Abstract
Takotsubo syndrome (TTS) is a poorly recognized heart disease that was initially regarded as a benign condition. Recently, it has been shown that TTS may be associated with severe clinical complications including death and that its prevalence is probably underestimated. Since current guidelines on TTS are lacking, it appears timely and important to provide an expert consensus statement on TTS. The clinical expert consensus document part I summarizes the current state of knowledge on clinical presentation and characteristics of TTS and agrees on controversies surrounding TTS such as nomenclature, different TTS types, role of coronary artery disease, and etiology. This consensus also proposes new diagnostic criteria based on current knowledge to improve diagnostic accuracy.
Keywords: Takotsubo syndrome, Broken heart syndrome, Takotsubo definition, Acute heart failure, Consensus statement, InterTAK Diagnostic Criteria
Outline
History 2033
Nomenclature 2033
Epidemiology 2034
Symptoms and signs 2035
Diagnostic criteria 2035
Pathophysiology 2036
Sympathetic stimulation 2036
Potential pathophysiological effects of enhanced sympathetic stimulation 2036
Plaque rupture 2036
Multi-vessel epicardial spasm 2036
Microcirculatory dysfunction 2036
Catecholamine toxicity on cardiomyocytes 2037
Activation of myocardial survival pathways 2037
Predisposition and risk factors 2038
Hormonal factors 2038
Genetic factors 2038
Psychiatric and neurologic disorders 2038
Triggers 2039
Emotional stressors 2039
Physical stressors 2039
Absence of identifiable causes 2039
Types of takotsubo syndrome 2039
Chronobiology 2042
References 2042
History
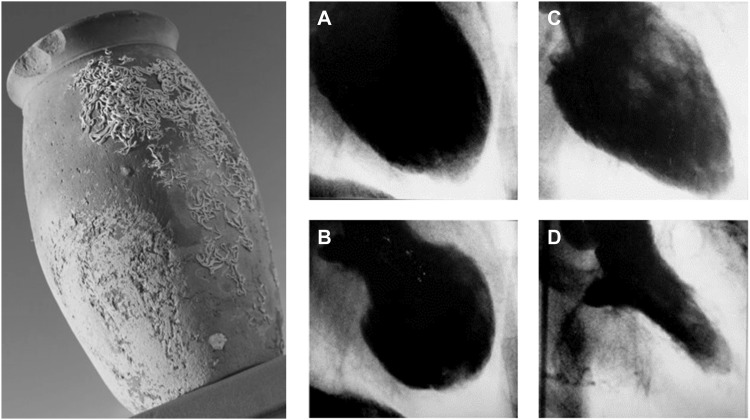
The term takotsubo syndrome (TTS) was first introduced when Sato et al.1 published their report of five cases in a Japanese medical textbook in 1990. The first TTS case of this series was managed in 1983 in the Hiroshima City Hospital (Figure 1). A 64-year-old female presented with acute chest pain consistent with acute myocardial infarction (AMI), typical electrocardiographic (ECG) changes, but normal coronary arteries and an unusual appearance of the left ventricle (LV) with a narrow neck and apical ballooning during systole. Interestingly, the marked wall motion abnormalities on left ventriculography disappeared after 2 weeks. Over time TTS was more frequently diagnosed in Japan. Therefore, it was first assumed that this disorder only affected people of Asian descent, as TTS was completely unknown to the Western world until the first cases were published from French and American research groups in the late 1990s.2,3 Desmet et al.4 introduced the first patient case series in Caucasians using the term ‘takotsubo’.
Figure 1.
Historical Japanese octopus trap (left). Courtesy of Dr Templin, University Hospital Zurich, Zurich, Switzerland. Left ventriculogram of the first reported case of takotsubo syndrome. Diastole (A) and systole (B) during the acute phase of takotsubo syndrome. Recovery of left ventricular wall motion abnormality two weeks after the event (C and D). Courtesy of Dr Dote, Hiroshima City Asa Hospital, Hiroshima, Japan.
Takotsubo syndrome gained international awareness among researcher and physicians when Wittstein et al.5 reported their findings in the New England Journal of Medicine in 2005. Since then TTS has been more frequently recognized worldwide but still remains an underappreciated and often misdiagnosed disorder.6,7
Nomenclature
Takotsubo syndrome derived its name from the Japanese word for octopus trap, due to the shape of the LV at the end of systole and has been described under a remarkable number of different names in the literature including ‘broken heart syndrome’, ‘stress cardiomyopathy’, and ‘apical ballooning syndrome’.8 No single term precisely describes the heterogeneous ventricular appearance with which this syndrome can occur. To date, consensus has not been reached on the nomenclature. The term ‘takotsubo’ is widely used in acknowledgement of the Japanese physicians who initially described this disorder.1 However, in contrast to other cardiomyopathies that are usually not transient in nature, TTS is characterized by a temporary wall motion abnormality of the LV and shares common features with acute coronary syndrome (ACS) [similar symptoms at presentation, ECG abnormalities, elevated cardiac biomarkers as well as a comparable in-hospital mortality with ST-segment elevation myocardial infarction (STEMI) and non-STEMI] specifically in terms of a microvascular ACS form.9 Among different etiologies of heart failure such as coronary artery disease (CAD), tachyarrhtyhmias etc. TTS includes a wide spectrum of emotional or physical triggers resulting also in left ventricular dysfunction. Therefore, it is best described as a ‘syndrome’ and the term ‘takotsubo syndrome’ seems most appropriate.9,10,11
Epidemiology
Since the initial report by Japanese cardiologists 25 years ago, TTS has been increasingly recognized in diverse countries across six continents. Takotsubo syndrome is estimated to represent approximately 1–3%12,13 of all and 5–6%14 of female patients presenting with suspected STEMI. The Nationwide Inpatient Sample discharge records from 2008 using the International Classification of Diseases revealed that TTS accounts for 0.02% of hospitalizations in the United States.15 Recurrence rate of TTS is estimated to be 1.8% per-patient year.16 Based on the published literature about 90%16,17 of TTS patients are women with a mean age of 67–70 years,16,18 and around 80% are older than 50 years (Figure 2).16 Women older than 55 years have a five-fold greater risk of developing TTS than women younger than 55 years and a 10-fold greater risk than men.15 With growing awareness of TTS, male patients are diagnosed more often, especially after a physical triggering event.19 TTS has also been described in children20,21 with the youngest reported TTS patient being a premature neonate born in the 28th gestational week.22 Current data on racial differences are inconsistent and large-scale studies are lacking. However, it has been reported that TTS seems to be uncommon in African–Americans and Hispanics,23 while most of the cases reported in the United States have been Caucasians.15,24 Furthermore, it has been reported that patients of African-American descent have more in-hospital complications such as respiratory failure, stroke and require more frequently mechanical ventilation compared to Caucasians and Hispanics.25 With regard to ECG differences, it has been shown that QT prolongation as well as T-wave inversion are more often reported in African-American women with TTS. 26 Of note, regarding gender differences the TTS prevalence in men appears to be higher in Japan.19 The prevalence of TTS appears to be higher in patients with non-emotional triggers admitted to intensive care units.27 Moreover, it is likely that subclinical TTS cases remain undetected, especially in non-percutaneous coronary intervention centres.28
Figure 2.
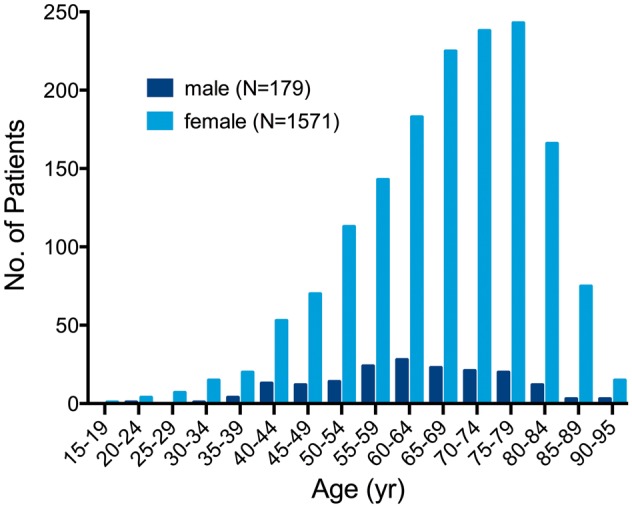
Age and sex distribution of patients with takotsubo syndrome. Reprinted with permission from Templin et al.16
Symptoms and signs
The most common symptoms of TTS are acute chest pain, dyspnoea, or syncope and thus indistinguishable from AMI at the first glance.16 However, in some patients, TTS may be diagnosed incidentally by new ECG changes or a sudden elevation of cardiac biomarkers. Clinical manifestation of TTS induced by severe physical stress may be dominated by the manifestation of the underlying acute illness. In this regard, patients with ischaemic stroke or seizure-triggered, TTS had less frequent chest pain,29,30 which could be explained by impaired consciousness, neurologic complications, or a sudden haemodynamic deterioration. In contrast, patients with emotional stress factors had a higher prevalence of chest pain and palpitations.31 Importantly, a subset of TTS patients may present with symptoms arising from its complications, e.g. heart failure, pulmonary oedema, stroke, cardiogenic shock, or cardiac arrest.
Diagnostic criteria
The diagnosis of TTS is often challenging because its clinical phenotype may closely resemble AMI regarding ECG abnormalities and biomarkers.32 While a widely established non-invasive tool allowing a rapid and reliable diagnosis of TTS is currently lacking, coronary angiography with left ventriculography is considered the gold standard diagnostic tool to exclude or confirm TTS.
Abe et al.33 introduced the first diagnostic criteria for TTS in 2003. One year later, a dedicated group of cardiologists from the Mayo Clinic proposed their diagnostic criteria.34 In 2006, the American College of Cardiology and American Heart Association classified TTS as a primary acquired cardiomyopathy.35 In 2008, the revised version of the Mayo Clinic Diagnostic Criteria was published incorporating neurogenic stunned myocardium.32 Furthermore, the authors defined different TTS sub-types and highlighted that obstructive coronary lesions may occasionally be present concomitantly.32 The Mayo Clinic Diagnostic Criteria are the most widely known, but exceptions to the rule [e.g. the presence of CAD] are poorly appreciated among physicians and cardiologists. More recently, other research groups have proposed slightly different criteria for TTS, i.e. the Japanese Guidelines,36 the Gothenburg criteria,37 the Johns Hopkins criteria,38 the Tako-tsubo Italian Network proposal,39 the criteria of the Heart Failure Association (HFA) TTS Taskforce of the European Society of Cardiology (ESC),10 as well as the criteria recommended by Madias.40 Thus, there is a lack of a worldwide consensus.41 Based on current knowledge, we have developed new international diagnostic criteria (InterTAK Diagnostic Criteria, Table 1) for the diagnosis of TTS that may help to improve identification and stratification of TTS. The most important changes with accompanying rationale include:
Table 1.
International Takotsubo Diagnostic Criteria (InterTAK Diagnostic Criteria)
| 1. | Patients show transienta left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or midventricular, basal, or focal wall motion abnormalities. Right ventricular involvement can be present. Besides these regional wall motion patterns, transitions between all types can exist. The regional wall motion abnormality usually extends beyond a single epicardial vascular distribution; however, rare cases can exist where the regional wall motion abnormality is present in the subtended myocardial territory of a single coronary artery (focal TTS).b |
| 2. | An emotional, physical, or combined trigger can precede the takotsubo syndrome event, but this is not obligatory. |
| 3. | Neurologic disorders (e.g. subarachnoid haemorrhage, stroke/transient ischaemic attack, or seizures) as well as pheochromocytoma may serve as triggers for takotsubo syndrome. |
| 4. | New ECG abnormalities are present (ST-segment elevation, ST-segment depression, T-wave inversion, and QTc prolongation); however, rare cases exist without any ECG changes. |
| 5. | Levels of cardiac biomarkers (troponin and creatine kinase) are moderately elevated in most cases; significant elevation of brain natriuretic peptide is common. |
| 6. | Significant coronary artery disease is not a contradiction in takotsubo syndrome. |
| 7. | Patients have no evidence of infectious myocarditis.b |
| 8. | Postmenopausal women are predominantly affected. |
Wall motion abnormalities may remain for a prolonged period of time or documentation of recovery may not be possible. For example, death before evidence of recovery is captured.
