Abstract
The clinical expert consensus statement on takotsubo syndrome (TTS) part II focuses on the diagnostic workup, outcome, and management. The recommendations are based on interpretation of the limited clinical trial data currently available and experience of international TTS experts. It summarizes the diagnostic approach, which may facilitate correct and timely diagnosis. Furthermore, the document covers areas where controversies still exist in risk stratification and management of TTS. Based on available data the document provides recommendations on optimal care of such patients for practising physicians.
Keywords: Takotsubo syndrome, Broken heart syndrome, Acute heart failure, Consensus statement, Diagnostic algorithm
Outline
Diagnostic workup 2048
Electrocardiogram 2048
ST-segment elevation 2049
T-wave inversion and QT interval prolongation 2049
Other electrocardiographic findings 2050
InterTAK Diagnostic Score 2050
Biomarkers 2051
Markers of myocardial necrosis 2051
B-type natriuretic peptide and N-terminal prohormone of brain natriuretic peptide 2051
Other potential biomarkers 2051
Imaging 2051
Coronary angiography and ventriculography 2051
Echocardiography 2051
Cardiac computed tomography angiography 2052
Cardiac magnetic resonance imaging 2053
Cardiac nuclear imaging 2053
Perfusion imaging 2053
Metabolic imaging 2053
Sympathetic nervous imaging 2053
Complications and outcomes 2053
Arrhythmias 2055
Ventricular arrhythmias 2055
Other cardiac arrhythmias 2055
Recurrence 2055
Therapeutic management 2056
Pre-hospital treatment 2056
Acute treatment 2056
Long-term treatment 2057
Future directions 2058
Key questions 2058
Prospective approaches 2058
References 2058
Diagnostic workup
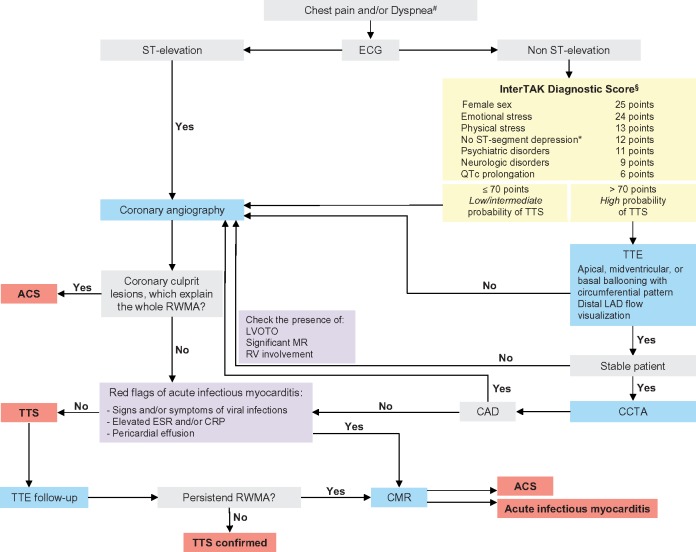
A diagnostic algorithm for takotsubo syndrome (TTS) is proposed by the expert committee (Figure 1). Patients presenting with ST-segment elevation should undergo urgent coronary angiography (CAG) with left ventriculography to exclude acute myocardial infarction (AMI). In patients with non ST-segment elevation the InterTAK Diagnostic Score can be considered. While an InterTAK Score ≤70 points suggests a low to intermediate probability of TTS, a score ≥70 indicates a high probability for the presence of TTS. Patients with a low probability should undergo CAG with left ventriculography, while in patients with a high score transthoracic echocardiography (TTE) should be considered. In the absence of a circumferential ballooning pattern CAG is recommended. In stable patients with circumferential ballooning pattern coronary computed tomography angiography (CCTA) is favoured to exclude coronary artery disease (CAD). In unstable patients, typical complications of TTS such as left ventricular outflow tract obstruction (LVOTO) should be determined with TTE and CAG to safely rule out AMI. In patients with normal coronaries on CCTA or CAG and typical ballooning patterns without ‘red flags’ of acute infectious myocarditis TTS is the most likely diagnosis and can be confirmed after follow-up echocardiography. In case of positive ‘red flags’ of acute infectious myocarditis cardiac magnetic resonance (CMR) should be performed to confirm the diagnosis.
Figure 1.
Diagnostic algorithm of takotsubo syndrome. #Applied to patients who are seeking medical emergency departments with e.g. chest pain and/or dyspnoea. §The InterTAK Diagnostic Score did not include patients with pheochromocytoma induced takotsubo syndrome in which atypical pattern are more frequently noted. *Except in lead aVR. ACS, acute coronary syndrome; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance; CRP, c-reactive protein; ECG, electrocardiogram; ESR, erythrocyte sedimentation rate; InterTAK, International Takotsubo Registry; LAD, left anterior descending coronary artery; LVOTO, left ventricular outflow tract obstruction; MR, mitral regurgitation; QTc, QT-time corrected for heart rate; RV, right ventricle; RWMA, regional wall motion abnormality; TTE, transthoracic echocardiography; TTS, takotsubo syndrome.
Electrocardiogram
The initial electrocardiogram (ECG) is abnormal in most patients with TTS usually demonstrating ischaemic ST-segment elevation, T-wave inversion, or both.1–4 In the InterTAK Registry, ST-segment elevation was present in 44%, ST-segment depression in 8%, T-wave inversion in 41%, and left bundle branch block in 5%.2 As in acute coronary syndrome (ACS), the ECG in TTS demonstrates temporal evolution typically with resolution of initial ST-segment elevation (if present), followed by progressive T-wave inversion and QT interval prolongation over several days, with subsequent gradual resolution of T-wave inversion and QT interval prolongation over days to weeks.5–8 The initial and subsequent ECG findings are influenced by several variables, including the geographic pattern of left ventricular (LV) ballooning, presence or absence of right ventricular (RV) ballooning, time from symptom onset to presentation, presence of myocardial oedema, and recovery rate of myocardial cellular function.
ST-segment elevation
As with ST-segment elevation myocardial infarction (STEMI), the location and extent of ST-segment elevation in TTS corresponds to the anatomic location of myocardial injury, most often the mid and apical LV segments.2 Consequently, ST-segment elevation usually involves precordial, lateral, and apical ECG leads, closely resembling that of anterior STEMI due to left anterior descending coronary occlusion.9,10 Lead -aVR (inverse of aVR) representing +30° in the frontal plane is generally aligned with the LV apex, and can be assembled with other leads to create an ‘ECG map’, useful in comparing the ST-segment elevation pattern of TTS with that of anterior STEMI.3,9,11,12 ST-segment elevation in TTS is centred on precordial leads V2–V5 and limb leads II and aVR, whereas in anterior STEMI the ST-segment elevation centres on precordial leads V1–V4 and limb leads I and aVL. Several ECG criteria with high sensitivity and specificity have been proposed to reliably distinguish TTS from anterior STEMI.3,12–14 Most focus on ST-segment elevation in the precordial leads, particularly lead V1, as ST-segment elevation in this lead is less pronounced in TTS than in anterior STEMI.9,10,12 ST-segment elevation limited to the inferior leads (II, III, aVF) is distinctly uncommon in TTS. Despite these differences, overlap exists and an urgent coronary angiogram is necessary to differentiate TTS from STEMI with certainty.9–11
T-wave inversion and QT interval prolongation
Progressive T-wave inversion and QT interval prolongation is a common ECG finding in TTS. In patients with delayed presentation, these changes may be present on admission in the absence of ST-segment elevation, and can be the only detectable ECG changes and therefore important for the diagnosis. The geographic distribution of T-wave inversion closely parallels that of ST-segment elevation and may be an electrophysiological manifestation of myocardial stunning. In TTS, T-wave inversion is often more prominent and more broadly distributed than in ACS. Furthermore, T-wave inversion is associated with presence of myocardial oedema, and may persist for several months even after LV contractile recovery, thus leaving an electrophysiological footprint of the TTS event.5,15–19 QT interval prolongation provides a substrate for torsades de pointes ventricular tachycardia and may be a prognostic marker for sudden cardiac death16,17
Other electrocardiogram findings
Anterior Q-waves (or poor R-wave progression) without accompanying ST-segment elevation or T-wave inversion, a pattern sometimes referred to as ‘anterior infarction, age indeterminate’ occurs with some frequency in TTS. Pathologic Q-waves are less frequently encountered in TTS than anterior STEMI (15% vs. 69%).14 In TTS, as in anterior STEMI, Q-waves may occur in the acute phase, and regress rapidly with R-wave re-appearance, consistent with electrical stunning.20,21 Both J-wave and/or fragmented QRS complexes have been reported acutely, the former associated with death from cardiac causes and/or ventricular tachyarrhythmia.22 Low QRS voltage likely representing myocardial oedema is prevalent in TTS.23 Left bundle branch block is present in around 5% of patients.2 ST-segment depression is uncommon, occurring in fewer than 10% of TTS patients but in over 30% of ACS patients,2 therefore, the presence of ST-segment depression may suggest ACS.
InterTAK Diagnostic Score
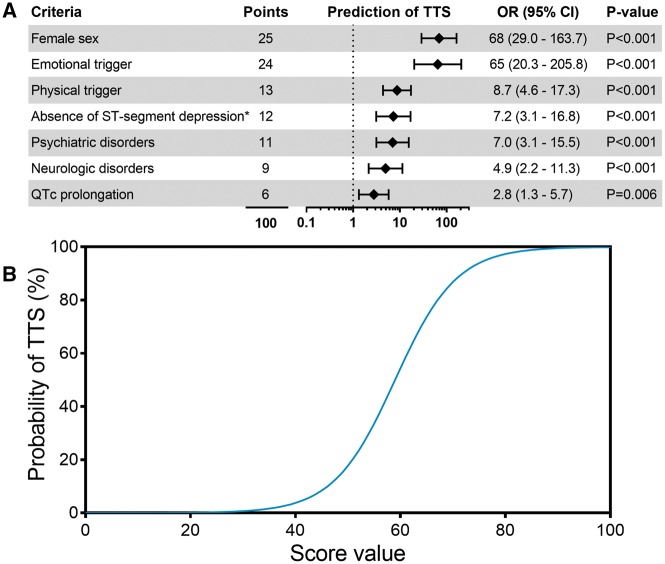
The InterTAK Diagnostic Score was developed by the International Takotsubo Registry to provide clinicians a model to assess the likelihood of TTS diagnosis. The criteria that make up the InterTAK Diagnostic Score are based on clinical features and ECG to predict the probability of the presence of TTS and to distinguish TTS from ACS (Figure 1, Figure 2A).24 The InterTAK Diagnostic Score comprises seven parameters [female sex, emotional trigger, physical trigger, absence of ST-segment depression (except in lead aVR), psychiatric disorders, neurologic disorders, and QT prolongation] ranked by their diagnostic importance with a maximum attainable score of 100 points (Figure 2A).24 All parameters can be easily obtained in the emergency department and do not require an imaging modality.24
Figure 2.
InterTAK Diagnostic Score. Predictors for diagnosing takotsubo syndrome by multiple logistic regression analysis. Odds ratios of the parameters female sex, emotional trigger, physical trigger, absence of ST-segment depression, psychiatric disorders, neurologic disorders, and QTc prolongation, which were chosen to build the InterTAK Diagnostic Score. *Except in lead aVR (A). Sigmoid curve shows the estimated prevalence of takotsubo syndrome in clinical practice (B). Modified and reprinted with permission from Ghadri et al.24. CI, confidence interval; OR, odds ratio; QTc, QT-time corrected for heart rate; TTS, takotsubo syndrome.
