Abstract
Background
The goal of this study was to conduct a preliminary network analysis (using graph-theory measures) of intrinsic functional connectivity in adult smokers, with an exploration of sex differences in smokers.
Methods
Twenty-seven adult smokers (13 males; mean age = 35) and 17 sex and age-matched controls (11 males; mean age = 35) completed a blood oxygen level-dependent resting state functional magnetic resonance imaging experiment. Data analysis involved preprocessing, creation of connectivity matrices using partial correlation, and computation of graph-theory measures using the Brain Connectivity Toolbox. Connector hubs and additional graph-theory measures were examined for differences between smokers and controls and correlations with nicotine dependence. Sex differences were examined in a priori regions of interest based on prior literature.
Results
Compared to nonsmokers, connector hubs in smokers emerged primarily in limbic (parahippocampus) and salience network (cingulate cortex) regions. In addition, global influence of the right insula and left nucleus accumbens was associated with higher nicotine dependence. These trends were present in male but not female smokers.
Conclusions
Network communication was altered in smokers, primarily in limbic and salience network regions. Network topology was associated with nicotine dependence in male but not female smokers in regions associated with reinforcement (nucleus accumbens) and craving (insula), consistent with the idea that male smokers are more sensitive to the reinforcing aspects of nicotine than female smokers.
Implications
Identifying alterations in brain network communication in male and female smokers can help tailor future behavioral and pharmacological smoking interventions. Male smokers showed alterations in brain networks associated with the reinforcing effects of nicotine more so than females, suggesting that pharmacotherapies targeting reinforcement and craving may be more efficacious in male smokers.
Introduction
Nicotine-dependent cigarette smoking continues to be a critical public health issue with adverse economic, medical, and psychiatric outcomes. Understanding the neurobiological underpinnings of nicotine dependence can lead to more effective smoking cessation treatments. Recent advances in neuroimaging methodology, such as resting state functional magenetic resonance imaging and functional connectivity analysis, have the potential to elucidate critical aspects of brain network communication that may be altered in nicotine dependence.1
Alterations in functional connectivity in chronic smokers involve connectivity both within and between regions associated with executive control, default mode, salience, and limbic networks, according to a recent review by Fedota and Stein.1 They note that both increased and decreased connectivity have been reported across a range of different analysis techniques, but more studies report reduced rather than increased connectivity in chronic smoking. However, increased connectivity, particularly with respect to salience network and limbic regions, is associated with greater cue reactivity. For example, Janes et al.2 showed that insula-dorsal anterior cingulate intrinsic functional connectivity was correlated with increased cue reactivity in a number of different brain regions. In addition, nicotine withdrawal alters default mode, salience, and executive control network connectivity which may play a role in cognitive dysfunction and relapse.3,4
Fewer studies have used graph-theory approaches to examine altered functional connectivity in nicotine dependence. In contrast to seed-based and independent components analysis approaches, graph-theory metrics capture higher level and more abstract aspects of network communication by not only defining the connections of a given region, but also weighting or modifying those connections based on other network connections.5,6 Although first-order connectivity analyses have revealed important differences between nicotine-dependent (ND) subjects and controls, higher-order network properties may reveal additional markers of individual differences in nicotine dependence.
Of those studies that have used graph theory to study chronic nicotine use some interesting, albeit mixed, findings have emerged. For example, Breckel et al.7 report no differences in network measures of global efficiency or clustering in ND smokers versus controls. In direct contrast, Lin et al.8 reported differences between smokers and nonsmokers in similar graph-theory measures as well as correlations between these network measures and years of cigarette use. They concluded that smokers not only exhibit lower network efficiency, but also show a shift from internally directed thought (weaker local efficiency in the default mode network vs. nonsmokers) to externally driven processing (higher local efficiency in visual regions vs. nonsmokers). Li et al.9 examined functional connectivity strength in smokers and nonsmokers with slow and normal nicotine metabolic rate, based on CYP2A6 genotype. In that study, both the dorsal ACC and ventral striatum showed increased network connectivity strength in normal-genotype smokers and the connectivity of the insula biased network connectivity in these regions.
Collectively, these initial findings suggest a potential functional brain network profile of ND. However, graph-theory measures are just starting to be applied to nicotine addiction. The present study extends this investigation by examining differences between smokers and nonsmokers using graph-theory measures that have not yet been examined: connector hubs, provincial hubs, and eigenvector centrality. Clustering coefficient, which has been used in prior studies,7,8,10 is also included. This study also examines which measures are more sensitive to a clinically relevant variable, nicotine dependence, as measured by the Fagerstrom Test for Nicotine Dependence (FTND).11 Finally, this study explores sex differences in functional network topology in smokers given that female smokers derive more reward from the non-nicotine behavioral stimuli (eg, ritual of smoking, manipulation of cigarettes, sensory cues) than the pharmacological effects of nicotine12,13 compared to male smokers. In addition, negative mood and affect play a greater role in abstinence induced craving in female smokers compared to male smokers.14 Also, some recent studies have reported significant sex differences in intrinsic brain organization in smokers.15,16 A common theme is that in the brain regions and networks investigated, female smokers show stronger functional connectivity than male smokers. Specifically, Wetherill et al.15 reported that female smokers showed stronger connectivity of the amygdala with the anterior cingulate, insula, and inferior parietal lobule/cortex (IPC). Beltz et al.16 reported greater default mode network connectivity in female smokers. Zhang et al.17 showed that female smokers had stronger basal nucleus of Meynert connectivity than male smokers. However, to our knowledge, no study has examined sex differences in smokers using graph-theory measures. The present study explores these sex differences.