Cardiac magnetic resonance imaging is recommended to exclude infectious myocarditis and diagnosis confirmation of takotsubo syndrome.
(i) Pheochromocytoma is a neuroendocrine tumour derived from enterochromaffin cells of the adrenal gland that may lead to a ‘catecholamine storm’ with LV dysfunction, ECG abnormalities, and increased biomarkers as well as hypercontraction of sarcomeres and contraction band necrosis indistinguishable from TTS.42
Notwithstanding, most of the diagnostic criteria have excluded pheochromocytoma as a specific cause of TTS.32–34,36,37,40 The Japanese criteria emphasize that pheochromocytoma is a TTS-like myocardial dysfunction.36 Pheochromocytoma is also included as a secondary cause of TTS in the diagnostic criteria of the HFA of the ESC.10
(ii) Concomitant CAD is reported with a prevalence ranging from 10–29%.16,43,44 In this regard, patients with TTS and obstructive CAD are often misdiagnosed as classical ACS and differentiation can be challenging.45 Therefore, the presence of CAD should not be considered as an exclusion criterion as acknowledged by the modified Mayo Clinic Diagnostic Criteria.32 In such patients, the wall motion abnormalities usually extend beyond the territory of the involved coronary artery. Furthermore, TTS may co-exist with ACS46 and it has been reported that ACS itself may trigger TTS.47–50
(iii) There are rare cases in which the regional wall motion abnormality corresponds to the distribution of a single coronary artery.16,32,51 This holds true for the focal TTS type mostly involving an anterolateral segment.16,51 Therefore, the criteria should not exclude cases in which the wall motion abnormalities are restricted to the distribution of a single coronary artery. In this situation, a clear differentiation of TTS, ACS, or myocarditis requires cardiac magnetic resonance imaging demonstrating myocardial oedema rather than late gadolinium enhancement in case of TTS.52
Pathophysiology
Sympathetic stimulation
The precise pathophysiological mechanisms of TTS are incompletely understood, but there is considerable evidence that sympathetic stimulation is central to its pathogenesis. An identifiable emotionally or physically triggering event precipitates the syndrome in most cases,16 and TTS has been associated with conditions of catecholamine excess (e.g. pheochromocytoma,53 central nervous system disorders54) and activated specific cerebral regions.55 Clinical features of TTS and the various ballooning patterns can be caused by intravenous administration of catecholamines and beta-agonists.56 Although it has been shown that patients with TTS triggered by emotional stress have markedly elevated levels of catecholamines compared to patients with Killip Class III myocardial infarction,5 others57 could not replicate this finding most likely due to methodological issues. In line with a sympathetic stimulation, elevated norepinephrine levels in the coronary sinus have been found in TTS patients, suggesting an increase in the local release of myocardial catecholamines.58 Accordingly, analyses of heart rate variability have also demonstrated a sympathetic predominance and marked depression of parasympathetic activity during the acute phase.59 Microneurographic studies confirmed increased muscle sympathetic nerve activity and decreased spontaneous baroreflex control of sympathetic tone in some TTS patients,60 as did myocardial scintigraphy using 123I-metaiodobenzylguanidine.61 Furthermore, abnormalities in myocardial sympathetic function can persist for months after recovery of LV systolic function.62 These abnormalities appear to induce an interstitial mononuclear inflammatory response and occasionally contraction band necrosis.5
Several animal models have also supported the central role of adrenergic stimulation in TTS.63–65 In rats, LV apical ballooning can be provoked by immobilization stress and attenuated by alpha- and beta-receptor blockade.66 Furthermore, in a more recent and novel rat model, it was possible to demonstrate that the administration of different catecholamines instigates the various ventricular ballooning patterns by an afterload-dependent mechanism.67
Potential pathophysiological effects of enhanced sympathetic stimulation
Although enhanced sympathetic stimulation is central to TTS, the mechanism by which catecholamine excess precipitates myocardial stunning in the variety of regional ballooning patterns that characterize this syndrome is unknown. Several hypotheses have been proposed as follows:
Plaque rupture
It has been suggested that transient ischaemia induced by plaque rupture followed by rapid lysis may cause myocardial stunning in patients with apparent non-obstructed CAD at angiography. Indeed, eccentric atherosclerotic plaques in the mid-portion of the left anterior descending (LAD) coronary artery have been reported, but intravascular ultrasound and optical coherence tomography have failed to identify ruptured plaques in the vast majority of TTS patients.68–70 Furthermore, this explanation is very unlikely as patients with TTS exhibit wall motion abnormalities extending beyond single coronary vascular territories and also sometimes include the right ventricle. In addition, the apical ballooning phenotype is known to occur in the absence of a wraparound LAD and this coronary anatomical variant is not more prevalent in TTS than in the control group.71
Multi-vessel epicardial spasm
Sympathetically mediated epicardial spasm has been proposed as a potential cause in TTS. Takotsubo syndrome may be associated with endothelial dysfunction and other conditions of abnormal vasomotor function such as migraine or Raynaud’s phenomenon.72 Similarly, endothelium-dependent dilation is reduced after emotional stress and prevented by endothelin antagonists.73 At presentation, patients with TTS have marked impairment in brachial artery flow-mediated dilation compared to those with infarction or healthy controls, which gradually improves over several weeks.74 In the early recovery period, predisposition to coronary vasospasm using intracoronary acetylcholine was demonstrated in some, but not all TTS patients.75 Furthermore, it has been suggested that the pattern of LV dysfunction in patients with TTS may require involvement of specific coronary side branches.76 Similarly, myocardial bridging in the LAD has been considered.77 Although epicardial coronary vasoconstriction may contribute to TTS in a subset of patients,1,78 the vast majority of patients do not show any evidence of epicardial spasm even with use of provocative agents.
Furthermore, endothelial dysfunction is often associated with oxidative stress, and studies suggest that this may play a role in myocardial dysfunction in TTS. A recent study by Zhang et al.79 found that hydrogen sulfide relieved cardiac dysfunction in animal models by decreasing oxidative stress. It has been reported that the level of oxidative stress correlates to the extent of myocardial dysfunction in TTS patients in the acute recovery phase. Nanno et al.80 measured 8-hydroxy-2’-deoxyguanosine (8-OHdG) and norepinephrine levels in TTS patients compared with AMI patients. They found that 8-OHdG levels changed proportionately with wall motion score and plasma levels of norepinephrine were twice as high in TTS patients as in AMI patients.
Microcirculatory dysfunction
Catecholamines and endothelin exert their vasoconstrictor effects primarily in the coronary microvasculature where α1-receptors81 and endothelin receptor type A predominate suggesting that acute microcirculatory dysfunction may have a central role in TTS. Furthermore, acutely TTS exhibits decreased microRNA (miRNA) 125a-5p as well as increased plasma levels of its target endothelin-1 in line with the microvascular spasms hypothesis.82 Microvascular blood flow may be reduced in the acute phase of TTS as is coronary flow reserve.83–87 Similarly, increased thrombolysis in myocardial infarction (TIMI) frame counts and abnormal grades of TIMI myocardial perfusion have been noted.11,88
In the acute phase, intravenous administration of adenosine has been shown to transiently improve myocardial perfusion, wall motion score index, and left ventricular ejection fraction (LVEF) in TTS, suggesting that intense microvascular constriction plays a major role in the pathophysiology.89 In addition, the notion of acute microcirculatory dysfunction in TTS as a contributing pathophysiological factor secondary to enhanced sympathetic stimulation is supported by endomyocardial biopsies revealing apoptosis of microvascular endothelial cells.90 Microcirculatory dysfunction in the acute phase of TTS is transient and its recovery appears to correlate with improved myocardial function.
Cold pressor testing 1–3 years after the acute episode results in an elevation of catecholamines and transient apical and mid-LV wall motion abnormalities.91 Mental stress or reactive hyperaemia result in lower vasomotor responses, but higher catecholamine levels in women with TTS compatible with impaired vascular reactivity and endothelial function.92 Similarly, in women with a history of TTS coronary vasomotion to acetylcholine is impaired.93 Impaired microvascular endothelial function was observed in virtually all patients with TTS.
Catecholamine toxicity on cardiomyocytes
Transient LV dysfunction in TTS could also result from direct effects of catecholamines on cardiomyocytes. Endomyocardial biopsies revealed occasional contraction band necrosis, which is generally observed in clinical settings of extreme catecholamine production such as pheochromocytoma or subarachnoid haemorrhage, associated with hypercontracted sarcomeres, dense eosinophilic transverse bands, and interstitial mononuclear inflammation as a reflection of myocyte injury.38 Catecholamines can decrease myocyte viability through cyclic adenosine monophosphate (cAMP) mediated Ca2+ overload as it may occur in TTS. Sarcoplasmic-Ca2+-adenosine-triphosphatase (SERCA2a) gene expression is downregulated and that of sarcolipin upregulated, while phospholamban is dephosphorylated in TTS.94 Thus, an increased phospholamban/SERCA2a ratio could result in contractile dysfunction due to decreased Ca2+-affinity.95 Indeed, intense G-protein stimulated β1-adrenergic receptor signalling modulates gene expression via the cAMP responsive element binding protein-1 and nuclear factor of activated T-cells signalling pathways.95
In rodent heart failure models, administration of isoproterenol yields apical fibrosis,96 and abnormalities of apical contraction and metabolism,97 features known to occur in dysfunctional apical segments during the acute phase in TTS using fludeoxyglucose-positron emission tomographic studies.98,99 In animal models, intracellular lipid droplets accumulate in cardiomyocytes in response to high doses of catecholamines100 as in endomyocardial biopsies of TTS patients during the acute phase, but not after recovery.101 In a rat model of TTS myocardial perfusion in dysfunctional segments appears preserved, challenging microvascular spasm as a primary mediator.102
In the mammalian LV β-adrenergic receptor density is highest in the apex, while sympathetic innervation is the lowest63,103–105 suggesting that it may be more sensitive to high levels of catecholamines which may reduce not only coronary blood flow, but at high levels paradoxically also exert negative inotropic effects63,104 due to a ‘molecular switch’ of the β2-adrenergic receptor from the positive inotropic Gs to the negatively inotropic Gi pathway.106–108 Since the β2-adrenoceptor is linked via Gi activation to stimulation of endothelial nitric oxide (NO) synthase, it seems possible, that peroxynitrate mediated nitrosative stress could lead to negative inotropy and inflammation in TTS. Indeed, TTS patients have markers of increased NO signalling109 and post-mortem hearts of TTS patients also demonstrate markers of increased nitrosative stress.110 Peroxynitrite release would also result in activation of poly(ADP-ribose)-transferase-1, which might contribute to the myocardial energetic impairment, which has recently been reported in patients with TTS.111 Endomyocardial biopsies in patients with TTS further suggest that these anti-apoptotic pathways are activated acutely.112 A polymorphism of the G-protein receptor kinase 5 (GRK5) gene L41Q that blunts β2-Gi trafficking appears common in TTS.113 On the other hand, a larger study failed to support the conclusions of this study.114
In summary, current evidence suggests that TTS is caused by an acute release of catecholamines from either sympathetic nerves, the adrenal medulla, or as drug therapy and occurs primarily in subjects with increased susceptibility of the coronary microcirculation and of cardiac myocytes to the stress hormones leading to prolonged but transient LV dysfunction with secondary myocardial inflammation.
