Short abstract
Objective
This study was performed to examine the working memory (WM) encoding and retrieval abilities in patients with major depressive disorder (MDD) and determine whether a mood-congruent memory effect is present.
Methods
The modified Sternberg WM paradigm with positive, negative, and neutral emotional pictures was used to investigate the WM abilities of 26 patients with MDD and 26 healthy controls (HCs).
Results
No significant difference in picture WM was found between the MDD and HC groups; however, the accuracy of picture position WM was significantly lower and the response time was significantly longer in the MDD than HC group, regardless of the picture or position WM. Additionally, in the MDD group, the accuracy of negative picture/position WM was significantly higher than that of positive picture/position WM.
Conclusions
These results suggest that in patients with MDD, spatial WM impairment was more severe than object WM. In addition, these patients’ WM retrieval was impaired, resulting in a decrease in WM retrieval ability, which may be an important cause of the slow thought in patients with MDD. Moreover, patients with depression have a mood-congruent memory effect, which may be an important factor in the occurrence and maintenance of depression.
Keywords: Depression, working memory (WM), emotion, WM encoding, WM retrieval, Sternberg WM paradigm
Introduction
At present, the study of depression focuses largely on emotional processing, but patients with depression may have disorders of other cognitive functions such as attention, memory, and executive function.1–3 Many studies have shown that patients with depression not only have emotional disorders but may also have associated cognitive impairment,4–7 which manifests as functional damage of executive function, language, and working memory (WM).8–12 WM is a system that provides temporary information storage and limited processing for cognitive information processing; it is also the basis of many cognitive functions such as speech comprehension, reasoning, problem solving, and learning.13 Imaging studies have shown that executive dysfunction of the prefrontal cortex can decrease the WM ability of patients with major depressive disorder (MDD)14 as shown by an increased WM error rate and prolonged response time.15 Schatzberg et al.16 found that patients with depression had significantly impaired attention and word memory; however, a study of word memory by Fossati et al.17 showed that patients with depression had normal recall and recognition but impaired free recall. Harvey et al.5 used the n-back WM paradigm to study word memory and found that the accuracy of memory was lower and the response time was significantly longer in patients with depression than in healthy controls (HCs). Likewise, Rose and Ebmeier18 performed a symbolic n-back experimental paradigm study and found that the accuracy of memory was lower and the response time was significantly longer in patients with depression than in HCs. In a study of spatial WM, Lavric et al.19 used tasks of word n-back WM and spatial n-back WM and found that negative emotion had no effect on word memory; it only affected the spatial WM. Li et al.20 also showed that negative emotion had no effect on the word memory task; it only affected spatial WM, causing a decline in spatial WM retention ability. However, other studies have shown that both word and spatial WM are impaired in patients with MDD.21 Taken together, the results of these studies suggest that there is still no agreement on whether the WM of patients with depression is impaired.
The mood-congruent memory effect refers to the concept that emotional information that is consistent with the current emotional state is easier to remember.21–23 In a study on the mood-congruent memory effect performed by inducing different emotions in the study subjects, those subjects with negative states had more accurate memory than those with positive states24; that is, people who are in a negative state more easily remember negative information.25 An important feature of patients with depression is persistent dysthymia and a chronically negative state. One study showed that compared with HCs, patients with depression more frequently remembered the negative stimulus material that was consistent with their negative mood and less frequently remembered the positive stimulus material that was not consistent with their mood.26 The results of a meta-analysis showed that compared with the control group, patients with depressive disorders were more likely to remember the negative stimulus consistent with their negative moods but were less likely to remember the positive stimulus inconsistent with their moods.27 Our previous studies on emotional picture WM in patients with mild depression demonstrated that the memory accuracy of negative pictures was significantly higher than that of positive pictures and that the memory accuracy of the position of negative pictures was also significantly higher than that of the position of positive pictures, demonstrating the existence of the mood-congruency effect.28 The enhancement of memory of negative material that is consistent with mood and the impairment of memory of positive material that is inconsistent with mood may be a manifestation of cognitive control in patients with depression.29,30 In our study, we not only investigated the difference in WM between the MDD and HC groups but also investigated whether the MDD group exhibited a mood-congruent WM effect.28
WM also involves cognitive activities including memory encoding, preservation, and retrieval.31 However, not enough research on the WM ability in patients with MDD has yet been performed, and few studies on both emotional picture WM and spatial WM have been carried out. The Sternberg paradigm is a classic experimental paradigm used in WM studies and serves as an experimental scheme for studying human short-term memory32 (called the Sternberg experiment or the Sternberg WM experiment by later researchers). In the present study, we used an improved Sternberg WM paradigm and pictures that readily induce emotions in patients with MDD and HCs to examine the object and spatial WM in patients with MDD.