Methods
Subjects
Twenty-seven ND tobacco smokers (n = 13 males; n = 14 females) and 17 healthy nonsmoking (HC) controls (n = 11 males; n = 6 females) participated at Yale University. This study was approved by the Yale University School of Medicine Human Investigation Committee. Participants provided written informed consent and were recruited by word of mouth, posters, and television and newspaper advertisements. Participants completed a single neuroimaging session and the data were collected as part of ongoing neuroreceptor imaging studies in tobacco smokers. Eligibility was determined as follows: a medical examination including a physical examination, electrocardiogram, serum chemistries, thyroid function studies, complete blood count, urinalysis, and urine toxicology screening was completed. Participants had no history of significant medical illness or major head trauma. The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID) was administered to rule out Axis I Disorders including substance and alcohol dependence but not nicotine dependence in tobacco smokers.
All smokers except one male and two females completed the FTND.11 Tobacco smokers were required to have smoked ≥10 cigarettes daily for at least 1 year, confirmed by plasma cotinine levels >150 ng/mL, urine cotinine levels >100 ng/mL, and carbon monoxide levels >11 at intake. Smokers were not abstinent at the time of the scanning session and were asked to smoke within the hour prior to the start of the scanning session. HC subjects were never-smokers defined as fewer than 100 cigarettes in lifetime and zero cigarettes in the last year. Exclusion criteria were as follows: metal implants, major medical conditions, current/history of an Axis I psychiatric disorder, a head injury, pregnant/nursing, individuals unable to remain abstinent from substances of abuse.
Functional Neuroimaging Data Acquisition
Two consecutive resting state functional magnetic resonance imaging scans were collected on a Siemens TIM Trio 3T scanner using a 12-channel head coil and single-shot gradient echo echo-planar imaging (repetition time = 3.4 seconds, echo time = 30 ms, flip angle = 85°, field of view = 210 mm, 51 2.5-mm thick AC-PC parallel slices with whole brain coverage). Each scan lasted 5.7 minutes and yielded 100 volumes. A high-resolution T1-weighted MPRAGE (TR = 2530 ms, TE = 2.77 ms, flip angle = 7°, field of view = 256 mm2, 1.0-mm thick slices) was also acquired.
Resting State Functional Magnetic Resonance Imaging Data Preprocessing
Each of the two time-series for a subject was first motion corrected (MCFLIRT) using rigid body alignment and the middle volume as a reference volume. Then each of the time-series was registered to the Montreal Neurological Institute (MNI) template resampled at 3 mm resolution before the two time-series were concatenated. Slice timing correction and spatial filtering (full width half maximum = 7.5 mm), were applied to the concatenated time-series, which was then submitted to multiple regression using FSL to remove effects of global signal and head motion. Regressors included global signal (extracted from gray matter, white matter and cerebrospinal fluid masks, which were created using FMRIB's Software Library (FSL’s) FAST tissue segmentation tool), and 6 head motion parameters. The residual image from this regression step was then band-pass filtered (0.009–0.08 Hz) using Analysis of Functional Images (AFNI). The spatially normalized image was then parcellated using a 294 region atlas—the 264 regions from Power et al.18 with 30 additional subcortical regions (amygdala, hippocampus, striatum). Each region of interest (ROI) was represented by a 10-mm diameter sphere. The blood oxygen level-dependent signal time-series was extracted in each of the 294 ROIs using FSL’s “Featquery” function.
Connectome Measures
Prior to computing the 294 × 294 functional connectivity matrix, the censored timepoints (identified with “fsl_motion_outliers”) were removed from the time-series for each subject18 in MatLab (R2012a). Across the sample, the percent of timepoints removed ranged from 5%–29%. Smokers (M = 14.2%, SD = 6%) and HC (M = 14.3%, SD = 6%) were not different in terms of percent of timepoints removed, t(42) = −0.05, p = .96, nor were male (M = 15%, SD = 6%) and female (M = 14%, SD = 6%) smokers different, t(25) = 0.45, p = .66. Given that none of the groups were different on this measure, no subjects were removed from analyses. Notably, FTND was not correlated with percent timepoints removed, rho = .31, p = .14.
