Abstract
Pathology and in vivo imaging studies have identified superficial plaque erosion as a frequent and important mechanism underlying acute coronary syndromes (ACS). In contrast with plaque rupture, the pathophysiological mechanisms leading to plaque erosion remain poorly understood. The advent of intravascular imaging techniques, particularly optical coherence tomography, has aided understanding of this mode of ACS in vivo by complementing previous insights from pathology studies. Appreciation of the distinct biological and clinical mechanisms of plaque erosion points to the possibility of tailored management strategies for patients presenting with ACS.
Keywords: Acute coronary syndrome , Atherosclerosis , Intact fibrous cap , Intravascular imaging , Optical coherence tomography , Plaque erosion
History and pathology data
Ischaemic heart disease remains a major source of morbidity and mortality worldwide. Patients with acute coronary syndromes (ACS) can have a wide spectrum of clinical presentations as well as imaging and pathology findings.1 While coronary thrombi had traditionally been thought to arise from plaque rupture in most cases,2–4 patients presenting with ACS exhibit a range of culprit lesion morphologies. Recognition of the second most common pathophysiological mechanism, plaque erosion, emerged initially from pathology reviews of patients with sudden cardiac death (SCD), or fatal acute myocardial infarction (AMI),2,5–7 a concept confirmed more recently by in vivo intravascular imaging studies.
While understanding of the pathophysiology of plaque rupture has become well established, the mechanisms leading to plaque erosion have remained less well understood.8 As medical therapy has proven effective for ‘stabilization’ of lipid-rich atheromatous plaques, further understanding of the mechanisms underlying plaque erosion and the development of targeted treatments has gained interest in recent years.
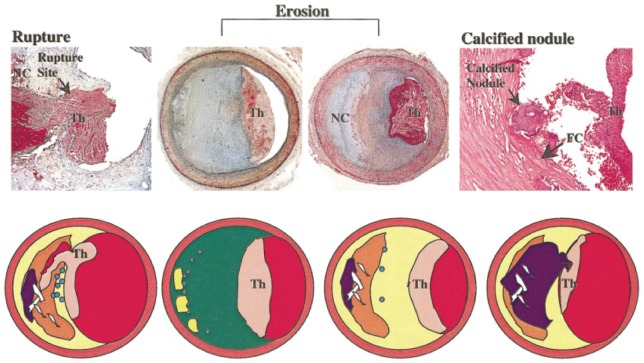
Three principal mechanisms for ACS have been proposed: (i) Plaque erosion, the focus of this article, involves the formation of thrombus in an area of endothelial denudation adjacent to an atherosclerotic plaque without disruption of the fibrous cap overlying a superficial lipid-rich necrotic core; (ii) Plaque rupture, the most common mechanism, commonly complicates lipid-rich atherosclerotic plaques with thin fibrous cap (Figure 1). Metalloproteinases contribute to fibrous cap rupture and exposure of the necrotic core to the vessel lumen leading to platelet activation and thrombus formation. In a small subset, plaque disruption occurs at the site of a calcified nodule.9,10 Plaques can rupture with or without signs of systemic inflammation1; and (iii) In some cases, ACS can also occur without apparent thrombus.
Figure 1.
Left: a thin cap fibroatheroma with ruptured fibrous cap and luminal thrombi is shown. A large necrotic core communicates with the lumen. Middle: erosion occurs over lesions rich in proteoglycans and smooth muscle cells. Thrombus is attached in an area that lacks endothelium. These lesions have few inflammatory cells, in particular of mononuclear leukocytes. Right: a calcified nodule is protruding into the lumen through a disrupted thin fibrous cap (adapted from Virmani et al.10). NC, necrotic core; Th, thrombi.
An early study by van der Wal et al.5 reported pathology findings of 20 patients who had died of AMI: 12 patients had features consistent with plaque rupture, while the other eight had evidence of superficial erosion without rupture. A subsequent series of 50 patients similarly showed a prevalence of plaque erosion in 44% and plaque rupture in 56% of patients with SCD.6 One of the largest studies, by Arbustini et al.,7 sought to evaluate the prevalence of plaque erosion by examining pathological specimens of almost 300 consecutive patients with AMI at a single institution. This study found an acute thrombus in nearly all patients (98%), and identified plaque erosion in 25% of cases, with a higher predominance in women (37.4%) than in men (18.5%), and no significant difference in location or distribution of infarction and thrombus.7 Overall, 11 autopsy studies to date have described plaque erosion (Table 1). Taken together, they have demonstrated that, on average, the prevalence of plaque erosion associated with coronary thrombosis is 31%.14
Table 1.