Based on the findings of previous studies, we put forward the following hypotheses. First, the WM ability is impaired in patients with MDD, which would be shown in this study by significantly lower memory accuracy and a longer reaction time during both picture and position WM in patients with MDD than in HCs. Second, patients with MDD have a mood-congruent WM effect, which would be shown in this study by significantly higher memory accuracy of negative pictures and their positions than that of positive pictures and their positions.
Materials and methods
Ethics statement
All subjects provided signed informed consent to participate in this study. The study was approved by the Ethics Committee of Beijing Anding Hospital, Capital Medical University, China.
Subjects
Equal numbers of patients with MDD and HCs matched for age, sex, and years of education were included in this study. The subjects in the MDD group were outpatients who first visited the Anding Hospital of Capital Medical University, and those in the HC group were recruited by poster advertisements. Patients with depression were assessed using the Mini-International Neuropsychiatric Interview (MINI) and the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Patients diagnosed with depression were also required to complete the 17-item Hamilton Depression Rating Scale (HAM-D) and Beck Depression Inventory (BDI). The inclusion criteria for the MDD group were as follows: age of 18 to 60 years, right-handedness, having met the DSM-IV diagnostic criteria for depression, a diagnosis of MDD according to an HAM-D score of ≥18 and BDI score of ≥14, not taking any antidepressant drugs and no history of antidepressant treatment within 2 weeks of entering the group, no color blindness or other eye disease, normal or corrected-to-normal vision, and ability to finish the experiment. The exclusion criteria were as follows: DSM-IV diagnosis of a schizoaffective mental disorder, bipolar disorder, intellectual disability, dysmnesia, or other cognitive disorder; serious anxiety symptoms or panic attacks; suicidal ideation or attempted suicide; a history of epilepsy or febrile convulsions, cranial trauma, or severe unstable somatic disease; a diagnosis of diabetes, thyroid disease, hypertension, heart disease, or other systemic disease; and mental disorders caused by substance abuse (alcohol, drugs, etc.).
The inclusion criteria for the HC group were as follows: age range of 18 to 60 years, right-handedness, not taking any drugs that affect the functioning of the nervous system, no history of depression or other psychiatric disorders, no alcohol or other substance dependence, no color blindness or other eye disease, normal vision or corrected vision, and BDI score of <4.
Experimental materials
Three types of positive, neutral, and negative pictures among a total of 60 images were used in our experiment. All pictures were obtained from the International Affective Picture System. The average valence and arousal of the positive pictures was 7.31 ± 0.44 and 5.54 ± 0.44, respectively; the average valence and arousal of the negative pictures was 2.79 ± 0.51 and 5.97 ± 0.44, respectively; and the average valence and arousal of the neutral pictures was 5.18 ± 0.17 and 3.23 ± 0.22, respectively. The results of an independent-samples t-test with multiple comparison correction showed the following results: the valence of the positive pictures was significantly greater than that of the negative pictures [t(118) = 101.569, p < 0.005], while the arousal of the positive and negative pictures was not significantly different [t(118) = −2.052]; both the valence and arousal of the positive pictures were significantly greater than those of the neutral pictures [valence: t(118) = 47.516, p < 0.005; arousal: t(118) = 36.437, p < 0.005]; the valence of the negative pictures was significantly lower than that of the neutral pictures [t(118) = −56.621, p < 0.005]; and the arousal of the negative pictures was significantly greater than that of the neutral pictures [t(118) = 43.191, p < 0.005]. After processing by Picture Manager software (Microsoft, Redmond, WA, USA), the size, grayscale, and resolution ratio of all images were consistent.
