | 1118 | (99.0) | 12 | (1.0) | 24 | (14–43) |
| 5–9 yrs | 7.0 | 293 | (95.8) | 13 | (4.2) | 114 | (66–196) |
| 10–14 yrs | 12.4 | 175 | (93.6) | 12 | (6.4) | 222 | (126–390) |
| ≥15 yrs | 17.5 | 122 | (89.7) | 14 | (10.3) | 422 | (250–712) |
| Time since last employment | |||||||
| <5 yrs | 0.3 | 100 | (93.5) | 7 | (6.5) | 39 | (19–83) |
| 5–14 yrs | 10.3 | 157 | (91.3) | 15 | (8.7) | 92 | (55–152) |
| 15–24 yrs | 20.7 | 227 | (93.4) | 16 | (6.6) | 112 | (69–183) |
| ≥25 yrs | 42.6 | 1288 | (99.0) | 13 | (1.0) | 62 | (36–106) |
CI, confidence interval; MRR, mortality rate ratio; NA, not applicable.
*Median value within each exposure category mortality.
†Per 100,000 person-years.
The cumulative hazard function of asbestosis death is presented in Figure 2. Mortality increased constantly with age and most asbestosis deaths were observed among subjects aged 70 years or more.
FIGURE 2.
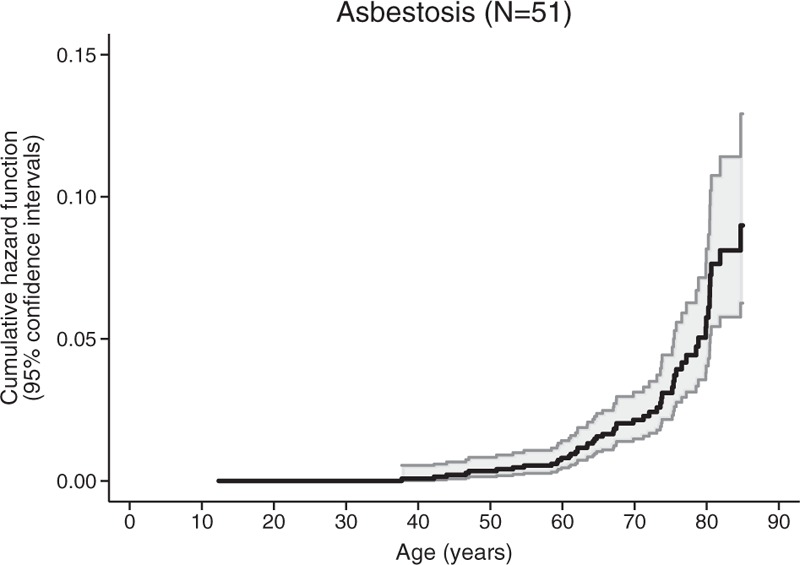
Nelson-Aalen cumulative hazard function for death due to asbestosis.
The results of the Cox models fitted to analyze the role of the selected predictors in determining the risk of death from asbestosis are presented in Table 2. The HR by sex was higher among males only when the temporal pattern of exposure was not taken into account (adjusted HR 1.2, 95% CI, 0.7 to 2.2). Age at first exposure was a predictor of the risk, as we observed the highest HR among subjects aged 35 years or more at first employment (HR 2.7, 95% CI, 1.1 to 6.4). The risk of asbestosis death was much lower for those who were employed first after 1968 (HR 0.3, 95% CI, 0.1 to 0.8). Duration of employment is a well-known determinant of the risk of asbestosis; however, our estimates show that the association was attenuated when other temporal factors of exposure were considered (eg, the HR for durations of at least 15 years decreased from 8.5 to 3.2 after adjustment for year of first exposure, TSLE, and sex). Compared with the crude MR, the association between TSLE and asbestosis mortality was reversed when modeled against age; indeed, we observed the lowest HR for TSLE of 25 years or more (adjusted HR 0.3, 95% CI, 0.1 to 0.9).
TABLE 2.