The connectivity matrix was a weighted adjacency matrix representing a fully connected undirected graph. Each matrix element reflected the partial correlation between two discrete resting state functional magnetic resonance imaging time-series while controlling for all other time-series. A shrinkage operation was used19 because the number of timepoints in a single time-series did not exceed the number of ROIs.
The Brain Connectivity Toolbox20 implemented in MatLab was used to compute all graph-theory measures. First, modularity was determined in the HC group. Modularity is a measure that quantifies the degree of organization of a network into densely interconnected communities. Each network node is assigned to a specific community or module.21 Communities have high intramodular connections relative to intermodular connections. Community structure (ie, the assignment of nodes to modules) was determined by running the Louvain algorithm22 1000 times per subject and determining the consensus modularity for each subject from the 1000 samples. These consensus affiliation vectors were then submitted to the agreement function to attain an HC group community structure. The HC community structure was used to calculate within-module degree z-score (DEGREE-Z) and diversity coefficient (DIV*, which included both positive and negative edge weights6) for each subject in the study, including smokers. DEGREE-Z provides an index of nodes that have high centrality within a module (provincial hubs)23 whereas DIV* yields a measure of connector hubs that enable communication across modules. By using the HC community structure for all subjects, the goal was to determine the extent to which nodes that play a central role in network communication (connector and provincial hubs) in a typical age-matched network were different in smokers.
In addition to the two graph-theory metrics used to establish connector and provincial hubs, eigenvector centrality (EVC) and clustering coefficient (CC) were computed. EVC is a spectral, self-referential measure of centrality.24 A node with a high EVC is connected to other nodes with high eigenvector score (https://sites.google.com/site/bctnet/, accessed September 20, 2017). EVC considers connections to influential nodes to be more important than connections to marginal nodes. CC is a local measure of segregation representing the fraction of a node’s neighbors that are also neighbors of each others; these patterns effectively form triangles around the node.20,25
Analysis of Group Differences
The primary analysis examined group differences (smokers, HC) in connector and provincial hub regions that were identified using the HC modularity partition, as described above, for smokers and HC. A Shapiro-Wilk test was conducted to test for violations of normality for the dependent variables and outliers were defined as values that were more than three times the interquartile range. Outliers were removed before conducting t tests and analyses of covariance (ANCOVAs) with education as a covariate. Independent samples t tests using Levene’s test were conducted in each connector and provincial hub region. Spearman rank correlations were also conducted between FTND and DIV*, DEGREEZ, EVC, and CC for each hub region only in smokers because HC did not have FTND scores (24 of the 27 smokers had FTND scores). Outliers were not removed for Spearman rank correlations. Results are reported using Holm-corrected26 α levels to account for eight simultaneous tests for each network metric (ie, dependent variable) and statistic (eg, four connector hub regions for smokers and four connector hub regions for HC were examined simultaneously with t tests using DIV* as the dependent variable). Tests that were significant at an uncorrected α level of .05 are also noted in some cases.
Analysis of Sex Differences
This exploratory analysis examined sex differences in smokers using independent-samples t tests in a priori ROIs. Sex differences were not examined in HC subjects because the sample was smaller than that for smokers and there were only six females. Additional ROIs that have been reported in prior functional connectivity studies of sex differences in smokers were also explored if these a priori regions did not already emerge as hubs in the primary analysis. Wetherill et al.15 reported sex differences in smokers in connectivity of the amygdala with the anterior cingulate, insula, and inferior parietal lobule/cortex (IPC). Beltz et al.16 also reported sex differences in the IPC. We also examined the bilateral nucleus accumbens given its important role in reinforcing effects of drugs. Therefore, 10 a priori regions were explored: bilateral ACC (L:−11, 26, 25; R:12, 36, 20), amygdala (L: −28, −4, −22; R:28, −4, −22), insula (L: −35, 20, 1; R:34, 16, −8), accumbens (L: −10, 12, −7; R:10, 10, 8), and IPC (L: −45, −64, 35; R:52, −59, 36). Only 24 of the 27 smokers completed the FTND. Independent samples t tests with Levene’s test were conducted to test for sex differences in smokers. Correlations with FTND were conducted for each network metric for male and female smokers combined, then only those ROIs that showed significant group correlations were examined further by sex. Holm-corrected α levels were determined based on up to 10 simultaneous tests for each network metric and statistical test.