Experimental paradigm and experimental procedure
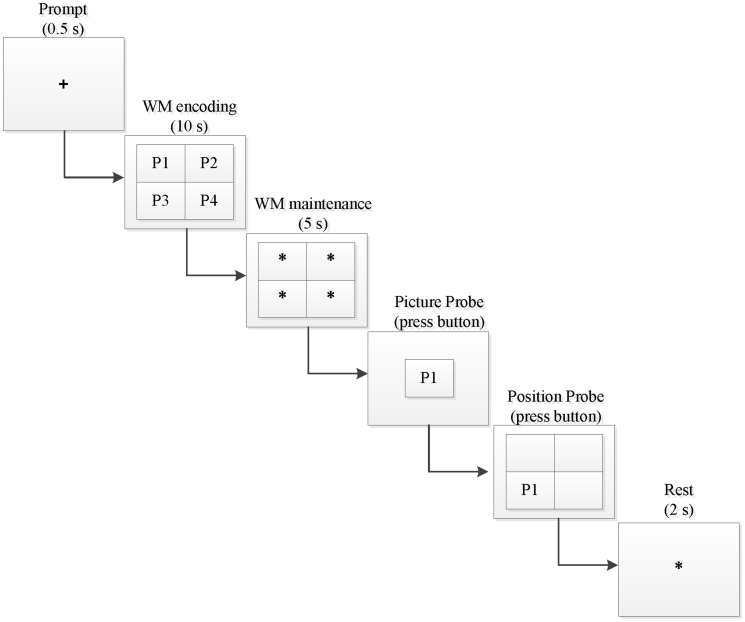
An improved Sternberg WM paradigm was used in this study. The traditional Sternberg experimental paradigm uses letters or numbers to detect non-spatial WM. In this study, we used emotional pictures instead of letters or numbers to test non-spatial WM and simultaneously examined the effects of the types of emotional pictures on WM; we also added a picture position target to detect the spatial WM. Each cue in the experiment involved four pictures of the same type (i.e., four positive, four negative, or four neutral), and the four pictures corresponded to four different positions (upper left, upper right, lower left, and lower right). To balance the position of the pictures, we used a pseudo-random presentation order. Thus, three types of picture cues were used in the experiment: positive, negative, and neutral. The type of target stimulus was the same as the cue. Two types of target stimuli were used: a picture target in the center of the screen and a picture location target that appeared in a certain position.
The experimental procedure is shown in Figure 1. First, a “+” symbol appeared in the center of the screen for 0.5 seconds, indicating that the cues would appear immediately. The screen then presented a group (four pictures, located at the upper left, upper right, lower left, and lower right) of emotional pictures (positive, negative, or neutral) as a prompt, and this group of pictures disappeared after 10 seconds. An asterisk (“*”) was then shown in place of each disappeared emotional image for 5 seconds. When the asterisk had disappeared, a picture of the emotional target was displayed in the center of the screen, and the participant’s task was to determine whether the picture had appeared in the previous cue. The participants responded by pressing one of two keys: the left key if it had appeared and the right key if it had not. If the participant pressed the left key, a picture depicting the emotion that had appeared in the prompt message was displayed in a random position of the four positions. The subject was then required to answer whether the picture had been in this position; he or she pressed the left key if it had appeared and the right key if it had not. If the subjects selected the right button, the picture position assessment would be ignored, and an asterisk would appear. The subjects would then have 2 seconds of rest time before entering the next trial.
Figure 1.
An example trial of the emotional WM task in our experiment. WM, working memory.
The whole experiment was divided into three stages: memory encoding, memory retention, and memory retrieval. The memory encoding phase was the 10 seconds from presentation of the emotional picture information to its disappearance. The memory retention phase was the 5 seconds from disappearance of the prompt message to appearance of the target information (i.e., the presentation time of the four asterisks). The memory extraction phase was the duration from presentation of the target information to the subject’s response.
Statistical analysis
Statistical analyses were conducted using SPSS software, version 21.0 (IBM Corp., Armonk, NY, USA). We employed a mixed-model repeated-measures analysis of variance with a 2 × 3 factorial design (group: depressed, control; within-subject factor stimulus material: positive, neutral, negative) to assess the accuracy and response time of the picture and position WM. Between-group comparisons of age and years of education were performed using an independent-samples t-test, and between-group comparison of the sex distribution was performed using a chi-square test. Pair-wise intragroup comparisons of the three emotional picture types were performed using paired-samples t-tests, and the Bonferroni method was applied to conduct multiple-comparison corrections.
According to the Bonferroni correction principle,33 if an experimenter is testing n independent hypotheses on a set of data, then the statistical significance level that should be used for each hypothesis separately is 1/n times what it would be if only one hypothesis were tested. For example, to test three independent hypotheses with the same data at the α = 0.05 significance level, the probability of error for the three tests is 1 − (1 − α)n = 1 − 0.8573 = 0.1427, which is unacceptable. Thus, instead of using a p value threshold of 0.05, one would use a stricter threshold of 0.017. To facilitate comparisons of our results, we still selected the α = 0.05 significance level, while the p-value of the paired-samples t-tests was multiplied by the number of multiple comparisons (i.e., p × 3).
Results
Demographic and clinical data analysis
A total of 52 subjects participated in the experiment: 26 patients with MDD and 26 HCs matched for age, sex, and years of education. The demographic and clinical data are shown in Table 1. There were no significant differences in sex (chi-square test), age (independent-samples t-test), or education years (independent-samples t-test) between the MDD and HC groups. The MDD group had significantly higher BDI scores than the HC group.
Table 1.