Hazard Ratio of Death Due to Asbestosis
| Crude Estimates | Adjusted Estimates | ||||
| Characteristic | Cases | HR | (95% CI) | HR | (95% CI) |
| Sex | |||||
| Female | 20 | 1.0 | (Ref.) | 1.0† | (Ref.)† |
| Male | 31 | 2.0 | (1.1–3.5) | 1.2† | (0.7–2.2)† |
| Age at first employment | |||||
| <25 yrs | 8 | 1.0 | (Ref.) | 1.0‡ | (Ref.)‡ |
| 25–34 yrs | 10 | 1.4 | (0.5–3.6) | 0.9‡ | (0.3–2.4)‡ |
| ≥35 yrs | 33 | 3.1 | (1.4–6.9) | 2.7‡ | (1.1–6.4)‡ |
| Year of first employment | |||||
| ≤1960 | 26 | 1.0 | (Ref.) | 1.0§ | (Ref.)§ |
| 1961–1968 | 20 | 0.7 | (0.4–1.3) | 0.9§ | (0.5–1.6)§ |
| ≥1969 | 5 | 0.3 | (0.1–0.7) | 0.3§ | (0.1–0.8)§ |
| Duration of employment* | |||||
| <5 yrs | 12 | 1.0 | (Ref.) | 1.0|| | (Ref.)|| |
| 5–9 yrs | 13 | 3.7 | (1.7–8.1) | 2.5|| | (1.1–5.8)|| |
| 10–14 yrs | 12 | 6.5 | (2.9–14.5) | 4.2|| | (1.8–9.9)|| |
| ≥15 yrs | 14 | 8.5 | (3.9–18.6) | 3.2|| | (1.3–7.6)|| |
| Time since last employment* | |||||
| <5 yrs | 7 | 1.0 | (Ref.) | 1.0‡ | (Ref.)‡ |
| 5–14 yrs | 15 | 1.0 | (0.4–2.5) | 1.2‡ | (0.5–3.2)‡ |
| 15–24 yrs | 16 | 0.4 | (0.2–1.2) | 0.7‡ | (0.2–2.0)‡ |
| ≥25 yrs | 13 | 0.1 | (0.0–0.3) | 0.3‡ | (0.1–0.9)‡ |
Estimates from Cox proportional hazards regression models with age as the main temporal axis
CI, confidence interval; HR, hazard ratio; Ref., reference category.
*Time-varying exposure.
†Estimates adjusted by year of first employment, duration of employment, and time since last employment (continuous variables).
‡Estimates adjusted by year of first employment (continuous variable), duration of employment (continuous variable), and sex.
§Estimates adjusted by duration of employment (continuous variable), time since last employment (continuous variable), and sex.
||Estimates adjusted by year of first employment (continuous variable), time since last employment (continuous variable), and sex.
The findings presented in Table 2 are confirmed by the estimates from the multivariable spline model presented in Figure 3. In the covariate selection process (backward selection), sex did not reach the significance threshold (P < 0.1) for the inclusion in the multivariable model. On the log scale, the HR of asbestosis death increased linearly with duration of employment and declined with increasing TSLE. In relation to year at first employment, we observed a strong and constant decline of the hazard after 1970. HRs of asbestosis at selected values of the continuous predictors included in the spline model are tabulated in Table 3.
FIGURE 3.
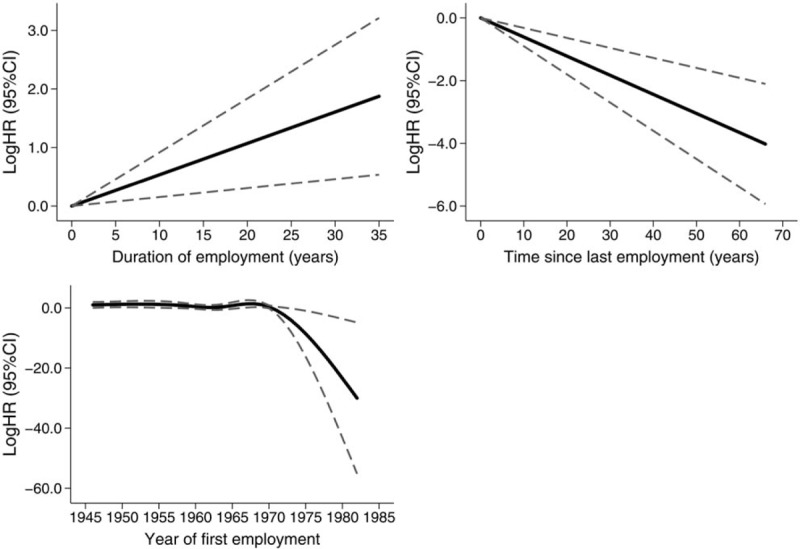
Hazard ratio of death due to asbestosis from a Cox regression model including duration of exposure, time since last exposure, and year of first exposure. Estimates from a cubic regression spline model selected through a backward selection strategy. CI, confidence intervals; HR, hazard ratios.
