Short abstract
This report describes a 66-year-old Caucasian male who acutely developed severe, bilateral impairment of visual acuity at 24 years of age. Leber’s hereditary optic neuropathy (LHON) was suspected but the diagnosis was not genetically confirmed until the age of 49 years when the primary LHON mutation m.3460G>A was detected. Since onset, visual acuity had slightly improved. The family history was positive for LHON (brother, two sisters of mother, female cousin) and genetically confirmed in his brother and one aunt. Since the age of 65 years, he had experienced recurrent vertigo. His cardiological history was positive for arterial hypertension, noncompaction, myocardial thickening, intermittent right bundle-branch-block (RBBB) and Wolff-Parkinson-White (WPW) syndrome. In addition to LHON, he presented with polyneuropathy, hyperCKaemia, carotid artery occlusion, and a history of stroke. Cardiological investigations at 66 years of age revealed mildly reduced systolic function, enlarged atria, and nonsustained ventricular tachycardias. He underwent an electrophysiological investigation, but radiofrequency ablation was ruled out due to a ‘bizarre’ cardiac conduction system. Instead, an implantable cardioverter defibrillator was proposed but refused by the patient. Since the vertigo did not resolve it was attributed to polyneuropathy. This case demonstrates that LHON may be associated with noncompaction, myocardial thickening, reduced systolic function, enlarged atria, RBBB, WPW syndrome and nonsustained ventricular tachycardias. WPW syndrome in LHON may require invasive antiarrhythmic treatment.
Keywords: Noncompaction, Leber’s hereditary optic neuropathy, genotype, phenotype, myopathy, hypertrophic cardiomyopathy, neuropathy
Introduction
Wolff-Parkinson-White (WPW) syndrome is a frequent cardiac manifestation of Leber’s hereditary optic neuropathy (LHON).1 In two series of 163 and 51 genetically-confirmed LHON patients, WPW syndrome occurred in 9% of each of them.1,2 WPW syndrome was also reported in a further patient with left ventricular hypertrabeculation/noncompaction (LVHT).3 This case report describes a patient with LHON, WPW syndrome and LVHT who had developed asymptomatic, nonsustained ventricular tachycardias.
Case report
The patient is a 66-year-old Caucasian male (height 186 cm; weight 98 kg) who acutely developed binocular visual disturbance at the age of 24 years. Visual acuity progressively declined within 1 month to seeing only contours. Upon ophthalmological work-up, LHON was suspected. Visual acuity slightly improved over the following years. The diagnosis was genetically confirmed by detection of the primary LHON mutation m.3460G>A at the age of 49 years. At the age of 60 years, he underwent right hip replacement therapy. At the age of 61 years, he experienced a speech disturbance for 4 days, which was attributed to an ischaemic stroke in the right frontoparietal and occipitoparietal regions with complete remission. Carotid ultrasound at that time revealed occlusion of the right carotid artery. A mild stenosis was diagnosed on the left carotid artery. Cardiovascular risk factors included arterial hypertension and hyperlipidaemia. Since the age of 65 years, he has experienced recurrent nonsystematic vertigo. He had a previous history of smoking 5 pack/years until the age of 61 years. His history was further positive for recurrent, sometimes traumatic falls. The family history was positive for LHON in his brother, the oldest sister of his mother, the second sister of his mother, and one female cousin. The mutation of the index case was also found in his brother and one aunt. The brother with LHON had LVHT, a complex-I defect on biochemical investigations of the muscle homogenate, was an alcoholic, had died suddenly, and had been previously described.3,4 The index patient’s mother had died from a ‘valve abnormality’. His brother had WPW syndrome as well.
At the age of 66 years, he was able to read letters with a height of approximately 4 cm and was able to watch television on an oversized screen. He was in a depressive mood since the death of his partner 5 years earlier and the sudden, unexplained death of his brother 1 year earlier, whom he found dead in his flat. Neurological examinations revealed markedly reduced visual acuity, positional tremor, past-pointing bilaterally, and reduced Achilles tendon reflexes (already present at age 50 years but not at age 24 years). Serum lactate, which was normal at age 24 years, was 2.5 mmol/l (normal range, 0.5–1.6 mmol/l), creatine kinase was 238 U/l (normal value, <190 U/l), and folic acid was 2.49 ng/ml (normal range, 4.6–18.7 ng/ml). Nerve conduction studies revealed a sensory polyneuropathy of the mixed type in the lower legs. Carotid ultrasound confirmed the previously diagnosed occlusion of the right carotid artery but could not confirm stenosis of the left carotid artery, where only plaques were found.