Activation of myocardial survival pathways
The severe wall motion abnormalities seen in TTS are transient suggesting that protective mechanisms are likely to operate to preserve myocardial integrity. Two different mechanisms might elicit myocardial protection. The first one is represented by adrenoceptor-related protective mechanisms. Indeed, supra-physiological levels of epinephrine trigger β2-adrenoceptor to switch from Gs to Gi coupling, thus causing a negative inotropic response, which limits the degree of acute myocardial injury in response to the catecholamine surge.107 The second mechanism is represented by the phosphoinositide 3-kinase/protein kinase B (AKT) survival pathway, which has been found to be transiently activated during the acute phase of TTS.112 AKT is critical for postnatal cardiac growth and coronary angiogenesis. Also, its downstream targets, especially the mechanistic target of rapamycin and glycogen synthase kinase 3 (GSK3), are well-established regulators of metabolism, proliferation, and cell survival. Cell survival is achieved through various mechanisms: (i) direct inhibition of apoptosis, (ii) inhibition of proapoptotic transcriptional factors, (iii) enhancement of anti-apoptotic transcriptional factors, and (iv) enhancement of cell metabolism by inhibition of the GSK3.
The demonstration that down-regulation of myocardial function is a protective mechanism caused by a severe reduction of perfusion is confirmed by several clinical studies showing ‘inverse perfusion-metabolism mismatch,’ which is typically observed during myocardial stunning.115
Predisposition and risk factors
Psychological and physical stressors are universal and affect virtually all individuals throughout their life. However, very few people develop TTS and even fewer experience recurrent episodes. These observations support the existence of risk factors that may make certain individuals more susceptible to TTS. Predisposition and risk factors for TTS are reviewed below:
Hormonal factors
The striking preponderance of postmenopausal females suggests a hormonal influence. Potentially, declining oestrogen levels after menopause increase the susceptibility to TTS in women.116 Indeed, women older than 55 years have an almost five-fold risk of developing TTS compared to those younger than 55 years.15 Oestrogens can influence vasomotor tone via up-regulation of endothelial NO synthase.117 Also, there is evidence that oestrogens can attenuate catecholamine-mediated vasoconstriction and decrease the sympathetic response to mental stress in perimenopausal women.118,119 In women with subarachnoid haemorrhage, low levels of oestradiol have been associated with an increased risk of LV wall motion abnormalities.120 In ovariectomized rats subjected to immobilization stress, ECG and contractile abnormalities can be induced and attenuated with oestrogen supplementation.121 However, systematic data demonstrating a clear link between oestrogen levels and the development of TTS are lacking so far.
Genetic factors
A genetic predisposition to TTS has been suggested by a report of five cases of familial TTS, two in mother-daughter pairs122,123 and three in pairs of sisters.124–126 Takotsubo syndrome does not appear to have a multigenerational Mendelian inheritance pattern. Hence, it is likely that a genetic predisposition (if present) may interact with environmental factors, polygenic aetiology and/or recessive susceptibility alleles. Polymorphisms in adrenergic genes indeed affect receptor function and downstream signalling, 127 and this raises the possibility that their distribution may differ in TTS patients. Indeed, functional variants of adrenergic receptor genes have been associated with the magnitude of cardiac dysfunction in patients with subarachnoid haemorrhage128 and pheochromocytoma,129 conditions which can trigger TTS.
β1-adrenergic receptor (amino acid position 389) and β2-adrenergic receptor (amino acid position 27) variants were associated with a greater release of troponin I and α2-adrenergic receptor deletion (del322–325) with reduced LVEF.128 However, α2c-adrenergic receptor and β1-adrenergic receptor polymorphisms do not seem to differ between TTS and controls.130 In contrast, a different distribution of β1-receptor polymorphisms Arg389Gly [homozygous arginine (Arg)/Arg] is more frequently found in TTS, while β2-receptor polymorphisms Gln27Glu [homozygous glutamine (Gln)/Gln] were found more frequently in healthy controls, and no difference was observed in the β2-receptor Arg16Gly variant between groups.131 Furthermore, similar genetic polymorphisms in the β1-adrenergic receptor and the β2-adrenergic receptor were noted in TTS and controls, while a higher frequency of rs17098707 polymorphism in the GRK5 gene was found in TTS patients.113 Unfortunately, these studies provide conflicting results and are limited in their gene-targeted approach and incomplete in genetic characterization of the complex adrenergic signalling network. Whole-exome sequencing in 28 TTS subjects revealed no difference in allele frequency or burden between TTS subjects and population controls.132 As such, these data do not provide strong evidence for a genetic predisposition in TTS, but lend support to genetic heterogeneity and a potential polygenic susceptibility conferring a cumulative effect on dysregulation of adrenergic pathways. Most of the published studies were conducted in small cohorts and much larger cohorts are required to evaluate the genetics of TTS comprehensively.
Borchert et al.133 have investigated a genetic predisposition for TTS by creating the first ‘takotsubo in a dish’ model by using TTS-specific induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). This model recapitulates some of the pathophysiology observed in patients during the acute phase of TTS allowing further exploration of underlying mechanisms.134 They found an overactive β-adrenergic pathway and higher sensitivity of catecholamines in TTS iPSC-CMs and TTS engineered heart muscle.133 Interestingly, receptor desensitization and different β1/β2-adrenoreceptor responses shed further light on the mechanisms of TTS. Based on this TTS-model future treatment targets should be identified to rescue patients with TTS.133,134
Psychiatric and neurologic disorders
A high prevalence of psychiatric and neurologic disorders has been reported in patients with TTS. In an age- and sex-matched comparison between patients with TTS and ACS, rates of psychiatric or neurologic disorders were substantially higher in TTS.16 In this regard, 27% had an acute, former, or chronic history of neurologic disorders and 42% had a psychiatric diagnosis with half of them suffering from depression.16 Indeed, anxiety and depression appear more common in TTS than in patients with STEMI or healthy controls135 and in a prospective study, the prevalence of depression and anxiety was 78%, much higher than in patients with ACS.136 Patients with TTS also appear to have a high prevalence of type-D-personality, which is characterized by negative emotions and social inhibition, and which has been associated with an increased cardiovascular risk.137 However, another study found no difference in the personality profile and stress coping skills between TTS patients and population controls.138,139 Interestingly, in a recent study comparing the signature of circulating miRNAs in TTS and STEMI, miR-16 and miR-26a, known to be associated with neuropsychiatric conditions, were significantly upregulated in TTS.82 Psychological disorders may thus have a pathogenic role. Of note, depressed patients have an exaggerated norepinephrine response to emotional stress,140 and a subset of patients has an increased spillover and decreased reuptake of norepinephrine. Similarly, patients with panic disorder and anxiety have a decreased catecholamine reuptake due to impairment of norepinephrine reuptake transporters.141 On the other hand, antidepressants, e.g. selective norepinephrine reuptake inhibitors, may facilitate myocardial stunning by increasing local levels of catecholamines.142 This increased sympathetic response to acute stress combined with greater cardiac sympathetic sensitivity may make patients with mood disorders and anxiety susceptible to stress-related cardiac dysfunction.
Takotsubo syndrome has been reported to occur after neurologic disorders especially stroke,143 subarachnoid haemorrhage,144 and seizures.29 Histopathological findings of autopsied patients with sudden unexpected death in epilepsy revealed contraction band necrosis,145 abnormalities also found in autopsied patients with TTS.146 It has been demonstrated that regions of the insular or posterior fossa are mainly affected in patients with ischaemic stroke and epileptic events.147 This suggests that neurologic or psychiatric conditions may serve as predisposing factors for the development of TTS. Furthermore, a heart-brain interaction has been proposed in TTS. In this regard, substantial structural differences between TTS and healthy controls have been shown including the limbic network comprising the insula, amygdala, cingulate cortex, and hippocampus, all of which are strongly involved in the control of emotional processing, cognition, and the autonomic nervous system.148
Triggers
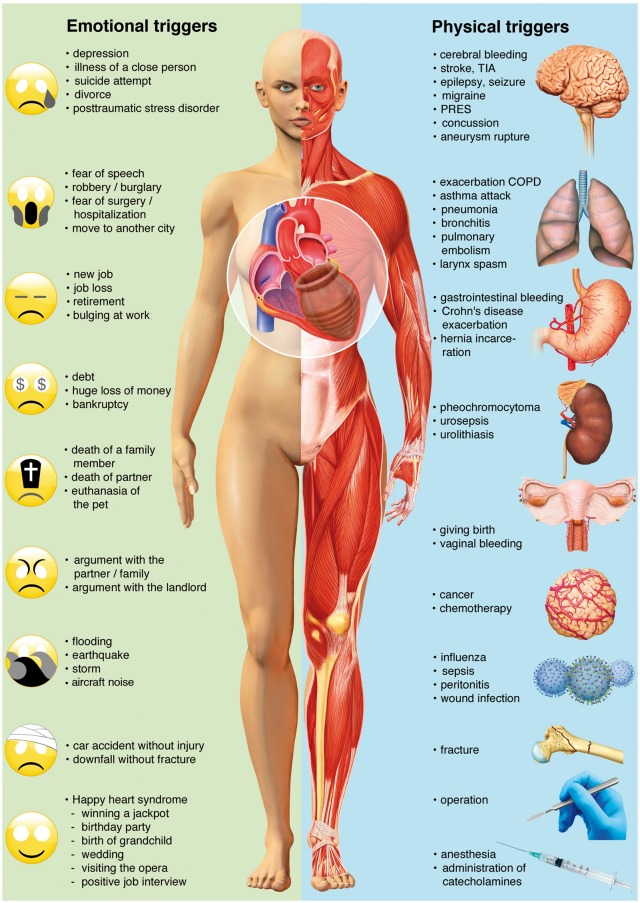
A hallmark of TTS is its association with a preceding stressful event. Initially, most reported triggers involved an emotional trauma.1 As TTS became more known, an association with physical stressors was also noted as well as TTS cases that occur in the absence of an evident stressor.16,149 A systematic illustration of preceding emotional and physical stressors is shown in Figure 3.
Figure 3.
Emotional and physical stress factors precipitating takotsubo syndrome. Reprinted, modified, and translated with permission from Schlossbauer et al.7 COPD, chronic obstructive pulmonary disease; PRES, posterior reversible encephalopathy syndrome; TIA, transient ischaemic attack.
Physical triggers are more common than emotional stress factors.16 Interestingly, male patients are more often affected from a physical stressful event, while in women an emotional trigger can be more frequently observed.16 Of note, precipitating triggers may represent a combination of emotional and physical issues16 (e.g. panic attack during a medical procedure), as well as environmental triggers such as long-term exposure to aircraft noise150. On the other hand, about one-third of patients presents without evidence of an identifiable preceding stressful event.151
In hospitalized patients, TTS may have an atypical presentation and manifest itself by tachycardia, hypotension, heart failure, elevation of cardiac biomarkers, or ECG abnormalities. It has been reported that patients with in-hospital TTS are more frequently males and have a higher prevalence of in-hospital death compared to patients with out-of-hospital TTS.152 This suggests that out-of-hospital TTS often occurs in the absence of a critical medical problem, while in-hospital TTS is preceded mainly by chronic comorbidities or acute medical illnesses.