Comparison of demographic differences between the two groups.
| MDD (n = 26) | HCs (n = 26) | p-value | |
|---|---|---|---|
| Sex, male:female | 11:15 | 12:14 | 0.812 |
| Age, years | 37.45 ± 10.86 | 36.98 ± 11.23 | 0.791 |
| Education years | 14.43 ± 4.10 | 15.12 ± 3.81 | 0.521 |
| HAM-D score | 23.68 ± 8.50 | ||
| BDI score | 16.28 ± 8.46 | 1.28 ± 1.46 | 0.000 |
Data are presented as n or mean ± standard deviation.
MDD, major depressive disorder; HCs, healthy controls; HAM-D, Hamilton Depression Rating Scale; BDI, Beck Depression Inventory
Comparison of accuracy and reaction time of picture WM between groups
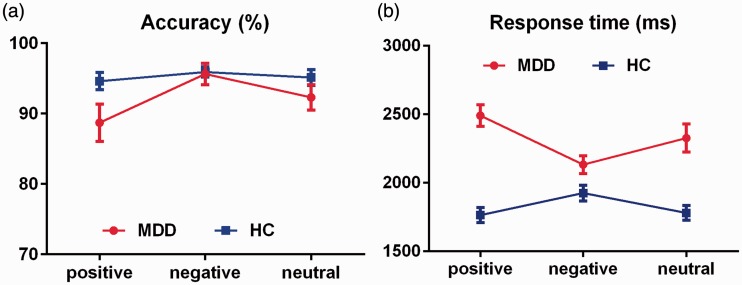
The accuracy and reaction time of picture WM in the MDD and HC groups are shown in Figure 2.
Figure 2.
Comparison of picture WM accuracy and reaction time between the groups. (a) Accuracy of picture WM. (b) Reaction time of picture WM. Error bars represent the standard error of the mean. WM, working memory; MDD, major depressive disorder; HC, healthy control.
Accuracy of picture WM
The accuracy of picture WM was analyzed by mixed variance 2 × 3 factorial analysis (group: MDD, HC; emotional category: positive, neutral, negative). The main effect of the group factor was not significant [F(1, 50) = 2.221, η2 = 0.043], the main effect of the emotional category was significant [F(1, 50) = 7.614, p < 0.05, η2 = 0.132], and the interaction between group and emotional category was significant [F(1, 50) = 4.285, p < 0.05, η2 = 0.079].
The two-sample t-test of different emotional valence between groups showed no significant difference between the groups, whether positive, negative, or neutral [positive: t(50) = −2.012, d = −0.569; negative: t(50) = −0.150, d = −0.043; neutral: t(50) = −1.322, d = −0.374].
The paired t-test for intragroup comparisons revealed the following. In the MDD group, the negative picture accuracy was significantly higher than the positive picture accuracy [positive vs. negative: t(25) = −2.775, p = 0.030, d = −0.639], but there were no significant differences between the positive and neutral pictures [t(25) = −2.487, d = −0.316] or between the negative and neutral pictures [t(25) = 2.170, d = 0.400]. In the HC group, pairwise comparison revealed no significant differences [positive vs. negative: t(25) = −1.729, d = −0.252; positive vs. neutral: t(25) =−1.443, d = −0.086; negative vs. neutral: t(25) = 1.140, d = 0.159].
Reaction time of picture WM
The reaction time of picture WM was assessed by mixed variance 2 × 3 factorial analysis (group: MDD, HC; emotional category: positive, neutral, negative). The main effect of the group factor was significant [F(1, 50) = 29.674, p < 0.005, η2 = 0.372], the main effect of the emotion category factor was not significant [F(1, 50) = 2.910, η2 = 0.055], and the interaction between group and emotional category was significant [F(1, 50) = 4.339, p < 0.05, η2 = 0.080].
The two-sample t-test of different emotional valence between groups showed that regardless of the positive, negative, or neutral reaction time, the reaction time was longer in the MDD than HC group [positive: t(50) = 7.534, p < 0.005, d = 2.131; negative: t(50) = 2.360, p < 0.05, d = 0.667; neutral: t(50) = 4.714, p < 0.005, d = 1.333].
The paired t-test for intragroup comparisons showed the following. In the MDD group, the negative picture memory retrieval reaction time was significantly shorter than the positive picture WM reaction time [t(25) = −10.725, p < 0.005, d = −0.982], but there were no significant differences between positive and neutral pictures [t(25) = 1.917, d = 0.359] or between negative and neutral pictures [t(25) = −2.323, d = −0.449]. In the HC group, the negative picture WM reaction time was significantly longer than the positive picture WM reaction time [negative vs. positive: t(25) = 5.253, p < 0.005, d = 0.562] and neutral picture WM reaction time [negative vs. neutral: t(25) = 4.084, p < 0.05, d = 0.510], but there was no significant difference between positive and neutral pictures [t(25) = −1.237, d = −0.059].