Depending on the disease prevalence this means that patients with 30 score points have a predicted probability of <1%, while patients with 50 points have a probability of 18%, and patients with a score value >70 points have a probability of ∼90% of suffering from TTS (Figure 2B).24
Biomarkers
Markers of myocardial necrosis
Virtually all cases of TTS exhibit evidence of myocardial necrosis. On admission, troponin values are usually equally elevated compared to ACS, however, peak values are substantially lower compared to the classical ACS.2 High admission troponin levels are a predictor for a worse in-hospital outcome.2 Typically, there is only a slight increase in creatine kinase.2 The extent of LV regional wall motion impairment generally greatly exceeds that of associated myocardial necrosis biomarkers, likely reflecting a large mass of reversibly injured (stunned) myocardium.
B-type natriuretic peptide and N-terminal prohormone of brain natriuretic peptide
Takotsubo syndrome is frequently associated with a substantial increase in the plasma levels of B-type natriuretic peptide (BNP) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) reaching its peak approximately 24–48 h after symptom onset25,26 as a reflection of regional LV dysfunction. A gradual return of BNP/NT-proBNP towards normal levels occurs within the next few months after presentation.27
The degree of NT-proBNP elevation appears directly related to: (i) the degree of sympathetic overactivation (as reflected by normetanephrine concentrations), (ii) peak C-reactive protein concentrations (suggesting that BNP release might be at least in part of inflammatory origin), and (iii) systolic LV dysfunction [as measured by wall motion score index (WMSI)].25 Peak NT-proBNP levels also vary with the extent of LV oedema as measured by CMR.28
Other potential biomarkers
Interleukin (IL)-6 levels appear less elevated while those of IL-7 are more elevated in TTS compared with AMI.29 However, differences between groups were small and unlikely to be of diagnostic utility.
Two recently published studies focused on the potential utility of the release and circulation of certain microRNAs (miRNAs) in association with TTS onset.30,31 Kuwabara et al.30 noted that elevation of circulating miR-133a appeared to represent an early consequence of myocardial injury, including TTS and AMI. However, subsequent analyses of cases of TTS (N = 36) and evolving STEMI (N = 27) suggested that the elevation of miR-133a was more marked in STEMI than in TTS. Furthermore, Jaguszewski et al. demonstrated that a unique signature including miR-1, miR-16, miR-26a, and miR-133a represents a robust biomarker on admission and can be used to differentiate TTS from STEMI patients.31 Furthermore, the up-regulation of miR-16 and miR-26a is known to be associated with stress- and affective disorders.32–34
Especially in patients with biventricular involvement, it has been shown that plasma concentrations of the stress-responsive cytokine growth differentiation factor-15 increased more rapidly after the onset of TTS.35
Imaging
Coronary angiography and ventriculography
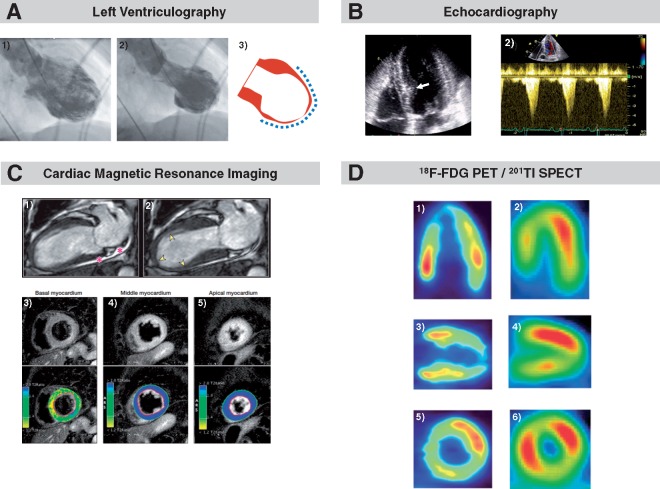
Although non-invasive imaging modalities are useful in the workup of patients with TTS, final differential diagnosis from ACS requires coronary angiogram, which is performed in the context of ST-elevation in primary percutaneous coronary intervention service. In case of suspected TTS with coexisting and significant CAD, careful comparison of CAG and biplane ventriculography in similar views is mandatory to search for a perfusion-contraction mismatch.36,37 This comparison is essential for distinguishing TTS from classical AMI in patients with wall motion abnormalities and obstructive CAD. In this regard, it has been reported that approximately one-third of patients with the classical apical ballooning show a small zone with preserved contractility in the most distal portion of the apex, which is described as the ‘apical nipple sign’.38 Furthermore, as LVOTO occurs in approximately 20% of patients with TTS,39 haemodynamic assessment for the presence of a pressure-gradient in the outflow tract as well as assessment of left ventricular end-diastolic pressure are recommended. Figure 3A demonstrates apical ballooning pattern on left ventriculography.
Figure 3.
Apical ballooning illustrated by different imaging modalities. Typical takotsubo type with apical ballooning pattern during diastole (A.1) and systole (A.2) on left ventriculography. Dashed lines indicate extent of wall motion abnormality (A.3). Modified and reprinted with permission from Templin et al.2 Apical four-chamber view obtained by echocardiography showing apical ballooning and left ventricular cavity with bulging of the basal interventricular septum (white arrow) (B.1). B.2 reveals left ventricular outflow tract obstruction by pulsed-wave Doppler interrogation. Modified and reprinted with permission from Merli et al.100 Apical ballooning as illustrated by cardiac magnetic resonance imaging. The asterisks indicate pericardial effusion (C.1) and yellow arrows (C.2) shows the region of akinesia. T2-weighted images on short-axis view demonstrates normal signal intensity of the basal myocardium (C.3) and global oedema of the mid and apical myocardium (C.4 and C.5). Modified and reprinted with permission from Eitel et al.82 Metabolic imaging with positron emission tomography and 18F-flurodeoxyglucose (D.1, D.3, D.5) demonstrates decreased uptake in the apex and midventricular segments. Perfusion imaging using single photon emission computed tomography with 201thallium chloride (D.2, D.4, D.6) shows a smaller perfusion defect in the apex and midventricular segments. Reprinted with permission from Yoshida et al.98
Echocardiography
Echocardiography is the most used imaging tool to assess changes in LV function such as symmetric regional wall motion abnormalities (RWMAs).4 Different variants can be identified with echocardiography which include:
Apical ballooning, hypo-, a-, or dyskinesia of mid-apical myocardial segments is typical, sometimes associated with hypokinetic mid-segments.2,40 The anterior or entire interventricular septum, inferior or midventricular anterolateral wall may also be involved.41,42 LV twisting on 2D speckle-tracking imaging is reduced or reversed to clockwise apical rotation and the rate of untwisting (a sensitive index of regional diastolic dysfunction) is reduced in the acute phase.43
Midventricular TTS featured by hypo-, a-, or dyskinesia of midventricular segments, most often resembling a cuff.2,40,44,45
Basal forms where only basal segments are involved2,40: This phenotype is rare and appears commonly in patients with subarachnoid haemorrhage,46 epinephrine-induced TTS47 or phaeochromocytoma.48
Focal TTS mostly involving an anterolateral segment has been described.2,40 Differentiating this unusual TTS type from ACS or myocarditis requires CMR.49
Right ventricular involvement is characterized by RV dilatation with hypo- to akinesia of the free wall and apex in its isolated form.50,51
In TTS, LV wall motion abnormalities extend beyond the distribution of a single coronary artery territory, therefore systolic dysfunction appears ‘circular’ at speckle-tracking echocardiography.52 A WMSI ≥1.75 with more than four dysfunctional segments identifies TTS with 83% sensitivity and 100% specificity.53 Doppler estimation of coronary artery flow ameliorates the diagnostic accuracy of wall motion abnormalities,54 whereas adenosine may lead to dramatic improvements of global and regional LV function.41
Intravenous ultrasound contrast agents facilitate wall motion assessment especially at the apex52 and constitute a useful method especially in patients in whom CAG is not performed, mainly due to active bleeding or other comorbid conditions that may imbalance the risk-benefit ratio of CAG (see Cardiac computed tomography angiography section). Myocardial opacification is reduced within dysfunctional segments with the transmural perfusion defects being more evident early after TTS onset.55 Coronary flow reserve, assessed by transthoracic Doppler echocardiography, is reduced to 1.6–2.6 at the levels of the right and left coronary arteries56,57 and correlates with indices of LV systolic but not diastolic function.58 In contrast to ischaemic cardiomyopathy, myocardial contraction does not improve with low-dose dobutamine at the early stages.59,60 However, it has also been demonstrated that low dose dobutamine stress echocardiography improved systolic left ventricular function by normalizing or improving the hypokinetic segments.61 Moreover, in STEMI, viable myocardial segments exhibit longitudinal shortening, while in TTS systolic lengthening (passive motion) is present initially and resolves at follow-up.62
Importantly, echocardiography allows detection of all acute TTS complications. In LV apical ballooning, basal segments are hyperkinetic and may cause dynamic LVOTO,63 mainly in patients with pre-existing septal bulge64 which further reduces stroke volume and is associated with mitral regurgitation (MR) due to systolic anterior motion of the mitral leaflet (Figure 3B).63,65 Severe MR may also result from leaflet tethering by displacement or dysfunction of papillary muscles. Mitral regurgitation is estimated to be present in 14–25% of TTS patients.66
Advanced echocardiographic techniques such as speckle-tracking imaging which reveals a paradoxical (dyskinetic) positive longitudinal systolic strain of biventricular mid-apical segments.67 Echocardiography also identifies covered rupture of the LV free wall68 as well as thrombus formation within a dysfunctional LV apex69 or within left-atrial appendage even in the absence of atrial fibrillation.70 Independent predictors of adverse outcomes include low left ventricular ejection fraction (LVEF), increased LV filling pressure, and moderate-to-severe MR at 4–6 weeks.71,72
At peak, apical and anteroseptal akinesis with basal hyper-contractility produces near-cavity obliteration.73
Typically, LV contractility recovers completely in 4–8 weeks.2,74 Some segments of the LV recover earlier than others, displaying increased apical rotation, LV twisting and untwisting and recovered global longitudinal strain.43,75 Resolution of LVOTO and MR76 occurs in parallel with myocardial functional recovery. During TTS recurrence, the LV ballooning pattern may resemble the initial event77 or alternatively, manifest as other variants.78
Cardiac computed tomography angiography
In the presence of life-threatening comorbid conditions such as terminal malignancy, intracranial bleeding, advanced age with frailty and bleeding diathesis, invasive CAG may pose a considerable risk for complications. In a recent study by Murugiah et al.,79 a substantial proportion of patients with TTS as a secondary diagnosis code did not undergo CAG. The reasons for not performing CAG were not described but likely included the mentioned life-threatening comorbid conditions. In such patients, non-invasive CCTA may be an appropriate alternative to CAG. Coronary computed tomography angiography provides information on both coronary artery anatomy and regional LV contraction. Assessment of LV contraction by CCTA requires image acquisition throughout the cardiac cycle and thus higher radiation exposure. In patients with suspected recurrent TTS and a previous CAG, CCTA may be a diagnostic alternative. Furthermore, CCTA may be considered instead of CAG in the following circumstances: in stable patients with low suspicion of ACS, suspected recurrent TTS, and patients with elevation of cardiac biomarkers or ECG changes in association with acute critical illnesses such as sepsis, intracranial disease (e.g. subarachnoid haemorrhage, ischaemic stroke), and other critical conditions known to be complicated by TTS.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging cannot be used easily in the acute setting of TTS, but is very useful in the subacute phase. In addition to identification of typical RWMAs, CMR allows precise quantification of RV and LV function, assessment of additional abnormalities/complications (i.e. pericardial and/or pleural effusion, LV and RV thrombi), and characterization of myocardial tissue (i.e. oedema, inflammation, necrosis/fibrosis) (Figure 3C).80,81 Recently, specific CMR criteria for TTS diagnosis at the time of acute presentation were established which include the combination of typical RWMAs, oedema, and the absence of evidence of irreversible tissue injury [late gadolinium enhancement (LGE)].82 While LGE is usually absent and predicts complete normalization of LV function, subtle fibrosis may be present and a sign of worse outcomes.83–85 In most TTS patients, myocardial oedema is present in regions with abnormal systolic function possibly due to inflammation, increased wall stress and/or transient ischemia82 and indicative of the extent and severity of tissue injury.86 CMR is superior to echocardiography for detection of RV involvement82 including isolated RV TTS87 which may negatively impact outcome.88 Importantly, absence of LGE in dysfunctional LV regions allows distinction between TTS and other conditions including ACS (subendocardial or transmural LGE corresponding to a vascular territory) and many cases of acute myocarditis (frequent, but not universal presence of epicardial or ‘patchy’ LGE). Therefore, CMR provides incremental value for the differential diagnosis, and therapeutic decision-making in patients with suspected TTS.89
Cardiac nuclear imaging
Both single photon emission computed tomography (SPECT, using 201thallium chloride or 99mtechnetium sestamibi) which provides semi-quantitative information and position emission tomography (PET, using 13N-ammonia 82Rubidium) which offers quantitative measurements, have been used in TTS for assessment of perfusion, metabolism, and innervation.
