Abstract
Background
Epidemiologic data suggest that diets rich in nuts have beneficial health effects, including reducing total and cause-specific mortality from cancer and heart disease. Although there is accumulating preclinical evidence that walnuts beneficially affect the gastrointestinal microbiota and gut and metabolic health, these relations have not been investigated in humans.
Objective
We aimed to assess the impact of walnut consumption on the human gastrointestinal microbiota and metabolic markers of health.
Methods
A controlled-feeding, randomized crossover study was undertaken in healthy men and women [n = 18; mean age = 53.1 y; body mass index (kg/m2): 28.8]. Study participants received isocaloric diets containing 0 or 42 g walnuts/d for two 3-wk periods, with a 1-wk washout between diet periods. Fecal and blood samples were collected at baseline and at the end of each period to assess secondary outcomes of the study, including effects of walnut consumption on fecal microbiota and bile acids and metabolic markers of health.
Results
Compared with after the control period, walnut consumption resulted in a 49–160% higher relative abundance of Faecalibacterium, Clostridium, Dialister, and Roseburia and 16–38% lower relative abundances of Ruminococcus, Dorea, Oscillospira, and Bifidobacterium (P < 0.05). Fecal secondary bile acids, deoxycholic acid and lithocholic acid, were 25% and 45% lower, respectively, after the walnut treatment compared with the control treatment (P < 0.05). Serum LDL cholesterol and the noncholesterol sterol campesterol concentrations were 7% and 6% lower, respectively, after walnut consumption compared with after the control treatment (P < 0.01).
Conclusion
Walnut consumption affected the composition and function of the human gastrointestinal microbiota, increasing the relative abundances of Firmicutes species in butyrate-producing Clostridium clusters XIVa and IV, including Faecalibacterium and Roseburia, and reducing microbially derived, proinflammatory secondary bile acids and LDL cholesterol. These results suggest that the gastrointestinal microbiota may contribute to the underlying mechanisms of the beneficial health effects of walnut consumption. This trial was registered at www.clinicaltrials.gov as NCT01832909.
Keywords: tree nuts, microbiome, deoxycholic acid, lithocholic acid, cardiovascular health
Introduction
The human gastrointestinal tract represents one of the most densely populated microbial communities on earth, containing as many microbial cells as there are human cells in the body and >150 times more bacterial genes than the human genome (1, 2). Among healthy adults, the large intestine contains the densest community of microorganisms, containing upward of 1011 microbial cells/g of colonic contents. Beneficial contributions of these microbes include salvaging energy by metabolizing nondigestable dietary fibers to produce butyrate, a preferential metabolite for colonocytes. However, the gastrointestinal microbiota can also contribute to deleterious effects, such as gastrointestinal and systemic inflammation and the development of noncommunicable diseases, including obesity, cardiovascular disease, and cancer (2–4).
Epidemiologic data suggest that a Western diet pattern, rich in saturated fat and simple sugars and devoid of an appreciable amount of dietary fiber, is associated with increased risk of obesity, cardiovascular disease, and colorectal and liver cancer (5, 6). Alternatively, diets rich in nuts, a source of dietary fiber and unsaturated fatty acids, are associated with a reduced risk of total and cause-specific mortality from cancer and heart disease (7). Habitual dietary patterns contribute to the composition and function of the human gastrointestinal microbiome (8). Importantly, short-term changes in diet can alter the microbiome: switching from a vegetarian diet to a diet high in saturated fat and processed meats rapidly (within 2–4 d) changed fecal microbial profiles, reduced fecal butyrate concentrations, and increased bile acid secretion in healthy adults (8, 9). Although the consumption of dietary fiber and prebiotics have been shown to differentially affect the human gastrointestinal microbiome (10–12), there is a dearth of knowledge on the impact of specific whole foods on the human microbiota.
Previously, a complete feeding trial that used a randomized controlled crossover design was completed to determine the metabolizable energy of walnuts when consumed as part of a typical American diet (13). The metabolizable energy of walnuts was 21% lower than that predicted by the Atwater values because of reduced digestibility of macronutrients. Because the nutrients that escape digestion in the small intestine enter the large intestine and provide substrates for fermentation by colonic microbiota, our objective was to elucidate the effects of the reduced digestibility of walnuts on the fecal microbiota, fecal bile acids, and metabolic markers of health in healthy adults.
Methods
This study is a follow-up to a previously conducted trial (www.clinicaltrials.gov; NCT01832909). Details of the design and results of the primary study, which assessed the metabolizable energy of walnuts by using bomb calorimetry of diets, walnuts, fecal, and urine samples, were reported elsewhere (13). Herein, secondary outcomes, including fecal microbiota and bile acid concentrations, and metabolic health variables are reported. Briefly, a randomized, crossover, controlled-feeding study with two 3-wk dietary interventions was conducted at the Beltsville Human Nutrition Research Center. The fully controlled dietary feeding intervention provided the same base diet during each treatment condition; the base diet was unsupplemented during the control condition and supplemented with 1.5 servings (42 g) of walnut halves and pieces daily during the walnut treatment condition. This amount is consistent with the FDA qualified health claim for walnut consumption on coronary artery disease. The base diet provided 17% of energy from protein, 29% of energy from fat, and 54% of energy from carbohydrates. The base diet foods were reduced in equal proportions during the walnut treatment condition to achieve isocaloric food intake during the 2 diet periods. Diets were scaled according to the energy requirements of the study participants for weight maintenance throughout the study. Participants completed both of the dietary intervention conditions with a 1-wk compliance break between each condition. The treatment order was randomized by dividing participants by sex and by using a random-number generator. Participants completed daily questionnaires for dietary deviations, medications, unusual exercise, and general well-being.