Pathology studies of plaque erosion and patient clinical characteristics (adapted from White et al.14)
| Study | Number of cases | Female | Average age (years) | Rupture (%) | Erosion (%) |
|---|---|---|---|---|---|
| van der Wal et al.5 | 20 thrombus | n/r | 63 | 60 | 40 |
| Farb et al.6 | 96 SCD/50 thrombus | 32% | Rupture: 53 | 56 | 44 |
| Erosion: 44 | |||||
| Burke et al.22 | 113 SCD/59 thrombus | 0% | 50 | 69 | 31 |
| Burke et al.23 | 51 SCD/26 thrombus | 100% | Rupture: 58 | 31 | 69 |
| Erosion: 45 | |||||
| Arbustini et al.7 | 298 MI/291 thrombus | 37% | Rupture: 68 | 75 | 25 |
| Erosion: 70 | |||||
| Kolodgie et al.11 | 49 culprit plaques | 0% (Rupture) | Rupture: 46 | 22 | 41 |
| 45% (Erosion) | Erosion: 41 | ||||
| 28% (Stable) | Stable: 47 | ||||
| Burke et al.24 | 457 SCD/224 thrombus | n/r | n/r | 69 | 31 |
| Sato et al.12 | 31 MI/23 thrombus | 13% | Rupture: 70 | 78 | 22 |
| Erosion: 68 | |||||
| Schwartz et al.13 | 44 SCD | 14% | 51 | 57 | 43 |
| Kramer et al.25 | 345 SCD/181 thrombus | 11% (Rupture) | Rupture: 52 | 71 | 29 |
| 26% (Erosion) | Erosion: 43 | ||||
| Tavora et al.26 | 314 SCD/170 thrombus | 19% | ≈50 | 70 | 30 |
MI, myocardial infarction; n/r, not reported; SCD, sudden cardiac death.
Pathobiology of plaque erosion
As mentioned above, unlike plaque rupture, plaque erosion remains poorly characterized.14 In general, plaque erosion occurs over lesions rich in proteoglycans and smooth muscle cells with local absence of intimal endothelial cells.6,15 Breaches in endothelial integrity, probably related to local flow perturbation, implicate the exposure of the underlying collagen as one nidus for thrombus formation. Further pathology studies by Durand et al.15 support this mechanism for thrombus formation by demonstrating thrombus formation at sites of experimental endothelial loss provoked by induction of apoptosis with intravascular staurosporin. Cellular expression of the histocompatibility antigen HLA-DR indicates immunological activation typically stimulated by the T cell cytokine interferon gamma.16 Early observations showed numerous T cells and macrophages with strong HLA-DR antigen expression at the site of erosion as well as strong HLA-DR expression on smooth muscle cells in adjacent tissues.5,6,10,11,14 Subsequent work by Sugiyama et al.17 provided mechanistic insight into endothelial cell desquamation at areas of plaque erosion by demonstrating that endothelial cell death and desquamation via apoptosis or oncosis pathways could result from exposure hypochlorous acid, an oxidant species elaborated by myeloperoxidase. Macrophages within the atherosclerotic plaque sub-endothelial region can express this enzyme.17 Subsequent studies also showed that thrombus overlying plaque erosion had higher concentrations of myeloperoxidase-positive cells than that observed in ruptured plaques.18,19 Polymorphonuclear leukocytes contain abundant myeloperoxidase. Co-culture of endothelial cells with polymorphonuclear leukocytes can induce endothelial cell injury and death.18,20,21 Eroded plaques have a higher concentration of extracellular matrix molecules, such as hyaluronan and versican, with considerably less decorin and biglycan typically seen in plaques that exhibit morphological characteristics associated with stability. Furthermore, a cell surface receptor for hyaluronan, CD44, localizes prominently in eroded vs. ruptured or stable plaques, pointing again to a distinct mechanistic pathway for erosion.5,6,11,14 These findings contrast sharply with the pathobiology of plaque rupture which associates with local and systemic inflammation, activation of both innate and adaptive immunity, and thrombosis triggered by tissue factor. Plaque rupture involves macrophages much more than granulocytes. Hence, the inflammatory cell types in ruptured vs. eroded plaques differ diametrically.