Results
Subject Demographics
Smokers and controls were not different in age, t(31) = −0.08, p = .94 (smokers: M = 35.1, SD = 8.6; controls: M = 35.4, SD = 11.4). Education level was recorded in 12 of the 17 HC subjects and in 19 of the 27 smokers (coded as 1 for some high school up to 5 for graduate degree). In this subsample, education was higher in HC than in smokers, t(29) = −3.7, p = .001 (HC: M = 3.8, SD = 1.2; smokers: M = 2.4, SD = 0.9). Data on cigarettes per day were available in 23 smokers. Male and female smokers were not different in age, t(31) = −0.95, p = .35 (males: M = 36.8, SD = 10.2; females: M = 33.7, SD = 8.5), FTND scores, t(22) = −1.6, p = .12 (males: M = 6.3, SD = 2.8; females: M = 4.7, SD = 1.9), cigarettes per day, t(21) = 0.81, p = .43 (males: M = 14.5, SD = 7.7; females: M = 12.3, SD = 5.2) or education, t(17) = 1.1, p = .272 (males: M = 2.7, SD = 1.1; females: M = 2.2, SD = 0.63).
Community Structure and Modularity
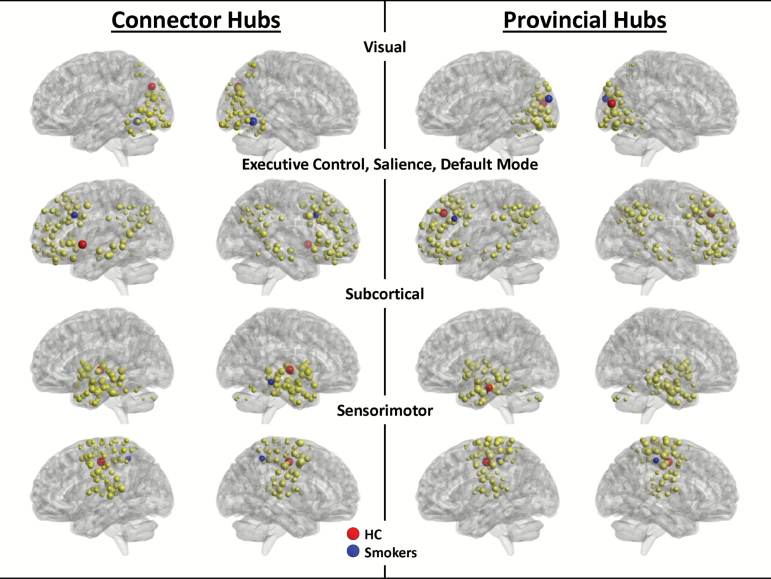
To define hubs, the community structure was first established in HC, which is illustrated in Figure 1. Four modules emerged. (1) Visual (VIS): visual cortex or aspects of the temporal lobe typically involved in high-level visual processing. (2) Executive control (EC), salience network, default mode network: cortical regions primarily in lateral frontal and parietal cortex often associated with dorsal and ventral attention networks, default mode (medial parietal, posterior cingulate, lateral inferior parietal, dorsomedial prefrontal) and salience networks (insular/opercular, anterior cingulate). (3) Subcortical (SUB): regions in the medial temporal lobe, cerebellum, striatum, and thalamus. (4) Sensorimotor (SM): primary and secondary motor and sensory cortex. In each module, however, there were nodes that might normally be associated with other networks established in the literature. Therefore, the labels assigned to these modules reflect the majority of nodes included in each module, but not the totality of nodes.
Figure 1.
Community structure in healthy controls, with connector and provincial hubs in controls and smokers. Four modules emerged from the analysis of community structure in healthy controls: visual, executive control/salience/default mode, subcortical, and sensorimotor. The nodes in each of those modules are shown in yellow, with connector hubs (left) and provincial hubs (right) in each subject group, healthy controls (red), smokers (blue).
Connector and Provincial Hubs in Healthy Controls and Smokers
Connector hubs were defined as nodes within each module with the highest DIV* scores. Provincial hubs were defined as nodes within each module with the highest DEGREE-Z scores (Figure 1; Table 1). For the most part, hubs in smokers differed from HC hubs except that the left hippocampus was a provincial hub for both HC and smokers, albeit in a slightly different location. Table 1 also notes the rank for each hub by subject group. The rankings indicate whether a network or provincial hub in one group was actually a “close second” (or third, etc) in the other group. The greatest difference (according to rank) was the EC provincial hub for smokers (left middle frontal cortex), which ranked 47th in HC. However, seven hubs were ranked in the top 5 for both groups, suggesting some agreement in hub topology between smokers and HC.
Table 1.