Participants
Study participants were recruited from the Washington, DC area. Interested volunteers completed a health history questionnaire; were measured for height, weight, and blood pressure; and provided fasting blood and urine samples for the analysis of lipids, glucose, a comprehensive metabolic panel, and complete blood count and urinalysis. Potential volunteers were excluded for any of the following reasons: <25 or >75 y of age, BMI (kg/m2) of <20 or >38, fasting glucose ≥126 mg/dL, blood pressure ≥160/100 mm Hg, fasting total serum blood cholesterol ≥280 mg/dL, and fasting serum TGs ≥300 mg/dL; if they were smokers or allergic to walnuts; if they abused alcohol; if they had kidney disease, liver disease, gout, hyperthyroidism, untreated or unstable hypothyroidism, certain cancers, gastrointestinal disease, pancreatic disease, diabetes requiring medication, unstable body weight over the past 12 mo, malabsorption syndrome; or if they were women who were pregnant, lactating, or had given birth in the past 12 mo. All of the study procedures were reviewed and approved by the Institutional Review Board of the Medstar Research Institute and were in accord with the Declaration of Helsinki. All of the participants provided written consent forms.
Sample collection and analyses
Blood samples were collected at the beginning and end of each treatment condition after a 12-h overnight fast. Serum samples were assayed to assess total cholesterol, LDL cholesterol, HDL cholesterol, TGs, and glucose by using enzymatic procedures (Vitros 5,1; Ortho-Clinical Diagnostics, Inc.); and inflammatory markers (IL-6, C-reactive protein [CRP], serum amyloid A [SAA], soluble adhesion molecules [soluble intercellular adhesion molecule (sICAM) and soluble vascular cell adhesion molecule (sVCAM)]) were measured by using sandwich-type immunoassay methods (Meso Scientific Discovery). Plasma noncholesterol sterol (squalene, desmosterol, lathosterol, cholestenol, campesterol, and β-sitosterol) concentrations were quantified by GC and expressed relative to total cholesterol (14, 15).
Fecal samples were collected at the beginning and end of each dietary period. Upon collection, samples were homogenized in a bag, placed in aliquots, and stored at –80°C at the Beltsville Human Nutrition Research Center before overnight shipping on dry ice to the University of Illinois. Fecal DNA was isolated with the use of a PowerLyzer PowerSoil DNA Isolation Kit (MoBio Laboratories) according to the manufacturer's instructions. Bacterial (16S V4 region, 505f/806r) (16), archaeal (349f/806r), and fungal (ITS1–ITS4) (17) genes were amplified on a Fluidigm Access Array then sequenced on a MiSeq with the use of v3 reagents (Illumina, Inc.) in the WM Keck Center for Biotechnology, University of Illinois, as previously described (18).
Sequence data were analyzed with QIIME 1.8.0; sequences were clustered into operational taxonomic units (OTUs) with the use of closed reference OTU picking against the 13-8 Greengenes database (97% similarity) for the bacterial and archaeal sequences (19). Fungal OTUs were generated with the use of the open reference OTU-picking algorithm against the UNITE OTUs ITS 12_11 reference database. Additional quality filtering (20) was undertaken before rarefying the bacterial sequences to an even sampling depth of 35,957 sequences/sample to assess α and β diversity measures as well as the impact of treatment on OTU relative abundances. Predicted bacterial functional capacity was assessed with the use of PICRUSt, version 1.1.1, and the KEGG Pathway Database (21).
Freeze-dried fecal aliquots were shipped overnight to the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University for analysis of bile acids. Briefly, total lipids were extracted overnight by using a modified Folch method (22) with nor-deoxycholic acid (0.4 μg/μL; Steraloids) as the internal standard. Samples were analyzed on an ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry UHPLC-QTOF MS (Agilent Technologies) equipped with a 1290 series LC system and a 6550 iFunnel QTOF MS detector. Samples were injected onto the column (Zorbax Eclipse Plus C18; 2.1 × 50 mm, 1.8 μm) maintained at 25°C, and the bile acids eluted with the use of a 40-min isocratic mobile phase at 0.25 mL/min, from 10 mM ammonium acetate and 0.1% ammonium hydroxide in 100% methanol (pH = 7.5). The primary (cholic and chenodeoxycholic acids) and secondary (lithocholic and deoxycholic acids) bile acids were identified on the basis of molecular formula, retention times, and mass (MassHunter Qualitative Analysis software) and quantified (micrograms per milligram) with the use of the respective standard curves. Interassay CVs were 1.9% for cholic acid, 5.4% for chenodeoxycholic acid, 2.4% for deoxycholic acid and 4.5% for lithocholic acid.