Emotional stressors
Psychological triggers represent a range of traumatic emotions including grief (e.g. death of a family member, friend, or pet), interpersonal conflicts (e.g. divorce or family estrangement), fear and panic (e.g. robbery, assault, or public speaking), anger (e.g. argument with a family member or landlord), anxiety (e.g. personal illness, childcare, or homelessness), financial or employment problems (e.g. gambling loss, business failure, or job loss), or embarrassment (e.g. legal proceedings, infidelity, incarceration of family member, defeat in a competitive event).149 Natural disasters such as earthquakes153,154 and floods155 are also associated with an increase in TTS events. However, emotional triggers are not always negative as positive emotional events can also provoke TTS (e.g. surprise birthday party, winning a jackpot, and positive job interview)156 as shown in Figure 3. This entity has been described as the 'happy heart syndrome.156
Physical stressors
Physical stressors may be related to physical activities (for instance heavy gardening157 or sports158), medical conditions, or procedures such as acute respiratory failure (e.g. asthma,159 end-stage chronic obstructive lung disease160), pancreatitis,161 cholecystitis,162 pneumothorax,163 traumatic injury,164 sepsis,165 thyrotoxicosis,166 malignancy also including chemotherapy167 and radiotherapy,168 pregnancy,169 Caesarean section,170 lightning strike,171 near drowning,172 hypothermia,173 cocaine,174 alcohol175 or opiate withdrawal,176 and carbon-monoxide poisoning.177 Exogenous drugs in terms of catecholamines56,178 and sympathomimetic drugs56,179 may also act as triggers for TTS including dobutamine stress testing,180 electrophysiological testing181 (with isoproterenol or epinephrine) and beta-agonists for asthma or chronic obstructive lung disease.179,182 Also, acute coronary artery obstruction might act also as a trigger for TTS.47
Nervous system conditions (e.g. stroke,143 head trauma,183 migraine72, intracerebral haemorrhage,184 or seizures29) also represent an important trigger in the acute onset of TTS.
Endogenous catecholamine spillover related to pheochromocytoma serves as a distinct physical trigger.
Absence of identifiable causes
Recognition that TTS may occur spontaneously has demonstrated the inappropriateness of the term ‘stress cardiomyopathy’ to describe the entire spectrum of TTS. Whether the clinical course differs for this subset is unknown, and levels of catecholamines and related hormones have not been reported.
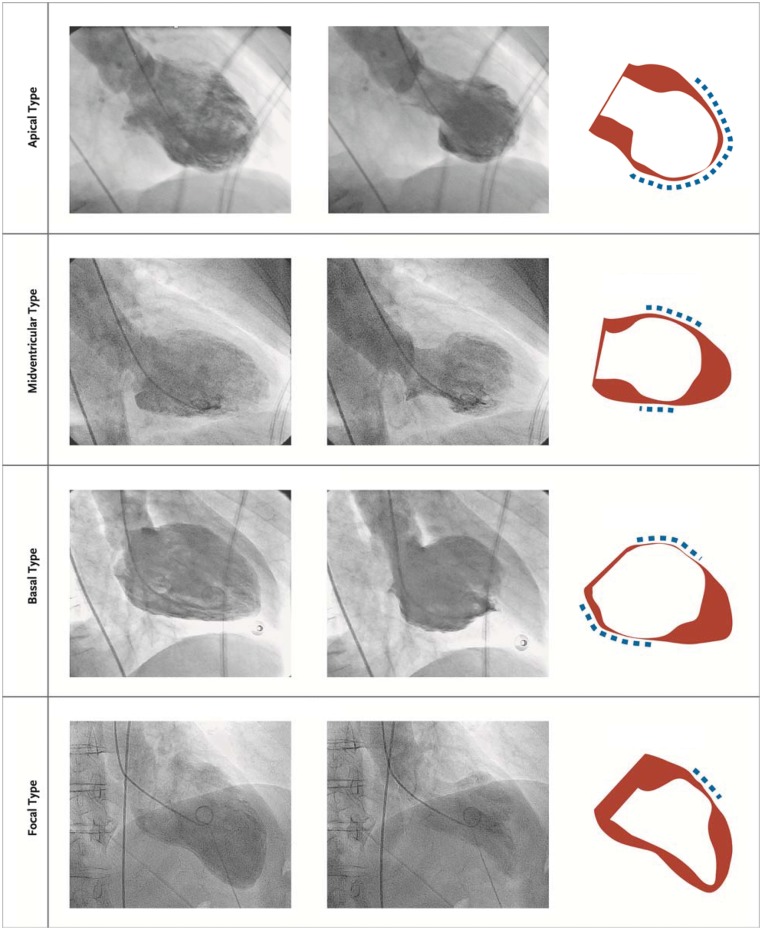
Types of takotsubo syndrome
Although several anatomical TTS variants have been described four major types can be differentiated based on the distribution of regional wall motion abnormalities as shown in Figure 4.16,51 The most common TTS type and widely recognized form is the (i) apical ballooning type also known as the typical TTS form, which occurs in the majority of cases.16,51 Over the past years, atypical TTS types have been increasingly recognized.51 These include the (ii) midventricular, (iii) basal, and (iv) focal wall motion patterns.51 Recently, it has been demonstrated that patients suffering from atypical TTS have a different clinical phenotype.51 These patients are younger, suffer more often from neurologic comorbidities, have lower brain natriuretic peptide values, a less impaired LVEF, and more frequent ST-segment depression compared to typical TTS.51,185 In-hospital complication rate is similar between typical and atypical types, while 1-year mortality is higher in typical TTS.51 After adjustment for confounders, LVEF <45%, atrial fibrillation, neurologic disorders but not TTS phenotype were independent predictors of death.51 Beyond 1-year, long-term mortality is similar in typical and atypical TTS phenotypes, therefore, patients should be equally monitored and treated.51 The basal phenotype has been reported to be associated with the presence of pheochromocytoma,186 epinephrine-induced TTS,178 or subarachnoid haemorrhage187 consequently, these conditions should be considered in this particular setting.
Figure 4.
The four different types of takotsubo syndrome during diastole (left column) and systole (middle column). The right column depicts diastole in red and systole in white. The blue dashed lines demonstrate the region of the wall motion abnormality. Reprinted and modified with permission from Templin et al.16
Besides the four major TTS types, other morphological variants have been described including the biventricular (apical type and right ventricular involvement),19 isolated right ventricular,188,189 and global form.190 Global hypokinesia as a manifestation of TTS is difficult to prove given the very broad differential diagnoses including conditions such as tachycardia-induced cardiomyopathy. Right ventricular involvement is present in about one-third of TTS patients and may be a predictor for worse outcome.191 The true prevalence of the isolated right ventricular form is unknown since little attention is paid to the right ventricle in daily clinical echo routine.
Patients with recurrent TTS can demonstrate different wall motion patterns at each event,192,193 suggesting that left ventricular adrenergic receptor distribution does not explain different TTS types.
Chronobiology
A growing body of evidence reveals that acute cardiovascular events are not distributed randomly over time, but instead depend on the time of day, day of the week, and months/season of the year.194–197 Several studies have investigated chronobiological features of TTS. Two studies reported a peak in the morning198,199 and afternoon hours,200 while others failed to show a statistically significant temporal pattern.201 Two studies observed the highest frequency on Monday196,202 and a third investigation has not found a weekly variation.198 Most conducted studies reported a summer preference for TTS,24,203 while one study reported a winter peak.204 Hence, conflicting results about the chronobiological pattern of TTS exist.
Funding
J.R.G. has received a research grant “Filling the gap” from the University of Zurich.
Conflict of interest: none declared.
References
- 1. Sato H. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm In: Kodama K, Haze K,, Hori M, eds. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo: Kagakuhyoronsha Publishing Co; 1990. p56–64; (Article in Japanese). [Google Scholar]
- 2. Pavin D, Le Breton H, Daubert C.. Human stress cardiomyopathy mimicking acute myocardial syndrome. Heart 1997;78:509–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharkey SW, Shear W, Hodges M, Herzog CA.. Reversible myocardial contraction abnormalities in patients with an acute noncardiac illness. Chest 1998;114:98–105. [DOI] [PubMed] [Google Scholar]
- 4. Desmet WJ, Adriaenssens BF, Dens JA.. Apical ballooning of the left ventricle: first series in white patients. Heart 2003;89:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC.. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–548. [DOI] [PubMed] [Google Scholar]
- 6. Templin C, Napp LC, Ghadri JR.. Takotsubo syndrome: underdiagnosed, underestimated, but understood? J Am Coll Cardiol 2016;67:1937–1940. [DOI] [PubMed] [Google Scholar]
- 7. Schlossbauer SA, Ghadri JR, Templin C.. Takotsubo-Syndrom—ein häufig verkanntes Krankheitsbild. Praxis (Bern 1994) 2016;105:1185–1192. [DOI] [PubMed] [Google Scholar]
- 8. Sharkey SW, Lesser JR, Maron MS, Maron BJ.. Why not just call it tako-tsubo cardiomyopathy: a discussion of nomenclature. J Am Coll Cardiol 2011;57:1496–1497. [DOI] [PubMed] [Google Scholar]
- 9. Luscher TF, Templin C.. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur Heart J 2016;37:2816–2820. [DOI] [PubMed] [Google Scholar]
- 10. Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E.. Current state of knowledge on takotsubo syndrome: a position statement from the taskforce on takotsubo syndrome of the heart failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
- 11. Pelliccia F, Sinagra G, Elliott P, Parodi G, Basso C, Camici PG. Takotsubo is not a cardiomyopathy. Int J Cardiol 2018;254:250–253. [DOI] [PubMed] [Google Scholar]
- 12. Prasad A, Dangas G, Srinivasan M, Yu J, Gersh BJ, Mehran R, Stone GW.. Incidence and angiographic characteristics of patients with apical ballooning syndrome (takotsubo/stress cardiomyopathy) in the HORIZONS-AMI trial: an analysis from a multicenter, international study of ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2014;83:343–348. [DOI] [PubMed] [Google Scholar]
- 13. Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, Wright RS, Rihal CS.. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004;94:343–346. [DOI] [PubMed] [Google Scholar]
- 14. Redfors B, Vedad R, Angeras O, Ramunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D, Libungan B, Shao Y, Albertsson P, Stone GW, Omerovic E.. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction—a report from the SWEDEHEART registry. Int J Cardiol 2015;185:282–289. [DOI] [PubMed] [Google Scholar]
- 15. Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL.. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J 2012;164:66–71 e1. [DOI] [PubMed] [Google Scholar]
- 16. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF.. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 17. Schneider B, Athanasiadis A, Stollberger C, Pistner W, Schwab J, Gottwald U, Schoeller R, Gerecke B, Hoffmann E, Wegner C, Sechtem U.. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int J Cardiol 2013;166:584–588. [DOI] [PubMed] [Google Scholar]
- 18. Roshanzamir S, Showkathali R.. Takotsubo cardiomyopathy a short review. Curr Cardiol Rev 2013;9:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aizawa K, Suzuki T.. Takotsubo cardiomyopathy: Japanese perspective. Heart Fail Clin 2013;9:243–247. [DOI] [PubMed] [Google Scholar]
- 20. Berton E, Vitali-Serdoz L, Vallon P, Maschio M, Gortani G, Benettoni A.. Young girl with apical ballooning heart syndrome. Int J Cardiol 2012;161:e4–e6. [DOI] [PubMed] [Google Scholar]
