Short abstract
Purpose
To determine the best platelet function test for in-stent tissue protrusion following carotid artery stenting (CAS).
Methods
Patients who underwent CAS were recruited prospectively in this observational study. Combination of aspirin 100 mg/day and clopidogrel 75 mg/day was administered for a minimum of 7 days prior to procedure. Platelet aggregation was measured by light transmittance aggregometry (LTA) following stimulation by adenosine diphosphate (ADP), collagen, and thrombin receptor activating peptide (TRAP) and by the point of care assay, VerifyNow which measures aspirin and thienopyridine reaction units.
Results
In-stent tissue protrusion with maximum projection area of ≥1 mm2 was detected by optical coherence tomography (OCT) in 10/28 (36%) patients. Baseline characteristics were not significantly different between the two in-stent size groups (i.e., ≥1 mm2 vs. <1 mm2) but after stimulation by collagen at 10 and 20 μg/ml, platelet reactivity as measured by LTA was significantly higher in the ≥1 mm2 group compared with the <1 mm2 group. No other differences in platelet function were detected.
Conclusions
Collagen-induced platelet reactivity was related to in-stent tissue protrusion size following CAS.
Keywords: Carotid artery stenting, in-stent tissue protrusion, optical coherence tomography, light transmittance aggregometry, VerifyNow
Introduction
Despite the development of devices to reduce the risk of ischemic events in carotid artery stenting (CAS), thromboembolism, intra- or post-operatively, remains a common complication.1,2 Several factors including manoeuvres of the intraluminal catheter, presence of atherosclerotic aortic arch plaques, balloon angioplasty and stent deployment may cause peri-procedural embolization. Importantly, in-stent tissue protrusion (plaque or thrombus) is a common cause of post-procedural complications.3 Although digital subtraction angiography, 3D computed tomography (CT) angiography, duplex sonography and intravascular ultrasound are commonly used to detect the presence of in-stent tissue protrusions, their diagnostic resolution can be inadequate for accurate evaluation of the inner surface of the stent. 4 Optical coherence tomography (OCT) is a non-contact, light-based imaging method with an excellent resolution; its axial resolution is approximately 10 μm. 5,6 Therefore, OCT easily facilitates the visualisation of plaque or thrombus characteristics.
Dual antiplatelet therapy with clopidogrel and aspirin has been shown to contribute to the reduction of ischemic events associated with CAS 7,8 and has become a standard pre-treatment medication for this procedure.9,10 However, one of the limitations of antiplatelet therapy is the wide inter-individual variability of platelet inhibition.11–13 Indeed, concerns have been raised regarding the poor response to antiplatelet therapy in the fields of neurovascular and cardiovascular stenting. For example, although patients with high residual platelet reactivity (HRP) under clopidogrel treatment are known to be at the risk of thromboembolism,14–16 adjustment of the dose of antiplatelet agent based on platelet reactivity failed to show clinical superiority over administration of a standard dose of dual antiplatelet therapy in both cardiovascular stenting and CAS.17–21 Therefore, although platelet reactivity may be an indicator of thromboembolic events, it is uncertain whether HRP can be used reliably as a therapeutic target.
The gold standard technique for measuring platelet aggregation is light transmittance aggregometry (LTA) which permits the use of several agonists at different doses.22 However, LTA has several limitations and is time-consuming because it entails centrifugation of blood and preparation of the agonists. Moreover, the role of LTA in establishing platelet function for peri-procedural ischemic complications in the neurovascular field has not been fully elucidated.23
Point-of-care (POC) platelet function assays are simple, convenient and widely used tests of platelet aggregation. 22 However, the tests have some limitations, including single-dose administration and are specific for single agonists. Importantly, platelets may be activated through several receptor-mediator pathways involving not only P2Y12 receptors but also thrombin and glycoprotein VI (GPVI) receptors. Therefore, some POC assays may not be sensitive enough to evaluate platelet function correctly because of the specificity of the agonist.
In this study, we investigated the relationship between the presence of in-stent tissue protrusion detected by OCT and platelet reactivity assessed by LTA and the VerifyNow system which is a POC assay.