Statistical analysis
Differences in serum (lipids, glucose, inflammatory markers, and sterols) and fecal (microbial abundances and bile acids) measures at the end of each dietary treatment period were analyzed with the use of a mixed-effects ANOVA with treatment as the fixed effect and participant and period as the random effects. Statistical analysis was conducted in SAS version 9.4 (SAS Institute, Inc.). The UNIVARIATE procedure and Shapiro-Wilk statistic were used to test for data normality, and logarithmic transformations were utilized as needed. Changes from baseline scores were calculated in the walnut group to assess relations between change in bacterial abundances and change in serum cholesterol concentrations. Bivariate correlations were conducted to assess the relations between bacterial abundances and secondary bile acid concentrations at the end of the walnut consumption period. Pearson's correlations were used for normally distributed variables and Spearman correlation for nonnormally distributed variables. Wilcoxon’s rank-sum tests were conducted to compare predicted bacterial butanoate metabolism (KEGG 00650) and secondary bile acid biosynthesis (KEGG 00121) gene counts at the end of the walnut and control periods. Linear discriminant analysis effect size (LEfSE; Galaxy version 1.0) was used to assess KEGG Pathway Hierarchy Level 2 and 3 metabolism data. For all of the analyses, P ≤ 0.05 was considered significant and P ≤ 0.10 was considered to be a trend.
Results
All 18 participants, 10 men and 8 women, who were randomly assigned completed the study (Supplemental Figure 1). Participants had a mean ± SEM BMI of 28.8 ± 0.9 and were aged 53.1 ± 2.2 y (Table 1).
TABLE 1.
Baseline characteristics of the 10 men and 8 women who consumed control and walnut diets, each for 3 wk1
| Characteristics | Mean ± SEM | Range |
|---|---|---|
| Age, y | 53.1 ± 2.2 | 35.0–67.8 |
| BMI, kg/m2 | 28.8 ± 0.9 | 20.2–34.9 |
| Serum LDL cholesterol, mg/dL | 121 ± 6.4 | 65.5–175 |
| Serum HDL cholesterol, mg/dL | 51.4 ± 2.9 | 29.1–77.9 |
| Serum TGs, mg/dL | 116 ± 12.7 | 39.2–203 |
| Serum glucose, mg/dL | 92.7 ± 1.7 | 89.4–116 |
| Systolic blood pressure, mm Hg | 117 ± 3.4 | 98–144 |
| Diastolic blood pressure, mm Hg | 71.4 ± 2.2 | 60–90 |
1Serum analytes were assessed after an overnight fast. Adapted with permission from reference 13.
The mean ± SD bacterial OTU was 88,880 ± 47,427, with a maximum of 288,079 counts and a minimum of 35,957 counts/sample. At the phylum level, the majority of sequences were dominated by Firmicutes, Bacteroidetes, and Actinobacteria (84.4%, 6.5%, and 5.4%, respectively). Thirty-five families were identified. Six families (Lachnospiraceae, Ruminococcaceae, an undefined family in the Clostridales order, Bacteroidaceae, Bifodobacteriaceae, and Verrucomicrobiaceae) comprised >90% of all families. Seventy-two genera were classified, with 22 representing >96% of sequences and 15 representing <1%; Bacteroides, Blautia, Coprococcus, Ruminococcus, unclassified Ruminococcaceae, unclassified Clostridiales, and unclassified Lachnospiraceae represented 74% of sequences. The mean ± SD archaeal OTU was 19,205 ± 42,075, with a maximum of 163,351 counts and a minimum of 3 counts/sample. Treatment did not affect archaea abundances. Fungal OTUs were present in lower abundances with a mean ± SD of 854 ± 2765 counts/sample. The minimum was 1 OTU (13 samples) and the maximum was 17,404 sequences. Treatment did not affect fungal abundances.
Walnut consumption did not affect bacterial α diversity measures, including phylogenetic diversity, observed OTUs, or richness and evenness. For β diversity, weighted principal coordinates analysis of UniFrac distances between samples based on their 97% OTU composition and abundances showed that bacterial communities were affected by walnut consumption (P = 0.03). At the phylum level, compared with the end of the control period, walnut consumption increased (P = 0.04) the relative abundance of Firmicutes and decreased (P = 0.02) the relative abundance of Actinobacteria (Table 2). Among bacterial genera, Faecalibacterium, Clostridium, Roseburia, and Dialister were increased (P < 0.05) at the end of the walnut intervention period compared with the control ( Table 2). Alternatively, the relative abundances of Ruminococcus, Dorea, Oscillospira, and Bifidobacterium were lower (P < 0.05) at the end of the control diet period than at the end of the walnut diet period (Table 2). The number of predicted bacterial butanoate metabolism genes was not different at the end of the walnut period (488,366 ± 168,601) compared with after the control period (570,743 ± 151,243) (P = 0.11).
TABLE 2.