Perfusion imaging
Mild reduction of perfusion in dysfunctional segments using myocardial perfusion scintigraphy has been noted in some studies, while others reported normal perfusion.90–94 However, ‘myocardial thinning’ in involved segments during the acute phase of TTS may lead to a reduction in isotope counts because of the partial volume effect, which may mimic reduction of perfusion on SPECT, but following correction for this factor on PET, blood flow in the thinned regions (typically in the apex) is indeed maintained, while the normally functioning (basal) segments show hyper-perfusion.95
Metabolic imaging
The role of metabolic imaging in the clinical setting has not been determined and it has been performed mainly for research purpose of investigating the pathophysiology of TTS, although it can provide additional information about the diseased myocardium. Both SPECT using 123I-β-methyl-iodophenyl pentadecanoic acid (which reflects fatty-acid) and PET using 18F-flourodeoxyglucose (which reflect glucose utilization) often show reduced metabolic activity in the impaired regions, while myocardial perfusion is often (near) normal.96,97 An example with apical TTS is shown in Figure 3D, revealing severely reduced 18F-flourodeoxyglucose uptake in the apex despite only slightly reduced perfusion as assessed with 201thallium chloride.98 Possibly, glucose utilization is disturbed due to insulin resistance as a consequence of high levels of circulating catecholamines.99
Sympathetic nervous imaging
Myocardial uptake of 123I-metaiodobenzylguanidine (123I-MIBG, imaged with SPECT) reflects myocardial sympathetic innervation 123I-MIBG is reduced for months in dysfunctional segments whereas perfusion is almost normal, consistent with regional disturbance of sympathetic neuronal activity.101 In the subacute phase of TTS, 123I-MIBG SPECT can be combined with SPECT perfusion imaging to exclude ACS where both perfusion and innervation are reduced. Position emission tomography imaging has also been used in TTS for assessment of cardiac innervation with the use 11C hydroxyephedrine.102
Complications and outcomes
Although TTS is generally considered a benign disease, contemporary observations show that rates of cardiogenic shock and death are comparable to ACS patients treated according to current guidelines.2,103–108 While TTS is a reversible condition, hemodynamic and electrical instability during the acute phase expose patients to the risk of serious adverse in-hospital events which occur in approximately one-fifth of TTS patients (Figure 4).2 This substantial incidence of life-threatening complications necessitates close monitoring and early intervention in unstable TTS patients with risk stratification at diagnosis allowing triage to appropriate care.66 Parameters predicting adverse in-hospital outcome include: physical trigger, acute neurologic or psychiatric diseases, initial troponin >10× upper reference limit, and admission LVEF <45%.2 Furthermore, male patients have an up to three-fold increased rate of death and major adverse cardiac and cerebrovascular events (MACCE)109 and more often had an underlying critical illness, further contributing to the higher mortality.2 Sobue et al.110 demonstrated that physical triggers and male gender are independent risk factors of in-hospital mortality in TTS. Data from the Tokyo Coronary Care Unit Network revealed that high values of BNP and white blood cell counts were also linked to higher rates of in-hospital complications.111 Complications included cardiac death, pump failure (Killip grade ΙΙ), sustained ventricular tachycardia or ventricular fibrillation (VT/VF), and advanced atrioventricular block (AV-block). In the study by Takashio et al.112 the magnitude and extent of ST-segment elevation with ECG were found to be independent predictors of in-hospital adverse events. However, those findings were not confirmed by others. Common in-hospital complications include cardiac arrhythmias,113 LVOTO,64 cardiogenic shock,2 ventricular thrombus,114 pulmonary oedema,115 ventricular septal defect,116 and free wall rupture.117 In addition, to the demographic parameter of age 75, echocardiographic parameters that predict adverse in-hospital outcome (acute heart failure, cardiogenic shock, and in-hospital mortality) include LVEF, E/e’ ratio, and reversible moderate to severe MR. However, only reversible moderate to severe MR was an independent predictor when considering cardiogenic shock and death as the composite outcome in this study, in addition to heart rate. Moreover, it has been demonstrated that high heart rate and low systolic blood pressure are associated with increased mortality in TTS.118 Along with the Charlson comorbidity index and systolic pulmonary artery pressure, RV involvement is an independent predictor of acute heart failure and of a composite endpoint including adverse events, such as acute heart failure, cardiogenic shock, and in-hospital mortality.119
Figure 4.
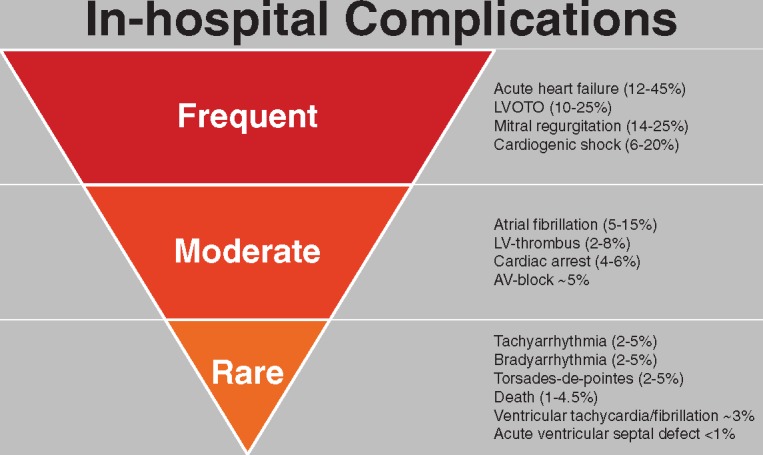
Overview of in-hospital complications according to their prevalence. AV, atrioventricular block; LV, left ventricle; LVOTO, left ventricular outflow tract obstruction.
Data on long-term survival are scarce. In 2007, Elesber et al.120 reported that long-term mortality did not differ between a TTS population and an age-, gender-, birth-, year-, and race-matched population. While Sharkey et al.121 found that all-cause mortality during follow-up exceeded a matched general population with most deaths occurring in the first year. More recently, it has been reported that long-term mortality of patients with TTS122 is similar to (Figure 5A) patients with CAD.103 TTS patient data from the Swedish Angiography and Angioplasty Registry (SCAAR) from 2009 to 2013 were compared to data from patients with and without CAD, and demonstrated that mortality rates for TTS were worse than in patients without CAD and comparable to those of patients with CAD.103 In the largest TTS registry to date, death rates are estimated to be 5.6% and rate of MACCE 9.9% per-patient year (Figure 5B),2 suggesting that TTS is not a benign disease. A recent study found that patients with the typical TTS type have a comparable outcome to patients presenting with the atypical type even after adjustment for confounders, suggesting that both patient groups should be equally monitored in the long-term.40 On the other hand, 1-year mortality differs between the two groups, as it is driven by clinical factors including atrial fibrillation, LVEF on admission <45%, and neurologic disorders, rather than by TTS type.40 In a smaller study, predictive factors of long-term mortality in TTS were male sex, Killip class III/IV, and diabetes mellitus.122 The prognostic role of diabetes mellitus is controversial, as it is postulated that it may exert a protective effect in TTS, given that the prevalence of diabetes mellitus in TTS is lower than expected for an age- and sex-matched population.123 Some studies, though limited by their population size and experimental design, suggest that patients with diabetes mellitus have a more favourable in-hospital and 1-year outcome.124,125
Figure 5.
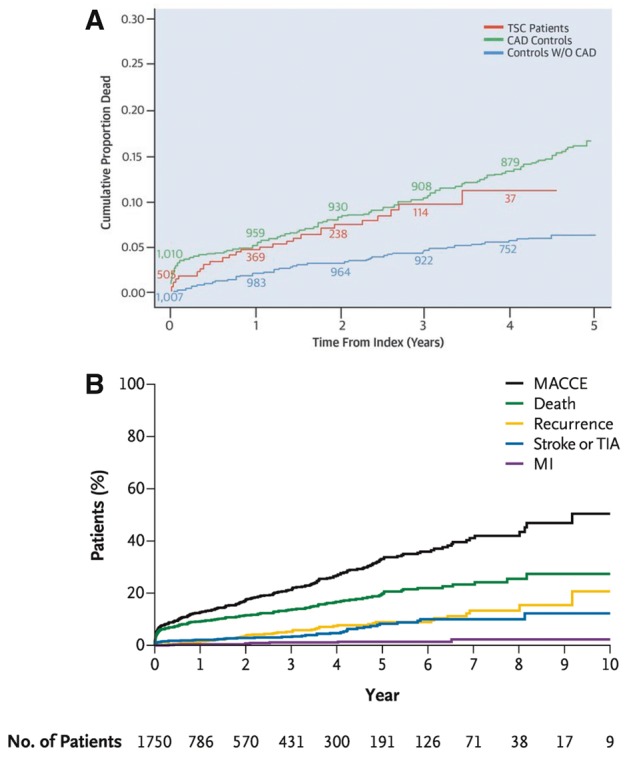
Long-term outcome (5-years) of patients with TTS compared to patients with and without CAD (A). Long-term outcome (10-years) of patients with TTS (B). MACCE refers to a composite of death from any cause, recurrence of takotsubo syndrome, stroke or transient ischaemic attack, or myocardial infarction. CAD, coronary artery disease; MACCE, major adverse cardiac and cerebrovascular event; MI, myocardial infarction; TIA, transient ischaemic attack; TSC, takotsubo stress cardiomyopathy. Reprinted with permission from Tornvall et al.103 and Templin et al.2
Arrhythmias
Cardiac arrhythmias are important determinants of short-term clinical outcome.