- 21. Otillio JK, Harris JK, Tuuri R.. A 6-year-old girl with undiagnosed hemophagocytic lymphohistiocytosis and takotsubo cardiomyopathy: a case report and review of the literature. Pediatr Emerg Care 2014;30:561–565. [DOI] [PubMed] [Google Scholar]
- 22. Rozema T, Klein LR.. Takotsubo cardiomyopathy: a case report and literature review. Cardiol Young 2016;26:406–409. [DOI] [PubMed] [Google Scholar]
- 23. Nascimento FO, Larrauri-Reyes MC, Santana O, Pérez-Caminero M, Lamas GA.. Comparison of stress cardiomyopathy in hispanic and non-hispanic patients. Rev Esp Cardiol (Engl Ed) 2013;66:67–68. [DOI] [PubMed] [Google Scholar]
- 24. Regnante RA, Zuzek RW, Weinsier SB, Latif SR, Linsky RA, Ahmed HN, Sadiq I.. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol 2009;103:1015–1019. [DOI] [PubMed] [Google Scholar]
- 25. Franco E, Dias A, Koshkelashvili N, Pressman GS, Hebert K, Figueredo VM. Distinctive electrocardiographic features in African Americans diagnosed with takotsubo cardiomyopathy. Ann Noninvasive Electrocardiol 2016;21:486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qaqa A, Daoko J, Jallad N, Aburomeh O, Goldfarb I, Shamoon F. Takotsubo syndrome in African American vs. non-African American women. West J Emerg Med 2011;12:218–223. [PMC free article] [PubMed] [Google Scholar]
- 27. Park JH, Kang SJ, Song JK, Kim HK, Lim CM, Kang DH, Koh Y.. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest 2005;128:296–302. [DOI] [PubMed] [Google Scholar]
- 28. Ghadri JR, Ruschitzka F, Luscher TF, Templin C.. Takotsubo cardiomyopathy: still much more to learn. Heart 2014;100:1804–1812. [DOI] [PubMed] [Google Scholar]
- 29. Stollberger C, Wegner C, Finsterer J.. Seizure-associated Takotsubo cardiomyopathy. Epilepsia 2011;52:e160–e167. [DOI] [PubMed] [Google Scholar]
- 30. Jung JM, Kim JG, Kim JB, Cho KH, Yu S, Oh K, Kim YH, Choi JY, Seo WK.. Takotsubo-like myocardial dysfunction in ischemic stroke: a hospital-based registry and systematic literature review. Stroke 2016;47:2729–2736. [DOI] [PubMed] [Google Scholar]
- 31. Song BG, Yang HS, Hwang HK, Kang GH, Park YH, Chun WJ, Oh JH.. The impact of stressor patterns on clinical features in patients with tako-tsubo cardiomyopathy: experiences of two tertiary cardiovascular centers. Clin Cardiol 2012;35:E6–E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prasad A, Lerman A, Rihal CS.. Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 33. Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H.. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol 2003;41:737–742. [DOI] [PubMed] [Google Scholar]
- 34. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS.. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858–865. [DOI] [PubMed] [Google Scholar]
- 35. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 36. Kawai S, Kitabatake A, Tomoike H.. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J 2007;71:990–992. [DOI] [PubMed] [Google Scholar]
- 37. Schultz T, Shao Y, Redfors B, Sverrisdottir YB, Ramunddal T, Albertsson P, Matejka G, Omerovic E.. Stress-induced cardiomyopathy in Sweden: evidence for different ethnic predisposition and altered cardio-circulatory status. Cardiology 2012;122:180–186. [DOI] [PubMed] [Google Scholar]
- 38. Wittstein IS. Stress cardiomyopathy: a syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol 2012;32:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parodi G, Citro R, Bellandi B, Provenza G, Marrani M, Bossone E; Tako-Tsubo Italian Network. Revised clinical diagnostic criteria for tako-tsubo syndrome: the Tako-tsubo Italian Network proposal. Int J Cardiol 2014;172:282–283. [DOI] [PubMed] [Google Scholar]
- 40. Madias JE. Why the current diagnostic criteria of takotsubo syndrome are outmoded: a proposal for new criteria. Int J Cardiol 2014;174:468–470. [DOI] [PubMed] [Google Scholar]
- 41. Scantlebury DC, Prasad A.. Diagnosis of takotsubo cardiomyopathy. Circ J 2014;78:2129–2139. [DOI] [PubMed] [Google Scholar]
- 42. Samuels MA. The brain-heart connection. Circulation 2007;116:77–84. [DOI] [PubMed] [Google Scholar]
- 43. Winchester DE, Ragosta M, Taylor AM.. Concurrence of angiographic coronary artery disease in patients with apical ballooning syndrome (tako-tsubo cardiomyopathy). Catheter Cardiovasc Interv 2008;72:612–616. [DOI] [PubMed] [Google Scholar]
- 44. Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakama Y, Maruhashi T, Kagawa E, Dai K, Matsushita J, Ikenaga H.. Prevalence of incidental coronary artery disease in tako-tsubo cardiomyopathy. Coron Artery Dis 2009;20:214–218. [DOI] [PubMed] [Google Scholar]
- 45. Napp LC, Ghadri JR, Bauersachs J, Templin C.. Acute coronary syndrome or takotsubo cardiomyopathy: the suspect may not always be the culprit. Int J Cardiol 2015;187:116–119. [DOI] [PubMed] [Google Scholar]
- 46. Haghi D, Papavassiliu T, Hamm K, Kaden JJ, Borggrefe M, Suselbeck T.. Coronary artery disease in takotsubo cardiomyopathy. Circ J 2007;71:1092–1094. [DOI] [PubMed] [Google Scholar]
- 47. Y-Hassan S. Takotsubo syndrome triggered by acute coronary syndrome in a cohort of 20 patients: an often missed diagnosis. Int J Cardiol Res 2015;02:28–33. [Google Scholar]
- 48. Y-Hassan S, Böhm F.. The causal link between spontaneous coronary artery dissection and takotsubo syndrome: a case presented with both conditions. Int J Cardiol 2016;203:828–831. [DOI] [PubMed] [Google Scholar]
- 49. Redfors B, Ramunddal T, Shao Y, Omerovic E.. Takotsubo triggered by acute myocardial infarction: a common but overlooked syndrome? J Geriatr Cardiol 2014;11:171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Napp LC, Ghadri JR, Cammann VL, Bauersachs J, Templin C.. Takotsubo cardiomyopathy: completely simple but not so easy. Int J Cardiol 2015;197:257–259. [DOI] [PubMed] [Google Scholar]
- 51. Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Luscher TF, Templin C; International Takotsubo Registry. Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome: data from the International Takotsubo Registry. JAMA Cardiol 2016;1:335–340. [DOI] [PubMed] [Google Scholar]
- 52. Kato K, Kitahara H, Fujimoto Y, Sakai Y, Ishibashi I, Himi T, Kobayashi Y.. Prevalence and clinical features of focal takotsubo cardiomyopathy. Circ J 2016;80:1824–1829. [DOI] [PubMed] [Google Scholar]
- 53. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagege A, Amar L.. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart 2013;99:1438–1444. [DOI] [PubMed] [Google Scholar]
- 54. Finsterer J, Wahbi K.. CNS disease triggering Takotsubo stress cardiomyopathy. Int J Cardiol 2014;177:322–329. [DOI] [PubMed] [Google Scholar]
- 55. Suzuki H, Matsumoto Y, Kaneta T, Sugimura K, Takahashi J, Fukumoto Y, Takahashi S, Shimokawa H.. Evidence for brain activation in patients with takotsubo cardiomyopathy. Circ J 2014;78:256–258. [DOI] [PubMed] [Google Scholar]
- 56. Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS.. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol 2009;53:1320–1325. [DOI] [PubMed] [Google Scholar]
- 57. Y-Hassan S, Henareh L.. Plasma catecholamine levels in patients with takotsubo syndrome: implications for the pathogenesis of the disease. Int J Cardiol 2015;181:35–38. [DOI] [PubMed] [Google Scholar]
- 58. Kume T, Kawamoto T, Okura H, Toyota E, Neishi Y, Watanabe N, Hayashida A, Okahashi N, Yoshimura Y, Saito K, Nezuo S, Yamada R, Yoshida K.. Local release of catecholamines from the hearts of patients with tako-tsubo-like left ventricular dysfunction. Circ J 2008;72:106–108. [DOI] [PubMed] [Google Scholar]
- 59. Ortak J, Khattab K, Barantke M, Wiegand UK, Bansch D, Ince H, Nienaber CA, Bonnemeier H.. Evolution of cardiac autonomic nervous activity indices in patients presenting with transient left ventricular apical ballooning. Pacing Clin Electrophysiol 2009;32:S21–S25. [DOI] [PubMed] [Google Scholar]
- 60. Vaccaro A, Despas F, Delmas C, Lairez O, Lambert E, Lambert G, Labrunee M, Guiraud T, Esler M, Galinier M, Senard JM, Pathak A.. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS One 2014;9:e93278.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burgdorf C, von Hof K, Schunkert H, Kurowski V.. Regional alterations in myocardial sympathetic innervation in patients with transient left-ventricular apical ballooning (Tako-Tsubo cardiomyopathy). J Nucl Cardiol 2008;15:65–72. [DOI] [PubMed] [Google Scholar]
- 62. Verberne HJ, van der Heijden DJ, van Eck-Smit BL, Somsen GA.. Persisting myocardial sympathetic dysfunction in takotsubo cardiomyopathy. J Nucl Cardiol 2009;16:321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O'Gara P, Stuckey DJ, Nikolaev VO, Diakonov I, Pannell L, Gong H, Sun H, Peters NS, Petrou M, Zheng Z, Gorelik J, Lyon AR, Harding SE.. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 2012;126:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shao Y, Redfors B, Scharin Tang M, Mollmann H, Troidl C, Szardien S, Hamm C, Nef H, Boren J, Omerovic E.. Novel rat model reveals important roles of beta-adrenoreceptors in stress-induced cardiomyopathy. Int J Cardiol 2013;168:1943–1950. [DOI] [PubMed] [Google Scholar]
- 65. Sachdeva J, Dai W, Kloner RA.. Functional and histological assessment of an experimental model of Takotsubo's cardiomyopathy. J Am Heart Assoc 2014;3:e000921.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ueyama T, Kasamatsu K, Hano T, Yamamoto K, Tsuruo Y, Nishio I.. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: a possible animal model of ′tako-tsubo′ cardiomyopathy. Circ J 2002;66:712–713. [DOI] [PubMed] [Google Scholar]
- 67. Redfors B, Ali A, Shao Y, Lundgren J, Gan LM, Omerovic E.. Different catecholamines induce different patterns of takotsubo-like cardiac dysfunction in an apparently afterload dependent manner. Int J Cardiol 2014;174:330–336. [DOI] [PubMed] [Google Scholar]
- 68. Delgado GA, Truesdell AG, Kirchner RM, Zuzek RW, Pomerantsev EV, Gordon PC, Regnante RA.. An angiographic and intravascular ultrasound study of the left anterior descending coronary artery in takotsubo cardiomyopathy. Am J Cardiol 2011;108:888–891. [DOI] [PubMed] [Google Scholar]