Fecal bacterial phyla and genera of adults who consumed control and walnut diets, each for 3 wk, in a crossover design1
| Percentage of sequences | ||||
|---|---|---|---|---|
| Phyla and genera | Control | Walnut | SEM | P |
| Firmicutes | 82.4 | 87.2 | 2.83 | 0.04 |
| Blautia | 8.33 | 8.23 | 0.82 | 0.82 |
| Coprococcus | 7.56 | 6.99 | 0.74 | 0.25 |
| Ruminococcus | 6.19 | 5.17 | 0.85 | <0.01 |
| Dorea | 3.21 | 2.58 | 0.39 | <0.01 |
| Streptococcus | 2.07 | 1.98 | 1.03 | 0.80 |
| Faecalibacterium | 1.45 | 2.77 | 0.59 | 0.02 |
| Roseburia | 0.79 | 1.32 | 0.29 | <0.01 |
| Clostridium | 0.72 | 1.07 | 0.13 | 0.01 |
| Oscillospira2 | 0.51 | 0.37 | 0.06 | 0.04 |
| Dialister | 0.28 | 0.73 | 0.25 | 0.04 |
| Lactobacillus | 0.28 | 0.46 | 0.36 | 0.34 |
| Lachnospira | 0.23 | 0.26 | 0.06 | 0.63 |
| Anaerostipes | 0.22 | 0.26 | 0.05 | 0.30 |
| Bacteroidetes | 5.72 | 5.20 | 2.10 | 0.62 |
| Bacteroides | 4.88 | 4.60 | 1.81 | 0.77 |
| Parabacteroides | 0.13 | 0.16 | 0.07 | 0.48 |
| Actinobacteria | 8.19 | 5.00 | 1.90 | 0.02 |
| Bifidobacterium | 6.43 | 3.97 | 1.83 | 0.03 |
| Collinsella2 | 0.10 | 0.05 | 0.26 | 0.07 |
| Verrucomicrobia | 3.26 | 1.77 | 0.96 | 0.41 |
| Akkermansia | 3.26 | 1.77 | 0.96 | 0.41 |
| Proteobacteria | 0.23 | 0.58 | 0.13 | 0.13 |
| Firmicutes:Bacteroidetes3 | 1.64 | 1.73 | 0.21 | 0.54 |
1Values are least-square means ± SEMs unless otherwise indicated; n = 18. Treatment effects were evaluated with the use of a mixed-model ANOVA.
2Values are mean log-normalized sequence abundances ± SEs.
3Values are least-square means ± SEMs of log-transformed Firmicutes-to-Bacteroidetes ratio.
Primary bile acids were not affected by treatment. Microbially derived secondary bile acids, deoxycholic acid and lithocholic acid, were reduced by 25% and 45%, respectively, in the walnut group compared with the control (P < 0.01) (Table 3). The number of predicted bacterial biosynthesis of secondary bile acids genes tended (P = 0.09) to be lower at the end of the walnut period (36,331 ± 12,256) than at the end of the control period (42,714 ± 11,605).
TABLE 3.
Fecal bile acid concentration of freeze-dried feces collected from adults who consumed control and walnut diets, each for 3 wk1
| Control | Walnut | SEM | P | |
|---|---|---|---|---|
| Cholic acid, µg/mg | 0.32 | 0.28 | 0.09 | 0.54 |
| Chenodeoxycholic acid, µg/mg | 0.20 | 0.15 | 0.05 | 0.19 |
| Deoxycholic acid, µg/mg | 2.61 | 1.96 | 0.23 | <0.01 |
| Lithocholic acid, µg/mg | 0.76 | 0.41 | 0.06 | <0.01 |
1Values are least-square means ± pooled SEMs; n = 18 participants in a crossover design. Treatment effects were evaluated with the use of a mixed-model ANOVA.
Serum total cholesterol concentrations were lower (P = 0.03) by 7 mg/dL (4% reduction) and LDL-cholesterol concentrations were lower (P < 0.01) by 9 mg/dL (7% reduction) after walnut consumption compared with the control (Table 4). Other metabolic variables were unaffected by walnut consumption. Serum campesterol was lower (P = 0.01) by 10 μmol/mmol (6% reduction), and lathosterol concentrations tended to be lower (P = 0.06) during the walnut period compared with the control (Table 5). There were no associations between the change in total cholesterol or LDL cholesterol and microbes affected by walnut consumption (Table 6). Lithocholic acid concentrations were positively associated with the relative abundances of Dorea (r = 0.46, P = 0.05) (Figure 1A) at the end of the walnut treatment period and tended to be negatively associated with Roseburia (r = −0.42, P = 0.08) (Figure 1B). There were no associations between deoxycholic acid concentrations and bacteria affected by walnut consumption (Table 7). Other predicted bacterial metabolism gene counts were unaffected by walnut consumption (Supplemental Tables 1 and 2).
TABLE 4.
Serum analytes of adults who consumed control and walnut diets, each for 3 wk1
| Control | Walnut | SEM | P | |
|---|---|---|---|---|
| Cholesterol, mg/dL | 194 | 187 | 7.1 | 0.03 |
| LDL cholesterol, mg/dL | 117 | 108 | 6.5 | <0.01 |
| HDL cholesterol, mg/dL | 48.0 | 48.5 | 3.0 | 0.51 |
| TGs, mg/dL | 92.4 | 89.8 | 8.2 | 0.35 |
| Glucose, mg/dL | 99.7 | 99.5 | 1.6 | 0.79 |
| IL-6, pg/mL | 1.43 | 1.36 | 0.18 | 0.32 |
| CRP, ng/mL | 3330 | 3830 | 1290 | 0.37 |
| SAA, ng/mL | 5490 | 6104 | 2790 | 0.52 |
| sICAM, ng/mL | 336 | 334 | 16.4 | 0.68 |
| sVCAM, ng/mL | 503 | 502 | 32.6 | 0.86 |
1Values are least-square means ± pooled SEMs; n = 18 participants in a crossover design. Treatment effects were evaluated with the use of a mixed-model ANOVA. CRP, C-reactive protein; SAA, serum amyloid A; sICAM, soluble intercellular adhesion molecule; sVCAM, soluble vascular cell adhesion molecule.