Ventricular arrhythmias
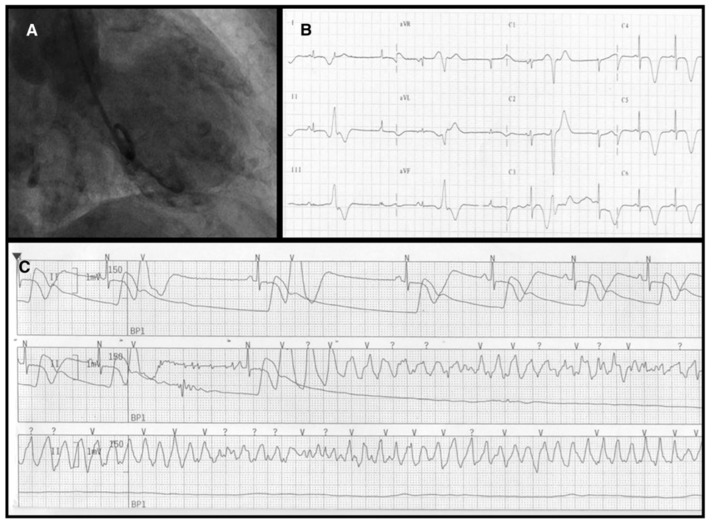
Life-threatening ventricular arrhythmias, such as torsades de pointes, VT, or VF occur in 3.0–8.6% and are a frequent cause of death.2,126–128 Life-threatening ventricular arrhythmias occur most often in the subacute phase (i.e. hospital days 2–4) and coincide with anterolateral T-wave inversion and QT-interval prolongation.129 QTc prolongation at admission occurs in up to half of the patients.2 Most malignant arrhythmic episodes are associated with a QTc >500 ms, with pause-dependent torsades de pointes degenerating into VF.127,128,130 . Figure 6 shows a patient with apical ballooning (A). On the third day of hospitalization giant negative T-waves, marked QT prolongation, and ‘R on T’ premature ventricular beats were noted on ECG (B). Furthermore, pause-dependent (‘long-short sequence’) torsade-de-pointes/ventricular fibrillation requiring electrical cardioversion were recorded by telemetry (C).128 Accordingly, TTS should be regarded as a type of acquired long QT syndrome with risk for malignant arrhythmic events.128,130
Figure 6.
Arrhythmic complication in takotsubo syndrome. Left ventriculography (antero-posterior view) showing the typical apical ballooning pattern with akinesia of the mid-apical segments and hyperkinesia of the basal segment (A). A 12-lead electrocardiogram recorded at the third day of hospitalization showing giant negative T-waves in leads aVL, L1, L2, aVF and V4–V6, marked QT prolongation (QTc = 552 ms) and ‘R on T’ premature ventricular beats (B). Telemetry recording of a pause-dependent (‘long-short sequence’) torsade-de-pointes/ventricular fibrillation, which required electrical cardioversion (C). Reprinted with permission from Migliore et al.130
Cardiac magnetic resonance findings reveal an association between transient myocardial oedema, as evidenced by T2-weighted sequences, and dynamic T-wave inversion and QT prolongation.131,132 Thus, myocardial oedema may contribute to transmural or regional (i.e. from the apex to the base of the LV) repolarization inhomogeneity. QT prolongation thus reflects the delayed and dispersed ventricular repolarisation that predisposes to local re-excitation and eventually to torsade de pointes or VF.131–133
Rarely, cardiac arrest is the initial presentation of TTS unrelated to QT interval prolongation. The mechanism of these potentially lethal arrhythmias is probably distinct from that encountered during the subacute phase where acute catecholamine toxicity and/or myocardial ischaemia play a primary role. In some cases, TTS may not represent the trigger for tachyarrhythmias, but rather the consequence of the stress of cardiac arrest and/or resuscitation, which may include administration of epinephrine.134
Other cardiac arrhythmias
New-onset paroxysmal or persistent atrial fibrillation occurs in 4.7%, sinus-node dysfunction in 1.3%, and AV-block in 2.9%, most likely due to neuro-autonomic imbalance, catecholamine stress, and increased vagal tone.127,130,134
Recurrence
Patients who survive the initial event have a second event in approximately 5% of cases, mostly occurring 3 weeks to 3.8 years after the first event.135 Recurrent TTS afflicts men and women and may occur at any age including in childhood.78,136,137 Both the triggering event and the ballooning pattern may differ during recurrent events.78 Some have postulated that an index TTS event may protect the affected LV regions from recurrent involvement through a mechanism akin to ischaemic ‘pre-conditioning’.138 However, detailed review of published cases and clinical experience suggest that there are frequent examples of recurrence in which the ballooning pattern is similar between episodes, thereby making this hypothesis unlikely.
Therapeutic management
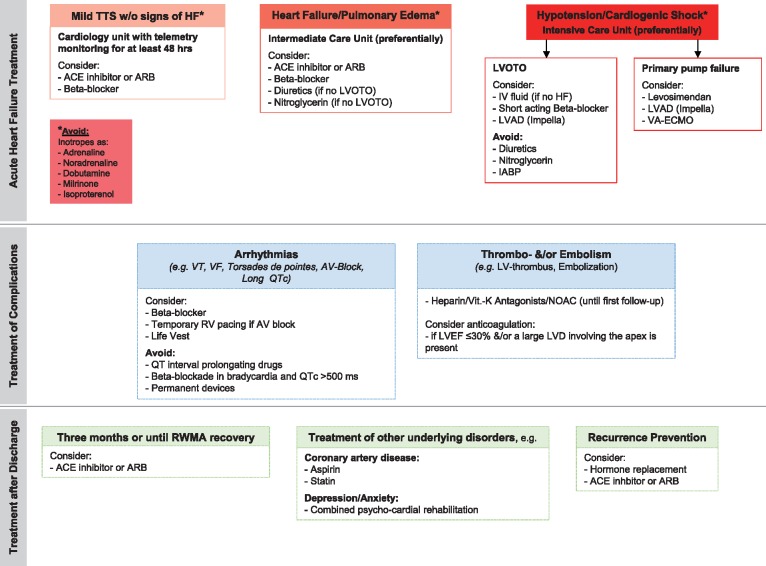
Guidelines regarding TTS management are lacking as no prospective randomized clinical trials have been performed in this patient population. Therapeutic strategies are therefore based on clinical experience and expert consensus (evidence level C). Table 1 reviews current data on medical management of TTS patients139 based of retrospective analysis,2,140,141 meta-analysis,142,144 and case series.143Figure 7 summarizes a proposed therapeutic management approach for patients with TTS.
Table 1.
Overview of retrospective analyses, meta-analyses, and case series of medical management for takotsubo syndromea
| Authors | Study design | No. of patients | Outcome measures | Follow-up time | Medication | Effect |
|---|---|---|---|---|---|---|
| Santoro et al.143 | Case series | 13 | Adverse events | During hospitalization | Levosimendan | Probably beneficial |
| Isogai et al.140 | Retrospective | 2110 | Mortality | 30 days | β-Blockers | Not beneficial |
| Dias et al.141 | Retrospective | 206 | MACE | During hospitalization | Antiplatelet | Beneficial |
| β-Blockers | Not beneficial | |||||
| Statins | Not beneficial | |||||
| ACEI | Not beneficial | |||||
| Templin et al.2 | Retrospective | 1118 | Mortality | 1 year | β-Blockers | Not beneficial |
| ACEI/ARB | Beneficial | |||||
| Santoro et al.142 | Meta-analysis | 511 | Recurrence | 24–60 months | β-Blockers | Not beneficial |
| ACEI/ARB | Not beneficial | |||||
| Aspirin | Not beneficial | |||||
| Statins | Not beneficial | |||||
| Singh et al.144 | Meta-analysis | 847 | Recurrence | 19–33 months | β-Blockers | Not beneficial |
| ACEI/ARB | Beneficial |
Reprinted with permission from Kato et al.139
ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin-receptor blocker; MACE, major adverse cardiac event.
Figure 7.
Management of takotsubo syndrome. ACE, angiotensin-converting-enzyme; ARB, angiotensin-receptor blocker; AV-block, atrioventricular block; HF, heart failure; IABP, intra-aortic balloon pump; IV, intravenous; LV, left ventricle; LVAD, left ventricular assist device; LVD, left ventricular dysfunction; LVEF, left ventricular ejection fraction; LVOTO, left ventricular outflow tract obstruction; NOAC, novel oral anticoagulant; QTc, QT-time corrected for heart rate; RV, right ventricle; TTS, takotsubo syndrome; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Pre-hospital treatment
As TTS is clinically difficult to distinguish from ACS, upon first presentation patients should be transferred to a cardiology unit with imaging capabilities and a cardiac catheterization laboratory and receive guideline based treatment of ACS,105–108 in particular aspirin, heparin, and if required morphine and oxygen. Patients with cardiogenic shock or post cardiac arrest require intensive care. Electrocardiogram monitoring is essential as a prolonged QT-interval may trigger malignant ventricular arrhythmias (torsades de pointes) and AV-block may occur.
Acute treatment
Takotsubo syndrome patients with cardiogenic shock, in particular those with apical ballooning should be promptly evaluated for the presence of LVOTO, which occurs in about 20% of cases.39 This should be performed during angiography with LV pressure recording during careful retraction of the pigtail catheter from the LV apex beyond the aortic valve. Similarly, a pressure gradient can be detected and quantified using Doppler echocardiography using continuous wave Doppler.145 Particularly, when using catecholamines serial Doppler studies should be considered to detect an evolving pressure gradient. In TTS patients treated with catecholamine drugs a 20% mortality has been reported81; although this may represent a selection bias due to the initial presentation of the patients. Recently, it has been suggested that the Ca2+-sensitizer levosimendan could be used safely and effectively in TTS as an alternative inotrope to catecholamine agents.143 Furthermore, beta-blockers may improve LVOTO, but are contraindicated in acute and severe heart failure with low LVEF, hypotension, and in those with bradycardia. Although evidence is unproven, TTS patients with LVOTO may benefit from the If channel inhibitor ivabradine.146,147
As catecholamine levels are elevated in TTS, beta-blockers seem to be reasonable until full recovery of LVEF, but trials supporting this hypothesis are lacking. Animal experiments have shown that apical ballooning is attenuated after administration of drugs with both alpha- and beta-adrenoceptor blocking properties.148 In an animal model, intravenous metoprolol improved epinephrine-induced apical ballooning.149 However, due to the potential risk of pause-dependent torsades de pointes, beta-blockers should be used cautiously, especially in patients with bradycardia and QTc >500 ms. Angiotensin-converting-enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) may potentially facilitate LV recovery. Diuretics are indicated in patients with pulmonary oedema. In addition, nitroglycerin is useful to reduce LV and RV filling pressures and afterload in the case of acute heart failure; however, the administration of nitroglycerin in the presence of LVOTO has been found to worsen the pressure gradient and therefore should be avoided in this scenario (see Figure 7).
QT-interval prolonging drugs should be used cautiously in the acute phase because of the risk to induce torsades de pointes or ventricular tachycardia and fibrillation.
Severe LV dysfunction with extended apical ballooning entails the risk of an LV thrombus and subsequent systemic embolism. Although evidence is lacking, anticoagulation with intravenous/subcutaneous heparin would appear to be appropriate in such patients and post-discharge oral anticoagulation or antiplatelet therapy may be considered on an individual, per-patient basis. As LV dysfunction and ECG abnormalities are reversible, an implantable cardioverter-defibrillator for primary or secondary prevention is of uncertain value in TTS patients experiencing malignant ventricular arrhythmias.130,150 In case of excessive prolongation of the QT interval or life-threatening ventricular arrhythmias a wearable defibrillator (life vest) could be considered.151 The residual risk of malignant arrhythmic events after recovery from TTS is unknown. A temporary transvenous pacemaker is appropriate for those with haemodynamically significant bradycardia.
Long-term treatment
The use of ACEi or ARB was associated with improved survival at 1-year follow-up even after propensity matching.2 In contrast, there was no evidence of any survival benefit for the use of beta-blockers.2 Moreover, one-third of patients experienced a TTS recurrence during beta-blockade2 suggesting that other receptors such as alpha-receptors, that are more prevalent in the coronary microcirculation, might be involved.
