Abstract
Introduction
Prenatal nicotine exposure (PNE) from maternal cigarette smoking is linked to developmental deficits, including impaired auditory processing, language, generalized intelligence, attention, and sleep. Fetal brain undergoes massive growth, organization, and connectivity during gestation, making it particularly vulnerable to neurotoxic insult. Nicotine binds to nicotinic acetylcholine receptors, which are extensively involved in growth, connectivity, and function of developing neural circuitry and neurotransmitter systems. Thus, PNE may have long-term impact on neurobehavioral development. The purpose of this study was to compare the auditory K-complex, an event-related potential reflective of auditory gating, sleep preservation and memory consolidation during sleep, in infants with and without PNE and to relate these neural correlates to neurobehavioral development.
Methods
We compared brain responses to an auditory paired-click paradigm in 3- to 5-month-old infants during Stage 2 sleep, when the K-complex is best observed. We measured component amplitude and delta activity during the K-complex.
Results
Infants with PNE demonstrated significantly smaller amplitude of the N550 component and reduced delta-band power within elicited K-complexes compared to nonexposed infants and also were less likely to orient with a head turn to a novel auditory stimulus (bell ring) when awake.
Conclusions
PNE may impair auditory sensory gating, which may contribute to disrupted sleep and to reduced auditory discrimination and learning, attention re-orienting, and/or arousal during wakefulness reported in other studies.
Implications
Links between PNE and reduced K-complex amplitude and delta power may represent altered cholinergic and GABAergic synaptic programming and possibly reflect early neural bases for PNE-linked disruptions in sleep quality and auditory processing. These may pose significant disadvantage for language acquisition, attention, and social interaction necessary for academic and social success.
Introduction
Recent data show that at least 22% of pregnant women smoke in the first trimester and 14% continue to smoke throughout the third trimester.1 Prenatal smoking increases risk for low birth weight, prematurity, still birth, sudden infant death,2,3 substance abuse,4 and deficits in behavioral and cognitive development including impulsivity, auditory and visual sensory processing,5,6 attention,7 language skills,8 and notably, sleep/waking abnormalities.9–11 Therefore, it is important to learn more about the mechanisms that may underlie these negative outcomes.
Of the more than 4000 chemicals contained in cigarette smoke, nicotine is considered the primary reinforcing substance,12,13 with well-documented neurotoxic effects on fetal brain development.14 It readily crosses the placental barrier into fetal circulation and brain,15 where it binds to nicotinic acetylcholine receptors (nAChR) that are extensively distributed throughout the fetal nervous system. Diverse regional and temporal expression and nAChR subtype profiles permit widespread developmental influence across time and brain structures during gestation, allowing acetylcholine (ACh) to play a crucial role in directing and modulating maturation of neural circuitry in brain stem,16,17 subcortical,18 and cortical systems.5,19 The nAChRs are also critically important in formation of sensory–cortical connections such as thalamus-to-auditory cortex circuitry and for central catecholamine pathways and the crucial switch in GABA-ergic systems from excitatory to inhibitory functioning during fetal development. Animal models show substantial prenatal nicotine exposure (PNE)-induced disrupted development of central auditory processing networks and impaired auditory discrimination in early life as well as impaired ACh signaling in response to auditory stimuli which persists into adulthood.9,20
The impact of nicotine on brain development during gestation may manifest as deficits in attention and auditory processing after birth.5,7 In the current study we used electroencephalographic (EEG) recording to study the auditory sensory processing as a function of PNE in 3- to 5-month-old infants. Here we report results from examination of the elicited K-complex in Stage 2 sleep, which we hypothesized would be impaired in infants with PNE compared to nonexposed infants. The K-complex is a modality-independent, sleep-specific event-related potential (ERP). It displays the same scalp topography in response to external or internal stimuli,21 and is detectable in the first month of life,22 making it an appropriate tool for study of infants. The auditory K-complex is posited to be involved with gating of auditory information and arousal inhibition during sleep23 and is considered analogous to the orienting response during wakefulness.24 The N550 component is unique to, and considered most indicative of, the elicited K-complex response.25,26
Methods
Participants
Forty-eight healthy singleton infants and their mothers were recruited from prenatal clinics and the community (PNE = 24 exposed to maternal prenatal cigarette smoking and 24 born to nonsmoking mothers who did not reside with a smoker during pregnancy).