TABLE 3.
Hazard Ratios of Asbestosis Death at Selected Values of the Studied Predictors
| Variable and Value | HR | (95% CI) |
| Duration of exposure | ||
| 0 | 1.0 | (Ref.) |
| 5 | 1.3 | (1.1–1.6) |
| 10 | 1.7 | (1.2–2.5) |
| 20 | 2.9 | (1.4–6.3) |
| 30 | 5.0 | (1.6–15.7) |
| Time since last employment | ||
| 0 | 1.0 | Ref. |
| 10 | 0.5 | (0.4–0.7) |
| 20 | 0.3 | (0.2–0.5) |
| 30 | 0.2 | (0.1–0.4) |
| 40 | 0.1 | (0.0–0.3) |
| Year of first employment* | ||
| 1970 | 1.4 | (0.9–2.4) |
| 1972 | 0.1 | (0.0–1.1) |
| 1974 | 0.0 | (0.0–0.6) |
| 1976 | 0.0 | (0.0–0.2) |
| 1978 | 0.0 | (0.0–0.1) |
Adjusted estimates from the spline regression model presented in Figure 3.
CI, confidence interval; HR, hazard ratio; Ref., reference value.
*Year of first employment was centered on its mean (1962.7).
We conducted two series of sensitivity analyses, one extending our case definition to other pneumoconiosis and the other by applying competing risks regression models. All the estimates from these models were consistent with those presented in Table 2 (data not shown).
DISCUSSION
We presented data on the role of the temporal pattern of employment on the risk of death from asbestosis in a cohort of asbestos textile workers. We document that the risk increased with longer duration and decreased substantially with TSLE. As expected, the higher burden of asbestosis was observed among subjects employed first before 1969.
Our study population includes subjects who had been employed in an asbestos textile industry where very high environmental levels of exposure to asbestos were measured.7,8 As a result, we observed an overall incidence of asbestosis death that was almost as high as data reported from historical cohorts of insulation workers.14 We observed a log-linear increase in the hazard ratio of asbestosis mortality by exposure duration. Our findings are broadly in line with those reported in the medical literature, although the shape of the association (ie, log-linear or log-quadratic) is still controversial.15–17 We observed that the risk of asbestosis death declined among subjects first employed after 1970; this is consistent with available environmental monitoring data and knowledge on the exposure–risk relationship.7,8,15–17 Although we observed the higher crude MR among subjects with TSLE between 15 and 24 years, the Cox regression models demonstrated a declining hazard with increasing TSLE. This is in apparent contrast with a UK study, whose findings, which may be affected by ecological fallacy, apparently support the hypothesis that the risk of asbestosis continues to increase for decades after cessation of exposure.5
Our study supports the hypothesis that the risk of death due to asbestosis is higher shortly after cessation of exposure, though it remains elevated for decades after the cessation of the exposure (in our cohort, 13 out of 51 asbestosis deaths were observed 25 or more years after cessation of employment). A surprising finding of our study is that the risk of death due to asbestosis was higher among subjects who were employed for the first time after age 35 years. Again, this observation is apparently not in line with the results of the ecological study from the United Kingdom, in which the mortality pattern due to asbestosis seemed determined by asbestos exposures which occurred early in life.5 To our best knowledge, we are the first to report data on the risk of death due to asbestosis by age at first exposure. Thus, more studies on this topic are necessary to replicate our findings.
We observed a higher rate of asbestosis in males compared with females, although the confidence intervals of the estimates were overlapping. The observed differences can be attributed to the different intensity of exposure to asbestos. An environmental monitoring campaign carried out in 1968 and 1969 demonstrated a high variation in fibers concentration within the factory, ranging from more than 100 fibers per cm3 (carding department) to less than 5 fibers per cm3 (texture and stranded wire making departments).8 Sex segregation in employment is likely to have occurred in that factory during the 1960s and the 1970s; however, we could not test this hypothesis, as we did not have access to information on job titles and tasks.