The prevalence of recurrent TTS is relatively low, consequently conducting randomised trials of pharmacological agents to prevent recurrence is challenging. Beta-blocker therapy after hospital discharge does not appear to prevent recurrence,2,144 whereas ACEi or ARB are associated with a lower prevalence of recurrence. The significance of this observation remains uncertain and requires validation in other cohorts.
If concomitant coronary atherosclerosis is present, aspirin and statins are appropriate. As TTS mainly occurs in postmenopausal women oestrogen supplementation in those with recurrence is questionable. In an animal model oestrogen supplementation partially attenuated TTS,152 and chronic oestrogen supplementation after oophorectomy improved the condition.153
Psychiatric disorders (e.g. depression, anxiety) are common in TTS patients2,154 and those might benefit from a combined psycho-cardiologic rehabilitation.155 Whether anti-depressants or other psychiatric drugs might provide clinical benefit in such patients is controversial.
Future directions
Over recent years research has shown that TTS is a much more heterogeneous condition than previously thought. Originally believed to be a benign disease, studies have shown that TTS has morbidity and mortality rates that are comparable to those of ACS.2,103 TTS can affect many others aside from postmenopausal women with an emotional trigger,2,135 as originally described, and can present as an atypical type rather than apical ballooning.40 Still, there is much more to be uncovered surrounding TTS and the underlying pathophysiology of the syndrome.156
Key questions
Many questions need further investigation: Why are women affected predominantly? What is the role of triggering factors in stress responses of the heart? Why do different TTS phenotypes exist? Which patients are vulnerable to TTS or prone to recurrence? Is there a genetic predisposition to TTS? What is the exact pathogenesis of TTS? Are there specific treatment options in the acute stage of this life-threatening syndrome or to prevent recurrence? Additional research needs to be conducted to answer these important questions.
Prospective approaches
The link between the brain and heart seems to play a key role in TTS. Additionally, studies on circulating miRNAs suggest there could be a genetic aspect to the pathophysiology of TTS, and the predominance of female patients suggests that TTS could be related to sex hormones and the endocrine system. Takotsubo syndrome is more than a cardiac disease, and it requires a new and interdisciplinary approach to increase awareness among not only cardiologists, but physicians at large. To establish evidence based strategies for effective TTS treatment, randomized prospective trials will be necessary utilizing a large number of patients from multicentre international consortia.
Funding
J.R.G. has received a research grant “Filling the gap” from the University of Zurich.
Conflict of interest: none declared.
References
- 1. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E.. Apical ballooning syndrome or Takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–1529. [DOI] [PubMed] [Google Scholar]
- 2. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF.. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 3. Frangieh AH, Obeid S, Ghadri JR, Imori Y, D'Ascenzo F, Kovac M, Ruschitzka F, Luscher TF, Duru F, Templin C, Inter TAKC.. ECG criteria to differentiate between Takotsubo (Stress) cardiomyopathy and myocardial infarction. J Am Heart Assoc 2016;5:e003418.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad A, Lerman A, Rihal CS.. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 5. Bennett J, Ferdinande B, Kayaert P, Wiyono S, Goetschalkx K, Dubois C, Sinnaeve P, Adriaenssens T, Coosemans M, Desmet W.. Time course of electrocardiographic changes in transient left ventricular ballooning syndrome. Int J Cardiol 2013;169:276–280. [DOI] [PubMed] [Google Scholar]
- 6. Mitsuma W, Kodama M, Ito M, Tanaka K, Yanagawa T, Ikarashi N, Sugiura K, Kimura S, Yagihara N, Kashimura T, Fuse K, Hirono S, Okura Y, Aizawa Y.. Serial electrocardiographic findings in women with Takotsubo cardiomyopathy. Am J Cardiol 2007;100:106–109. [DOI] [PubMed] [Google Scholar]
- 7. Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakamura S, Yoshida M, Mitsuba N, Hata T, Sato H.. Time course of electrocardiographic changes in patients with Tako-Tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J 2004;68:77–81. [DOI] [PubMed] [Google Scholar]
- 8. Kosuge M, Kimura K.. Electrocardiographic findings of Takotsubo cardiomyopathy as compared with those of anterior acute myocardial infarction. J Electrocardiol 2014;47:684–689. [DOI] [PubMed] [Google Scholar]
- 9. Sharkey SW. Electrocardiogram mimics of acute ST-segment elevation myocardial infarction: insights from cardiac magnetic resonance imaging in patients with Tako-Tsubo (stress) cardiomyopathy. J Electrocardiol 2008;41:621–625. [DOI] [PubMed] [Google Scholar]
- 10. Bybee KA, Motiei A, Syed IS, Kara T, Prasad A, Lennon RJ, Murphy JG, Hammill SC, Rihal CS, Wright RS.. Electrocardiography cannot reliably differentiate transient left ventricular apical ballooning syndrome from anterior ST-segment elevation myocardial infarction. J Electrocardiol 2007;40:38.e1–6. [DOI] [PubMed] [Google Scholar]
- 11. Chao T, Lindsay J, Collins S, Woldeyes L, Joshi SB, Steinberg DH, Satler LF, Kent KM, Suddath WO, Pichard AD, Waksman R.. Can acute occlusion of the left anterior descending coronary artery produce a typical ‘Takotsubo’ left ventricular contraction pattern? Am J Cardiol 2009;104:202–204. [DOI] [PubMed] [Google Scholar]
- 12. Kosuge M, Ebina T, Hibi K, Morita S, Okuda J, Iwahashi N, Tsukahara K, Nakachi T, Kiyokuni M, Ishikawa T, Umemura S, Kimura K.. Simple and accurate electrocardiographic criteria to differentiate Takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol 2010;55:2514–2516. [DOI] [PubMed] [Google Scholar]
- 13. Tamura A, Watanabe T, Ishihara M, Ando S, Naono S, Zaizen H, Abe Y, Yano S, Shinozaki K, Kotoku M, Momii H, Kadokami T, Kadota J.. A new electrocardiographic criterion to differentiate between Takotsubo cardiomyopathy and anterior wall ST-segment elevation acute myocardial infarction. Am J Cardiol 2011;108:630–633. [DOI] [PubMed] [Google Scholar]
- 14. Ogura R, Hiasa Y, Takahashi T, Yamaguchi K, Fujiwara K, Ohara Y, Nada T, Ogata T, Kusunoki K, Yuba K, Hosokawa S, Kishi K, Ohtani R.. Specific findings of the standard 12-lead ECG in patients with ‘Takotsubo’ cardiomyopathy: comparison with the findings of acute anterior myocardial infarction. Circ J 2003;67:687–690. [DOI] [PubMed] [Google Scholar]
- 15. Kosuge M, Ebina T, Hibi K, Iwahashi N, Tsukahara K, Endo M, Maejima N, Nagashima Z, Suzuki H, Morita S, Umemura S, Kimura K.. Differences in negative T waves between Takotsubo cardiomyopathy and reperfused anterior acute myocardial infarction. Circ J 2012;76:462–468. [DOI] [PubMed] [Google Scholar]
- 16. Behr ER, Mahida S.. Takotsubo cardiomyopathy and the long-QT syndrome: an insult to repolarization reserve. Europace 2009;11:697–700. [DOI] [PubMed] [Google Scholar]
- 17. Matsuoka K, Okubo S, Fujii E, Uchida F, Kasai A, Aoki T, Makino K, Omichi C, Fujimoto N, Ohta S, Sawai T, Nakano T.. Evaluation of the arrhythmogenecity of stress-induced ‘Takotsubo cardiomyopathy’ from the time course of the 12-lead surface electrocardiogram. Am J Cardiol 2003;92:230–233. [DOI] [PubMed] [Google Scholar]
- 18. Migliore F, Zorzi A, Marra MP, Basso C, Corbetti F, De Lazzari M, Tarantini G, Buja P, Lacognata C, Thiene G, Corrado D, Iliceto S.. Myocardial edema underlies dynamic T-wave inversion (Wellens' ECG pattern) in patients with reversible left ventricular dysfunction. Heart Rhythm 2011;8:1629–1634. [DOI] [PubMed] [Google Scholar]
- 19. Migliore F, Zorzi A, Perazzolo Marra M, Iliceto S, Corrado D.. Myocardial edema as a substrate of electrocardiographic abnormalities and life-threatening arrhythmias in reversible ventricular dysfunction of Takotsubo cardiomyopathy: imaging evidence, presumed mechanisms, and implications for therapy. Heart Rhythm 2015;12:1867–1877. [DOI] [PubMed] [Google Scholar]
- 20. Sclarovsky S, Nikus K.. The electrocardiographic paradox of Tako-Tsubo cardiomyopathy-comparison with acute ischemic syndromes and consideration of molecular biology and electrophysiology to understand the electrical-mechanical mismatching. J Electrocardiol 2010;43:173–176. [DOI] [PubMed] [Google Scholar]
- 21. Namgung J. Electrocardiographic findings in Takotsubo cardiomyopathy: ECG evolution and its difference from the ECG of acute coronary syndrome. Clin Med Insights Cardiol 2014;8:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimizu M, Nishizaki M, Yamawake N, Fujii H, Sakurada H, Isobe M, Hiraoka M.. J wave and fragmented QRS formation during the hyperacute phase in Takotsubo cardiomyopathy. Circ J 2014;78:943–949. [DOI] [PubMed] [Google Scholar]
- 23. Madias JE. Transient attenuation of the amplitude of the QRS complexes in the diagnosis of Takotsubo syndrome. Eur Heart J Acute Cardiovasc Care 2014;3:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghadri JR, Cammann VL, Jurisic S, Seifert B, Napp LC, Diekmann J, Bataiosu DR, D'Ascenzo F, Ding KJ, Sarcon A, Kazemian E, Birri T, Ruschitzka F, Luscher TF, Templin C; InterTAK co-investigators. A novel clinical score (InterTAK Diagnostic Score) to differentiate Takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail 2017;19:1036–42. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen TH, Neil CJ, Sverdlov AL, Mahadavan G, Chirkov YY, Kucia AM, Stansborough J, Beltrame JF, Selvanayagam JB, Zeitz CJ, Struthers AD, Frenneaux MP, Horowitz JD.. N-terminal pro-brain natriuretic protein levels in Takotsubo cardiomyopathy. Am J Cardiol 2011;108:1316–1321. [DOI] [PubMed] [Google Scholar]
- 26. Morita E, Yasue H, Yoshimura M, Ogawa H, Jougasaki M, Matsumura T, Mukoyama M, Nakao K.. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993;88:82–91. [DOI] [PubMed] [Google Scholar]
- 27. Akashi YJ, Musha H, Nakazawa K, Miyake F.. Plasma brain natriuretic peptide in Takotsubo cardiomyopathy. QJM 2004;97:599–607. [DOI] [PubMed] [Google Scholar]