- 69. Pawlowski T, Mintz GS, Kulawik T, Gil RJ.. Virtual histology intravascular ultrasound evaluation of the left anterior descending coronary artery in patients with transient left ventricular ballooning syndrome. Kardiol Pol 2010;68:1093–1098. [PubMed] [Google Scholar]
- 70. Eitel I, Stiermaier T, Graf T, Moller C, Rommel KP, Eitel C, Schuler G, Thiele H, Desch S.. Optical coherence tomography to evaluate plaque burden and morphology in patients with Takotsubo syndrome. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoyt J, Lerman A, Lennon RJ, Rihal CS, Prasad A.. Left anterior descending artery length and coronary atherosclerosis in apical ballooning syndrome (Takotsubo/stress induced cardiomyopathy). Int J Cardiol 2010;145:112–115. [DOI] [PubMed] [Google Scholar]
- 72. Scantlebury DC, Prasad A, Rabinstein AA, Best PJ.. Prevalence of migraine and Raynaud phenomenon in women with apical ballooning syndrome (Takotsubo or stress cardiomyopathy). Am J Cardiol 2013;111:1284–1288. [DOI] [PubMed] [Google Scholar]
- 73. Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF, Noll G.. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation 2002;105:2817–2820. [DOI] [PubMed] [Google Scholar]
- 74. Vasilieva E, Vorobyeva I, Lebedeva A, Urazovskaya I, Kalinskaya A, Skrypnik D, Shpektor A.. Brachial artery flow-mediated dilation in patients with tako-tsubo cardiomyopathy. Am J Med 2011;124:1176–1179. [DOI] [PubMed] [Google Scholar]
- 75. Tsuchihashi K, Ueshima K, Uchida T, Oh-Mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I; Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11–18. [DOI] [PubMed] [Google Scholar]
- 76. Angelini P, Monge J, Simpson L.. Biventricular takotsubo cardiomyopathy: case report and general discussion. Tex Heart Inst J 2013;40:312–315. [PMC free article] [PubMed] [Google Scholar]
- 77. Migliore F, Maffei E, Perazzolo Marra M, Bilato C, Napodano M, Corbetti F, Zorzi A, Andres AL, Sarais C, Cacciavillani L, Favaretto E, Martini C, Seitun S, Cademartiri F, Corrado D, Iliceto S, Tarantini G.. LAD coronary artery myocardial bridging and apical ballooning syndrome. JACC Cardiovasc Imaging 2013;6:32–41. [DOI] [PubMed] [Google Scholar]
- 78. Fiol M, Carrillo A, Rodriguez A, Herrero J, Garcia-Niebla J.. Left ventricular ballooning syndrome due to vasospasm of the middle portion of the left anterior descending coronary artery. Cardiol J 2012;19:314–316. [DOI] [PubMed] [Google Scholar]
- 79. Zhang Z, Jin S, Teng X, Duan X, Chen Y, Wu Y.. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 2017;67:10–25. [DOI] [PubMed] [Google Scholar]
- 80. Nanno T, Kobayashi Y, Oda S, Ishiguchi H, Myoren T, Miyazaki Y, Suetomi T, Ono M, Mochizuki M, Oda T, Okuda S, Yamada J, Okamura T, Yano M.. A Marked increase in myocardial oxidative stress associated with sympathetic hyperactivity is related to transient myocardial dysfunction in patients with takotsubo cardiomyopathy. Circulation 2015;132:A14124. [Google Scholar]
- 81. Cohen RA, Shepherd JT, Vanhoutte PM.. Prejunctional and postjunctional actions of endogenous norepinephrine at the sympathetic neuroeffector junction in canine coronary arteries. Circ Res 1983;52:16–25. [DOI] [PubMed] [Google Scholar]
- 82. Jaguszewski M, Osipova J, Ghadri JR, Napp LC, Widera C, Franke J, Fijalkowski M, Nowak R, Fijalkowska M, Volkmann I, Katus HA, Wollert KC, Bauersachs J, Erne P, Luscher TF, Thum T, Templin C.. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ghadri JR, Dougoud S, Maier W, Kaufmann PA, Gaemperli O, Prasad A, Luscher TF, Templin C.. A PET/CT-follow-up imaging study to differentiate takotsubo cardiomyopathy from acute myocardial infarction. Int J Cardiovasc Imaging 2014;30:207–209. [DOI] [PubMed] [Google Scholar]
- 84. Meimoun P, Malaquin D, Sayah S, Benali T, Luycx-Bore A, Levy F, Zemir H, Tribouilloy C.. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr 2008;21:72–77. [DOI] [PubMed] [Google Scholar]
- 85. Rigo F, Sicari R, Citro R, Ossena G, Buja P, Picano E.. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: a Doppler transthoracic echo study. Ann Med 2009;41:462–470. [DOI] [PubMed] [Google Scholar]
- 86. Kume T, Akasaka T, Kawamoto T, Yoshitani H, Watanabe N, Neishi Y, Wada N, Yoshida K.. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J 2005;69:934–939. [DOI] [PubMed] [Google Scholar]
- 87. Cuisset T, Quilici J, Pankert M, Fourcade L, Poyet R, Lambert M, Bonnet JL.. Usefulness of index of microcirculatory resistance to detect microvascular dysfunction as a potential mechanism of stress-induced cardiomyopathy (Tako-tsubo syndrome). Int J Cardiol 2011;153:e51–e53. [DOI] [PubMed] [Google Scholar]
- 88. Elesber A, Lerman A, Bybee KA, Murphy JG, Barsness G, Singh M, Rihal CS, Prasad A.. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J 2006;152:469 e9–413. [DOI] [PubMed] [Google Scholar]
- 89. Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, Rebuzzi AG, Crea F.. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical ballooning or tako-tsubo syndrome. Eur Heart J 2010;31:1319–1327. [DOI] [PubMed] [Google Scholar]
- 90. Uchida Y, Egami H, Uchida Y, Sakurai T, Kanai M, Shirai S, Nakagawa O, Oshima T.. Possible participation of endothelial cell apoptosis of coronary microvessels in the genesis of Takotsubo cardiomyopathy. Clin Cardiol 2010;33:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Barletta G, Del Pace S, Boddi M, Del Bene R, Salvadori C, Bellandi B, Coppo M, Saletti E, Gensini GF.. Abnormal coronary reserve and left ventricular wall motion during cold pressor test in patients with previous left ventricular ballooning syndrome. Eur Heart J 2009;30:3007–3014. [DOI] [PubMed] [Google Scholar]
- 92. Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A.. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol 2010;56:1840–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Patel SM, Lerman A, Lennon RJ, Prasad A.. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (takotsubo/stress cardiomyopathy). Eur Heart J Acute Cardiovasc Care 2013;2:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nef HM, Mollmann H, Troidl C, Kostin S, Voss S, Hilpert P, Behrens CB, Rolf A, Rixe J, Weber M, Hamm CW, Elsasser A.. Abnormalities in intracellular Ca2+ regulation contribute to the pathomechanism of tako-tsubo cardiomyopathy. Eur Heart J 2009;30:2155–2164. [DOI] [PubMed] [Google Scholar]
- 95. Nef HM, Mollmann H, Akashi YJ, Hamm CW.. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol 2010;7:187–193. [DOI] [PubMed] [Google Scholar]
- 96. Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol 1985;17:291–306. [DOI] [PubMed] [Google Scholar]
- 97. Heather LC, Catchpole AF, Stuckey DJ, Cole MA, Carr CA, Clarke K.. Isoproterenol induces in vivo functional and metabolic abnormalities: similar to those found in the infarcted rat heart. J Physiol Pharmacol 2009;60:31–39. [PubMed] [Google Scholar]
- 98. Christensen TE, Bang LE, Holmvang L, Ghotbi AA, Lassen ML, Andersen F, Ihlemann N, Andersson H, Grande P, Kjaer A, Hasbak P.. Cardiac (9)(9)mTc sestamibi SPECT and (1)(8)F FDG PET as viability markers in takotsubo cardiomyopathy. Int J Cardiovasc Imaging 2014;30:1407–1416. [DOI] [PubMed] [Google Scholar]
- 99. Rendl G, Rettenbacher L, Keinrath P, Altenberger J, Schuler J, Heigert M, Pichler M, Pirich C.. Different pattern of regional metabolic abnormalities in takotsubo cardiomyopathy as evidenced by F-18 FDG PET-CT. Wiener Klin Wochenschr 2010;122:184–185. [DOI] [PubMed] [Google Scholar]
- 100. Shao Y, Redfors B, Stahlman M, Tang MS, Miljanovic A, Mollmann H, Troidl C, Szardien S, Hamm C, Nef H, Boren J, Omerovic E.. A mouse model reveals an important role for catecholamine-induced lipotoxicity in the pathogenesis of stress-induced cardiomyopathy. Eur J Heart Fail 2013;15:9–22. [DOI] [PubMed] [Google Scholar]
- 101. Nef HM, Mollmann H, Kostin S, Troidl C, Voss S, Weber M, Dill T, Rolf A, Brandt R, Hamm CW, Elsasser A.. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J 2007;28:2456–2464. [DOI] [PubMed] [Google Scholar]
- 102. Redfors B, Shao Y, Wikstrom J, Lyon AR, Oldfors A, Gan LM, Omerovic E.. Contrast echocardiography reveals apparently normal coronary perfusion in a rat model of stress-induced (Takotsubo) cardiomyopathy. Eur Heart J Cardiovasc Imaging 2014;15:152–157. [DOI] [PubMed] [Google Scholar]
- 103. Kawano H, Okada R, Yano K.. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 2003;18:32–39. [DOI] [PubMed] [Google Scholar]
- 104. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE.. Stress (Takotsubo) cardiomyopathy–a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clini Pract Cardiovasc Med 2008;5:22–29. [DOI] [PubMed] [Google Scholar]
- 105. Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, Okino H.. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res 1993;27:192–198. [DOI] [PubMed] [Google Scholar]
- 106. Communal C, Colucci WS, Singh K.. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta -adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. J Biol Chem 2000;275:19395–19400. [DOI] [PubMed] [Google Scholar]
- 107. Heubach JF, Ravens U, Kaumann AJ.. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human beta2-adrenoceptors overexpressed in mouse heart. Mol Pharmacol 2004;65:1313–1322. [DOI] [PubMed] [Google Scholar]
- 108. Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP.. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A 2001;98:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nguyen TH, Neil CJ, Sverdlov AL, Ngo DT, Chan WP, Heresztyn T, Chirkov YY, Tsikas D, Frenneaux MP, Horowitz JD.. Enhanced NO signaling in patients with Takotsubo cardiomyopathy: short-term pain, long-term gain? Cardiovasc Drugs Ther 2013;27:541–547. [DOI] [PubMed] [Google Scholar]
- 110. Surikow SY, Raman B, Licari J, Singh K, Nguyen TH, Horowitz JD.. Evidence of nitrosative stress within hearts of patients dying of Tako-tsubo cardiomyopathy. Int J Cardiol 2015;189:112–114. [DOI] [PubMed] [Google Scholar]