Comparison of accuracy and reaction time of picture position WM between groups
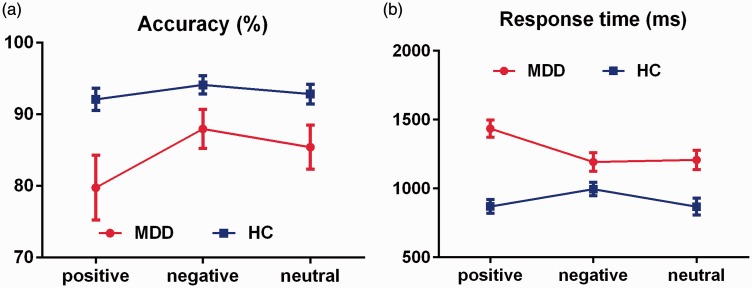
The accuracy and reaction time of picture position WM in the MDD and HC groups are shown in Figure 3.
Figure 3.
Comparison of picture position WM accuracy and reaction time between the groups. (a) Accuracy of picture position WM. (b) Reaction time of picture position WM. Error bars represent the standard error of the mean. WM, working memory; MDD, major depressive disorder; HC, healthy control.
Accuracy of picture position WM
The accuracy of picture position WM was assessed by mixed variance 2 × 3 factorial analysis (group: MDD, HC; emotion category: positive, neutral, negative). The main effect of the group factor was significant [F(1, 50) = 6.174, p < 0.05, η2 = 0.110], the main effect of the emotional category factor was significant [F(1, 50) = 8.344, p < 0.05, η2 = 0.143], and the interaction between group and emotional category was significant [F(1, 50) = 4.818, p < 0.05, η2 = 0.088].
The two-sample t-test of different emotional valence between the groups showed that regardless of the positive, negative, or neutral reaction time, the accuracy of picture position WM was lower in the MDD than HC group [positive: t(50) = −2.564, p < 0.05, d = −0.725; negative: t(50) =−2.028, d = 0.573; neutral: t(50) = −2.208, p < 0.05, d = 0.624].
The paired t-test for within-group comparisons showed the following. In the MDD group, the negative picture position WM accuracy was significantly higher than the positive picture position WM accuracy [t(25) = −2.633, p = 0.042, d = −0.438] and the neutral picture position WM accuracy was significantly higher than the positive picture position WM accuracy [t(25) =−2.590, p = 0.048, d = −0.291], but there was no significant difference between the negative and neutral picture position WM accuracy [t(25) = 1.548, d = 0.176]. In the HC group, the pairwise comparison revealed no significant differences [positive vs. negative: t(25) = −1.873, d = −0.285; positive vs. neutral: t(25) = −1.806, d = −0.104; negative vs. neutral: t(25) = 1.308, d = 0.191].
Reaction time of picture position WM
The reaction time of picture position WM was analyzed by mixed variance 2 × 3 factorial analysis. The main effect of the group factor was significant [F(1, 50) = 21.626, p < 0.005, η2 = 0.302], the main effect of the emotional category factor was significant [F(1, 50) = 11.332, p < 0.005, η2 = 0.185], and the interaction between the group and emotion category was significant [F(1, 50) = 10.991, p < 0.005, η2 = 0.180].
The two-sample t-test of different emotional valence between the groups showed that regardless of the positive, negative, or neutral reaction time, the reaction time of picture position WM was longer in the MDD than HC group [positive: t(50) = 7.061, p < 0.005, d = 1.997; negative: t(50) = 2.361, p < 0.005, d = 0.668; neutral: t(50) = 3.664, p < 0.005, d = 1.036].
The paired t-test for intragroup comparisons showed the following. In the MDD group, the reaction time of the positive picture position WM was significantly longer than that of negative picture position WM [positive vs. negative: t(25) = 7.532, p < 0.005, d = 0.745] and neutral picture position WM [positive vs. neutral: t(25) = 4.325, p < 0.005, d = 0.684], but there was no significant difference between negative and neutral pictures [t(25) = −0.442, d = −0.045]. In the HC group, the reaction time of the negative picture position WM was significantly shorter than that of the positive pictures [positive vs. negative: t(25) = −4.195, p < 0.005, d = −0.511] but longer than that of the neutral pictures [negative vs. neutral: t(25) = 2.904, p = 0.024, d = 0.463]. There was no significant difference between the positive and neutral pictures [t(25) = 0.040, d = 0.006].