Procedures
Third Trimester Visit
Mothers were screened for cigarette smoking during pregnancy with the Time Line Follow Back (TLFB) interview,27 which assessed the average number of cigarettes smoked per day for each day in pregnancy. The mean number of cigarettes per day in each trimester was derived from these values. Maternal expiratory carbon monoxide (CO) and carboxyhemoglobin levels were assessed by breathalyzer (pico+ Smokerlyzer, Bedfont, United Kingdom), and maternal urine was collected for assay of the nicotine metabolite, cotinine (reported as ng cotinine per mg creatinine).
Postpartum Visit
Mothers returned with their infants for developmental and EEG assessments between 3 and 5 months postpartum. Maternal postpartum tobacco smoke exposure was assessed with expiratory CO and carboxyhemoglobin and urinary cotinine. The Cognitive, Language, and Motor scales and subscales of the Bayley III Scales of Infant and Toddler Development32 were conducted to assess infant development (full results to be reported elsewhere). Infants then underwent the EEG protocol during sleep.
Recording of EEG
At approximately 3–5 months, infants attended an EEG session at a time compatible with nap schedule. Single-lead gold electrodes were placed on the scalp and face according to the 10–20 International System. The EEG was recorded from Cz; HEOG and VEOG electrodes recorded bilateral eye movements; right mastoid served as reference. EEG data were recorded on a Synamps system (1000 Hz, 0.1 Hz high-pass and 100 Hz low-pass recording filters) and captured and analyzed using NeuroScan 4.4 software. The K-complex consists of P200, N350, N550, and P900 components. The N350 component is maximal at the Cz electrode, and N550 and P900 are maximal at Fz28. However, since this protocol was originally designed to measure the P50 component during rapid eye movement (REM) sleep (see Hunter et al., 2008, for full protocol description29), the Fz electrode was not included. Therefore, all components of the K-complex were acquired using the Cz electrode.
Auditory stimuli were presented binaurally at 85 dB from two speakers placed 50.8 cm to the left and right of the infant’s head. Identical paired clicks (duration 0.04 ms, intrapair interval 500 ms, and interpair interval 10 seconds) were presented throughout the session, per the auditory P50 protocol.30
Analyses of continuous EEG data were limited to Stage 2 sleep because the K-complex does not occur during REM,21 and those occurring in Stages 3 and 4 blend with existing delta waves. Appearance of sleep spindles indicated Stage 2 sleep onset; larger delta waves signified Stage 3 sleep.31 We required approximately ≥7 minutes of Stage 2 sleep to ensure sufficient trials.
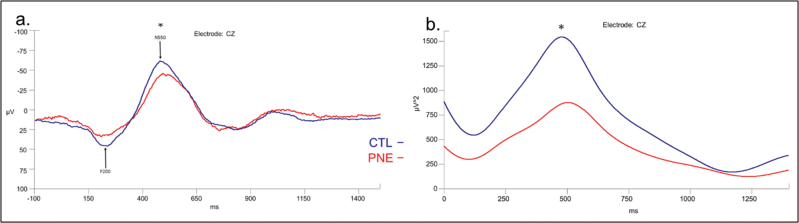
Data were epoched to include 100 ms before to 1400 ms after the first click within each pair. Epochs with artifacts exceeding ± 150 μVs were removed from the analysis. Epochs were examined individually. Epochs with visible elicited K-complexes were included in further analysis. All accepted events for each participant were averaged, creating a K-complex waveform for each individual. A 30-Hz low-pass filter with a 12-dB roll off was applied to each average waveform. Automatic peak detection identified the four components of the K-complex for each average waveform. A grand average was created for each group (CTL and PNE), and these were overlaid into one figure (Figure 1). We compared groups on (a) the peak-to-peak amplitude of the K-complex ERP components, as the amplitude of one can alter the amplitude of subsequent peaks and (b) power of the delta band in the K-complex waveform, as delta is the main spectral contributor.
Figure 1.