Patients and methods
Patients
This prospective, observational study involved all patients who were admitted to our institution from May 2014 to June 2016 with a diagnosis of internal carotid artery stenosis and underwent CAS. The indications for CAS were carotid artery stenosis ≥50% in symptomatic patients and ≥ 80% in asymptomatic patients as detected by digital subtraction angiography. 24
Patients received antiplatelet therapy (i.e., aspirin 100 mg/day and clopidogrel 75 mg/day) for a minimum of seven days before the procedure. Baseline characteristics of the patients and morphological characteristics of the lesions were recorded by two investigators [M.T. and M.M.]. The study was approved by the institutional ethics committee at the hospital and informed consent was obtained from all patients.
Blood sampling and preparation of human platelet-rich plasma
Prior to the CAS, blood samples were collected by two investigators [M.T. and M.M.] and placed in containers with 1/10 volume of 3.2% sodium citrate. Platelet-rich plasma was obtained by centrifugation at 155g for 12 minutes and platelet-poor plasma was prepared from residual blood by centrifugation at 1,400g for 5 minutes.
Platelet function tests
These assays were performed by two investigators [M.T. and Y.E.] Platelet aggregation in citrated platelet-rich plasma was analysed by a light transmittance aggregometer (PA-200 Kowa Co. Ltd., Tokyo, Japan) at 37°C with a stirring speed of 800 rpm. Adenosine diphosphate (ADP) (Sigma-Aldrich, St. Louis, MO), collagen (Takeda Austria GmbH, Linz, Austria) and thrombin receptor-activating peptide (TRAP) (Bachem AG, Budendorf, Switzerland) were used to induce aggregation. Each agonist was used at three concentrations (ADP: 3, 10, and 20 μM; collagen: 3, 10, and 20 μg/ml; TRAP: 10, 20, and 30 μM). The platelet-rich plasma and platelet-poor plasma transmittance percentages were recorded as 0% and 100%, respectively. Aggregation was recorded as a percentage of the maximum transmittance.
In addition, the VerifyNow rapid platelet function assay (Accumetrics, San Diego, CA) was also used to assess platelet function. This is a platelet function assay designed to measure directly the effects of drugs on the P2Y12 receptor. 22 Two assays were used, one using aspirin whose results are expressed as Aspirin Reaction Units (ARU) and the other thienopyridine (i.e., clopidogrel; the PRUTest) whose results are expressed as P2Y12 Reaction Units (PRU).
MRI analysis
Magnetic resonance imaging (MRI) using a 1.5-T system (Intera Achieva Nova Dual, Philips Medical Systems, The Netherlands) equipped with standard neck array coils was performed before the CAS procedure. T1 weighted images (T1WI) of the carotid artery, including the minimum lumen area, were acquired. For plaque evaluation, the relative signal intensity of the plaque from T1WI was compared with that of the sternocleidomastoid muscle. Plaques with signal intensity ratio (SIR) of ≥1.25 were defined as high SIR plaques and were at higher risk for cerebral embolism during CAS procedures. 25,26
In addition, all patients underwent pre-operative diffusion-weighted imaging (DWI) of the brain. A second brain scan was taken within 72 hours after the CAS at which time only newly appearing lesions were regarded as ischemic lesions. The MRI findings were evaluated blindly by an independent neuroradiologist.
CAS procedures
All CAS procedures were performed under local anaesthesia administered through the femoral artery. The procedures were performed by a neuro-interventional team that consisted of three investigators [M. T., Y.E. and Y. E.]. A bolus injection of heparin (100U/kg) was administered immediately before the procedure to increase activated clotting time to a minimum of 250 seconds. A 9F Mo.Ma device (Medtronic, Minneapolis, MN) with two compliant balloons was navigated into the affected vessel; the distal balloon was placed in the external carotid artery, while the proximal balloon was placed in the common carotid artery. Two types of stents were placed in the stenotic lesions: an open-cell stent (Precise [Johnson & Johnson, Cordis, Minneapolis, MN] or Protégé [Covidien, Mansfield, MA] or a closed-cell stent (Wallstent [Boston Scientific, Natick, MA]). The selection of the type of stent was determined at the discretion of the attending physicians.
Post-dilatation was performed after stent deployment when residual stenosis was >30%.
Optical Coherence Tomography
Following the CAS procedures, OCT imaging was performed using the Terumo Intravascular Optical frequency domain imaging (OFDI) system (LUNAWAVE, Terumo Corp., Tokyo, Japan). For this procedure, an imaging catheter (FastView, Terumo Corp., Tokyo, Japan) was inserted into the carotid artery beyond the distal end of the stent. Scanning was performed from the distal to the proximal portion of the stent using a built-in automatic pullback system while the common and external carotid arteries were occluded by using the balloons.