- 111. Dawson DK, Neil CJ, Henning A, Cameron D, Jagpal B, Bruce M, Horowitz J, Frenneaux MP.. Tako-Tsubo cardiomyopathy: a heart stressed out of energy? JACC Cardiovasc Imaging 2015;8:985–987. [DOI] [PubMed] [Google Scholar]
- 112. Nef HM, Mollmann H, Hilpert P, Troidl C, Voss S, Rolf A, Behrens CB, Weber M, Hamm CW, Elsasser A.. Activated cell survival cascade protects cardiomyocytes from cell death in Tako-Tsubo cardiomyopathy. Eur J Heart Fail 2009;11:758–764. [DOI] [PubMed] [Google Scholar]
- 113. Spinelli L, Trimarco V, Di Marino S, Marino M, Iaccarino G, Trimarco B.. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur J Heart Fail 2010;12:13–16. [DOI] [PubMed] [Google Scholar]
- 114. Figtree GA, Bagnall RD, Abdulla I, Buchholz S, Galougahi KK, Yan W, Tan T, Neil C, Horowitz JD, Semsarian C, Ward MR.. No association of G-protein-coupled receptor kinase 5 or beta-adrenergic receptor polymorphisms with Takotsubo cardiomyopathy in a large Australian cohort. Eur J Heart Fail 2013;15:730–733. [DOI] [PubMed] [Google Scholar]
- 115. Vitale C, Rosano GM, Kaski JC.. Role of coronary microvascular dysfunction in takotsubo cardiomyopathy. Circ J 2016;80:299–305. [DOI] [PubMed] [Google Scholar]
- 116. Ueyama T, Kasamatsu K, Hano T, Tsuruo Y, Ishikura F.. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann N Y Acad Sci 2008;1148:479–485. [DOI] [PubMed] [Google Scholar]
- 117. Sader MA, Celermajer DS.. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res 2002;53:597–604. [DOI] [PubMed] [Google Scholar]
- 118. Komesaroff PA, Esler MD, Sudhir K.. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab 1999;84:606–610. [DOI] [PubMed] [Google Scholar]
- 119. Sung BH, Ching M, Izzo JL Jr, Dandona P, Wilson MF.. Estrogen improves abnormal norepinephrine-induced vasoconstriction in postmenopausal women. J Hypertens 1999;17:523–528. [DOI] [PubMed] [Google Scholar]
- 120. Sugimoto K, Inamasu J, Hirose Y, Kato Y, Ito K, Iwase M, Sugimoto K, Watanabe E, Takahashi A, Ozaki Y.. The role of norepinephrine and estradiol in the pathogenesis of cardiac wall motion abnormality associated with subarachnoid hemorrhage. Stroke 2012;43:1897–1903. [DOI] [PubMed] [Google Scholar]
- 121. Ueyama T, Ishikura F, Matsuda A, Asanuma T, Ueda K, Ichinose M, Kasamatsu K, Hano T, Akasaka T, Tsuruo Y, Morimoto K, Beppu S.. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J 2007;71:565–573. [DOI] [PubMed] [Google Scholar]
- 122. Cherian J, Angelis D, Filiberti A, Saperia G.. Can takotsubo cardiomyopathy be familial? Int J Cardiol 2007;121:74–75. [DOI] [PubMed] [Google Scholar]
- 123. Kumar G, Holmes DR Jr, Prasad A.. “Familial” apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol 2010;144:444–445. [DOI] [PubMed] [Google Scholar]
- 124. Ikutomi M, Yamasaki M, Matsusita M, Watari Y, Arashi H, Endo G, Yamaguchi JI, Ohnishi S.. Takotsubo cardiomyopathy in siblings. Heart Vessels 2014;29:119–122. [DOI] [PubMed] [Google Scholar]
- 125. Pison L, De Vusser P, Mullens W.. Apical ballooning in relatives. Heart 2004;90:e67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Caretta G, Robba D, Vizzardi E, Bonadei I, Raddino R, Metra M.. Tako-tsubo cardiomyopathy in two sisters: a chance finding or familial predisposition? Clin Res Cardiol 2015;104:614–616. [DOI] [PubMed] [Google Scholar]
- 127. Dorn GW., 2nd Adrenergic signaling polymorphisms and their impact on cardiovascular disease. Physiol Rev 2010;90:1013–1062. [DOI] [PubMed] [Google Scholar]
- 128. Zaroff JG, Pawlikowska L, Miss JC, Yarlagadda S, Ha C, Achrol A, Kwok PY, McCulloch CE, Lawton MT, Ko N, Smith W, Young WL.. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke 2006;37:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gujja KR, Aslam AF, Privman V, Tejani F, Vasavada B.. Initial presentation of pheochromocytoma with Takotsubo cardiomyopathy: a brief review of literature. J Cardiovasc Med 2010;11:49–52. [DOI] [PubMed] [Google Scholar]
- 130. Sharkey SW, Maron BJ, Nelson P, Parpart M, Maron MS, Bristow MR.. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J Cardiol 2009;53:53–57. [DOI] [PubMed] [Google Scholar]
- 131. Vriz O, Minisini R, Citro R, Guerra V, Zito C, De Luca G, Pavan D, Pirisi M, Limongelli G, Bossone E.. Analysis of beta1 and beta2-adrenergic receptors polymorphism in patients with apical ballooning cardiomyopathy. Acta Cardiol 2011;66:787–790. [DOI] [PubMed] [Google Scholar]
- 132. Goodloe AH, Evans JM, Middha S, Prasad A, Olson TM.. Characterizing genetic variation of adrenergic signalling pathways in Takotsubo (stress) cardiomyopathy exomes. Eur J Heart Fail 2014;16:942–949. [DOI] [PubMed] [Google Scholar]
- 133. Borchert T, Hübscher D, Guessoum CI, Lam T-DD, Ghadri JR, Schellinger IN, Tiburcy M, Liaw NY, Li Y, Haas J, Sossalla S, Huber MA, Cyganek L, Jacobshagen C, Dressel R, Raaz U, Nikolaev VO, Guan K, Thiele H, Meder B, Wollnik B, Zimmermann W-H, Lüscher TF, Hasenfuss G, Templin C, Streckfuss-Bömeke K.. Catecholamine-dependent beta-adrenergic signaling in a pluripotent stem cell model of Takotsubo cardiomyopathy. J Am Coll Cardiol 2017;70:975–991. [DOI] [PubMed] [Google Scholar]
- 134. Lyon A. Stress in a dish: exploring the mechanisms of Takotsubo syndrome. J Am Coll Cardiol 2017;70:992–995. [DOI] [PubMed] [Google Scholar]
- 135. Summers MR, Lennon RJ, Prasad A.. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J Am Coll Cardiol 2010;55:700–701. [DOI] [PubMed] [Google Scholar]
- 136. Delmas C, Lairez O, Mulin E, Delmas T, Boudou N, Dumonteil N, Biendel-Picquet C, Roncalli J, Elbaz M, Galinier M, Carrié D.. Anxiodepressive disorders and chronic psychological stress are associated with Tako-Tsubo cardiomyopathy-new physiopathological hypothesis. Circ J 2013;77:175–180. [DOI] [PubMed] [Google Scholar]
- 137. Compare A, Bigi R, Orrego PS, Proietti R, Grossi E, Steptoe A.. Type D personality is associated with the development of stress cardiomyopathy following emotional triggers. Ann Behav Med 2013;45:299–307. [DOI] [PubMed] [Google Scholar]
- 138. Scantlebury DC, Rohe DE, Best PJ, Lennon RJ, Lerman A, Prasad A.. Stress-coping skills and neuroticism in apical ballooning syndrome (Takotsubo/stress cardiomyopathy). Open Heart 2016;3:e000312.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Summers MR, Dib C, Prasad A.. Chronobiology of Tako-tsubo cardiomyopathy (apical ballooning syndrome). J Am Geriatr Soc 2010;58:805–806. [DOI] [PubMed] [Google Scholar]
- 140. Mausbach BT, Dimsdale JE, Ziegler MG, Mills PJ, Ancoli-Israel S, Patterson TL, Grant I.. Depressive symptoms predict norepinephrine response to a psychological stressor task in Alzheimer's caregivers. Psychosom Med 2005;67:638–642. [DOI] [PubMed] [Google Scholar]
- 141. Alvarenga ME, Richards JC, Lambert G, Esler MD.. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med 2006;68:8–16. [DOI] [PubMed] [Google Scholar]
- 142. Neil CJ, Chong CR, Nguyen TH, Horowitz JD.. Occurrence of Tako-Tsubo cardiomyopathy in association with ingestion of serotonin/noradrenaline reuptake inhibitors. Heart Lung Circ 2012;21:203–205. [DOI] [PubMed] [Google Scholar]
- 143. Scheitz JF, Mochmann HC, Witzenbichler B, Fiebach JB, Audebert HJ, Nolte CH.. Takotsubo cardiomyopathy following ischemic stroke: a cause of troponin elevation. J Neurol 2012;259:188–190. [DOI] [PubMed] [Google Scholar]
- 144. Inamasu J, Ganaha T, Nakae S, Ohmi T, Wakako A, Tanaka R, Kuwahara K, Kogame H, Kawazoe Y, Kumai T, Hayakawa M, Hirose Y.. Therapeutic outcomes for patients with aneurysmal subarachnoid hemorrhage complicated by Takotsubo cardiomyopathy. Acta Neurochir (Wien) 2016;158:885–893. [DOI] [PubMed] [Google Scholar]
- 145. Natelson BH, Suarez RV, Terrence CF, Turizo R.. Patients with epilepsy who die suddenly have cardiac disease. Arch Neurol 1998;55:857–860. [DOI] [PubMed] [Google Scholar]
- 146. Yoshida T, Hibino T, Kako N, Murai S, Oguri M, Kato K, Yajima K, Ohte N, Yokoi K, Kimura G.. A pathophysiologic study of tako-tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J 2007;28:2598–2604. [DOI] [PubMed] [Google Scholar]
- 147. Blanc C, Zeller M, Cottin Y, Daubail B, Vialatte AL, Giroud M, Bejot Y.. Takotsubo cardiomyopathy following acute cerebral events. Eur Neurol 2015;74:163–168. [DOI] [PubMed] [Google Scholar]
- 148. Hiestand T, Hänggi J, Klein C, Topka MS, Jaguszewski M, Ghadri JR, Lüscher TF, Jäncke L, Templin C.. Takotsubo Syndrome Associated With Structural Brain Alterations of the Limbic System. J Am Coll Cardiol 2018;71:809–811. [DOI] [PubMed] [Google Scholar]
- 149. Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ.. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–341. [DOI] [PubMed] [Google Scholar]
- 150. Münzel T, Knorr M, Schmidt F, von Bardeleben S, Gori T, Schulz E. Airborne disease: a case of a Takotsubo cardiomyopathie as a consequence of nighttime aircraft noise exposure. Eur Heart J. 2016;37:2844 [Epub 19 July 2016]. [DOI] [PubMed] [Google Scholar]
- 151. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E.. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–1529. [DOI] [PubMed] [Google Scholar]
- 152. Isogai T, Yasunaga H, Matsui H, Tanaka H, Ueda T, Horiguchi H, Fushimi K.. Out-of-hospital versus in-hospital Takotsubo cardiomyopathy: analysis of 3719 patients in the Diagnosis Procedure Combination database in Japan. Int J Cardiol 2014;176:413–417. [DOI] [PubMed] [Google Scholar]
- 153. Tagawa M, Nakamura Y, Ishiguro M, Satoh K, Chinushi M, Kodama M, Aizawa Y.. Transient left ventricular apical ballooning developing after the Central Niigata Prefecture Earthquake: two case reports. J Cardiol 2006;48:153–158. [PubMed] [Google Scholar]
- 154. Chan C, Elliott J, Troughton R, Frampton C, Smyth D, Crozier I, Bridgman P.. Acute myocardial infarction and stress cardiomyopathy following the Christchurch earthquakes. PLoS One 2013;8:e68504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Butterly SJ, Indrajith M, Garrahy P, Ng AC, Gould PA, Wang WY.. Stress-induced takotsubo cardiomyopathy in survivors of the 2011 Queensland floods. Med J Aust 2013;198:109–110. [DOI] [PubMed] [Google Scholar]