TABLE 5.
Serum noncholesterol sterol concentration of adults who consumed control and walnut diets, each for 3 wk1
| Control | Walnut | SEM | P | |
|---|---|---|---|---|
| Squalene, 100 μmol/mmol cholesterol | 15.9 | 14.0 | 1.87 | 0.25 |
| Desmosterol, 100 μmol/mmol cholesterol | 105 | 97.9 | 8.49 | 0.29 |
| Lathosterol, 100 μmol/mmol cholesterol | 183 | 169 | 26.8 | 0.06 |
| Cholestanol, 100 μmol/mmol cholesterol | 331 | 342 | 17.1 | 0.47 |
| Campesterol, 100 μmol/mmol cholesterol | 169 | 159 | 19.8 | 0.01 |
| β-Sitosterol, 100 μmol/mmol cholesterol | 75.4 | 77.9 | 10.7 | 0.14 |
1Values are least-square means ± pooled SEMs; n = 18 participants in a crossover design. Treatment effects were evaluated with the use of a mixed-model ANOVA.
TABLE 6.
Associations between fecal microbiota and serum cholesterol and LDL cholesterol in the walnut treatment group1
| Cholesterol, mg/dL | LDL cholesterol, mg/dL | |||
|---|---|---|---|---|
| Genera, % of sequences | ρ | P | ρ | P |
| Ruminococcus | −0.39 | 0.10 | −0.33 | 0.19 |
| Dorea | −0.03 | 0.89 | −0.13 | 0.62 |
| Faecalibacterium | 0.26 | 0.30 | 0.31 | 0.21 |
| Roseburia | 0.14 | 0.58 | 0.10 | 0.70 |
| Clostridium | 0.20 | 0.43 | −0.02 | 0.94 |
| Oscillospira | −0.21 | 0.41 | −0.39 | 0.11 |
| Dialister | −0.20 | 0.42 | −0.16 | 0.52 |
| Bifidobacterium | 0.17 | 0.49 | 0.03 | 0.92 |
1Relations between change from baseline fecal bacterial sequence abundances that were significantly affected by walnut consumption and serum total cholesterol and LDL cholesterol concentrations were assessed in the walnut treatment group (n = 18) with the use of bivariate correlations (Spearman's ρ).
FIGURE 1.
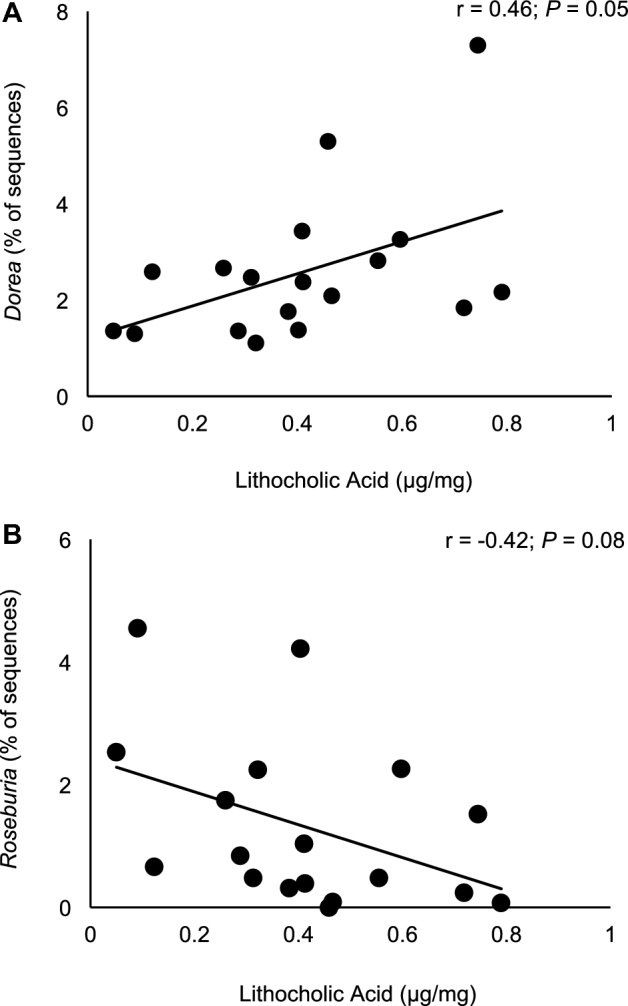
Bivariate correlations between fecal Dorea (A) and Roseburia (B) sequence abundances and lithocholic acid concentrations at the end of the walnut treatment period.
TABLE 7.