(a) Difference in peak-to-peak amplitude between P200 and N550 components of K-complex by exposure group, adjusted for postmenstrual age at electroencephalogram (EEG). CTL = 17 drug-free infants, PNE = 16 with prenatal nicotine exposure. F = 3.74, p = .036. (b) Delta power of K-complex measured at the Cz electrode, adjusted for postmenstrual age at EEG, by group; F = 3.76, p = .035.
The EEG data were analyzable for 33 of the 48 infants who attended the EEG visit (68.75%; PNE = 16, CTL = 17). Reasons for excluding subjects’ data from analyses were primarily poor recording quality/artifact and movement (4 CTL and 4 PNE), software or equipment malfunction (2 CTL and 2 PNE), and insufficient duration of Stage 2 sleep necessary for K-complex identification (3 CTL). Included subjects did not differ from excluded subjects on maternal education, marital status, or group; however, mothers of included infants were older (30.1 ± 0.7 vs 27.1 ± 0.7 years), t(2,31) = 2.62, p < .05.
Orienting Response
Since the K-complex during sleep is posited as analogous to the orienting response in awake infants, we operationalized behavioral orienting using 2 items included in the Bayley III Scale of Infant and Toddler Development assessment conducted prior to EEG at the postpartum visit: a bell ring and wrinkling paper. Each stimulus was positioned lateral and slightly behind the awake and alert infant’s head as per assessment instructions.32 Orienting was defined as a head turn toward the stimulus.
Statistical Analyses
Group differences were tested using two-sample t-tests for continuous variables and chi-square statistic for categorical values. Primary analyses of covariance (ANCOVA) tested group differences in K-complex component amplitude and delta band power, adjusted for postmenstrual age at EEG (days post-conception) to account for the wide age range at testing. Maternal education was added to significant models to test group effects with both covariates in the model. Inhomogeneity of variance was tested with the Welch test.
Results
Group characteristics are displayed in Table 1. Groups did not differ on ethnicity, gender, postmenstrual age at EEG (days since conception), or months since birth at EEG. Maternal education (χ2 = 12.1, p < .01) and gestational age at birth, t(2, 31) = 4.04, p = .0004, were significantly lower for PNE (mean ± standard error [SE]: 280 ± 1.9, range: 266–294) than CTL (270 ± 1.4, 261–278) groups, t(2, 31) = 4.04, p = .0004. However, all were born at >37 weeks gestation. Two females in the PNE group had low birth weight (2155 and 2353 g), but none reached the very low birth weight threshold (<1500 g). As expected, mean maternal expiratory CO and carboxyhemoglobin were higher in the PNE group and did not differ from third trimester to 3- to 5-month postnatal visit (paired t-test = 0.19, p= .85). Mean maternal urinary cotinine in third trimester was also higher in PNE (mean ± SE: 104.5 ± 29.8; range: 31–520) than CTL (1.4 ± 0.44, 0.3–5.3) groups. Maternal smoking rates ranged from 0 to 20 cigarettes per day in each trimester in the PNE group as reported by TLFB interview. Consistent with other studies, number of cigarettes per day dropped in each trimester.33,34
Table 1.