Study Strengths and Limitations
The main strength of our study is the large number of deaths attributed to asbestosis (N = 51). Another important characteristic of our study population is the distribution of the duration of employment—with many workers employed for less than 5 years. This allowed us to create an optimal contrast between long and short exposure durations. An additional important strength of our study is the long follow-up period and the small number of individuals lost at follow-up. We believe that our study provides novel information on the role of the temporal patterns of exposure to asbestos in determining the risk of asbestosis. Indeed, we relied on individual data that allow direct inference on the pathophysiological process.
However, our study presents a few limitations. We did not have individual data on the intensity of exposure to asbestos; hence, we interpreted the calendar period as a proxy of the intensity of the exposure—in line with the environmental monitoring data available.7 Moreover, the analysis of duration of employment in our cohort could be affected by confounding by intensity of exposure, which was not fully accounted for by the introduction of the calendar period in the multivariable regression models. The second limitation of our study is that we did not have access to incidence data and we could only investigate mortality. As asbestosis is a potentially lethal disease that may either progress or remain relatively stable,18 mortality does not necessarily reflect the incidence of the disease and factors associated with the probability of surviving might act as confounders in our analysis. Moreover, death can occur several years after the clinical onset of the disease19; thus, the TSLE that we have measured actually represents the upper bound estimate of the real time occurred between the last employment date and the incidence of the disease. Another limitation is that we did not have information on the validity of the diagnosis as we had solely access to official death records. According to widely applied criteria, any case of diffuse pulmonary fibrosis in a subject with a known history of substantial exposure to asbestos should be labeled as asbestosis.2,20 This definition assumes a null incidence of nonasbestos-related diffuse pulmonary fibrosis among asbestos workers, such as our cohort. However, interstitial pneumonia is probably less rare than generally thought and its incidence and prevalence have been reported to be increasing.1 In particular, smoking-related interstitial fibrosis is relatively common among subjects with a long history of smoking and its prevalence has been reported to be as high as 45% among subjects deceased from lung cancer.1,21 Thus, the ascertainment of asbestosis incidence or mortality based on register data—which includes cases diagnosed on broad and unspecific criteria—may lead to a substantial overestimation of the incidence of this disease. Bledsoe et al22 observed that more than 60% of the diagnoses of asbestosis based on clinical and radiological evidence were not confirmed by a histological examination. On the balance, we cannot exclude that some of the deaths attributed to asbestosis but not confirmed through a detailed histological examination in our cohort might actually be due to other forms of pulmonary fibrosis. We could not evaluate this aspect as we did not have information on smoking history and our case ascertainment was based only on death records. We could not evaluate this aspect as we did not have information on smoking history and our case ascertainment was based only on death records. Overdiagnosis of asbestosis would affect the results of internal comparisons, most likely leading to a bias toward the null because false positives will be less strongly associated with asbestos exposure than true positives. Any effect on the results of the analysis based on standardized mortality ratios (SMR), however, will depend on whether a similar bias operates in the population at large: if, as it is likely, overdiagnosis affects also asbestos-exposed workers in the general population, the results of the SMR analysis will not be biased. We did not have information on the natural history of asbestosis, and we could only investigate mortality. It is possible that some workers quit the employment at the factory due to the onset of the symptoms; this could partially explain why the incidence of asbestosis death was much higher soon after quitting the employment. However, this would not imply any increase in the incidence of asbestosis among subjects who retired without symptoms related to this disease. A further limitation of our study is that we did not have access to the complete occupational histories. We excluded from the analysis those subjects (N = 120) who were known to have worked in an asbestos industry before being hired at the textile factory, but we could not exclude any possible source of exposure to asbestos before or after this employment period. Another limitation of our study is the possible underreporting of asbestosis, particularly in the presence of a mild disease. Of note, we expect the quality of diagnosis, and the completeness of death records, to have improved over the time, also because of increasing reliability and availability of high-resolution computed tomography23,24; thus, the diagnostic sensitivity of death certificates should have been lower for those deaths occurred during the 1970s and the 1980s among subjects who had recently quit the job. If that were the case, the decrease in the risk of asbestosis death with increasing TSLE could be even larger than we have documented.
CONCLUSIONS
The role of the temporal pattern of exposure should always be assessed when investigating the individual risk of asbestosis. We observed that the risk of asbestosis death in the decades after cessation of the exposure to asbestos could be smaller than generally thought based on ecological studies.
Footnotes
AF and FSV equally contributed to this work.
This work was supported by internal funds of the participating institutions.
None of the authors has a direct financial interest related to this study. FSV, CLV, PB, and EP have acted as expert witnesses in litigations involving asbestos-related diseases.