- 28. Neil C, Nguyen TH, Kucia A, Crouch B, Sverdlov A, Chirkov Y, Mahadavan G, Selvanayagam J, Dawson D, Beltrame J, Zeitz C, Unger S, Redpath T, Frenneaux M, Horowitz J.. Slowly resolving global myocardial inflammation/oedema in Tako-Tsubo cardiomyopathy: evidence from T2-weighted cardiac MRI. Heart 2012;98:1278–1284. [DOI] [PubMed] [Google Scholar]
- 29. Pirzer R, Elmas E, Haghi D, Lippert C, Kralev S, Lang S, Borggrefe M, Kalsch T.. Platelet and monocyte activity markers and mediators of inflammation in Takotsubo cardiomyopathy. Heart Vessels 2012;27:186–192. [DOI] [PubMed] [Google Scholar]
- 30. Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T.. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 2011;4:446–454. [DOI] [PubMed] [Google Scholar]
- 31. Jaguszewski M, Osipova J, Ghadri JR, Napp LC, Widera C, Franke J, Fijalkowski M, Nowak R, Fijalkowska M, Volkmann I, Katus HA, Wollert KC, Bauersachs J, Erne P, Luscher TF, Thum T, Templin C.. A signature of circulating microRNAs differentiates Takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rinaldi A, Vincenti S, De Vito F, Bozzoni I, Oliverio A, Presutti C, Fragapane P, Mele A.. Stress induces region specific alterations in microRNAs expression in mice. Behav Brain Res 2010;208:265–269. [DOI] [PubMed] [Google Scholar]
- 33. Dwivedi Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. J Chem Neuroanat 2011;42:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O.. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 2010;329:1537–1541. [DOI] [PubMed] [Google Scholar]
- 35. Stiermaier T, Adams V, Just M, Blazek S, Desch S, Schuler G, Thiele H, Eitel I.. Growth differentiation factor-15 in Takotsubo cardiomyopathy: diagnostic and prognostic value. Int J Cardiol 2014;173:424–429. [DOI] [PubMed] [Google Scholar]
- 36. Napp LC, Ghadri JR, Bauersachs J, Templin C.. Acute coronary syndrome or Takotsubo cardiomyopathy: the suspect may not always be the culprit. Int J Cardiol 2015;187:116–119. [DOI] [PubMed] [Google Scholar]
- 37. Patel SM, Lennon RJ, Prasad A.. Regional wall motion abnormality in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): importance of biplane left ventriculography for differentiating from spontaneously aborted anterior myocardial infarction. Int J Cardiovasc Imaging 2012;28:687–694. [DOI] [PubMed] [Google Scholar]
- 38. Desmet W, Bennett J, Ferdinande B, De Cock D, Adriaenssens T, Coosemans M, Sinnaeve P, Kayaert P, Dubois C.. The apical nipple sign: a useful tool for discriminating between anterior infarction and transient left ventricular ballooning syndrome. Eur Heart J Acute Cardiovasc Care 2014;3:264–267. [DOI] [PubMed] [Google Scholar]
- 39. De Backer O, Debonnaire P, Gevaert S, Missault L, Gheeraert P, Muyldermans L.. Prevalence, associated factors and management implications of left ventricular outflow tract obstruction in Takotsubo cardiomyopathy: a two-year, two-center experience. BMC Cardiovasc Disord 2014;14:147.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Luscher TF, Templin C; International Takotsubo Registry. Differences in the clinical profile and outcomes of typical and atypical Takotsubo syndrome: data from the International Takotsubo Registry. JAMA Cardiol 2016;1:335–340. [DOI] [PubMed] [Google Scholar]
- 41. Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, Rebuzzi AG, Crea F.. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J 2010;31:1319–1327. [DOI] [PubMed] [Google Scholar]
- 42. Dewachter P, Tanase C, Levesque E, Nicaise-Roland P, Chollet-Martin S, Mouton-Faivre C, Benhamou D.. Apical ballooning syndrome following perioperative anaphylaxis is likely related to high doses of epinephrine. J Anesth 2011;25:282–285. [DOI] [PubMed] [Google Scholar]
- 43. Meimoun P, Passos P, Benali T, Boulanger J, Elmkies F, Zemir H, Clerc J, Luycx-Bore A.. Assessment of left ventricular twist mechanics in Tako-tsubo cardiomyopathy by two-dimensional speckle-tracking echocardiography. Eur J Echocardiogr 2011;12:931–939. [DOI] [PubMed] [Google Scholar]
- 44. Haghi D, Papavassiliu T, Fluchter S, Kaden JJ, Porner T, Borggrefe M, Suselbeck T.. Variant form of the acute apical ballooning syndrome (Takotsubo cardiomyopathy): observations on a novel entity. Heart 2006;92:392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hurst RT, Askew JW, Reuss CS, Lee RW, Sweeney JP, Fortuin FD, Oh JK, Tajik AJ.. Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol 2006;48:579–583. [DOI] [PubMed] [Google Scholar]
- 46. Shoukat S, Awad A, Nam DK, Hoskins MH, Samuels O, Higginson J, Clements SD Jr.. Cardiomyopathy with inverted Tako-Tsubo pattern in the setting of subarachnoid hemorrhage: a series of four cases. Neurocrit Care 2013;18:257–260. [DOI] [PubMed] [Google Scholar]
- 47. Y-Hassan S. Clinical features and outcome of epinephrine-induced takotsubo syndrome: Analysis of 33 published cases. Cardiovasc Revasc Med 2016;17:450–455. [DOI] [PubMed] [Google Scholar]
- 48. Naderi N, Amin A, Setayesh A, Pouraliakbar H, Mozaffari K, Maleki M.. Pheochromocytoma-induced reverse Tako-Tsubo with rapid recovery of left ventricular function. Cardiol J 2012;19:527–531. [DOI] [PubMed] [Google Scholar]
- 49. Kato K, Kitahara H, Fujimoto Y, Sakai Y, Ishibashi I, Himi T, Kobayashi Y.. Prevalence and clinical features of focal Takotsubo cardiomyopathy. Circ J 2016;80:1824–1829. [DOI] [PubMed] [Google Scholar]
- 50. Haghi D, Athanasiadis A, Papavassiliu T, Suselbeck T, Fluechter S, Mahrholdt H, Borggrefe M, Sechtem U.. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J 2006;27:2433–2439. [DOI] [PubMed] [Google Scholar]
- 51. Burgdorf C, Hunold P, Radke PW, Schunkert H, Kurowski V.. Isolated right ventricular stress-induced (‘Tako-Tsubo’) cardiomyopathy. Clin Res Cardiol 2011;100:617–619. [DOI] [PubMed] [Google Scholar]
- 52. Mansencal N, Pellerin D, Lamar A, Beauchet A, El Mahmoud R, Pilliere R, McKenna WJ, Dubourg O.. Diagnostic value of contrast echocardiography in Tako-Tsubo cardiomyopathy. Arch Cardiovasc Dis 2010;103:447–453. [DOI] [PubMed] [Google Scholar]
- 53. Citro R, Rigo F, Ciampi Q, D'Andrea A, Provenza G, Mirra M, Giudice R, Silvestri F, Di Benedetto G, Bossone E.. Echocardiographic assessment of regional left ventricular wall motion abnormalities in patients with Tako-Tsubo cardiomyopathy: comparison with anterior myocardial infarction. Eur J Echocardiogr 2011;12:542–549. [DOI] [PubMed] [Google Scholar]
- 54. Meimoun P, Clerc J, Vincent C, Flahaut F, Germain AL, Elmkies F, Zemir H, Luycx-Bore A.. Non-invasive detection of Tako-Tsubo cardiomyopathy vs. acute anterior myocardial infarction by transthoracic Doppler echocardiography. Eur Heart J Cardiovasc Imaging 2013;14:464–470. [DOI] [PubMed] [Google Scholar]
- 55. Abdelmoneim SS, Mankad SV, Bernier M, Dhoble A, Hagen ME, Ness SA, Chandrasekaran K, Pellikka PA, Oh JK, Mulvagh SL.. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J Am Soc Echocardiogr 2009;22:1249–1255. [DOI] [PubMed] [Google Scholar]
- 56. Meimoun P, Malaquin D, Sayah S, Benali T, Luycx-Bore A, Levy F, Zemir H, Tribouilloy C.. The coronary flow reserve is transiently impaired in Tako-Tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr 2008;21:72–77. [DOI] [PubMed] [Google Scholar]
- 57. Rigo F, Sicari R, Citro R, Ossena G, Buja P, Picano E.. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: a Doppler transthoracic echo study. Ann Med 2009;41:462–470. [DOI] [PubMed] [Google Scholar]
- 58. Meimoun P, Malaquin D, Benali T, Boulanger J, Zemir H, Tribouilloy C.. Transient impairment of coronary flow reserve in Tako-Tsubo cardiomyopathy is related to left ventricular systolic parameters. Eur J Echocardiogr 2008;10:265–270. [DOI] [PubMed] [Google Scholar]
- 59. Fujiwara S, Takeishi Y, Isoyama S, Aono G, Takizawa K, Honda H, Otomo T, Mitsuoka M, Itoh Y, Terashima M, Kubota I, Meguro T.. Responsiveness to dobutamine stimulation in patients with left ventricular apical ballooning syndrome. Am J Cardiol 2007;100:1600–1603. [DOI] [PubMed] [Google Scholar]
- 60. Piérard L, Picano E.. Myocardial viability In Picano E, ed. Stress Echocardiography: Fifth, Completely Revised and Updated Edition. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009, pp. 273–294. [Google Scholar]
- 61. Uznanska B, Plewka M, Wierzbowska-Drabik K, Chrzanowski L, Kasprzak JD.. Early prediction of ventricular recovery in Takotsubo syndrome using stress and contrast echocardiography. Med Sci Monit 2009;15:CS89–CS94. [PubMed] [Google Scholar]
- 62. Meimoun P, Abouth S, Boulanger J, Luycx-Bore A, Martis S, Clerc J.. Relationship between acute strain pattern and recovery in Tako-Tsubo cardiomyopathy and acute anterior myocardial infarction: a comparative study using two-dimensional longitudinal strain. Int J Cardiovasc Imaging 2014;30:1491–1500. [DOI] [PubMed] [Google Scholar]
- 63. Chandrasegaram MD, Celermajer DS, Wilson MK.. Apical ballooning syndrome complicated by acute severe mitral regurgitation with left ventricular outflow obstruction–case report. J Cardiothorac Surg 2007;2:14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. El Mahmoud R, Mansencal N, Pilliere R, Leyer F, Abbou N, Michaud P, Nallet O, Digne F, Lacombe P, Cattan S, Dubourg O.. Prevalence and characteristics of left ventricular outflow tract obstruction in Tako-Tsubo syndrome. Am Heart J 2008;156:543–548. [DOI] [PubMed] [Google Scholar]
- 65. Parodi G, Del Pace S, Salvadori C, Carrabba N, Olivotto I, Gensini GF; Tuscany Registry of Tako-Tsubo Cardiomyopathy. Left ventricular apical ballooning syndrome as a novel cause of acute mitral regurgitation. J Am Coll Cardiol 2007;50:647–649. [DOI] [PubMed] [Google Scholar]
- 66. Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E.. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
- 67. Vizzardi E, Bonadei I, Piovanelli B, Bugatti S, D'Aloia A.. Biventricular Tako-Tsubo cardiomyopathy: usefulness of 2D speckle tracking strain echocardiography. J Clin Ultrasound 2014;42:121–124. [DOI] [PubMed] [Google Scholar]
- 68. Ishida T, Yasu T, Arao K, Kawakami M, Saito M.. Images in cardiovascular medicine. Bedside diagnosis of cardiac rupture by contrast echocardiography. Circulation 2005;112:e354–e355. [DOI] [PubMed] [Google Scholar]