Discussion
The main purpose of this study was to investigate the difference in WM ability between patients with MDD and HCs and determine whether the patients with MDD had a mood-congruent memory effect through emotional pictures and positions. Before beginning the study, we considered that if there were no significant differences in memory accuracy between the MDD and HC groups, then the encoding and retrieval functions of the patients with MDD were normal; however, if the reaction time was significantly longer in the MDD than HC group, then the retrieval function of patients with MDD was impaired. If the MDD group showed significantly lower memory accuracy than the HC group but no significant difference in the reaction time, then the encoding/maintenance function of patients with MDD was impaired. Finally, if the MDD group showed significantly lower memory accuracy and a significantly longer reaction time than the HC group, then both the encoding/maintenance and retrieval functions of the patients with MD were impaired.
First, with regard to the emotional picture WM, the results of this study showed no significant difference in memory accuracy between the MDD group and the HC group. Instead, only the WM response time of the MDD group was significantly longer than that of HC group, which was not exactly consistent with our first hypothesis (the picture WM of the MDD group was impaired). However, the results are consistent with those reported by Harvey et al.7 and Rose and Ebmeier,18 who used the n-back WM paradigm for word memory. Their studies showed that the memory accuracy was lower and the response time was significantly longer in patients with depression than in HCs. The difference in the results may be due to differences in the research purposes. Our study mainly investigated the effect of emotional WM, and we used emotional pictures as experimental materials. Our results suggest that the encoding/maintenance function of object WM in patients with MDD is normal but that the memory retrieval function is impaired.
Second, our findings of picture position WM showed that spatial WM impairment (which supports our first hypothesis and is also consistent with previous studies) and negative mood are typical characteristics of patients with depression. In 2003, Lavric et al.19 used the word n-back WM task and spatial n-back WM task and found that negative emotion had no effect on the performance of the word memory task, whereas it affected the spatial WM. In 2005, Li et al.20 also showed that negative emotion had no effect on the memory task; it only had an effect on the spatial WM, indicating that the spatial WM retention decreased. These studies suggest that negative mood affects spatial WM because both negative emotions and spatial WM have right-hemisphere dominance, and the functional overlap of the cerebral cortex causes them to interact with each other.27 Weiland-Fiedler et al.4 suggested that depression causes damage to the right hemisphere; thus, the spatial WM associated with the right hemisphere is more severely affected. Lavric et al.19 considered that the influence of emotion on memory may not be characterized by regional competition in the cerebral cortex but by competition for attention resources. Spatial WM needs more attention resources than word memory, which results in impaired spatial WM.19
Neuroimaging studies performed by Posner and Petersen34 and Berryhill35 showed that the posterior parietal cortex of the human brain is related to attention, episodic memory, and WM. Cabeza et al.36 found that the posterior parietal cortex is composed of the dorsal parietal cortex (DPC) and ventral parietal cortex (VPC). The DPC is related to top-down cued memory retrieval, whereas the VPC is related to bottom-up non-cued memory retrieval; furthermore, DPC activity is related to the rapid response of cue extraction.37 In our study of cued WM, the patients’ DPC function might have been impaired or a functional connectivity disorder might have been present between the DPC and medial temporal lobe, leading to insufficient top-down attention resource allocation and significant prolongation of the spatial WM response time (memory retrieval time). However, one of the main symptoms of depression is persistent dysthymia, which is caused by unconscious automatic processing of negative events in patients with depression (rumination). This automatic extraction process is a bottom-up non-cued memory extraction process that is related to the VPC. Although cued memory retrieval and non-cued memory retrieval involve different posterior parietal cortexes because of the lack of reasonable allocation of time resources in cognitive control,38,39 the attentional resources of cued memory retrieval are insufficient, thus significantly prolonging the spatial WM response time (memory retrieval time).
In our previous study, we examined the picture WM and picture position WM ability of patients with first-onset depression and untreated patients with mild depression, and we only found that the spatial WM reaction time of the patients with mild depression was significantly longer than that of the HC group; no other significant differences were found between patients with mild depression and HCs.28 However, the present study not only showed that the picture WM was impaired in patients with MDD but also that the accuracy and response time of picture position WM in these patients were impaired. These findings may indicate that WM impairment is associated with depressive symptoms.
Additionally, for both picture WM and picture position WM, the negative WM accuracy was significantly higher than the positive WM accuracy in the MDD group, suggesting that patients with MDD have a mood-congruent memory effect; this is consistent with our second hypothesis. This result is also consistent with our previous findings on patients with mild depression,28 which may indicate that the mood-congruent memory effect is a typical feature of depression.