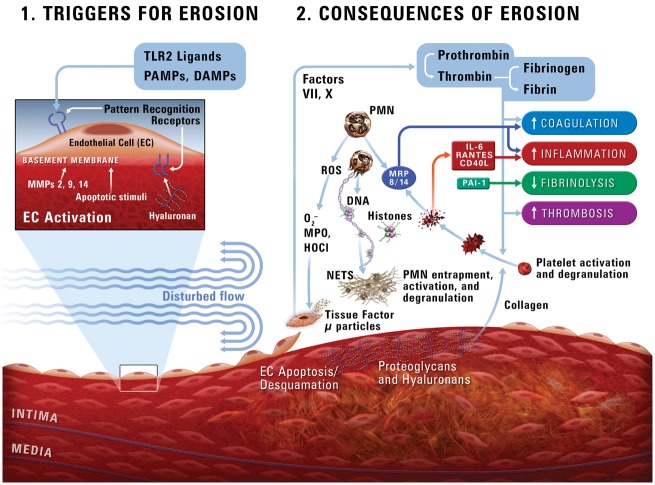
Recent in vitro experiments by Quillard et al.21 demonstrated that Toll-like receptor-2 (TLR-2) activation can drive endothelial cell damage and may contribute to denudation in areas of superficial plaque erosion (Figure 2). Understanding of the key role of neutrophils in erosion continues to evolve. Eroded plaques commonly contain neutrophil extracellular traps (NETs). Further, NETs can potentiate endothelial cell stress and stimulate apoptosis, and denudation of the endothelial monolayer. Neutrophil and NETs accumulation, and levels of TLR-2 correlate with apoptotic endothelial cells seen in eroded plaques in contrast with traditional ‘vulnerable’ or ruptured plaques. Neutrophil extracellular traps may thus contribute to the pathogenesis of plaque erosion.21 These web-like structures consist of neutrophilic proteins (nuclear, cytoplasmic, and granular) and decondensed chromatin that entrap platelets, fibrin strands, and promote thrombosis.21,27–29
Figure 2.
A schema of a potential pathophysiological pathway to superficial erosion (adapted from Quillard et al.21). PAMPs, pathogen-associated molecular patterns; DAMPs, danger-associated molecular patterns; TLR2, Toll-like receptor-2; MMP, matrix metalloproteinase; HOCl, hypochlorous acid; MPO, myeloperoxidase; NETs, neutrophil extracellular traps.
In summary, eroded plaques have an irregular surface with a discontinuity in the endothelial cell monolayer without fibrous cap rupture and generally lack a necrotic core. These features differ distinctly from the morphological characteristics of ruptured plaques.10,14
Plaque erosion appears to associate primarily with platelet-rich ‘white’ thrombi.12,13 In contrast, plaque rupture exposes various thrombogenic substrates including tissue factor that stimulate the coagulation cascade and fibrillar collagens that activate platelets, yielding mixed fibrin/erythrocyte-rich (‘red’) and platelet-rich (‘white’) thrombi. Indeed, in vivo study after successful thrombolysis showed that the predominant component of residual thrombus was platelets in erosion and erythrocytes in rupture.30
In vivo diagnosis and intravascular imaging
The advent of intravascular imaging permits more detailed evaluation and better understanding of the morphological aspects discussed above, as well as the ability to diagnose these findings in vivo and correlate them with clinical presentations. Optical coherence tomography (OCT), with a resolution of 10–20 µm, made it possible to visualize directly microscopic features of a plaque including fibrous cap, microvessels, thrombus, inflammatory cells, and cholesterol crystals in addition to macroscopic plaque morphology. Occasionally thrombus, particularly red thrombus, at the culprit lesion interferes with evaluation of underlying plaque morphology. In such cases, repeated aspiration thrombectomy or several days of antithrombotic treatment may improve visualization of the underlying plaque.