Bivariate correlations between fecal bacterial sequence abundances and secondary bile acid concentrations at the end of the walnut consumption period among the 18 adult participants
| Deoxycholic acid, µg/mg | Lithocholic acid, µg/mg | |||
|---|---|---|---|---|
| Genera, % of sequences | r | P | r | P |
| Ruminococcus | 0.07 | 0.77 | 0.08 | 0.77 |
| Dorea | 0.25 | 0.32 | 0.46 | 0.05 |
| Faecalibacterium | −0.27 | 0.28 | −0.25 | 0.33 |
| Roseburia | −0.32 | 0.19 | −0.42 | 0.08 |
| Clostridium | −0.29 | 0.25 | −0.21 | 0.41 |
| Oscillospira | −0.15 | 0.55 | 0.07 | 0.78 |
| Dialister | −0.24 | 0.34 | −0.17 | 0.49 |
| Bifidobacterium | −0.13 | 0.62 | −0.12 | 0.63 |
Discussion
To our knowledge, this is the first study to examine the impact of walnut consumption on the human fecal microbiota and bacterial metabolites and metabolic health. Specifically, in a completely controlled dietary intervention, the addition of 42 g walnut halves/d increased the relative abundance of Firmicutes species in the Clostridium clusters XIVa and IV, including Faecalibacterium, Roseburia, and Clostridium. These results are supported by preclinical studies indicating that walnuts increased the relative abundances of Firmicutes, including the genera Clostridium (23) and Roseburia (24). In vitro work has shown that Faecalibacterium and Roseburia spp. display butyryl CoA:acetate CoA transferase activity and ferment fiber to produce butyrate (25–27). Although in vitro fermentation of walnuts has not been investigated to our knowledge, finely ground almonds have been shown to increase butyrate concentrations in vitro (28). However, because our results also showed a reduction in the relative abundance of Bifidobacteria, a microbe that produces acetate, a necessary metabolite for butyrate production through butyryl CoA:acetate CoA transferase enzyme activity, walnut consumption may not have resulted in an overall increase in butyrate concentrations. Furthermore, our results showed that there was not a significant difference in predicted bacterial butyrate metabolism gene abundances between the walnut and control groups. Additional in vitro and in vivo research is necessary to determine if walnuts increase microbial butyrate production and gastrointestinal butyrate concentration, respectively.
The human liver synthesizes 2 primary bile acids, cholic acid and chenodeoxycholic acid, which are converted to secondary bile acids—deoxycholic and lithocholic acid, respectively—by intestinal bacteria (29). Western diets, which are rich in saturated fat and low in dietary fiber, result in increased amounts of secondary bile acids in the colon and biliary pool (30), and evidence suggests that deoxycholic and lithocholic acids are toxic bile acids associated with gastrointestinal diseases and colon cancer (31, 32). The gastrointestinal microbiota has been shown to regulate the metabolism and synthesis of bile acids via the nuclear bile acid receptor farnesoid X receptor (33). Dietary fatty acid composition has also been shown to differentially affect the interactions between bile acids and the gastrointestinal microbiota (34). Herein, we report significant reductions in microbially derived, proinflammatory secondary bile acids (e.g., deoxycholic acid and lithocholic acid) after walnut consumption, and a trend for a reduction in predicted bacterial secondary bile acid biosynthesis gene abundances in the walnut group compared with the control group (P = 0.09). Both reduced secondary bile acid concentrations and increased Faecalibacterium have been shown to reduce gut inflammation (31, 35). Furthermore, a rodent study showed that walnut consumption reduces colon tumor development and size and led to changes in the cecal microbiota (36). Thus, the findings in this report provide additional support for the connections between diet, the gastrointestinal microbiota, and colonic health.
Related to the effects on walnut consumption on cardiovascular health, similar to previous reports our results show that walnut consumption reduced total and LDL-cholesterol concentrations (37). Although walnut consumption reduced total and LDL-cholesterol concentrations and changed the composition of the fecal microbiota, there were no significant relations between microbes affected by walnut consumption and total and LDL cholesterol. These results suggest that total and LDL cholesterol are not directly related to gastrointestinal microbes, which is consistent with a cross-sectional study in 893 subjects that reported that neither LDL cholesterol nor total cholesterol were related to the microbiota (5, 6). Interestingly, Dorea and Roseburia tended to be related to the concentration of the secondary bile acid lithocholic acid, suggesting that the associations between the gastrointestinal microbiota and cardiovascular health may be related to the microbial metabolism of bile acids and subsequent signally through farnesoid X receptor.
PUFAs have antimicrobial properties, including the inhibition of bacterial FA synthesis (38), growth, and mucus adhesion (39). n–3 FA supplementation modulated rodent fecal microbiota by suppressing growth of bacteria associated with obesity and inflammation (40). PUFAs can also modulate the gastrointestinal microbiota due to differing abilities of bacteria to metabolize long-chain FAs. For example, in vitro studies have shown that Roseburia spp. are involved in the process of converting linoleic acid to its conjugated form (41–43). Thus, the increase in Roseburia in the present study may be related to its ability to metabolize FAs.