Participant and ERP characteristics by exposure group
| CTL | PNE | |||||
|---|---|---|---|---|---|---|
| Mean | SE | Range | Mean | SE | Range | |
| Gestational age at birth (days)a | 279.9 | 1.9 | 266–294 | 270.4 | 1.4 | 261–278 |
| Postmenstrual age at EEGb | 418.4 | 3.3 | 400–446 | 419.4 | 5.6 | 381–455 |
| Age at EEG, months | 4.61 | 0.5 | 4.0–5.5 | 4.96 | 0.8 | 3.9–6.2 |
| Birth weight, gramsc | 3610.5 | 118 | 2778–4423 | 3130.6 | 156 | 2155–4111 |
| Male, n (%) | 7 (.44) | 7 (.47) | ||||
| Nonwhite | 4 (.25) | 4 (.24) | ||||
| 3rd Trimester Maternal expired CO, ppmd | 1.67 | 0.14 | 1.0–2.0 | 9.6 | 2.5 | 1.0–33.5 |
| 3rd Trimester Maternal COHb, %d | 1.00 | 0.08 | 0.8–1.8 | 2.2 | 0.4 | 0.8–6.0 |
| Cigarettes/day Trimester 1e | 0 | 14.1 | 1.9 | 5–20 | ||
| Cigarettes/day Trimester 2e | 0 | 7.7 | 2.0 | 0–20 | ||
| Cigarettes/day Trimester 3e | 0 | 5.5 | 1.4 | 0–10 | ||
| Maternal Cotinine 3rd Trimesterf,g | 1.97 | 0.44 | 0.3–5.3 | 104.5 | 29.8 | 31–520 |
| Maternal Postpartum expired CO, ppma | 2.27 | 1.15 | 1.0–7.0 | 9.7 | 1.4 | 1.0–26.0 |
| K-Complex Characteristics | ||||||
| % of acceptable ERP epochsg | 28.1 | 2.3 | 9.5–41 | 25.3 | 2.3 | 15–42 |
| Peak-to-peak amplitudeh, µVg | 122.04 | 11.9 | 55.8–216.2 | 102.0 | 6.8 | 65.6–161.2 |
| Delta power of N550, µV^2g | 1574.3 | 343 | 231–5496 | 907.4 | 89.6 | 479–1789.5 |
Abbreviations: CTL, drug free control, n = 17; PNE, prenatal nicotine exposure n = 16; SE, standard error of the mean; CO, carbon monoxide; PPM, parts per million; Hb, hemoglobin
a p < .001.
bdays since conception;
c p <.09,
d p < .01, Gestational age at birth, birthweight, Maternal COHb, CO, and cotinine results of 2-sample t-test, df: 2, 31.
eTime Line Follow Back Interview.
f Maternal urinary cotinine (ng cotinine per mg creatinine).
g[AQ: Please provide significance for “g.”]
hPeak-to-peak difference between P200 and N550 components of elicited K-complex, adjusted for gestational age at EEG.
Group Differences in Elicited K-Complex Characteristics
Peak-to-peak differences between the P200 and N550 amplitude were significantly smaller for PNE (mean ± SE: 102 ± 6.8, range: 65.6–161.2) compared to CTL (122 ± 11.9, 55.8–216.2) groups (F = 3.74, p = .036), using postmenstrual age at EEG as a covariate (Figure 1a).
A significant group difference in peaks for the N550 component was found (F = 3.76; p = .035) with the PNE group exhibiting reduced delta band power (mean ± SE: 907.4 ± 89.6, range: 479–1789.5) relative to CTLs (1574.3 ± 343, 231.5–5496) after adjustment of postmenstrual age at EEG (Figure 1b). After adding maternal education as a covariate, group differences remained marginally significant (PNE: 750.8 ± 273; CTL: 1,624.44 ± 282; p = .0518). Mean group differences were marginal (p = .075) after adjustment for inhomogeneity of variances identified by the Welch test.
The PNE group also displayed significantly fewer acceptable epochs from which to analyze an elicited K-complex (25.3 ± 2.3, range: 15–42) compared to CTL (28.1 ± 2.3, 9.5–41; Table 1).
Orienting Response in Awake Infants
Significantly fewer PNE (50%) compared to CTL (94.75%) infants oriented to the bell ring while awake (χ2 = 7.58, df = 1, p = .006), with significant differences persisting after adjusting for postmenstrual age and maternal education (Group effect F = 5.24, p = .03). However, groups did not differ in response to the wrinkling paper stimulus.