In vivo prevalence of plaque erosion
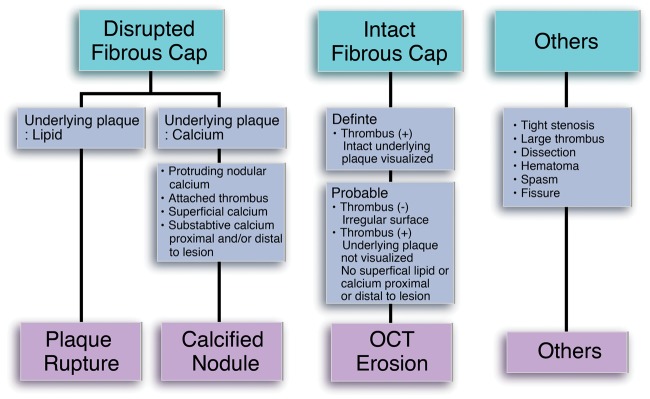
In 2005, Hayashi et al.31 reported that 39% of patients with AMI had erosion identified by coronary angioscopy (Table 2). Patients with erosion, as opposed to those with rupture, more frequently presented with ‘pre-infarction’ angina and had smaller infarct size. Kubo et al.32 studied 30 patients presenting with ACS and reported superficial erosion in 23% of cases by OCT. Kusama et al.33 reported that 37% of patients with acute anterior myocardial infarction lacked signs of culprit plaque rupture by intravascular ultrasound. Those studies, however, did not use clear diagnostic criteria for erosion. Visualization of the endothelial monolayer (1–5 µm) is below the resolution of OCT (10–15 µm). This recognition spurred the development of new criteria for the OCT diagnosis of plaque erosion (Figure 3).34 The presence of an intact fibrous cap at the culprit site differentiates erosion from rupture. Unlike autopsy studies, the in vivo diagnosis of erosion requires consideration of other factors such as the effects of antithrombotic therapy and limitations of OCT. Optical coherence tomography usually shows fibrous cap fracture in ruptured plaques unambiguously (Figure 4). Plaque erosion is a diagnosis of exclusion. The absence of fibrous cap rupture and a cavity in a patient with ACS suggests the diagnosis of plaque erosion, especially if accompanied by thrombus or an irregular intimal surface. Some groups have consequently proposed to refer to this imaging finding as ‘intact fibrous cap’ rather than as erosion. A Supplementary material online, Figure S1 includes additional OCT images of erosion.
Table 2.
In vivo prevalence of plaque rupture and plaque erosion in patients with acute coronary syndromes and acute myocardial infarction
| Author | Presentation | Number | Modality | Plaque with rupture (%) | Plaque without rupture (%) | Others |
|---|---|---|---|---|---|---|
| Hayashi et al.31 | AMI | 107 (72) | Angioscopy | 61 | 39 | |
| Kubo et al.32 | AMI | 30 | OCT | 73 | 23 | |
| Kusama et al.33 | Anterior MI | 91 | IVUS | 59 | 41 | |
| Ozaki 2011 | ACS | 57 (35) | CT | 71 | 29 | |
| Jia et al.34 | ACS | 126 (104) | OCT | 44 | 31 | 8% (CN) |
| Higuma et al.35 | AMI | 112 | OCT | 64 | 27 | 8% (CN) |
| Saia et al.36 | AMI | 140 (97) | OCT | 65 | 33 | 2% (SCAD) |
| Niccoli et al.37 | ACS | 139 | OCT | 59 | 41 | |
| Yonetsu et al.38 | ACS | 318 | OCT | 44 | 41 | 15%a |
| Kajander et al.39 | AMI | 93 | OCT | 49 | 44 | 7% (CN) |
| Kwon et al.41 | ACS | 133 | OCT | 68 | 32 |
Number: a total number of patients enrolled in the study. The number in parenthesis is the number of patients included in analysis.
ACS, acute coronary syndromes; AMI, acute myocardial infarction; CN, calcified nodule; IVUS, intravascular ultrasound; OCT, optical coherence tomography; SCAD, spontaneous coronary artery dissection.
Massive thrombus.
Figure 3.
An intact fibrous cap separates erosion from rupture or calcified nodule. In lesions without an intact fibrous cap, other characteristics of the underlying plaque further categorize the atheroma disruption as due to rupture or to penetration by a calcified nodule (adapted from Jia et al.34). OCT, optical coherence tomography.
Figure 4.
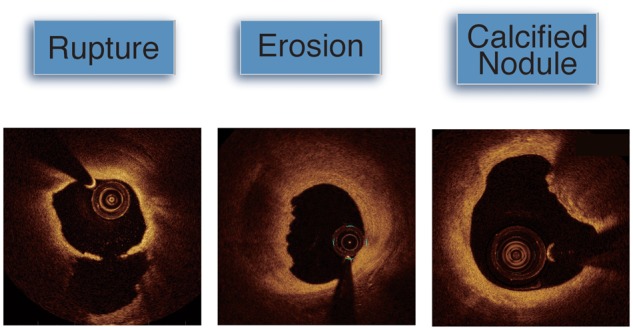
Optical coherence tomography imaging of a ruptured plaque shows circumferential superficial lipid and ruptured fibrous cap at 6 o’clock communicating with an empty cavity. A plaque complicated by erosion shows preserved vascular structure with larger lumen and the appearance of a platelet-rich thrombus. A calcific nodule resides at 1–3 o’clock. Such deposits are often seen in the area of substantive calcification.