Hub Regions for Each Subject Group
| Group | Hub type | Module | Region | MNI | Rank | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | HC | Smokers | ||||
| HC | Connector | ECa | L opercular | −51 | 8 | −2 | 1 | 31 |
| SMb | L mid-cingulate | 0 | −15 | 47 | 1 | 2 | ||
| SUBc | R Putamen | 31 | −14 | 2 | 1 | 3 | ||
| VISd | R fusiform | 27 | −59 | 9 | 1 | 20 | ||
| Provincial | EC | L DMPFC | −2 | 38 | 36 | 1 | 5 | |
| SM | L mid-cingulate | 0 | −15 | 47 | 1 | 13 | ||
| SUB | L hippocampus | −28 | −18 | −20 | 1 | 8 | ||
| VIS | R superior occipital | −18 | −84 | 13 | 1 | 2 | ||
| Smoker | Connector | EC | L ACC | −5 | 18 | 34 | 20 | 1 |
| SM | R precuneus | 4 | −48 | 51 | 20 | 1 | ||
| SUB | R parahippocampus | 27 | −37 | −13 | 34 | 1 | ||
| VIS | L cuneus | −16 | −77 | 34 | 3 | 1 | ||
| Provincial | EC | L middle frontal | −42 | 25 | 30 | 47 | 1 | |
| SM | R postcentral | 47 | −30 | 49 | 35 | 1 | ||
| SUB | L hippocampus | −24 | −12 | −24 | 2 | 1 | ||
| VIS | L occipital pole | −24 | −91 | 10 | 5 | 1 | ||
EC = executive control; HC = healthy nonsmoking controls; SM = sensorimotor; SUB = subcortical; VIS = Visual.
a107 regions.
b67 regions.
c72 regions.
d48 regions.
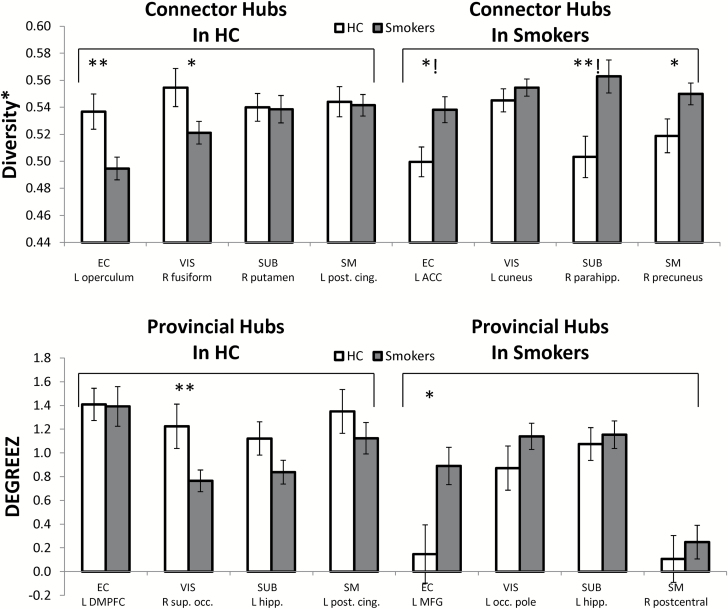
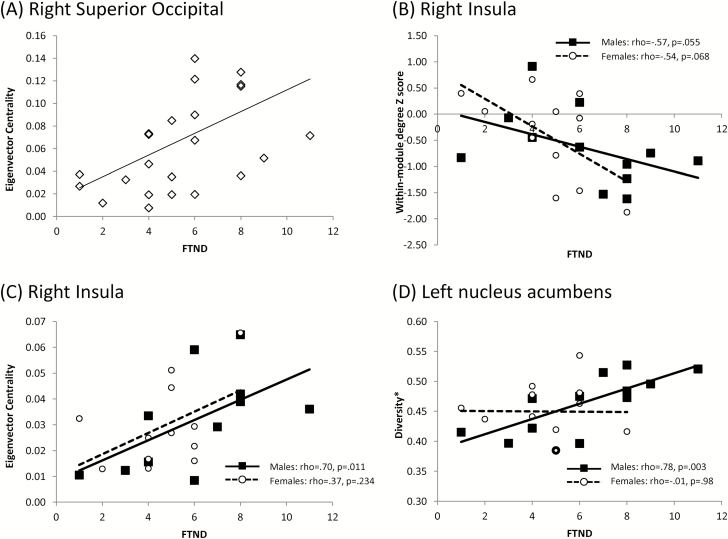
The primary analysis was an independent samples t test comparing smokers and HC in the hub regions listed in Table 1. Group differences emerged in the EC, SUB and VIS modules (Figure 2). For the EC module, the left operculum was a stronger connector hub (higher DIV*) in healthy controls, t(42) = 2.2, p = .007. For the SUB module, the right parahippocampal gyrus was a stronger connector hub (higher DIV*) in smokers, t(40) = −4.0, p = .0001 (2 outliers were removed). For the VIS module, the right superior occipital cortex was a stronger provincial hub (higher DEGREEZ) in healthy controls, t(42) = 2.9, p = .006). In addition, EVC was positively correlated with FTND in this region, (Figure 3A; rho = .553, p = .005).
Figure 2.