Discussion
Our study revealed reduced amplitude and delta band power within K-complexes elicited during Stage 2 (non-REM) sleep in response to repeated auditory stimuli in infants with PNE compared with CTL; also, significantly fewer PNE infants oriented with a head turn response to a bell ring stimulus while awake. These findings indicate that the PNE infants exhibited reduced neural responses to auditory information and perhaps a reduced ability to gate such repeated auditory stimuli in sleep. The analogue of this auditory K-complex during sleep, behavioral orienting to novel sounds while awake, was also reduced in the PNE group. Both sensory gating and orienting to environmental sounds are essential for survival and early interaction with caretakers, thus neural circuits underlying these processes typically appear functional very early in postnatal development.22,35 Therefore, our findings may reflect impaired sensory gating of auditory information in infants with PNE and are consistent with work showing that central auditory processing is vulnerable to disruption as a result of perinatal nicotine exposure.20 These results are also in line with longitudinal reports from the Ottawa Prenatal Prospective Study showing impaired performance of tasks requiring auditory processing in infants,36 children,37,38 and adolescents39,40 with PNE. In addition, these findings parallel reported PNE-related deficits in another marker of auditory sensory gating in infants—the P50 ERP detected during REM sleep,30 which is notably linked to later deficits in attention in early childhood.41
Our finding of reduced delta activity in elicited K-complexes suggests dysregulated sleep maintenance with possible disruption of information processing that occurs during sleep.42 Delta activity is typically increased during each K-complex compared to periods surrounding it and is accompanied by reduced neuronal firing, network activity, and broadband EEG power in other frequencies. These brief cortical “down-states” may facilitate transition into deeper slow-wave sleep and be involved in sleep preservation, synaptic homeostasis, and memory consolidation.25 Greater delta activity and slow wave sleep are accompanied by a relative reduction in central acetylcholine (ACh) compared to REM sleep, and prevention of this drop in ACh interferes with sleep-induced consolidation of declarative memory in humans.43
In rodent studies, nicotine administration at a time equivalent to human gestation disrupts postnatal sleep–wake cycles, causing premature neural cell differentiation and disrupted cholinergic and monoaminergic neurotransmitter systems that regulate sleep and vigilance. Central messenger RNA expression of the nAChR is also upregulated, which may be a compensatory response to desensitization by repeated nicotine stimulation of nAChR’s during gestation.44 The altered K-complex activity we observed may also reflect aberrant maturation of GABAergic neurons in thalamo-cortical pathways responsible for the relay of sensory information to the cortex45,46 as well as altered synaptic plasticity within the hippocampus,47 which may then disrupt the long-term memory consolidation essential for learning and memory that occurs during sleep.42,48,49
Limitations of the study include the small sample size, which restricted power to test the effects of other factors which may have influenced infant brain development and behavior (e.g., gender, birth weight, SES, and perinatal environment). Importantly, we are unable to identify or biologically confirm nicotine as the sole source of our findings, since preclinical studies show that other constituents of cigarette smoke exert significant detrimental effects on placental structure/function and fetal development.50–52 We measured self-report of cigarette smoking and two biological indicators of maternal exposure during third trimester as well as maternal exposure at the time of infant EEG test (expired CO-Hb and CO). However, we did not measure exposures to other drugs of abuse during pregnancy. This is a significant limitation, which substantially impacts our ability to conclude that our findings are due, completely or in part, to fetal exposure to maternal cigarette smoking. Future studies should include drug testing for additional teratogenic drugs including alcohol, marijuana, stimulants and opiates, and test for other potential influences on auditory processing. Moreover, we only assessed group differences in auditory-elicited K-complexes and did not measure spontaneous K-complexes or those evoked by other stimuli. Therefore, group differences in other types of K-complex responses cannot be ruled out. Measurement was limited to a single electrode at Cz, which may have underestimated the amplitude of N550 and P900. However, significant group differences were observed and K-complexes were detected in sufficient numbers of epochs using this configuration. Since mothers were not queried about infants’ sleep characteristics, we cannot generalize our findings to PNE-linked sleep problems at home. Nevertheless, PNE alters sleep–wake cycles reducing total and REM sleep in animals44 and is associated with sleep problems persisting through the first 12 years of life in humans.53
In summary, our findings support an early neural basis for PNE-linked disruptions in sleep quality and potential impairments in auditory processing and/or discrimination of relevant sounds. To the extent that PNE contributes to subtle or not so subtle lasting damage to developing neural circuits, the effects on productivity, achievement, and quality of life may be particularly burdensome. However, due to our study’s small sample size and limited power, replication and further detailed investigation in larger samples are needed to confirm these conclusions.
Funding
Funding was provided by NIH grant number R21DA03316 (KG).
Declaration of Interests
None declared.
Acknowledgements
We would like to thank Franc Donkers for his contributions to study design, Sharon Hunter for her generous contribution of the P50 protocol, Barbara Goldman for her help and expertise with developmental assessments, Pamela Beiler for her contributions to recruitment and data management, and Pilar Roa, Anna Evans and Jessica Cooper for data collection.