Evaluation of different WM (object and spatial) can objectively reflect the characteristics of WM impairment in patients with depression. The encoding/maintenance phase of WM directly affects the accuracy of the test. If the memory encoding/maintenance function is impaired, patients show lower accuracy. The retrieval function of WM can directly affect patients’ response time. If the memory retrieval function is impaired, the memory retrieval ability will decrease and the patients will have a longer response time. The results of this study showed that the accuracy of picture WM was not significantly different between the MDD and HC groups, suggesting that the encoding/maintenance function of the object WM of patients with MDD is not impaired. However, the picture memory response time of the MDD group was significantly longer than that of the HC group, indicating that the memory retrieval function of the MDD group was impaired; this leads to impairment of memory retrieval ability. The WM accuracy was significantly lower and the reaction time was significantly longer in the MDD than HC group, which may indicate that the encoding/maintenance and the memory retrieval functions of spatial WM are impaired in patients with MDD.
In conclusion, this study showed that the accuracy of object and spatial WM accuracy differed between the MDD and HC groups. However, the response time of both object and spatial WM were significantly longer in the MDD than HC group, which may be an important cause of the slow thought in patients with MDD. The limitations of this study are mainly due to its cross-sectional design, which may influence the explanation of causation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (61602017), the Beijing Natural Science Foundation (4164080), the Beijing Outstanding Talent Training Foundation (2014000020124G039), the National Basic Research Programme of China (2014CB744600), the “Rixin Scientist” Foundation of Beijing University of Technology (2017-RX(1)-03), the National Natural Science Foundation of China (61420106005), the International Science & Technology Cooperation Program of China (2013DFA32180), the Funding of Beijing Anding Hospital, Capital Medical University (YJ2015009), the Special Fund of Beijing Municipal Science and Technology Commission (Z151100003915117), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201607 and ZYLX201403), and the Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20151801).
References
- 1.Elliott R, Sahakian BJ, McKay AP, et al. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med 1996; 26: 975–989. [DOI] [PubMed] [Google Scholar]
- 2.Veiel HOF. A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol 1997; 19: 587–603. [DOI] [PubMed] [Google Scholar]
- 3.Hammar A Lund A andHugdahl K.. Selective impairment in effortful information processing in major depression. J Int Neuropsychol Soc 2003; 9: 954–959. [DOI] [PubMed] [Google Scholar]
- 4.Weiland-Fiedler P, Erickson K, Waldeck T, et al. Evidence for continuing neuropsychological impairments in depression. J Affect Dis 2004; 82: 253–258. [DOI] [PubMed] [Google Scholar]
- 5.Harvey PO, Le Bastard G, Pochon JB, et al. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res 2004; 38: 567–576. [DOI] [PubMed] [Google Scholar]
- 6.Monks PJ, Thompson JM, Bullmore ET, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord 2004; 6: 550–564. [DOI] [PubMed] [Google Scholar]
- 7.Harvey PO, Fossati P, Pochon JB, et al. Cognitive control and brain resources in major depression: An fMRI study using the n-back task. Neuroimage 2005; 26: 860–869. [DOI] [PubMed] [Google Scholar]
- 8.Den Hartog HM, Derix M, van Bemmel AL, et al. Cognitive functioning in young and middle-aged unmedicated out-patients with major depression: testing the effort and cognitive speed hypotheses. Psychol Med 2003; 33: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 9.Elderkin-Thompson V, Kumar A, Bilker WB, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol 2003; 18: 529–549. [DOI] [PubMed] [Google Scholar]
- 10.Ellwart T Rinck M andBecker ES. Selective memory and memory deficits in depressed inpatients. Depress Anxiety 2003; 17: 197–206. [DOI] [PubMed] [Google Scholar]
- 11.Gilboa-Schechtman E Erhard-Weiss D andJeczemien P.. Interpersonal deficits meet cognitive biases: memory for facial expressions in depressed and anxious men and women. Psychiatry Res 2002; 113: 279–293. [DOI] [PubMed] [Google Scholar]
- 12.Gorlyn M, Keilp JG, Grunebaum MF, et al. Neuropsychological characteristics as predictors of SSRI treatment response in depressed subjects. J Neural Transm (Vienna) 2008; 115: 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddeley A. Working memory. Science 1992; 255: 556–559. [DOI] [PubMed] [Google Scholar]
- 14.Pelosi L, Slade T, Blumhardt LD, et al. Working memory dysfunction in major depression: an event-related potential study. Clin Neurophysiol 2000; 111: 1531–1543. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz T, Perez-Serrano JM, Zaglul C, et al. Deficit of cognitive event-related potentials during a working task in patients with major depression. Actas Espanolas de Psiquiatria 2003; 31: 177–181. [PubMed] [Google Scholar]
- 16.Schatzberg AF, Posener JA, DeBattista C, et al. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry 2000; 157: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 17.Fossati P, Coyette F, Ergis AM, et al. Influence of age and executive functioning on verbal memory of inpatients with depression. J Affect Disord 2002; 68: 261–271. [DOI] [PubMed] [Google Scholar]
- 18.Rose EJ andEbmeier KP.. Pattern of impaired working memory during major depression. J Affect Disord 2006; 90: 149–161. [DOI] [PubMed] [Google Scholar]
- 19.Lavric A Rippon G andGray JR.. Threat-evoked anxiety disrupts spatial working memory performance: An attentional account. Cognit Ther Res 2003; 27: 489–504. [Google Scholar]
- 20.Li XB Li XY andLuo YJ. Selective effect of negative emotion on spatial and verbal working memory: An ERP study. Proceedings of the 2005 International Conference on Neural Networks and Brain, VOLS 2005; 1–3: 1284–1289.