Connector hub and provincial hub measures by group. (Top) Diversity* value for each connector hub identified in smokers and controls. The average value and standard error of the mean for each group is shown. (Bottom) Within module Degree Z score for each provincial hub identified in smokers and controls. The average value and standard error of the mean for each group is shown. Modules were: EC, executive control. SM, sensorimotor. SUB, subcortical. VIS, visual. ACC = anterior cingulate. Post. Cing. = posterior cingulate. Parahipp = parahippocampus. DMPFC = dorsomedial prefrontal cortex. Sup. Occ. = superior occipital. MFG = middle frontal gyrus. Occ. Pole = occipital pole. Hippo. = hippocampus. *group effect is significant at an uncorrected α of .05. **group effect is significant at a Holm-corrected α level.!group effect is significant in the ANCOVA controlling for education level.
Figure 3.
Correlations between nicotine dependence and graph-theory measures. (A) Eigenvector centrality and FTND scores in the right superior occipital cortex (group correlation: rho = .553, p = .005). (B) Within-module degree Z-score and FTND in the right insula (group correlation: rho = −.58, p = .003). (C) Eigenvector centrality and FTND in the right insula (group correlation: rho = .63, p = .001). (D) Diversity* and FTND in the left nucleus accumbens (group correlation: rho = .50, p = .012). In B–D, females are shown as hollow circles with dotted trend line and males are shown as solid squares with solid trend line. The correlations for males and females are noted in the legends for each plot. Note that data points in the same group with very similar values are depicted as points with thicker outlines.
Given that education level was significantly different in smokers and HC, a follow-up ANCOVA was conducted to examine group differences controlling for education level (covariate). The sample available for this analysis was smaller due to missing data on education level. However, it is important to know whether any of the effects isolated in the t tests above could be attributed to education. The group difference in DIV* in the right parahippocampus (connector hub in smokers for the SUB module) persisted when controlling for education, F(1, 29) = 14.8, p = .001. In addition, a marginally significant effect of DIV* in the left ACC (connector hub in smokers for the EC module) emerged as significant in the ANCOVA, F(1, 29) = 14.8, p = .001. The other two significant group differences (in the left operculum for DIV* and right superior occipital cortex for DEGREEZ) were not significant in the ANCOVAs.
Exploratory Analysis of Sex Differences in Smokers
Although the left ACC was selected as an a priori ROI for the exploratory analysis of sex differences, it emerged as a connector hub region in smokers. Therefore, it was not also examined as an a priori ROI, leaving nine a priori ROIs (using Holm correction, at least one test significant at α = 0.0056). None of the nine a priori ROIs showed sex differences in smokers, but there were two significant correlations with FTND at a corrected α level. In the right insula, DEGREEZ score was negatively correlated with FTND (Figure 3B; rho = −.58, p = .003) whereas EVC was positively correlated with FTND (Figure 3C; rho = .63, p = .0001). Correlations were further inspected by sex. DEGREEZ score correlations did not reach significance when broken down by sex (rho = −.57, p = .055 for males; rho = −.54, p = .068 for females), and EVC correlations were only marginally significant for male smokers (rho = .70, p = .011 for males; rho = .37, p = .234 for females). In the left nucleus accumbens, DIV* was positively correlated with FTND at an uncorrected level (Figure 3D; rho = .50, p = .012). This correlation showed a trend in male smokers (rho = .78, p = .003) but not female smokers (rho = −.01, p = .98).
Discussion
This preliminary study on differences in intrinsic functional connectivity between smokers and nonsmokers using graph-theory network measures indicates that smokers show altered connector and provincial hub topology. Network alterations emerged in limbic regions and occipital cortex, with trends in the salience network. Although sex differences in smokers did not emerge, the exploratory analysis indicated that global influence and hub connectivity of the insula and nucleus accumbens showed marginal associations with nicotine dependence in male but not female smokers.
Group Differences in Limbic System Topology
One of the present findings was higher connector hub connectivity of the right parahippocampus in smokers. This effect was significant in the whole sample as well as after controlling for education. Wylie et al.27 showed that acute nicotine exposure increases regional efficiency of limbic and paralimbic regions (including the parahippocampus) which enables the exchange of information between these regions and other brain regions. Lin et al.8 reported that the left parahippocampal cortex and right hippocampus had higher global efficiency in heavy smokers versus nonsmokers allowing for better network-wide communication via these regions. Similarly, as a connector hub, the parahippocampal cortex allows for communication across modules, enabling greater communication with other regions of the brain. The parahippocampal cortex serves as a critical relay between the hippocampus and association areas of the cortex. The hippocampus processes contextual information for retrieval of memories and is involved in conditioned responses to cues in substance dependence.28 The stronger influence of the right parahippocampal gyrus in nicotine dependence likely reflects the dominance of limbic system activity over other regulatory control systems.
Group Differences in Visual System Topology
Another finding was reduced provincial hub connectivity but marginally higher eigenvector centrality with greater nicotine dependence of the superior occipital cortex in smokers. However, the group difference did not persist after controlling for education. Whereas differences in smokers and controls have been reported in other studies8 the present finding was not very robust.