References
- 1. EPA. National survey on environmental management of asthma and children’s expsoure to environmental tobacco smoke., 2010. http://www.epa.gov/smokefree/pdfs/survey_fact_sheet.pdf.
- 2. Haglund B, Cnattingius S, Otterblad-Olausson P. Sudden infant death syndrome in Sweden, 1983-1990: season at death, age at death, and maternal smoking. Am J Epidemiol. 1995;142(6):619–624. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell EA, Ford RP, Stewart AW, Taylor BJ, Becroft DM, Thompson JM, Scragg R, Hassall IB, Barry DM, Allen EM, Roberts AP. Smoking and the sudden infant death syndrome. Pediatrics 1993;91(5):893–896. [PubMed] [Google Scholar]
- 4. Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38(7):892–899. [DOI] [PubMed] [Google Scholar]
- 5. Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84(1):30–44. [DOI] [PubMed] [Google Scholar]
- 6. Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23(5):421–430. [DOI] [PubMed] [Google Scholar]
- 7. Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56Suppl 1:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makin J, Fried PA, Watkinson B. A comparison of active and passive smoking during pregnancy: long-term effects. Neurotoxicol Teratol. 1991;13(1):5–12. [DOI] [PubMed] [Google Scholar]
- 9. Horst NK, Heath CJ, Neugebauer NM, Kimchi EY, Laubach M, Picciotto MR. Impaired auditory discrimination learning following perinatal nicotine exposure or β2 nicotinic acetylcholine receptor subunit deletion. Behav Brain Res. 2012;231(1):170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stone KC, LaGasse LL, Lester BM, et al. . Sleep problems in children with prenatal substance exposure: the Maternal Lifestyle study. Arch Pediatr Adolesc Med. 2010;164(5):452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibbs SM. Regulation of neuronal proliferation and differentiation by nitric oxide. Mol Neurobiol. 2003;27(2):107–120. [DOI] [PubMed] [Google Scholar]
- 12. Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl). 2006;184(3-4):274–285. [DOI] [PubMed] [Google Scholar]
- 13. Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl). 2004;175(2):134–142. [DOI] [PubMed] [Google Scholar]
- 14. Ginzel KH, Maritz GS, Marks DF, et al. . Critical review: nicotine for the fetus, the infant and the adolescent?J Health Psychol. 2007;12(2):215–224. [DOI] [PubMed] [Google Scholar]
- 15. Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8(6):384–395. [DOI] [PubMed] [Google Scholar]
- 16. Huang ZG, Griffioen KJ, Wang X, et al. . Differential control of central cardiorespiratory interactions by hypercapnia and the effect of prenatal nicotine. J Neurosci. 2006;26(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang ZG, Wang X, Dergacheva O, Mendelowitz D. Prenatal nicotine exposure recruits an excitatory pathway to brainstem parasympathetic cardioinhibitory neurons during hypoxia/hypercapnia in the rat: implications for sudden infant death syndrome. Pediatr Res. 2005;58(3):562–567. [DOI] [PubMed] [Google Scholar]
- 18. Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20(16):6106–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang K, Poytress BS, Chen Y, Leslie FM, Weinberger NM, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. Eur J Neurosci. 2006;24(3):857–866. [DOI] [PubMed] [Google Scholar]
- 21. Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response?J Sleep Res. 1999;8(4):273–280. [DOI] [PubMed] [Google Scholar]
- 22. Hughes JR. The development of the vertex sharp transient. Clin Electroencephalogr. 1998;29(4):183–187. [DOI] [PubMed] [Google Scholar]
- 23. Bastien CH, Ladouceur C, Campbell KB. EEG characteristics prior to and following the evoked K-Complex. Can J Exp Psychol. 2000;54(4):255–265. [DOI] [PubMed] [Google Scholar]
- 24. Colrain IM. The K-complex: a 7-decade history. Sleep. 2005;28(2):255–273. [DOI] [PubMed] [Google Scholar]
- 25. Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, Devinsky O, Kuzniecky R, Doyle W, Madsen JR, Bromfield E, Eross L, Halasz P, Karmos G, Csercsa R, Wittner L, Ulbert I. The human K-complex represents an isolated cortical down-state. Science 2009;324(5930):1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kállai I, Harsh J, Voss U. Attention to external stimuli during wakefulness and sleep: evoked 40-Hz response and N350. Psychophysiology. 2003;40(6):955–966. [DOI] [PubMed] [Google Scholar]