Using the newly established OCT diagnostic criteria, Jia et al.34 reported erosion in 31% of patients with ACS. Subsequent studies by Higuma et al.,35 Saia et al.,36 and Kajander et al.39 showed that the prevalence of erosion was 27%, 33%, and 44% in patients with AMI, respectively. Niccoli et al.,40 Yonetsu et al.,39 and Kwon et al.41 reported the prevalence of erosion in 24%, 41%, and 32% of ACS patients, respectively. Taken together, the prevalence of erosion as determined by OCT ranges between 30% and 40% in patients with ACS/AMI. As patients with ACS do not undergo intravascular imaging routinely, the studies cited above may underestimate the actual incidence of plaque erosion.42
Type of lesion and clinical presentations
In the first systematic OCT study, of 126 patients with ACS, plaque erosion associated with non-ST-segment elevation ACS (NSTE-ACS) clinical presentation in 61.5% of patients. In contrast, plaque rupture occurred more commonly in ST-segment elevation myocardial infarction (STEMI) in 70.9% of cases, but in only 29.1% of NSTE-ACS.34 Consistent with autopsy studies, patients with the culprit plaque erosion more frequently had NSTE-ACS. With less disruption of arterial integrity and larger lumens, patients with erosion might have non-occlusive thrombus or occlusive thrombus that could easily embolize distally. Four studies that included both STEMI and NSTE-ACS patients consistently showed higher incidence of NSTE-ACS in patients with plaque erosion (Table 3).
Table 3.
Incidence of ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndromes/unstable angina pectoris in patients with plaque erosion
| STEMI (%) | NSTE-ACS/UAP (%) | |
|---|---|---|
| Jia et al.34 | 38.5 | 61.5 |
| Niccoli et al.37 | 29.8 | 70.2 |
| Yonetsu et al.38 | 16 | 84 |
| Kwon et al.41 | 35 | 65 |
NSTE-ACS, non-ST-segment elevation acute coronary syndromes; STEMI, ST-segment elevation myocardial infarction; UAP, unstable angina pectoris.
Management of acute coronary syndromes due to superficial erosion and future directions
Current guidelines for the management of both STEMI and NSTE-ACS generally recommend early percutaneous coronary intervention (PCI) and stent implantation to achieve reperfusion and revascularization. Improved plaque imaging has shown the ability to distinguish the underlying pathophysiological mechanism of thrombosis, and thus offer the opportunity to tailor management.
Impact on outcome: erosion vs. rupture
Advances in culprit lesion imaging have improved understanding of the clinical features of erosion vs. rupture. A retrospective study by Higuma et al.35 showed that ACS associated with plaque rupture, has higher risk of larger infarction size, no-reflow phenomenon, and subsequently lower left ventricular function. A recent study by Yonetsu et al.38 showed that ACS patients with plaque erosion had better long-term prognosis than those with plaque rupture during a median follow-up of 576 days. Another study with 139 consecutive ACS patients (66% NSTE-ACS, 34% STEMI) also showed fewer major adverse cardiac events in patients with intact fibrous cap (14.0%) when compared with plaque rupture (39.0%) during up to 3 years of follow-up.37 The mechanisms that underlie the worse outcome of plaque rupture remain speculative and require further research.
Vascular response to stenting: erosion vs. rupture
Possible differences in responses to PCI in rupture vs. erosion remain poorly understood. Higuma et al.35 studied 112 patients with STEMI and found that those with plaque erosion had less microvascular damage and a trend towards less myocardial damage post-PCI. Saia et al.36 evaluated 140 consecutive patients with STEMI who underwent OCT pre-, immediately post, and 9-month post-DES implantation. They showed no differences in plaque morphology between erosion and rupture at follow-up. Both groups had similar late lumen loss, restenosis, neointimal area, malapposition, or stent strut coverage.36 Another study found more favourable immediate post-stent outcomes in plaque erosion than in plaque rupture in 141 patients with ACS.44 There were lower rates of stent malapposition (7.3% vs. 37.5%, P < 0.001), thrombus (14.6% vs. 59.4%, P < 0.001), protrusion (73.2% vs. 93.8%, P = 0.008), no-reflow and distal embolization in erosion vs. rupture. In contrast, a recent study of 65 patients with ACS who underwent 6-month follow-up OCT showed that plaque erosion (37% of cases) less favourable vascular healing following DES implantation at 6 months when compared with plaque rupture. The degree of neointima coverage was lower and a ratio of uncovered/covered struts was higher at 6 months in the plaque erosion compared with plaque rupture.45 While the mechanisms for these findings remain uncertain, greater release of platelet-derived growth factor from a larger thrombus burden following rupture might have contributed to better healing.