To remove blood from the vessel, during scanning, half-diluted contrast medium (Iomeron 300, Bracco, Milano, Italy) was infused continuously into the carotid arteries from the guiding catheter at a rate of 6 mL/s by a motor-driven injector. An in-stent tissue protrusion was defined as the presence of an intraluminal mass. The presence of in-stent tissue protrusion between the first and last frames in each cross section and the area of maximum projection was measured on the workstation of Terumo Intravascular OFDI system. All images were recorded digitally and analysed by two observers [M.T. and T.I.] who made a consensus evaluation.
Statistical analyses
Categorical variables were analysed using the χ2 test or Fisher’s exact test as appropriate. A probability value of <0.05 was considered statistically significant. All statistical analyses were performed using PASW Statistics software, version 18 (SPSS Japan, Tokyo, Japan). Statistical analyses were performed using PASW Statistics software (version 18; IBM SPSS, Japan, Tokyo, Japan) and a P-value < 0.05 was considered to indicate statistical significance.
Results
In total, 28 patients who underwent CAS during the study period were included in the study. Open-cell stents were used in 20 patients (71.4%) including Precise (n = 17) and Protégé (n = 3) stent types. The remaining eight patients received a closed-cell stent (Wallstent). All procedures were successfully completed and yielded adequate angiographic results. In addition, there were no technical or neurological complications associated with OCT.
In-stent tissue protrusion was detected by OCT in 23 patients (82.1%), with a mean maximum projection area of 0.94 mm2 (range, 0–4.6 mm2). The remaining five patients had no evidence of in-stent tissue protrusion. Ten patients (35.7%) had in-stent tissue protrusion area ≥1 mm2 (Table 1). Univariate analysis of the data showed no demographic, pre-procedural or post-operational factors were correlated with in-stent tissue protrusion size according to those with an area <1 mm2 and those with an area ≥1 mm2. Factors included the degree of original stenosis, T1 high plaque, the type of stent, requirement for post-dilatation, residual stenosis post-CAS and incidence of ischemic lesions on DWI post-CAS. Importantly, none of the 28 patients experienced a symptomatic stroke within 30 days post-procedure
Table 1.
Baseline characteristics of the patients according to the area of in-stent tissue protrusion
| Parameter | All patients |
Intraluminal area of in-stent tissue protrusion* |
|
|---|---|---|---|
| <1 mm2 | ≥1 mm2 | ||
| Patients | 28 | 18 | 10 |
| Age, years | 72.9 ± 6.3 | 73.2 ± 6.2 | 72.3 ± 6.8 |
| Sex, male | 16 (57) | 10 (55.6) | 6 (60) |
| Degree of stenosis, % | 77.4 ± 13.6 | 79.3 ± 12.9 | 74.3 ± 14.9 |
| Co-morbid conditions | |||
| Diabetes mellitus | 16 (57) | 9 (50) | 7 (70) |
| Hypertension | 8 (29) | 4 (22) | 4 (40) |
| Hyperlipidaemia | 16 (57) | 11 (61) | 5 (50) |
| Coronary artery disease | 12 (43) | 6 (33) | 6 (60) |
| Symptomatic stenosis | 16 (57) | 9 (50) | 7 (70) |
| Current cigarette use | 7 (25) | 3 (17) | 4 (40) |
| Plaque evaluation on carotid artery T1WI | |||
| High SIR plaques | 20 (71) | 13 (72) | 7 (70) |
| Stent | |||
| Open cell | 20 (71) | 13 | 7 |
| Closed cell | 8 (29) | 5 | 3 |
| Post-dilatation | 12 (43) | 9 (50) | 3 (33) |
| Residual stenosis after CAS, % | 14.9 ± 12.5 | 15.7 ± 13.9 | 13.3 ± 10.0 |
| Post-op ischemic brain lesions on DWI | 9 (32) | 6 (33) | 3 (30) |
| Laboratory parameters | |||
| Total cholesterol, mg/dl | 165.3 ± 56.7 | 160.4 ± 68.7 | 157.0 ± 15.4 |
| Triglycerides, mg/dl | 98.3 ± 28.9 | 100.9 ± 27.4 | 92.8 ± 33.1 |
| HDL cholesterol, mg/dl | 59.7± 30.1 | 64.3 ± 33.9 | 57.1 ± 23.7 |
| LDL cholesterol, mg/dl | 100.4 ± 42.7 | 93.8 ± 32.8 | 114.5 ± 58.7 |
| CRP, mg/dl | 0.7 ± 1.7 | 0.4 ± 0.6 | 1.3 ± 2.9 |
| Haemoglobin A1C, % | 6.0 ± 0.6 | 5.9 ± 0.5 | 6.4 ± 0.8 |
| Platelets, ×104/mm3 | 22.2 ± 5.2 | 21.0 ± 3.7 | 24.8 ± 7.1 |
Values are shown as mean ± standard deviation or n (%).