- 69. Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T.. Incidence and clinical significance of left ventricular thrombus in Tako-Tsubo cardiomyopathy assessed with echocardiography. QJM 2008;101:381–386. [DOI] [PubMed] [Google Scholar]
- 70. Buchholz S, Ward MR, Bhindi R, Nelson GI, Figtree GA, Grieve SM.. Cardiac thrombi in stress (Tako-Tsubo) cardiomyopathy: more than an apical issue? Mayo Clin Proc 2010;85:863–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Citro R, Rigo F, D'Andrea A, Ciampi Q, Parodi G, Provenza G, Piccolo R, Mirra M, Zito C, Giudice R, Patella MM, Antonini-Canterin F, Bossone E, Piscione F, Salerno-Uriarte J; Tako-Tsubo Italian Network Investigators. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in Tako-Tsubo cardiomyopathy. JACC Cardiovasc Imaging 2014;7:119–129. [DOI] [PubMed] [Google Scholar]
- 72. Tsuchihashi K, Ueshima K, Uchida T, Oh-Mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I; Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11–18. [DOI] [PubMed] [Google Scholar]
- 73. Margey R, Diamond P, McCann H, Sugrue D.. Dobutamine stress echo-induced apical ballooning (Takotsubo) syndrome. Eur J Echocardiogr 2009;10:395–399. [DOI] [PubMed] [Google Scholar]
- 74. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC.. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–548. [DOI] [PubMed] [Google Scholar]
- 75. Champ-Rigot L, Alexandre J, Grollier G, Milliez P.. Atypical Tako-tsubo syndrome: a morphologic variant or a step towards recovery? Int J Cardiol 2011;146:256–258. [DOI] [PubMed] [Google Scholar]
- 76. Brunetti ND, Ieva R, Rossi G, Barone N, De Gennaro L, Pellegrino PL, Mavilio G, Cuculo A, Di Biase M.. Ventricular outflow tract obstruction, systolic anterior motion and acute mitral regurgitation in Tako-Tsubo syndrome. Int J Cardiol 2008;127:e152–e157. [DOI] [PubMed] [Google Scholar]
- 77. Gogas BD, Antoniadis AG, Zacharoulis AA, Kolokathis F, Lekakis J, Kremastinos DT.. Recurrent apical ballooning syndrome ‘The masquerading acute cardiac syndrome’. Int J Cardiol 2011;150:e17–e19. [DOI] [PubMed] [Google Scholar]
- 78. Ghadri JR, Jaguszewski M, Corti R, Luscher TF, Templin C.. Different wall motion patterns of three consecutive episodes of Takotsubo cardiomyopathy in the same patient. Int J Cardiol 2012;160:e25–e27. [DOI] [PubMed] [Google Scholar]
- 79. Murugiah K, Wang Y, Desai NR, Spatz ES, Nuti SV, Dreyer RP, Krumholz HM.. Trends in short- and long-term outcomes for Takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail 2016;4:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Athanasiadis A, Schneider B, Sechtem U.. Role of cardiovascular magnetic resonance in Takotsubo cardiomyopathy. Heart Fail Clin 2013;9:167–176, viii. [DOI] [PubMed] [Google Scholar]
- 81. Templin C, Ghadri JR, Napp LC.. Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015;373:2689–2691. [DOI] [PubMed] [Google Scholar]
- 82. Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG.. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA 2011;306:277–286. [DOI] [PubMed] [Google Scholar]
- 83. Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA.. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 2008;51:2414–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rolf A, Nef HM, Mollmann H, Troidl C, Voss S, Conradi G, Rixe J, Steiger H, Beiring K, Hamm CW, Dill T.. Immunohistological basis of the late gadolinium enhancement phenomenon in Tako-Tsubo cardiomyopathy. Eur Heart J 2009;30:1635–1642. [DOI] [PubMed] [Google Scholar]
- 85. Heidary S, Patel H, Chung J, Yokota H, Gupta SN, Bennett MV, Katikireddy C, Nguyen P, Pauly JM, Terashima M, McConnell MV, Yang PC.. Quantitative tissue characterization of infarct core and border zone in patients with ischemic cardiomyopathy by magnetic resonance is associated with future cardiovascular events. J Am Coll Cardiol 2010;55:2762–2768. [DOI] [PubMed] [Google Scholar]
- 86. Eitel I, Friedrich MG.. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson 2011;13:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stahli BE, Ruschitzka F, Enseleit F.. Isolated right ventricular ballooning syndrome: a new variant of transient cardiomyopathy. Eur Heart J 2011;32:1821.. [DOI] [PubMed] [Google Scholar]
- 88. Kagiyama N, Okura H, Tamada T, Imai K, Yamada R, Kume T, Hayashida A, Neishi Y, Kawamoto T, Yoshida K.. Impact of right ventricular involvement on the prognosis of Takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 2016;17:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Eitel I, Behrendt F, Schindler K, Kivelitz D, Gutberlet M, Schuler G, Thiele H.. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J 2008;29:2651–2659. [DOI] [PubMed] [Google Scholar]
- 90. Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H.. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol 2003;41:737–742. [DOI] [PubMed] [Google Scholar]
- 91. Ghadri JR, Dougoud S, Maier W, Kaufmann PA, Gaemperli O, Prasad A, Luscher TF, Templin C.. A PET/CT-follow-up imaging study to differentiate Takotsubo cardiomyopathy from acute myocardial infarction. Int J Cardiovasc Imaging 2014;30:207–209. [DOI] [PubMed] [Google Scholar]
- 92. Ito K, Sugihara H, Kawasaki T, Yuba T, Doue T, Tanabe T, Adachi Y, Katoh S, Azuma A, Nakagawa M.. Assessment of ampulla (Takotsubo) cardiomyopathy with coronary angiography, two-dimensional echocardiography and 99mTc-tetrofosmin myocardial single photon emission computed tomography. Ann Nucl Med 2001;15:351–355. [DOI] [PubMed] [Google Scholar]
- 93. Cimarelli S, Imperiale A, Ben-Sellem D, Rischner J, Detour J, Morel O, Ohlmann P, Constantinesco A.. Nuclear medicine imaging of Takotsubo cardiomyopathy: typical form and midventricular ballooning syndrome. J Nucl Cardiol 2008;15:137–141. [DOI] [PubMed] [Google Scholar]
- 94. Cimarelli S, Sauer F, Morel O, Ohlmann P, Constantinesco A, Imperiale A.. Transient left ventricular dysfunction syndrome: patho-physiological bases through nuclear medicine imaging. Int J Cardiol 2010;144:212–218. [DOI] [PubMed] [Google Scholar]
- 95. Christensen TE, Ahtarovski KA, Bang LE, Holmvang L, Søholm H, Ghotbi AA, Andersson H, Vejlstrup N, Ihlemann N, Engstrøm T, Kjær A, Hasbak P.. Basal hyperaemia is the primary abnormality of perfusion in Takotsubo cardiomyopathy: a quantitative cardiac perfusion positron emission tomography study. Eur Heart J Cardiovasc Imaging 2015;16:1162–1169. [DOI] [PubMed] [Google Scholar]
- 96. Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Umemura T, Nakamura S, Yoshida M, Sato H.. Myocardial perfusion and fatty acid metabolism in patients with Tako-Tsubo-like left ventricular dysfunction. J Am Coll Cardiol 2003;41:743–748. [DOI] [PubMed] [Google Scholar]
- 97. Matsuo S, Nakajima K, Kinuya S, Yamagishi M.. Diagnostic utility of 123I-BMIPP imaging in patients with Takotsubo cardiomyopathy. J Cardiol 2014;64:49–56. [DOI] [PubMed] [Google Scholar]
- 98. Yoshida T, Hibino T, Kako N, Murai S, Oguri M, Kato K, Yajima K, Ohte N, Yokoi K, Kimura G.. A pathophysiologic study of Tako-Tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J 2007;28:2598–2604. [DOI] [PubMed] [Google Scholar]
- 99. Dorfman TA, Iskandrian AE.. Takotsubo cardiomyopathy: state-of-the-art review. J Nucl Cardiol 2009;16:122–134. [DOI] [PubMed] [Google Scholar]
- 100. Merli E, Sutcliffe S, Gori M, Sutherland GG.. Tako-Tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr 2006;7:53–61. [DOI] [PubMed] [Google Scholar]
- 101. Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Musha H, Sasaka K.. 123I-MIBG myocardial scintigraphy in patients with ‘Takotsubo’ cardiomyopathy. J Nucl Med 2004;45:1121–1127. [PubMed] [Google Scholar]
- 102. Prasad A, Madhavan M, Chareonthaitawee P.. Cardiac sympathetic activity in stress-induced (Takotsubo) cardiomyopathy. Nat Rev Cardiol 2009;6:430–434. [DOI] [PubMed] [Google Scholar]
- 103. Tornvall P, Collste O, Ehrenborg E, Jarnbert-Petterson H.. A case-control study of risk markers and mortality in Takotsubo stress cardiomyopathy. J Am Coll Cardiol 2016;67:1931–1936. [DOI] [PubMed] [Google Scholar]
- 104. Stiermaier T, Eitel C, Desch S, Fuernau G, Schuler G, Thiele H, Eitel I.. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care 2016;5:489–496. [DOI] [PubMed] [Google Scholar]
- 105. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J; Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 106. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Mario C. D, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D.. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 107. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; American College of C, American Heart Association Task Force on Practice G, Society for Cardiovascular A, Interventions, Society of Thoracic S, American Association for Clinical C. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 108. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 109. Brinjikji W, El-Sayed AM, Salka S.. In-hospital mortality among patients with Takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J 2012;164:215–221. [DOI] [PubMed] [Google Scholar]
- 110. Sobue Y, Watanabe E, Ichikawa T, Koshikawa M, Yamamoto M, Harada M, Ozaki Y.. Physically triggered Takotsubo cardiomyopathy has a higher in-hospital mortality rate. Int J Cardiol 2017;235:87–93. [DOI] [PubMed] [Google Scholar]
- 111. Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Konishi Y, Sakata K, Nagao K, Yamamoto T, Takayama M; Committee CCUNS. Characterization of predictors of in-hospital cardiac complications of Takotsubo cardiomyopathy: multi-center registry from Tokyo CCU Network. J Cardiol 2014;63:269–273. [DOI] [PubMed] [Google Scholar]
- 112. Takashio S, Yamamuro M, Kojima S, Izumiya Y, Kaikita K, Hokimoto S, Sugiyama S, Tsunoda R, Nakao K, Ogawa H.. Usefulness of SUM of ST-segment elevation on electrocardiograms (limb leads) for predicting in-hospital complications in patients with stress (Takotsubo) cardiomyopathy. Am J Cardiol 2012;109:1651–1656. [DOI] [PubMed] [Google Scholar]
- 113. Stiermaier T, Eitel C, Denef S, Desch S, Schuler G, Thiele H, Eitel I.. Significance of life-threatening arrhythmias in takotsubo cardiomyopathy. J Am Coll Cardiol 2015;65:2148–2150. [DOI] [PubMed] [Google Scholar]