Seminal prospective trials
Given the potential differences in vascular responses to PCI between plaque rupture and erosion and the association of plaque erosion with better overall clinical prognosis, a shift in management focusing on antithrombotic therapies rather than PCI in erosion cases merits consideration.42 Potential benefits include avoiding the risk of stent thrombosis and restenosis, and the need for prolonged dual antiplatelet therapy. An observational retrospective study showed that patients with OCT-defined plaque erosion who underwent aspiration thrombectomy remained asymptomatic for over 2 years. In this study, of 31 patients identified from four institutions, 40% were managed with dual antiplatelet therapy without PCI and 60% were managed with dual antiplatelet therapy plus PCI at the discretion of the treating clinicians. After over 2 years of follow-up, all patients remained asymptomatic regardless of stent implantation.46
The EROSION study was the first proof-of-concept study aimed at tailoring the treatment of ACS due to plaque erosion. This study showed the feasibility and initial safety of antithrombotic therapy without stenting in patients with plaque erosion.47 If OCT showed plaque erosion, and the residual vessel stenosis was <70% with TIMI grade 3 flow, patients received antithrombotic therapy without stenting (heparin for 3 days with concurrent aspirin and ticagrelor). Many patients also received glycoprotein IIb/IIIa antagonists, tirofiban. Plaque erosion was found in 25.4% of all cases, and at 1-month OCT follow-up, 78.3% of patients showed >50% decrease in thrombus volume (primary endpoint), and all but two patients remained free of major adverse cardiac events. While from a small, single-centre study, these data support further evaluation of therapeutic approaches tailored to the specific underlying mechanism of ACS.29
Clinical implications
Taken together, these findings suggest that pharmacological rather than mechanical intervention could provide an optimal treatment for patients with plaque erosion. This proposition calls for larger, randomized studies to affirm the pilot data and evaluate longer-term outcomes to test rigorously this novel management strategy for ACS due to plaque erosion. Ultimately, larger scale trials assessing the approach by which a combination of erosion-specific biomarkers40 and high resolution, non-invasive imaging that sharpens the ability to diagnose accurately plaque erosion, might pave the way towards practicing precision medicine based on pathophysiology of ACS. Confirmation of this concept in large-scale trials with longer follow-up could lead not only to a major shift in the management of over a million patients with ACS each year, but also to substantial saving of health care resources.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
NHLBI (NIH-R01 HL080472 to P.L.).
Conflict of interest: P.L. was supported by the RRM Charitable Fund. I-K.J. research was supported by the grant from Mr and Mrs Michael and Kathryn Park, and Mrs and Mr Gill and Allan Gray and also received educational grant and consulting fee from Abbott Vascular. All other authors declared no conflict of interest.