- 27. Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. [DOI] [PubMed] [Google Scholar]
- 28. Bastien C, Campbell K. The evoked K-complex: all-or-none phenomenon?Sleep. 1992;15(3):236–245. [DOI] [PubMed] [Google Scholar]
- 29. Hunter SK, Corral N, Ponicsan H, Ross RG. Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport. 2008;19(1):79–82. [DOI] [PubMed] [Google Scholar]
- 30. Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull. 2011;37(6):1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iber C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 32. Bayley N. Bayley Scales of Infant and Toddler Development, third edition San Antonio, TX: Harcourt Assessment, Inc; 2006. [Google Scholar]
- 33. Eiden RD, Homish GG, Colder CR, Schuetze P, Gray TR, Huestis MA. Changes in smoking patterns during pregnancy. Subst Use Misuse. 2013;48(7):513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fried PA, Barnes MV, Drake ER. Soft drug use after pregnancy compared to use before and during pregnancy. Am J Obstet Gynecol. 1985;151(6):787–792. [DOI] [PubMed] [Google Scholar]
- 35. Muir D, Field J. Newborn infants orient to sounds. Child Dev. 1979;50(2):431–436. [PubMed] [Google Scholar]
- 36. Fried PA, Makin JE. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicol Teratol. 1987;9(1):1–7. [DOI] [PubMed] [Google Scholar]
- 37. Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol. 1992;14(5):299–311. [DOI] [PubMed] [Google Scholar]
- 38. McCartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicol Teratol. 1994;16(3):269–276. [DOI] [PubMed] [Google Scholar]
- 39. Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20(3):293–306. [DOI] [PubMed] [Google Scholar]
- 40. Fried PA, Watkinson B, Siegel LS. Reading and language in 9- to 12-year olds prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol. 1997;19(3):171–183. [DOI] [PubMed] [Google Scholar]
- 41. Hutchison AK, Hunter SK, Wagner BD, Calvin EA, Zerbe GO, Ross RG. Diminished Infant P50 Sensory Gating Predicts Increased 40-Month-Old Attention, Anxiety/Depression, and Externalizing Symptoms. J Atten Disord. 2017;21(3):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. [DOI] [PubMed] [Google Scholar]
- 43. Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101(7):2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frank MG, Srere H, Ledezma C, O’Hara B, Heller HC. Prenatal nicotine alters vigilance states and AchR gene expression in the neonatal rat: implications for SIDS. Am J Physiol Regul Integr Comp Physiol. 2001;280(4):R1134–R1140. [DOI] [PubMed] [Google Scholar]
- 45. Caporro M, Haneef Z, Yeh HJ, et al. . Functional MRI of sleep spindles and K-complexes. Clin Neurophysiol. 2012;123(2):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crowley K, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002;11(2):129–140. [DOI] [PubMed] [Google Scholar]
- 47. Ognjanovski N, Maruyama D, Lashner N, Zochowski M, Aton SJ. CA1 hippocampal network activity changes during sleep-dependent memory consolidation. Front Syst Neurosci. 2014;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gais S, Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem. 2004;11(6):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8(4):331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Detmar J, Rennie MY, Whiteley KJ, et al. . Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. Am J Physiol Endocrinol Metab. 2008;295(2):E519–E530. [DOI] [PubMed] [Google Scholar]
- 51. Hall BJ, Cauley M, Burke DA, Kiany A, Slotkin TA, Levin ED. Cognitive and Behavioral Impairments Evoked by Low-Level Exposure to Tobacco Smoke Components: Comparison with Nicotine Alone. Toxicol Sci 2016;151(2):236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5(7):583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stone KC, High PC, Miller-Loncar CL, Lagasse LL, Lester BM. Longitudinal study of maternal report of sleep problems in children with prenatal exposure to cocaine and other drugs. Behav Sleep Med. 2009;7(4):196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]