*Univariate analysis showed that there were no statistically significant differences between groups in terms of demographic, pre/post-operational factors
T1WI, T1 weighted images; SIR, signal intensity ratio; CAS, carotid artery stenting; DWI, diffusion-weighted imaging; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein;
Results from platelet function tests taken pre-CAS, separated into two in-stent plaque size groups (i.e., <1 mm2 and ≥1 mm2) are shown in Table 2. Compared with the <1 mm2 group, the ≥1 mm2 group had significantly higher platelet reactivity induced by collagen at 10 μg/ml (P = 0.023) and at 20 μg/ml (P = 0.025). In contrast, the results of platelet reactivity induced by the other agonists using LTA (i.e., ADP and TRAP) and those measured by the VerifyNow assay were not significantly different between the two in-stent plaque size groups.
Table 2.
Comparison of maximum aggregation values according to the area of in-stent tissue protrusion
|
Intraluminal area of in-stent tissue protrusion |
Statistical significance | ||
|---|---|---|---|
| <1 mm2 | ≥1 mm2 | ||
| Patients | 18 | 10 | |
| LTA | |||
| ADP, % | |||
| 3 μM | 30.1 ± 12.5 | 33.2 ± 18.1 | ns |
| 10 μM | 47.7 ± 14.3 | 51.3 ± 19.2 | ns |
| 20 μM | 52.6 ± 15.9 | 57.9 ± 21.6 | ns |
| Collagen, % | |||
| 3 μg/mL | 25.4 ± 21.1 | 36.3 ± 15.0 | ns |
| 10 μg/mL | 42.7 ± 23.1 | 63.3 ± 18.4 | 0.023 |
| 20 μg/mL | 53.0 ± 24.0 | 73.9 ± 16.4 | 0.025 |
| TRAP, % | |||
| 10 μM | 15.8 ± 16.0 | 27.4 ± 19.2 | ns |
| 20 μM | 51.0 ± 25.4 | 62.4 ± 13.1 | ns |
| 30 μM | 66.4 ± 18.6 | 78.6 ± 10.2 | ns |
| VerifyNow assay | |||
| ARU | 469.8 ± 77.3 | 464.1 ± 115.0 | ns |
| PRU | 201.2 ± 61.8 | 179.6 ± 88.6 | ns |
Values are shown as means ± standard deviation
LTA, light transmittance aggregometry; TRAP, thrombin receptor-activating peptide; ADP, adenosine diphosphate; ARU, aspirin reaction units; PRU, P2Y12 reaction units
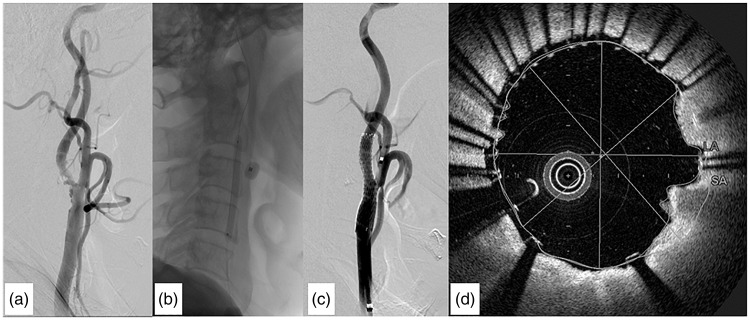
As an example of a typical case, the following account describes the detailed results from a 70-year-old man who experienced symptomatic right internal carotid artery stenosis and underwent CAS in our department. Based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria, 27 pre-operative digital subtraction angiography (DSA) showed 70% stenosis (Figure 1a). As shown by LTA, ADP-induced platelet aggregation was 17%, 31% and 33% for 3, 10 and 20 μM, respectively. Collagen-induced platelet aggregation was 23%, 43% and 57% for 3, 10 and 20 μg/ml, respectively. TRAP -induced platelet aggregation was 11%, 52% and 69% for 10, 20 and 30 μM, respectively. The VerifyNow assay results showed platelet aggregation was suppressed with ARU 350 and PRU 64. Following successful dilatation of the arterial lumen (Figure 1b) a Protégé stent was deployed (Figure 1c). An intraluminal thrombus was detected by OCT as a backscattering protrusion into the carotid lumen with a signal-intensity-free shadow and maximum projection area of 1.3 mm2 (Figure 1d).