In addition to providing a source of fiber and unsaturated FAs, walnuts are also high in ellagic acid. Gastrointestinal bacteria can metabolize ellagic acid to produce urolithins, which can enter the enterohepatic circulation and have anti-inflammatory and beneficial vascular effects (44–46). Walnut extract, including polyphenols and ellagic acid, has shown positive effects on endothelial health in vitro (47). To our knowledge, studies have only investigated the effects of walnut, tree nut, and polyphenol consumption on either the presence of urolithin byproducts through urinalysis or the microbiome through analysis of fecal samples (48, 49), whereas little is known about the roles of specific bacteria on polyphenol metabolism and urolithin production. Fermentation of pomegranate tannins including ellagic acid in vitro resulted in increased microbial fermentation, thereby reducing pH (50). Others have shown that Bifidobacterium fragilis is inhibited by the presence of ellagic acid (50), which could explain the decrease in the relative abundance of Bifidobacterium spp. after the walnut diet condition in the present study.
Although the age range for this study population was fairly broad (35–68 y), 13 of the 18 participants were aged ≥50 y. Thus, the characteristics of the microbiota would generally be reflective of a slightly older population. Because Faecalibacterium and Roseburia have been reported to be negatively associated with age and Oscillospira to be positively associated with age (51, 52), the consumption of walnuts may help to slow age-related changes in the microbiota. However, because semi-supercentenarians show enriched Bifidobacterium (52), and walnut consumption reduced the relative abundance of Bifidobacterium, additional research is necessary to assess the effects of walnut consumption and the microbiota in the context of aging.
The strength of this study is the use of a complete feeding, randomized, controlled crossover trial with a washout period to assess the impact of walnut consumption on physiologic and microbiological outcomes among human subjects. Study limitations include the lack of fecal fermentative end products, including short-chain FAs, and metagenomic analyses to assess the functional capacity of the microbiota.
In summary, walnut consumption affected the gastrointestinal microbiota and microbially derived secondary bile acids and reduced serum total and LDL cholesterol in healthy adults. These results suggest that the gastrointestinal microbiota may contribute to the underlying mechanisms of the beneficial health effects of walnut consumption, including cardiometabolic and gastrointestinal health.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—DJB and JAN: designed and conducted the research; NRM and AHL: conducted the bile acid assays; HMG, KSS, and HDH: conducted the microbiota analyses; HMG, RA, and HDH: analyzed the data; HDH: had primary responsibility for the final content; and all authors: wrote the manuscript and read and approved the final manuscript.
Notes
Supported by the USDA Agricultural Research Service and the California Walnut Commission.
Supplemental Figure 1 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Author disclosures: HMG, RA, NRM, and AHL, no conflicts of interest. HDH, KSS, and JAN were funded by the USDA Agricultural Research Service. DJB was funded by the USDA Agricultural Service and the California Walnut Commission.
References
- 1. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016;164:337–40. [DOI] [PubMed] [Google Scholar]
- 2. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55–60. [DOI] [PubMed] [Google Scholar]
- 3. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JA, Brandsma E, Marczynska J, Imhann F, Weersma RK, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res 2015;117:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 2012;3:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holscher HD, Caporaso JG, Hooda S, Brulc JM, Fahey GCJ, Swanson KS, Fahey GC Jr., Swanson KS. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr 2015;101:55–64. [DOI] [PubMed] [Google Scholar]
- 11. Holscher HD, Bauer LL, Vishnupriya G, Pelkman CL, Fahey GC, Swanson KS, Gourineni V, Pelkman CL, Fahey GC Jr., Swanson KS. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr 2015;145:2025–32. [DOI] [PubMed] [Google Scholar]
- 12. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017;8:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baer DJ, Gebauer SK, Novotny JA. Walnuts consumed by healthy adults provide less available energy than predicted by the Atwater factors. J Nutr 2015;9–13. [DOI] [PubMed] [Google Scholar]
- 14. Matthan NR. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J Lipid Res 2003;44:800–6. [DOI] [PubMed] [Google Scholar]
- 15. Matthan NR, Pencina M, LaRocque JM, Jacques PF, D'Agostino RB, Schaefer EJ, Lichtenstein AH. Alterations in cholesterol absorption/synthesis markers characterize Framingham Offspring Study participants with CHD. J Lipid Res 2009;50:1927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012;6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, et al. ; Fungal Barcoding Consortium . Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci USA 2012;109:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venable EB, Fenton KA, Braner VM, Reddington CE, Halpin MJ, Heitz SA, Francis JM, Gulson NA, Goyer CL, Bland SD, et al. Effects of feeding management on the equine cecal microbiota. J Equine Vet Sci 2017;49:113–21. [Google Scholar]