REFERENCES
- 1.Gulati M, Redlich CA. Asbestosis and environmental causes of usual interstitial pneumonia. Curr Opin Pulm Med 2015; 21:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tossavainen A. Asbestos, asbestosis, and cancer: the Helsinki criteria for diagnosis and attribution. Scand J Work Environ Health 1997; 23:311–316. [PubMed] [Google Scholar]
- 3.Corfiati M, Scarselli A, Binazzi A, et al. Epidemiological patterns of asbestos exposure and spatial clusters of incident cases of malignant mesothelioma from the Italian national registry. BMC Cancer 2015; 15:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Istituto Nazionale per l’Assicurazione contro gli Infortuni sul Lavoro (INAIL). Relazione Annuale 2015. Rome: INAIL. Available at: https://www.inail.it/cs/internet/comunicazione/pubblicazioni/rapporti-e-relazioni-inail/relazione_annuale_2015.html. Accessed June 22, 2016. [Google Scholar]
- 5.Darnton A, Hodgson J, Benson P, Coggon D. Mortality from asbestosis and mesothelioma in Britain by birth cohort. Occup Med 2012; 62:549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sluis-Cremer GK. Asbestos disease at low exposures after long residence times. Ann N Y Acad Sci 1991; 643:182–193. [DOI] [PubMed] [Google Scholar]
- 7.Pira E, Pelucchi C, Buffoni L, et al. Cancer mortality in a cohort of asbestos textile workers. Br J Cancer 2005; 92:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pira E, Pelucchi C, Piolatto PG, Negri E, Discalzi G, La Vecchia C. First and subsequent asbestos exposures in relation to mesothelioma and lung cancer mortality. Br J Cancer 2007; 97:1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pira E, Romano C, Violante FS, et al. Updated mortality study of a cohort of asbestos textile workers. Cancer Med 2016; 5:2623–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straif K, Benbrahim-Tallaa L, Baan R, et al. A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol 2009; 10:453–454. [DOI] [PubMed] [Google Scholar]
- 11.Aalen OO. Nonparametric inference for a family of counting processes. Ann Stat 1978; 6:701–726. [Google Scholar]
- 12.Royston P, Sauerbrei W. Multivariable modeling with cubic regression splines: a principled approach. Stata J 2007; 7:45. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 14.Selikoff IJ, Seidman H. Asbestos-associated deaths among insulation workers in the United States and Canada, 1967–1987. Ann N Y Acad Sci 1991; 643:1–14. [DOI] [PubMed] [Google Scholar]
- 15.Deng Q, Wang X, Wang M, Lan Y. Exposure-response relationship between chrysotile exposure and mortality from lung cancer and asbestosis. Occup Environ Med 2012; 69:81–86. [DOI] [PubMed] [Google Scholar]
- 16.Hein MJ, Stayner L, Lehman E, et al. Follow up study of chrysotile textile workers: cohort mortality and exposure-response. Occup Environ Med 2007; 64:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loomis D, Dement JM, Wolf SH, et al. Lung cancer mortality and fibre exposures among North Carolina asbestos textile workers. Occup Environ Med 2009; 66:535–542. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 2004; 170:691–715. [DOI] [PubMed] [Google Scholar]
- 19.Vehmas T, Pallasaho P, Piirilä P. Lung function predicts mortality: 10-year follow-up after lung cancer screening among asbestos-exposed workers. Int Arch Occup Environ Health 2013; 86:667–672. [DOI] [PubMed] [Google Scholar]
- 20.Wolff H, Vehmas T, Oksa P, Rantanen J, Vainio H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health 2015; 41:5–15. [DOI] [PubMed] [Google Scholar]
- 21.Katzenstein AL, Mukhopadhyay S, Zanardi C, Dexter E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol 2010; 41:316–325. [DOI] [PubMed] [Google Scholar]
- 22.Bledsoe JR, Christiani DC, Kradin RL. Smoking-associated fibrosis and pulmonary asbestosis. Int J Chron Obstruct Pulmon Dis 2014; 10:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naghavi M, Makela S, Foreman K, O’Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr 2010; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacchus L, Shah RD, Chung JH, et al. ACR Appropriateness Criteria Review ACR Appropriateness Criteria® Occupational Lung Diseases. J Thorac Imaging 2016; 31:W1–W3. [DOI] [PubMed] [Google Scholar]