Figure 1.
Representative images of carotid artery stenting (CAS) from a 70-year-old male patient. (a) Angiogram of the right carotid artery showing 70% stenosis. (b) Angiogram after CAS showing successful dilatation of the arterial lumen. (c) Angiogram after CAS showing successful implantation of a Protégé stent. (d) Intraluminal thrombus is detected by Optical coherence tomography (OCT) as a backscattering protrusion into the carotid lumen with signal-intensity-free shadowing.
Discussion
This study found that collagen-induced platelet reactivity at 10 and 20 μg/ml pre-procedure was related to in-stent tissue protrusion size following CAS. Collagen is known to play a pivotal role in platelet activation which is the first step in thrombus formation. 28 Balloon angioplasty and stent deployment are known to damage the endothelium of the vessel, resulting in cracks and dislodgment of the plaque.29 The exposed sub-endothelium that contains collagen interacts with platelets and can lead to further thrombus formation. Initially, platelets are stimulated by pathological sheer stress-activated GPIb/IX/V membrane receptor and by collagen-activated GPVI receptor.30 Activated GPVI causes upregulation of integrin activity,31 and leads to release of several platelet activators, such as ADP from dense granules and synthesis of thromboxane A2 from phospholipids via the arachidonic acid cascade.
In-stent tissue protrusion detected by OCT has been reported to be more common in open-cell stents than in closed-cell stents.32 In addition, in patients with unstable plaque, the size of an in-stent tissue protrusion with double-layer micromesh stent was smaller than that with conventional stent.33 The type of stent or the fragility of a plaque could affect the development of in-stent tissue protrusion. In this current study, the choice of stent was at the surgeon’s discretion and was based on plaque type, degree of tortuosity in the carotid artery and patient’s symptoms. Although this may have introduced an unintentional bias, our analysis showed that the type of stent and presence of post-CAS ischemic lesions detected by DWI did not differ according to the size of the in-stent tissue protrusion.
Several stimulators of platelet aggregation were used in this study. Collagen is a primary activator, whereas other agonists used in this study (i.e., ADP, TRAP, and arachidonic acid) were secondary activators. Although platelet function tests are able to evaluate the ability of platelets to aggregate under a variety of circumstances, they are commonly used as a means of monitoring the effects of antiplatelet therapy. 22 Specific agonists of antiplatelet agent receptors (e.g., ADP for the P2Y12 receptor) are useful tools in these assays but to predict the risk of thromboembolic events, primary platelet activators such as collagen may be more appropriate.
The study had several limitations. For example, the single-centre design, small number of patients and low rate of ischemic events precluded us from making definitive conclusions about the possible relationship between platelet function tests and in-stent tissue protrusion. Therefore, more studies are required using a large number of patients to clarify the role, if any, of platelet function tests on the presence of in-stent tissue protrusion.
Acknowledgements
The authors would like to thank Dr Takahiko Asano for his help in preparing the manuscript.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This study was supported by Grants-in-Aid for Scientific Research Grant Number 16K20001 and 16K20002 from Japan Society for the Promotion of Science.