- 19. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nature 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 2013;10:57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013;31:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 23. Nakanishi M, Chen Y, Qendro V, Miyamoto S, Weinstock E, Weinstock GM, Rosenberg DW. Effects of walnut consumption on colon carcinogenesis and microbial community structure. Cancer Prev Res 2016;9:692–703. [DOI] [PubMed] [Google Scholar]
- 24. Byerley LO, Samuelson D, Blanchard E, Luo M, Lorenzen BN, Banks S, Ponder MA, Welsh DA, Taylor CM. Changes in the gut microbial communities following addition of walnuts to the diet. J Nutr Biochem 2017;48:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS microbiology letters 2002;217:133–9. [DOI] [PubMed] [Google Scholar]
- 26. Duncan SH, Hold GL, Barcenilla A, Stewart CS, Flint HJ. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int J Syst Evol Microbiol 2002;52:1615–20. [DOI] [PubMed] [Google Scholar]
- 27. Duncan SH, Hold GL, Harmsen HJ, Stewart CS, Flint HJ. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol 2002;52:2141–6. [DOI] [PubMed] [Google Scholar]
- 28. Mandalari G, Nueno-Palop C, Bisignano G, Wickham MSJ, Narbad A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl Environ Microbiol 2008;74:4264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ridlon JM. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2005;47:241–59. [DOI] [PubMed] [Google Scholar]
- 30. Ochsenkühn T, Bayerdörffer E, Meining A, Schinkel M, Thiede C, Nüssler V, Sackmann M, Hatz R, Neubauer A, Paumgartner G. Colonic mucosal proliferation is related to serum deoxycholic acid levels. Cancer 1999;85:1664–9. [PubMed] [Google Scholar]
- 31. Parkin DM, Muir CS. Cancer incidence in five continents. IARC Sci Publ 1992;120:45–173. [PubMed] [Google Scholar]
- 32. Berg A. Nutrition, development and population growth. 29th ed Popul Bull; 1973;29:3–37. [PubMed] [Google Scholar]
- 33. Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013;17:225–35. [DOI] [PubMed] [Google Scholar]
- 34. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012;487:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014;12:661–72. [DOI] [PubMed] [Google Scholar]
- 36. Nakanishi M, Chen Y, Qendro V, Miyamoto S, Weinstock E, Weinstock GM, Rosenberg DW. Effects of walnut consumption on colon carcinogenesis and microbial community structure. Cancer Prev Res 2016;9:692–703. [DOI] [PubMed] [Google Scholar]
- 37. Kris-Etherton PM. Walnuts decrease risk of cardiovascular disease: a summary of efficacy and biologic mechanisms. J Nutr 2014;144(Suppl):547S–54S. [DOI] [PubMed] [Google Scholar]
- 38. Zheng CJ, Yoo J-S, Lee T-G, Cho H-Y, Kim Y-H, Kim W-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett 2005;579:5157–62. [DOI] [PubMed] [Google Scholar]
- 39. Kankaanpää PE, Salminen SJ, Isolauri E, Lee YK. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol Lett 2001;194:149–53. [DOI] [PubMed] [Google Scholar]
- 40. Yu H-N, Zhu J, Pan W, Shen S-R, Shan W-G, Das UN. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch Med Res 2014;45:195–202. [DOI] [PubMed] [Google Scholar]
- 41. Devillard E, McIntosh FM, Paillard D, Thomas NA, Shingfield KJ, Wallace RJ. Differences between human subjects in the composition of the faecal bacterial community and faecal metabolism of linoleic acid. Microbiology 2009;155:513–20. [DOI] [PubMed] [Google Scholar]
- 42. Gorissen L, Raes K, Weckx S, Dannenberger D, Leroy F, De Vuyst L, De Smet S. Production of conjugated linoleic acid and conjugated linolenic acid isomers by Bifidobacterium species. Appl Microbiol Biotechnol 2010;87:2257–66. [DOI] [PubMed] [Google Scholar]
- 43. Devillard E, McIntosh FM, Duncan SH, Wallace RJ. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J Bacteriol 2007;189:2566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toma F, Andre C. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J Agric Food Chem 2012;60:8930–40. [DOI] [PubMed] [Google Scholar]
- 45. Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 2013:1415–22. [DOI] [PubMed] [Google Scholar]
- 46. Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med 2010;31:513–39. [DOI] [PubMed] [Google Scholar]
- 47. Papoutsi Z, Kassi E, Chinou I, Halabalaki M, Skaltsounis LA, Moutsatsou P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br J Nutr 2008;99:715–22. [DOI] [PubMed] [Google Scholar]
- 48. Tulipani S, Llorach R, Jáuregui O, López-Uriarte P, Garcia-Aloy M, Bullo M, Salas-Salvadó J, Andrés-Lacueva C. Metabolomics unveils urinary changes in subjects with metabolic syndrome following 12-week nut consumption. J Proteome Res 2011;10:5047–58. [DOI] [PubMed] [Google Scholar]
- 49. Tuohy KM, Conterno L, Gasperotti M, Viola R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J Agric Food Chem 2012;60:8776–82. [DOI] [PubMed] [Google Scholar]
- 50. Ialonska DOB, Asimsetty SAGK, Chrader KEKS, Erreira DAF, Bialonska D, Kasimsetty SG, Schrader KK, Ferreira D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J Agric Food Chem 2009;57:8344–9. [DOI] [PubMed] [Google Scholar]
- 51. Wang F, Yu T, Huang G, Cai D, Liang X, Su H, Zhu Z, Li D, Yang Y, Shen P, et al. Gut microbiota community and its assembly associated with age and diet in Chinese centenarians. J Microbiol Biotechnol 2015;25:1195–204. [DOI] [PubMed] [Google Scholar]
- 52. Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, et al. Gut microbiota and extreme longevity. Curr Biol 2016;26:1480–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