- 156. Ghadri JR, Sarcon A, Diekmann J, Bataiosu DR, Cammann VL, Jurisic S, Napp LC, Jaguszewski M, Scherff F, Brugger P, Jancke L, Seifert B, Bax JJ, Ruschitzka F, Luscher TF, Templin C; InterTAK Co-investigators. Happy heart syndrome: role of positive emotional stress in takotsubo syndrome. Eur Heart J 2016;37:2823–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lousinha A, Gilkeson R, Bezerra H.. Left ventricular outflow tract obstruction and Takotsubo syndrome. Rev Port Cardiol 2012;31:49–51. [DOI] [PubMed] [Google Scholar]
- 158. Berry M, Roncalli J, Lairez O, Elbaz M, Carrie D, Galinier M.. Takotsubo cardiomyopathy in a squash player. Cardiol Res Pract 2009;2009:351621.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kotsiou OS, Douras A, Makris D, Mpaka N, Gourgoulianis KI.. Takotsubo cardiomyopathy: a known unknown foe of asthma. J Asthma 2017;54:880–886. [DOI] [PubMed] [Google Scholar]
- 160. Ghadri JR, Bataisou RD, Diekmann J, Luscher TF, Templin C.. First case of atypical takotsubo cardiomyopathy in a bilateral lung-transplanted patient due to acute respiratory failure. Eur Heart J Acute Cardiovasc Care 2015;4:482–485. [DOI] [PubMed] [Google Scholar]
- 161. Rajani R, Przedlacka A, Saha M, de Belder A.. Pancreatitis and the broken heart. Eur J Emerg Med 2010;17:27–29. [DOI] [PubMed] [Google Scholar]
- 162. Aggarwal V, Krantz MJ.. Migratory takotsubo cardiomyopathy in the setting of cholecystitis. Am J Med 2012;125:e5–e6. [DOI] [PubMed] [Google Scholar]
- 163. Gale M, Loarte P, Mirrer B, Mallet T, Salciccioli L, Petrie A, Cohen R.. Takotsubo cardiomyopathy in the setting of tension pneumothorax. Case Rep Crit Care 2015;2015:536931.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Elikowski W, Kudlinski B, Malek-Elikowska M, Foremska-Iciek J, Baszko A, Skrzywanek P.. [Takotsubo cardiomyopathy in a young woman after a traffic accident with blunt chest trauma]. Pol Merkur Lekarski 2016;40:372–376. [PubMed] [Google Scholar]
- 165. Y-Hassan S, Settergren M, Henareh L.. Sepsis-induced myocardial depression and takotsubo syndrome. Acute Cardiac Care 2014;16:102–109. [DOI] [PubMed] [Google Scholar]
- 166. Eliades M, El-Maouche D, Choudhary C, Zinsmeister B, Burman KD.. Takotsubo cardiomyopathy associated with thyrotoxicosis: a case report and review of the literature. Thyroid 2014;24:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Smith SA, Auseon AJ.. Chemotherapy-induced takotsubo cardiomyopathy. Heart Fail Clin 2013;9:233–242, x. [DOI] [PubMed] [Google Scholar]
- 168. Modi S, Baig W.. Radiotherapy-induced Tako-tsubo cardiomyopathy. Clin Oncol (R Coll Radiol) 2009;21:361–362. [DOI] [PubMed] [Google Scholar]
- 169. Brezina P, Isler CM.. Takotsubo cardiomyopathy in pregnancy. Obstet Gynecol 2008;112:450–452. [DOI] [PubMed] [Google Scholar]
- 170. Citro R, Lyon A, Arbustini E, Bossone E, Piscione F, Templin C, Narula J. Takotsubo syndrome after cesarean section: rare but possible. J Am Coll Cardiol 2018;71:1838–1839. [DOI] [PubMed] [Google Scholar]
- 171. Dundon BK, Puri R, Leong DP, Worthley MI.. Takotsubo cardiomyopathy following lightning strike. Emerg Med J 2008;25:460–461. [DOI] [PubMed] [Google Scholar]
- 172. Citro R, Patella MM, Bossone E, Maione A, Provenza G, Gregorio G.. Near-drowning syndrome: a possible trigger of tako-tsubo cardiomyopathy. J Cardiovasc Med 2008;9:501–505. [DOI] [PubMed] [Google Scholar]
- 173. Davin L, Legrand V, Legrand D.. A frozen heart. Eur Heart J 2009;30:1827.. [DOI] [PubMed] [Google Scholar]
- 174. Arora S, Alfayoumi F, Srinivasan V.. Transient left ventricular apical ballooning after cocaine use: is catecholamine cardiotoxicity the pathologic link? Mayo Clin Proc 2006;81:829–832. [DOI] [PubMed] [Google Scholar]
- 175. Stout BJ, Hoshide R, Vincent DS.. Takotsubo cardiomyopathy in the setting of acute alcohol withdrawal. Hawaii J Med Public Health 2012;71:193–194. [PMC free article] [PubMed] [Google Scholar]
- 176. Sarcon A, Ghadri JR, Wong G, Luscher TF, Templin C, Amsterdam E.. Takotsubo cardiomyopathy associated with opiate withdrawal. QJM 2014;107:301–302. [DOI] [PubMed] [Google Scholar]
- 177. Jung YS, Lee JS, Min YG, Park JS, Jeon WC, Park EJ, Shin JH, Oh S, Choi SC.. Carbon monoxide-induced cardiomyopathy. Circ J 2014;78:1437–1444. [DOI] [PubMed] [Google Scholar]
- 178. Y-Hassan S. Clinical features and outcome of epinephrine-induced takotsubo syndrome: analysis of 33 published cases. Cardiovasc Revasc Med 2016;17:450–455. [DOI] [PubMed] [Google Scholar]
- 179. Mendoza I, Novaro GM.. Repeat recurrence of takotsubo cardiomyopathy related to inhaled beta-2-adrenoceptor agonists. World J Cardiol 2012;4:211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Margey R, Diamond P, McCann H, Sugrue D.. Dobutamine stress echo-induced apical ballooning (Takotsubo) syndrome. Eur J Echocardiogr 2009;10:395–399. [DOI] [PubMed] [Google Scholar]
- 181. Collen J, Bimson W, Devine P.. A variant of Takotsubo cardiomyopathy: a rare complication in the electrophysiology lab. J Invasive Cardiol 2008;20:E310–E313. [PubMed] [Google Scholar]
- 182. Patel B, Assad D, Wiemann C, Zughaib M.. Repeated use of albuterol inhaler as a potential cause of Takotsubo cardiomyopathy. Am J Case Rep 2014;15:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Riera M, Llompart-Pou JA, Carrillo A, Blanco C.. Head injury and inverted Takotsubo cardiomyopathy. J Trauma 2010;68:E13–E15. [DOI] [PubMed] [Google Scholar]
- 184. Hjalmarsson C, Oras J, Redfors B.. A case of intracerebral hemorrhage and apical ballooning: an important differential diagnosis in ST-segment elevation. Int J Cardiol 2015;186:90–92. [DOI] [PubMed] [Google Scholar]
- 185. Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, Vonthein R, Schuler G, Thiele H, Eitel I.. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail 2016;18:650–656. [DOI] [PubMed] [Google Scholar]
- 186. Y-Hassan S. Clinical features and outcome of pheochromocytoma-induced Takotsubo syndrome: analysis of 80 published cases. Am J Cardiol 2016;117:1836–1844. [DOI] [PubMed] [Google Scholar]
- 187. Shoukat S, Awad A, Nam DK, Hoskins MH, Samuels O., Higginson J, Clements SD Jr.. Cardiomyopathy with inverted Tako-Tsubo pattern in the setting of subarachnoid hemorrhage: a series of four cases. Neurocrit Care 2013;18:257–260. [DOI] [PubMed] [Google Scholar]
- 188. Kagiyama N, Okura H, Kume T, Hayashida A, Yoshida K.. Isolated right ventricular takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:285.. [DOI] [PubMed] [Google Scholar]
- 189. Stahli BE, Ruschitzka F, Enseleit F.. Isolated right ventricular ballooning syndrome: a new variant of transient cardiomyopathy. Eur Heart J 2011;32:1821.. [DOI] [PubMed] [Google Scholar]
- 190. Elikowski W, Malek M, Lanocha M, Wroblewski D, Angerer D, Kurosz J, Rachuta K.. [Reversible dilated cardiomyopathy as an atypical form of takotsubo cardiomyopathy]. Pol Merkur Lekarski 2013;34:219–223. [PubMed] [Google Scholar]
- 191. Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG.. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011;306:277–286. [DOI] [PubMed] [Google Scholar]
- 192. Ghadri JR, Jaguszewski M, Corti R, Luscher TF, Templin C.. Different wall motion patterns of three consecutive episodes of takotsubo cardiomyopathy in the same patient. Int J Cardiol 2012;160:e25–e27. [DOI] [PubMed] [Google Scholar]
- 193. Hurst RT, Prasad A, Askew JW, Sengupta PP, Tajik AJ.. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging 2010;3:641–649. [DOI] [PubMed] [Google Scholar]
- 194. Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, Sobel BE, Willerson JT, Braunwald E.. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 195. Manfredini R, Portaluppi F, Zamboni P, Salmi R, Gallerani M.. Circadian variation in spontaneous rupture of abdominal aorta. Lancet 1999;353:643–644. [DOI] [PubMed] [Google Scholar]
- 196. Manfredini R, Manfredini F, Boari B, Bergami E, Mari E, Gamberini S, Salmi R, Gallerani M.. Seasonal and weekly patterns of hospital admissions for nonfatal and fatal myocardial infarction. Am J Emerg Med 2009;27:1097–1103. [DOI] [PubMed] [Google Scholar]
- 197. Willich SN, Lowel H, Lewis M, Hormann A, Arntz HR, Keil U.. Weekly variation of acute myocardial infarction. Increased Monday risk in the working population. Circulation 1994;90:87–93. [DOI] [PubMed] [Google Scholar]
- 198. Song BG, Oh JH, Kim HJ, Kim SH, Chung SM, Lee M, Kang GH, Park YH, Chun WJ.. Chronobiological variation in the occurrence of Tako-tsubo cardiomyopathy: experiences of two tertiary cardiovascular centers. Heart Lung 2013;42:40–47. [DOI] [PubMed] [Google Scholar]
- 199. Citro R, Previtali M, Bovelli D, Vriz O, Astarita C, Patella MM, Provenza G, Armentano C, Ciampi Q, Gregorio G, Piepoli M, Bossone E, Manfredini R.. Chronobiological patterns of onset of Tako-Tsubo cardiomyopathy: a multicenter Italian study. J Am Coll Cardiol 2009;54:180–181. [DOI] [PubMed] [Google Scholar]
- 200. Sharkey SW, Lesser JR, Garberich RF, Pink VR, Maron MS, Maron BJ.. Comparison of circadian rhythm patterns in Tako-tsubo cardiomyopathy versus ST-segment elevation myocardial infarction. Am J Cardiol 2012;110:795–799. [DOI] [PubMed] [Google Scholar]
- 201. Abdulla I, Kay S, Mussap C, Nelson GI, Rasmussen HH, Hansen PS, Ward MR.. Apical sparing in tako-tsubo cardiomyopathy. Intern Med J 2006;36:414–418. [DOI] [PubMed] [Google Scholar]
- 202. Manfredini R, Citro R, Previtali M, Vriz O, Ciampi Q, Pascotto M, Tagliamonte E, Provenza G, Manfredini F, Bossone E; Takotsubo Italian Network investigators. Monday preference in onset of takotsubo cardiomyopathy. Am J Emerg Med 2010;28:715–719. [DOI] [PubMed] [Google Scholar]
- 203. Aryal MR, Pathak R, Karmacharya P, Donato AA.. Seasonal and regional variation in Takotsubo cardiomyopathy. Am J Cardiol 2014;113:1592.. [DOI] [PubMed] [Google Scholar]
- 204. Eshtehardi P, Koestner SC, Adorjan P, Windecker S, Meier B, Hess OM, Wahl A, Cook S.. Transient apical ballooning syndrome–clinical characteristics, ballooning pattern, and long-term follow-up in a Swiss population. Int J Cardiol 2009;135:370–375. [DOI] [PubMed] [Google Scholar]