- 114. Icli A, Akilli H, Kayrak M, Aribas A, Ozdemir K.. Short-term warfarin treatment for apical thrombus in a patient with Takotsubo cardiomyopathy. Cardiovasc J Afr 2016;27:e12–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bharathi KS, Kulkarni S, Sadananda KS, Gurudatt CL.. Takotsubo cardiomyopathy precipitated by negative pressure pulmonary oedema following total thyroidectomy. Indian J Anaesth 2016;60:202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lu DY, Caplow J, Quatromoni N, Forde-McLean R, Owens AT.. Ventricular septal defect from Takotsubo syndrome. Case Rep Cardiol 2016;2016:2693062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jaguszewski M, Fijalkowski M, Nowak R, Czapiewski P, Ghadri JR, Templin C, Rynkiewicz A.. Ventricular rupture in Takotsubo cardiomyopathy. Eur Heart J 2012;33:1027.. [DOI] [PubMed] [Google Scholar]
- 118. Böhm M, Cammann VL, Ghadri JR, Ukena C, Gili S, Di Vece D, Kato K, Ding KJ, Szawan KA, Micek J, Jurisic S, D'Ascenzo F, Frangieh AH, Rechsteiner D, Seifert B, Ruschitzka F, Lüscher T, Templin C; InterTAK Collaborators. Interaction of systolic blood pressure and resting heart rate with clinical outcomes in takotsubo syndrome: insights from the International Takotsubo Registry. Eur J Heart Fail 2018; doi: 10.1002/ejhf.1162. [DOI] [PubMed] [Google Scholar]
- 119. Citro R, Bossone E, Parodi G, Rigo F, Nardi F, Provenza G, Zito C, Novo G, Vitale G, Prota C, Silverio A, Vriz O, D’andrea A, Antonini-Canterin F, Salerno-Uriarte J, Piscione F; Tako-tsubo Italian Network Investigators. Independent impact of RV involvement on in-hospital outcome of patients with Takotsubo syndrome. JACC Cardiovasc Imaging 2016;9:894–895. [DOI] [PubMed] [Google Scholar]
- 120. Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS.. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol 2007;50:448–452. [DOI] [PubMed] [Google Scholar]
- 121. Sharkey SW, Pink VR, Lesser JR, Garberich RF, Maron MS, Maron BJ.. Clinical profile of patients with high-risk Tako-Tsubo cardiomyopathy. Am J Cardiol 2015;116:765–772. [DOI] [PubMed] [Google Scholar]
- 122. Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, Vonthein R, Schuler G, Thiele H, Eitel I.. Long-term excess mortality in Takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail 2016;18:650–656. [DOI] [PubMed] [Google Scholar]
- 123. Madias JE. Low prevalence of diabetes mellitus in patients with Takotsubo syndrome: a plausible ′protective′ effect with pathophysiologic connotations. Eur Heart J Acute Cardiovasc Care 2016;5:164–170. [DOI] [PubMed] [Google Scholar]
- 124. Bill V, El-Battrawy I, Behnes M, Baumann S, Becher T, Elmas E, Hoffmann U, Haghi D, Fastner C, Kuschyk J, Papavassiliu T, Borggrefe M, Akin I. ‘Diabetes paradox’ in Takotsubo Cardiomyopathy. Int J Cardiol 2016;224:88–89. [DOI] [PubMed] [Google Scholar]
- 125. Dias A, Franco E, Rubio M, Koshkelashvili N, Bhalla V, Amanullah S, Hebert K, Figueredo VM.. Takotsubo Syndrome: does it matter if you have diabetes mellitus? Int J Cardiol 2016;224:398–399. [DOI] [PubMed] [Google Scholar]
- 126. Bonello L, Com O, Ait-Moktar O, Theron A, Moro PJ, Salem A, Sbragia P, Paganelli F.. Ventricular arrhythmias during Tako-tsubo syndrome. Int J Cardiol 2008;128:e50–e53. [DOI] [PubMed] [Google Scholar]
- 127. Syed FF, Asirvatham SJ, Francis J.. Arrhythmia occurrence with Takotsubo cardiomyopathy: a literature review. Europace 2011;13:780–788. [DOI] [PubMed] [Google Scholar]
- 128. Madias C, Fitzgibbons TP, Alsheikh-Ali AA, Bouchard JL, Kalsmith B, Garlitski AC, Tighe DA, Estes NA 3rd, Aurigemma GP, Link MS.. Acquired long QT syndrome from stress cardiomyopathy is associated with ventricular arrhythmias and torsades de pointes. Heart Rhythm 2011;8:555–561. [DOI] [PubMed] [Google Scholar]
- 129. Brown KH, Trohman RG, Madias C.. Arrhythmias in Takotsubo cardiomyopathy. Card Electrophysiol Clin 2015;7:331–340. [DOI] [PubMed] [Google Scholar]
- 130. Migliore F, Zorzi A, Peruzza F, Perazzolo Marra M, Tarantini G, Iliceto S, Corrado D.. Incidence and management of life-threatening arrhythmias in Takotsubo syndrome. Int J Cardiol 2013;166:261–263. [DOI] [PubMed] [Google Scholar]
- 131. Perazzolo Marra M, Zorzi A, Corbetti F, De Lazzari M, Migliore F, Tona F, Tarantini G, Iliceto S, Corrado D.. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens' ECG pattern) in Tako-Tsubo cardiomyopathy. Heart Rhythm 2013;10:70–77. [DOI] [PubMed] [Google Scholar]
- 132. Gasparetto N, Zorzi A, Perazzolo Marra M, Migliore F, Napodano M, Corrado D, Iliceto S, Cacciavillani L.. Atypical (mid-ventricular) Takotsubo syndrome in a survival of out-of-hospital ventricular fibrillation: cause or consequence? Int J Cardiol 2014;172:e51–e53. [DOI] [PubMed] [Google Scholar]
- 133. Zorzi A, Perazzolo Marra M, Migliore F, De Lazzari M, Tarantini G, Iliceto S, Corrado D.. Relationship between repolarization abnormalities and myocardial edema in atypical Tako-Tsubo syndrome. J Electrocardiol 2013;46:348–351. [DOI] [PubMed] [Google Scholar]
- 134. Ohkubo K, Watanabe I, Okumura Y, Ashino S, Kofune M, Nagashima K, Nakai T, Kasamaki Y, Hirayama A.. Functional atrioventricular conduction block in an elderly patient with acquired long QT syndrome: elucidation of the mechanism of block. J Electrocardiol 2011;44:353–356. [DOI] [PubMed] [Google Scholar]
- 135. Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ.. Natural history and expansive clinical profile of stress (Tako-Tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–341. [DOI] [PubMed] [Google Scholar]
- 136. Cattaneo M, Moccetti M, Pasotti E, Faletra F, Porretta AP, Kobza R, Gallino A.. Three recurrent episodes of apical-ballooning Takotsubo cardiomyopathy in a man. Circulation 2015;132:e377–e379. [DOI] [PubMed] [Google Scholar]
- 137. Srivastava NT, Parent JJ, Hurwitz RA.. Recurrent Takotsubo cardiomyopathy in a child. Cardiol Young 2016;26:410–412. [DOI] [PubMed] [Google Scholar]
- 138. Xu B, Williams PD, Brown M, Macisaac A.. Takotsubo cardiomyopathy: does recurrence tend to occur in a previously unaffected ventricular wall region? Circulation 2014;129:e339–e340. [DOI] [PubMed] [Google Scholar]
- 139. Kato K, Lyon AR, Ghadri JR, Templin C.. Takotsubo syndrome: aetiology, presentation and treatment. Heart 2017;103:1461–1469. [DOI] [PubMed] [Google Scholar]
- 140. Isogai T, Matsui H, Tanaka H, Fushimi K, Yasunaga H. Early β-blocker use and in-hospital mortality in patients with takotsubo cardiomyopathy. Heart 2016;102:1029–1035. [DOI] [PubMed] [Google Scholar]
- 141. Dias A, Franco E, Koshkelashvili N, Bhalla V, Pressman GS, Hebert K, Figueredo VM. Antiplatelet therapy in Takotsubo cardiomyopathy: does it improve cardiovascular outcomes during index event? Heart Vessels 2016;31:1285–1290. [DOI] [PubMed] [Google Scholar]
- 142. Santoro F, Ieva R, Musaico F, Ferraretti A, Triggiani G, Tarantino N, Di Biase M, Brunetti ND. Lack of efficacy of drug therapy in preventing takotsubo cardiomyopathy recurrence: a meta-analysis. Clin Cardiol 2014;37:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Santoro F, Ieva R, Ferraretti A, Ienco V, Carpagnano G, Lodispoto M, Di Biase L, Di Biase M, Brunetti ND.. Safety and feasibility of levosimendan administration in Takotsubo cardiomyopathy: a case series. Cardiovasc Ther 2013;31:e133–e137. [DOI] [PubMed] [Google Scholar]
- 144. Singh K, Carson K, Usmani Z, Sawhney G, Shah R, Horowitz J.. Systematic review and meta-analysis of incidence and correlates of recurrence of Takotsubo cardiomyopathy. Int J Cardiol 2014;174:696–701. [DOI] [PubMed] [Google Scholar]
- 145. Citro R, Lyon AR, Meimoun P, Omerovic E, Redfors B, Buck T, Lerakis S, Parodi G, Silverio A, Eitel I, Schneider B, Prasad A, Bossone E.. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr 2015;28:57–74. [DOI] [PubMed] [Google Scholar]
- 146. Munzel T, Knorr M, Schmidt F, von Bardeleben S, Gori T, Schulz E.. Airborne disease: a case of a Takotsubo cardiomyopathie as a consequence of nighttime aircraft noise exposure. Eur Heart J 2016;37:2844.. [DOI] [PubMed] [Google Scholar]
- 147. Madias JE. If channel blocker ivabradine vs. beta-blockers for sinus tachycardia in patients with Takotsubo syndrome. Int J Cardiol 2016;223:877–878. [DOI] [PubMed] [Google Scholar]
- 148. Ueyama T, Kasamatsu K, Hano T, Yamamoto K, Tsuruo Y, Nishio I.. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ‘Tako-Tsubo’ cardiomyopathy. Circ J 2002;66:712–713. [DOI] [PubMed] [Google Scholar]
- 149. Izumi Y, Okatani H, Shiota M, Nakao T, Ise R, Kito G, Miura K, Iwao H.. Effects of metoprolol on epinephrine-induced Takotsubo-like left ventricular dysfunction in non-human primates. Hypertens Res 2009;32:339–346. [DOI] [PubMed] [Google Scholar]
- 150. Stiermaier T, Rommel KP, Eitel C, Moller C, Graf T, Desch S, Thiele H, Eitel I.. Management of arrhythmias in patients with Takotsubo cardiomyopathy: is the implantation of permanent devices necessary? Heart Rhythm 2016;13:1979–1986. [DOI] [PubMed] [Google Scholar]
- 151. Peters S, Klein HU.. WCD LifeVest: risk stratification in a case of Tako-Tsubo cardiomyopathy with QT interval prolongation. Herz 2012;37:219–221. [DOI] [PubMed] [Google Scholar]
- 152. Ueyama T, Kasamatsu K, Hano T, Tsuruo Y, Ishikura F.. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann N Y Acad Sci 2008;1148:479–485. [DOI] [PubMed] [Google Scholar]
- 153. Ueyama T, Ishikura F, Matsuda A, Asanuma T, Ueda K, Ichinose M, Kasamatsu K, Hano T, Akasaka T, Tsuruo Y, Morimoto K, Beppu S.. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J 2007;71:565–573. [DOI] [PubMed] [Google Scholar]
- 154. Delmas C, Lairez O, Mulin E, Delmas T, Boudou N, Dumonteil N, Biendel-Picquet C, Roncalli J, Elbaz M, Galinier M, Carrié D.. Anxiodepressive disorders and chronic psychological stress are associated with Tako-Tsubo cardiomyopathy—new physiopathological hypothesis. Circ J 2013;77:175–180. [DOI] [PubMed] [Google Scholar]
- 155. Mayer KN, Ghadri J-R, Jaguszewski M, Scherff F, Saguner AM, Kazemian E, Baumann CR, Jenewein J, Tsakiris M, Lüscher TF, Brugger P, Templin C.. Takotsubo syndrome—a close connection to the brain: a prospective study investigating neuropsychiatric traits. IJC Metab Endocr 2016;12:36–41. [Google Scholar]
- 156. Ghadri JR, Ruschitzka F, Luscher TF, Templin C.. Takotsubo cardiomyopathy: still much more to learn. Heart 2014;100:1804–1812. [DOI] [PubMed] [Google Scholar]