References
- 1.Khan M andQureshi AI.. Factors associated with increased rates of post-procedural stroke or death following carotid artery stent placement: a systematic review. J Vasc Interv Neurol 2014; 7: 11–20. [PMC free article] [PubMed] [Google Scholar]
- 2.White CJ. Carotid artery stenting. J Am Coll Cardiol 2014; 64: 722–731. [DOI] [PubMed] [Google Scholar]
- 3.Kotsugi M, Takayama K, Myouchin K, et al. Carotid artery stenting: investigation of plaque protrusion incidence and prognosis. JACC Cardiovasc Interv 2017; 10: 824–831. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura S, Kawasaki M, Yamada K, et al. OCT of human carotid arterial plaques. JACC Cardiovasc Imaging 2011; 4: 432–436. [DOI] [PubMed] [Google Scholar]
- 5.Patwari P, Weissman NJ, Boppart SA, et al. Assessment of coronary plaque with optical coherence tomography and high-frequency ultrasound. Am J Cardiol 2000; 85: 641–644. [DOI] [PubMed] [Google Scholar]
- 6.Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002; 39: 604–609. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13: 767–771. [PubMed] [Google Scholar]
- 8.McKevitt FM, Randall MS, Cleveland TJ, et al. The Benefits of Combined Anti-platelet Treatment in Carotid Artery Stenting. Eur J Vasc Endovasc Surg 2005; 29: 522–527. [DOI] [PubMed] [Google Scholar]
- 9.Bates ER, Babb JD, Casey DE, Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting). J Am Coll Cardiol 2007; 49: 126–170. [DOI] [PubMed] [Google Scholar]
- 10.Enomoto Y andYoshimura S.. Antiplatelet therapy for carotid artery stenting. Interv Neurol 2013; 1: 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann N andHohlfeld T.. Clinical implications of aspirin resistance. Thromb Haemost 2008; 100: 379–390. [PubMed] [Google Scholar]
- 12.Serebruany VL, Steinhubl SR, Berger PB, et al. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45: 246–251. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran S, Wells KR, Lee VH, et al. Prevalence and risk factors for aspirin and clopidogrel resistance in cerebrovascular stenting. AJNR Am J Neuroradiol 2008; 29: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 2011; 306: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 15.Fifi JT, Brockington C, Narang J, et al. Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol 2013; 34: 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snoep JD, Hovens MM, Eikenboom JC, et al. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J 2007; 154: 221–231. [DOI] [PubMed] [Google Scholar]
- 17.Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016; 388: 2015–2022. [DOI] [PubMed] [Google Scholar]
- 18.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011; 305: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 19.Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol 2012; 59: 2159–2164. [DOI] [PubMed] [Google Scholar]
- 20.Collet JP, Cuisset T, Range G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012; 367: 2100–2109. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez A, Moniche F, Cayuela A, et al. Antiplatelet effects of clopidogrel dose adjustment (75 mg/d vs 150 mg/d) after carotid stenting. J Vasc Surg 2014; 60: 428–435. [DOI] [PubMed] [Google Scholar]
- 22.Paniccia R, Priora R, Liotta AA, et al. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015; 11:133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujimoto M, Enomoto Y, Kokuzawa J, et al. Diabetes mellitus and carotid artery plaques exhibiting high-intensity signals on MR angiography are related to increased platelet reactivity after carotid artery stenting. J Neurointerv Surg 2017; 9: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang EW, Chung J, Seo KD, et al. A protocol-based decision for choosing a proper surgical treatment option for carotid artery stenosis. J Cerebrovasc Endovasc Neurosurg 2015; 17:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida K, Narumi O, Chin M, et al. Characterization of carotid atherosclerosis and detection of soft plaque with use of black-blood MR imaging. AJNR Am J Neuroradiol 2008; 29:868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada K, Kawasaki M, Yoshimura S, et al. Prediction of silent ischemic lesions after carotid artery stenting using integrated backscatter ultrasound and magnetic resonance imaging. Atherosclerosis 2010; 208: 161–166. [DOI] [PubMed] [Google Scholar]
- 27.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. New Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 28.Kahn ML. Platelet-collagen responses: molecular basis and therapeutic promise. Semin Thromb Hemost 2004; 30:419–25. [DOI] [PubMed] [Google Scholar]
- 29.Farb A, Sangiorgi G, Carter AJ, et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999; 99: 44–52. [DOI] [PubMed] [Google Scholar]
- 30.Davi G andPatrono C.. Platelet activation and atherothrombosis. N Engl J Med 2007; 357: 2482–2494. [DOI] [PubMed] [Google Scholar]
- 31.Moroi M andJung SM.. Platelet glycoprotein VI: its structure and function. Thromb Res 2004; 114: 221–233. [DOI] [PubMed] [Google Scholar]
- 32.de Donato G, Setacci F, Sirignano P, et al. Optical coherence tomography after carotid stenting: rate of stent malapposition, plaque prolapse and fibrous cap rupture according to stent design. Eur J Vasc Endovasc Surg 2013; 45: 579–587. [DOI] [PubMed] [Google Scholar]
- 33.Yamada K, Yoshimura S, Miura M, et al. Potential of new generation double-layer micromesh stent for carotid artery stenting in patients with unstable plaque approximately A preliminary result using OFDI analysis approximately. World Neurosurg 2017; 105: 321–326. [DOI] [PubMed] [Google Scholar]