Group Differences in Salience Network Regions
Several findings in the present study indicated altered salience network topology in nicotine dependence. Although the analysis of modularity did not yield a unique module that was consistent with the salience network,29 the bulk of salience network regions were subsumed within a module that appeared to be an aggregate of salience, default mode and executive network regions. The salience network regions were more likely to be different between smokers and controls than regions associated with executive function and default mode networks. In smokers, the connector hub for the EC module was the left anterior cingulate which showed a significant group difference after controlling for education. Another major component of the salience network is the insula30 which showed greater global influence (higher EVC) and less modular influence (lower DEGREEZ) with higher nicotine dependence.
The anterior cingulate works closely with the insula and the intrinsic connectivity of the insula and anterior cingulate is well established.29 The insula receives many afferents from visceral organs and processes interoceptive information.31 This somatic information can be used to gauge internal bodily states which can then guide behavior. For example, physiological responses within the body during states of craving or high arousal are processed at a conscious level in the insula. This information can then be used to drive the behavioral or motoric response in a given situation (eg, approach or withdrawal). The anterior cingulate is often considered a critical hub region for guiding subsequent actions based on information processed in the insula.29
In smokers with higher nicotine dependence, the right insula was associated with a stronger global influence (EVC) and a weaker role as a provincial hub than in smokers with lower nicotine dependence. This is not surprising given that the right insula has been strongly associated with cue-induced craving in smokers.32,33 Other studies have also reported correlations between insula connectivity and FTND34 or between insula connectivity and craving ratings.35 We speculate that higher levels of nicotine dependence and years of smoking have primed the right insula to play a dominant role in intrinsic brain organization even in the resting state, where no external cues are presented. Craving to smoke (processed in the insula) may exert an overwhelming influence on action systems (guided by activity in the left anterior cingulate hub) to engage in behaviors in direct response to craving, such as smoking. We do not suggest that this profile necessarily reflects a tonic state of craving, but the global influence of the insula during the resting state may reflect its dominant role in influencing behavior in active behavioral states. In support, Claus et al.34 reported that the insula has more widespread connections with limbic, striatal, and somatosensory regions in response to visually presented smoking versus food cues in smokers, compared to orbitofrontal connections. Hence, the widespread connectivity of the insula appears to be present both during the resting state and in the context of incentive processing.
Sex Differences in Network Topology in Smokers
The regions of interest used to examine sex differences in the present study were based on prior studies showing sex differences in intrinsic connectivity in smokers: the insula, anterior cingulate, inferior parietal cortex, and amygdala.15,16 Although there were few group differences between male and female smokers in the present study, only males showed trends for associations between nicotine dependence and network topology. The correlation between FTND and global influence of the right insula and strength of connector hub connectivity (DIV*) of the left nucleus accumbens showed a trend in male but not female smokers. Both of these network measures reflect the capacity for a region to communicate broadly with other components of the brain. Connector hubs are a major locus of communication across functional brain modules and nodes with high EVC have connections with other nodes that are themselves highly connected. Hence, nodes that exhibit this property enable greater diffusion of information throughout the network. Greater nicotine dependence was thus associated with a more influential and connected right insula and left nucleus accumbens, and this trend was only present in male smokers.
Male smokers are more sensitive to the reinforcing and pharmacological effects of nicotine than female smokers, whereas female smokers find the sensory aspects of smoking (eg, smell, taste, impact on respiratory tract) more rewarding or arousing than male smokers.8,9,30–33 The factors that drive response to craving and withdrawal appear to be mirrored in brain network topology in male smokers: regions that process reinforcing effects and internal bodily states are associated with nicotine dependence in male but not female smokers.
One potential reason for finding no sex differences in a priori ROIs in the present study was that prior studies15,16 used functional connectivity analyses that relied on first-order connections of regions, or the correlation of time-series of regions. Graph-theory based connectivity measures use the first-order connections to compute higher-order aspects of connectivity. Hence, higher-order connectivity measures, like those used in the present study, may override or minimize subtle sex differences that have been detected with respect to first-order connections. Another more likely potential explanation for the lack of sex differences in the present study is the relatively small sample size. Wetherill et al.15 and Beltz et al.16 had almost twice as many participants.
Limitations
While this study included sex-matched healthy controls, the experiment was preliminary and conducted in a relatively small sample of nicotine-dependent smokers. Future research should aim to examine graph-theory properties in larger samples of male and female ND smokers and age- and education-matched control subjects. We attempted to control for group differences in education statistically, but a stronger approach would be to match groups on education level. Another potential limitation was that the community structure used to determine connector and provincial hubs was based on the healthy control group in this study and subsequent group comparisons included the same healthy control subjects. Future analyses could consider using a community structure derived from an independent sample.
Another limitation is the number of simultaneous statistical tests performed. Although we took measures to correct for multiple comparisons, an argument could be made for a more stringent correction based on the total number of statistical tests, rather than based on the number of tests per dependent variable. However, given the novel graph-theory measures examined and preliminary nature of this study, we adopted a less conservative approach. In addition, the trend correlations with FTND can be used as effect sizes when calculating statistical power for future studies.