- 21.Quraishi S andFrangou S.. Neuropsychology of bipolar disorder: a review. J Affect Disord 2002; 72: 209–226. [DOI] [PubMed] [Google Scholar]
- 22.Eich E andMacaulay D.. Are real moods required to reveal mood-congruent and mood-dependent memory? Psychol Sci 2000; 11: 244–248. [DOI] [PubMed] [Google Scholar]
- 23.Rothkopf JS andBlaney PH.. Mood congruent memory – the role of affective focus and gender. Cogn Emot 1991; 5: 53–64. [Google Scholar]
- 24.Watkins PC, Mathews A, Williamson DA, et al. Mood-congruent memory in depression – emotional priming or elaboration. J Abnorm Psychol 1992; 101: 581–586. [DOI] [PubMed] [Google Scholar]
- 25.Bower GH. Mood and memory. Am Psychol 1981; 36: 129–148. [DOI] [PubMed] [Google Scholar]
- 26.Storbeck J andClore GL.. With sadness comes accuracy; With happiness, false memory - Mood and the false memory effect. Psychol Sci 2005; 16: 785–791. [DOI] [PubMed] [Google Scholar]
- 27.Shackman AJ, Sarinopoulos I, Maxwell JS, et al. Anxiety selectively disrupts visuospatial working memory. Emotion 2006; 6: 40–61. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Zhong N, Lu S, et al. Cognitive behavioral performance of untreated depressed patients with mild depressive symptoms. Plos One 2016; 11: e0146356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matt GE Vazquez C andCampbell WK.. Mood-congruent recall of affectively toned stimuli – a meta-analytic review. Clin Psychol Rev 1992; 12: 227–255. [Google Scholar]
- 30.Joormann J andSiemer M.. Memory accessibility, mood regulation, and dysphoria: Difficulties in repairing sad mood with happy memories? J Abnorm Psychol 2004; 113: 179–188. [DOI] [PubMed] [Google Scholar]
- 31.Pinal D Zurrón M andDíaz F.. Effects of load and maintenance duration on the time course of information encoding and retrieval in working memory: from perceptual analysis to post-categorization processes. Front Hum Neurosci 2014; 8: 165. doi: 10.3389/fnhum.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sternberg S. (1966). High-speed scanning in human memory. Science 1966; 153: 652–654. [DOI] [PubMed] [Google Scholar]
- 33.Bonferroni CE. Teoria statistica delle classi e calcolo delle probabilità, Pubblicazioni del R Istituto Superiore di Scienze Economiche Commerciali di Firenze, 1936.
- 34.Posner MI andPetersen SE.. The attention system of the human brain. Annu Rev Neurosci 1990, 13, 25–42. [DOI] [PubMed] [Google Scholar]
- 35.Berryhill ME. Insights from neuropsychology: pinpointing the role of the posterior parietal cortex in episodic and working memory. Front Integr Neurosci 2012; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabeza R, Ciaramelli E, Olson IR, et al. The parietal cortex and episodic memory: an attentional account Nat Rev Neurosci 2008; 9: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burianová H, Ciaramelli E, Grady CL, et al. Top-down and bottom-up attention-to-memory: Mapping functional connectivity in two distinct networks that underlie cued and uncued recognition memory, NeuroImage 2012; 63: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 38.Hertel PT. Memory for emotional and nonemotional events in depression: A question of habit? New York: Oxford University Press, 2004, pp.186–216. [Google Scholar]
- 39.Beevers CG, Clasen P, Stice E, et al. Depression symptoms and cognitive control of emotion cues: a functional magnetic resonance imaging study. Neuroscience 2010; 167: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]