Supplementary Material
References
- 1. Crea F, Libby P.. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 2017;136:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J 1983;50:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies MJ, Thomas AC.. Plaque fissuring—the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J 1985;53:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R.. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 2013;34:719–728. [DOI] [PubMed] [Google Scholar]
- 5. van der Wal AC, Becker AE, van der Loos CM, Das PK.. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89:36–44. [DOI] [PubMed] [Google Scholar]
- 6. Farb A, Burke AP, Tang AL, Liang Y, Mannan P, Smialek J, Virmani R.. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996;93:1354–1363. [DOI] [PubMed] [Google Scholar]
- 7. Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, Virmani R.. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999;82:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;368:2004–2013. [DOI] [PubMed] [Google Scholar]
- 9. Falk E, Shah PK, Fuster V.. Coronary plaque disruption. Circulation 1995;92:657–671. [DOI] [PubMed] [Google Scholar]
- 10. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM.. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 11. Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, Virmani R.. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol 2002;22:1642–1648. [DOI] [PubMed] [Google Scholar]
- 12. Sato Y, Hatakeyama K, Yamashita A, Marutsuka K, Sumiyoshi A, Asada Y.. Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart 2005;91:526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartz RS, Burke A, Farb A, Kaye D, Lesser JR, Henry TD, Virmani R.. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: relation to epicardial plaque histopathology. J Am Coll Cardiol 2009;54:2167–2173. [DOI] [PubMed] [Google Scholar]
- 14. White SJ, Newby AC, Johnson TW.. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb Haemost 2016;115:509–519. [DOI] [PubMed] [Google Scholar]
- 15. Durand E, Scoazec A, Lafont A, Boddaert J, Hajzen AA, Addad F, Mirshahi M, Desnos M, Tedgui A, Mallat Z.. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation 2004;109:2503–2506. [DOI] [PubMed] [Google Scholar]
- 16. Warner SJ, Friedman GB, Libby P.. Regulation of major histocompatibility gene expression in human vascular smooth muscle cells. Arteriosclerosis 1989;9:279–288. [DOI] [PubMed] [Google Scholar]
- 17. Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P.. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol 2004;24:1309–1314. [DOI] [PubMed] [Google Scholar]
- 18. Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F.. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation 2010;122:2505–2513. [DOI] [PubMed] [Google Scholar]
- 19. Niccoli G, Dato I, Crea F.. Myeloperoxidase may help to differentiate coronary plaque erosion from plaque rupture in patients with acute coronary syndromes. Trends Cardiovasc Med 2010;20:276–281. [DOI] [PubMed] [Google Scholar]
- 20. Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ.. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P.. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J 2015;36:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary Risk Factors and Plaque Morphology in Men with Coronary Disease Who Died Suddenly. N Engl J Med 1997;336:1276–1282. [DOI] [PubMed] [Google Scholar]
- 23. Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of Risk Factors on the Mechanism of Acute Thrombosis and Sudden Coronary Death in Women. Circulation 1998;97:2110–2116. [DOI] [PubMed] [Google Scholar]
- 24. Burke AP, Farb A, Pestaner J, Malcom GT, Zieske A, Kutys R, Smialek J, Virmani R. Traditional Risk Factors and the Incidence of Sudden Coronary Death With and Without Coronary Thrombosis in Blacks. Circulation 2002;105:419–424. [DOI] [PubMed] [Google Scholar]
- 25. Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, Virmani R. Relationship of Thrombus Healing to Underlying Plaque Morphology in Sudden Coronary Death. J Am Coll Cardiol 2010;55:122–132. [DOI] [PubMed] [Google Scholar]
- 26. Tavora F, Cresswell N, Li L, Ripple M, Fowler D, Burke A. Sudden coronary death caused by pathologic intimal thickening without atheromatous plaque formation. Cardiovasc Pathol 2011;20:51–57. [DOI] [PubMed] [Google Scholar]
- 27. Döring Y, Soehnlein O, Weber C.. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res 2017;120:736–743. [DOI] [PubMed] [Google Scholar]
- 28. Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P.. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ Res 2017;121:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Libby P. Superficial erosion and the precision management of acute coronary syndromes: not one-size-fits-all. Eur Heart J 2017;38:801–803. [DOI] [PubMed] [Google Scholar]
- 30. Hu S, Yonetsu T, Jia H, Karanasos A, Aguirre AD, Tian J, Abtahian F, Vergallo R, Soeda T, Lee H, McNulty I, Kato K, Yu B, Mizuno K, Toutouzas K, Stefanadis C, Jang IK.. Residual thrombus pattern in patients with ST-segment elevation myocardial infarction caused by plaque erosion versus plaque rupture after successful fibrinolysis: an optical coherence tomography study. J Am Coll Cardiol 2014;63:1336–1338. [DOI] [PubMed] [Google Scholar]