Another limitation is that we did not measure ovarian hormone levels in our subjects. Both the default mode and executive control networks are functionally altered by ovarian hormones.36 Finally, the present study did not include measures of sensory and cue-related aspects of nicotine dependence which could be important in understanding individual and sex differences in the modulation of intrinsic network topology.
Conclusions
Despite these limitations, the present study used graph-theory measures that have not been used before to address differences between smokers and matched controls (eigenvector centrality, connector hubs and provincial hubs). Differences in large-scale functional network organization were apparent between smokers and controls and these differences largely centered around connectivity of the salience network, limbic regions, and visual cortex. One graph-theory measure, eigenvector centrality, was uniquely associated with variation in nicotine dependence. This higher-order measure of connectivity may thus be sensitive to clinically relevant aspects of nicotine dependence. Measures of hub connectivity, in contrast, were more instrumental in showing differences between smokers and controls. This information may be important in guiding the choice of graph theory measures in future studies of nicotine dependence.
Funding
This work was supported by National Institutes of Health grants P50DA016511-15, P50DA016511-15S1, K02DA031750, R01DA015577, and P50DA033945.
Declaration of Interests
None declared.
References
- 1. Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349(1):64–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janes AC, Farmer S, Peechatka AL, Frederick Bde B, Lukas SE. Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40(7):1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52(2):590–599. [DOI] [PubMed] [Google Scholar]
- 4. Janes AC, Farmer S, Frederick Bd, Nickerson LD, Lukas SE. An increase in tobacco craving is associated with enhanced medial prefrontal cortex network coupling. PLoS One. 2014;9(2):e88228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. 2017;20(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage. 2011;56(4):2068–2079. [DOI] [PubMed] [Google Scholar]
- 7. Breckel TP, Thiel CM, Giessing C. The efficiency of functional brain networks does not differ between smokers and non-smokers. Psychiatry Res. 2013;214(3):349–356. [DOI] [PubMed] [Google Scholar]
- 8. Lin F, Wu G, Zhu L, Lei H. Altered brain functional networks in heavy smokers. Addict Biol. 2015;20(4):809–819. [DOI] [PubMed] [Google Scholar]
- 9. Li S, Yang Y, Hoffmann E, Tyndale RF, Stein EA. CYP2A6 genetic variation alters striatal-cingulate circuits, network hubs, and executive processing in smokers. Biol Psychiatry. 2016;81(7):554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giessing C, Thiel CM, Alexander-Bloch AF, Patel AX, Bullmore ET. Human brain functional network changes associated with enhanced and impaired attentional task performance. J Neurosci. 2013;33(14):5903–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3–4):235–241. [DOI] [PubMed] [Google Scholar]
- 12. Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. Nicotine Tob Res. 2003;5(1):111–116. [DOI] [PubMed] [Google Scholar]
- 13. Perkins KA, Karelitz JL. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17(4):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addict Behav. 2013;38(2):1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict Behav. 2014;39(4):789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beltz AM, Berenbaum SA, Wilson SJ. Sex differences in resting state brain function of cigarette smokers and links to nicotine dependence. Exp Clin Psychopharmacol. 2015;23(4):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S, Hu S, Fucito LM, et al. . Resting-state functional connectivity of the basal nucleus of meynert in cigarette smokers: dependence level and gender differences. Nicotine Tob Res. 2017;19(4):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schäfer J, Strimmer K. A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat Appl Genet Mol Biol. 2005;4(1):Article32. [DOI] [PubMed] [Google Scholar]
- 20. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. [DOI] [PubMed] [Google Scholar]
- 21. Lancichinetti A, Fortunato S. Consensus clustering in complex networks. Sci Rep. 2012;2:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. Journal of Statistical Mechanics: Theory and Experiment. 2008;2008(10):P10008. [Google Scholar]
- 23. Guimerà R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433(7028):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lohmann G, Margulies DS, Horstmann A, et al. . Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One. 2010;5(4):e10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393(6684):440–442. [DOI] [PubMed] [Google Scholar]
- 26. Holm S. A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics. 1979; 6(2):65–70. [Google Scholar]
- 27. Wylie KP, Rojas DC, Tanabe J, Martin LF, Tregellas JR. Nicotine increases brain functional network efficiency. Neuroimage. 2012;63(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5-6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckert MA, Menon V, Walczak A, et al. . At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30(8):2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis?Trends Cogn Sci. 2005;9(12):566–571. [DOI] [PubMed] [Google Scholar]
- 32. Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Increased functional connectivity in an insula-based network is associated with improved smoking cessation outcomes. Neuropsychopharmacology. 2015;40(11):2648–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38(12):2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moran-Santa Maria MM, Hartwell KJ, Hanlon CA, et al. . Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict Biol. 2015;20(2):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage. 2014;90:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]