- 31. Hayashi T, Kiyoshima T, Matsuura M, Ueno M, Kobayashi N, Yabushita H, Kurooka A, Taniguchi M, Miyataka M, Kimura A, Ishikawa K.. Plaque erosion in the culprit lesion is prone to develop a smaller myocardial infarction size compared with plaque rupture. Am Heart J 2005;149:284–290. [DOI] [PubMed] [Google Scholar]
- 32. Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T.. Assessment of culprit lesion morphology in acute myocardial infarction. Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol 2007;50:933–939. [DOI] [PubMed] [Google Scholar]
- 33. Kusama I, Hibi K, Kosuge M, Nozawa N, Ozaki H, Yano H, Sumita S, Tsukahara K, Okuda J, Ebina T, Umemura S, Kimura K.. Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J Am Coll Cardiol 2007;50:1230–1237. [DOI] [PubMed] [Google Scholar]
- 34. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park S-J, Jang Y-S, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi S-Y, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients With Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J Am Coll Cardiol 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, Vergallo R, Minami Y, Ong DS, Lee H, Okumura K, Jang I-K.. A Combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2015;8:1166–1176. [DOI] [PubMed] [Google Scholar]
- 36. Saia F, Komukai K, Capodanno D, Sirbu V, Musumeci G, Boccuzzi G, Tarantini G, Fineschi M, Tumminello G, Bernelli C, Niccoli G, Coccato M, Bordoni B, Bezerra H, Biondi-Zoccai G, Virmani R, Guagliumi G.. Eroded versus ruptured plaques at the culprit site of STEMI: in vivo pathophysiological features and response to primary PCI. JACC Cardiovasc Imaging 2015;8:566–575. [DOI] [PubMed] [Google Scholar]
- 37. Niccoli G, Montone RA, Vito L Di, Gramegna M, Niccoli G, Montone RA, Vito L Di, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015;36:1377–1384. [DOI] [PubMed] [Google Scholar]
- 38. Yonetsu T, Lee T, Murai T, Suzuki M, Matsumura A, Hashimoto Y, Kakuta T. Plaque morphologies and the clinical prognosis of acute coronary syndrome caused by lesions with intact fibrous cap diagnosed by optical coherence tomography. Int J Cardiol 2016;203:766–774. [DOI] [PubMed] [Google Scholar]
- 39. Kajander OA, Pinilla-Echeverri N, Jolly SS, Bhindi R, Huhtala H, Niemelä K, Fung A, Vijayaraghavan R, Alexopoulos D, Sheth T.. Culprit plaque morphology in STEMI—an optical coherence tomography study: insights from the TOTAL-OCT substudy. EuroIntervention 2016;12:716–723. [DOI] [PubMed] [Google Scholar]
- 40. Niccoli G, Montone RA, Cataneo L, Cosentino N, Gramegna M, Refaat H, Porto I, Burzotta F, Trani C, Leone AM, Severino A, Crea F.. Morphological-biohumoral correlations in acute coronary syndromes: pathogenetic implications. Int J Cardiol 2014;171:463–466. [DOI] [PubMed] [Google Scholar]
- 41. Kwon JE, Lee WS, Mintz GS, Hong YJ, Lee SY, Kim KS, Hahn JY, Kumar KS, Won H, Hyeon SH, Shin SY, Lee KJ, Kim TH, Kim CJ, Kim SW. Multimodality Intravascular Imaging Assessment of Plaque Erosion versus Plaque Rupture in Patients with Acute Coronary Syndrome. Korean Circ J 2016;46:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ozaki Y, Okumura M, Ismail TF, Motoyama S, Naruse H, Hattori K, Kawai H, Sarai M, Takagi Y, Ishii J, Anno H, Virmani R, Serruys PW, Narula J. Coronary CT angiographic characteristics of culprit lesions in acute coronary syndromes not related to plaque rupture as defined by optical coherence tomography and angioscopy. Eur Heart J 2011;32:2814–2823. [DOI] [PubMed] [Google Scholar]
- 43. Braunwald E. Coronary plaque erosion: recognition and management. JACC Cardiovasc Imaging 2013;6:288–289. [DOI] [PubMed] [Google Scholar]
- 44. Hu S, Zhu Y, Zhang Y, Dai J, Li L, Dauerman H, Soeda T, Wang Z, Lee H, Wang C, Zhe C, Wang Y, Zheng G, Zhang S, Jia H, Yu B, Jang IK.. Management and outcome of patients with acute coronary syndrome caused by plaque rupture versus plaque erosion: an intravascular optical coherence tomography study. J Am Heart Assoc 2017;6:e004730.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu S, Wang C, Zhe C, Zhu Y, Yonetsu T, Jia H, Hou J, Zhang S, Jang IK, Yu B.. Plaque erosion delays vascular healing after drug eluting stent implantation in patients with acute coronary syndrome: an in vivo optical coherence tomography study. Catheter Cardiovasc Interv 2017;89:592–600. [DOI] [PubMed] [Google Scholar]
- 46. Prati F, Uemura S, Souteyrand G, Virmani R, Motreff P, Vito L, Di Biondi-Zoccai G, Halperin J, Fuster V, Ozaki Y, Narula J.. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc Imaging 2013;6:283–287. [DOI] [PubMed] [Google Scholar]
- 47. Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, Xu M, Yao Y, Hu S, Yamamoto E, Lee H, Zhang S, Yu B, Jang I-K.. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur Heart J 2017;38:792–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.