Cardiologically, the patient had a history of poorly controlled arterial hypertension since the age of 25 years. At 50 years of age, he started suffering from palpitations. At that time, electrocardiogram (ECG) showed a delta wave indicating WPW syndrome, signs of left ventricular hypertrophy and a right bundle-branch-block (RBBB). Echocardiography revealed an increased thickness of the left ventricular walls and LVHT affecting the apex and the posterior wall. Systolic function was normal. By Doppler-sonography, diastolic dysfunction (impaired relaxation) was diagnosed. Treatment with 20 mg lercanidipine, orally, twice daily and 32 mg candesartan, orally, daily was initiated. His history was negative for syncopes, atrial fibrillation, or resuscitation. The patient refused further cardiological follow-up.
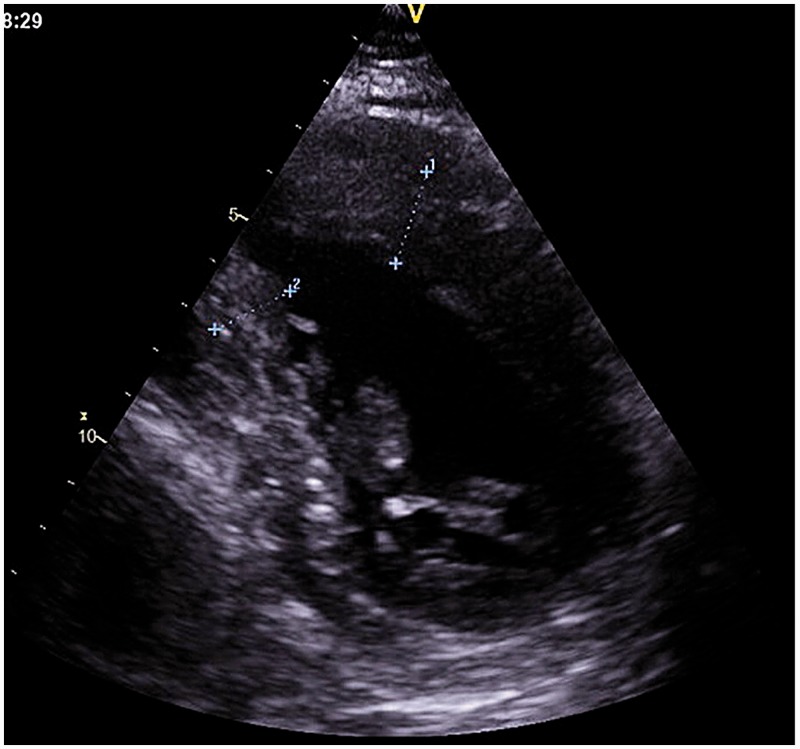
In May 2017, at the age of 66 years, the patient was admitted to the Second Medical Department with Cardiology and Intensive Care Medicine, Krankenanstalt Rudolfstiftung, Vienna, Austria because of increasing vertigo during the previous 1 year and agreed to undergo further cardiological investigations. The clinical cardiological examination was normal. Blood pressure was initially high but normal at discharge. Transthoracic echocardiography showed LVHT and myocardial thickening but normal systolic function. The ECG was unchanged showing RBBB, occasional ventricular ectopic beats, and WPW syndrome with a PQ-time of 104 ms and an intermittent delta wave. During telemetric monitoring, multiple ventricular ectopic beats and nonsustained ventricular tachycardia were observed. Echocardiographically, left ventricular wall thickening had increased and LVHT was only visible in the posterior wall (Figure 1). During stress-testing, the RBBB morphology of the QRS-complex disappeared at 55 Watts and recurred during rest. Considering these findings and the family history, it was decided to perform an electrophysiological investigation because of suspected left lateral pathway of the WPW syndrome. Electrophysiological investigations revealed a ‘bizarre’ cardiac conduction system, which is why the cardiologists refrained from undertaking radiofrequency ablation (RFA). Instead, implantation of an implantable cardioverter defibrillator (ICD) was proposed but refused by the patient. His last medications included: 50 mg trazodone, orally, daily; 100 mg acetyl-salicylic acid orally, daily; 20 mg lercanidipine, orally, twice daily; 32 mg candesartan, orally, daily; 2 mg lorazepam, orally, daily; 4 mg doxazosin, orally, three-times daily; 10 mg escitalopram, orally, daily; 10 mg ezetimibe, orally, daily; 1 mg rilmenidine, orally, twice daily; and 5 mg folic acid, orally, daily.
Figure 1.
Transthoracic echocardiography showing left ventricular hypertrabeculation/noncompaction at the posterior wall in the index case.
Discussion
The present patient with LHON is interesting because of the simultaneous occurrence of WPW syndrome with nonsustained ventricular tachycardias and LVHT. WPW syndrome has been repeatedly reported in patients with LHON and occurred with a frequency of 9% in a large Japanese cohort.1 This figure was confirmed in a Finnish study of 51 genetically-confirmed LHON patients.2 In this study, 30 patients carried the m.11778G>A mutation and 10 patients the m.3460G>A mutation.2 The remaining patients carried other LHON mutations. None of these previously reported patients had undergone RFA or implantation of an ICD. LVHT was not detected in any of the previously described patients with LHON, except for the brother of the present index case.3,4
In the present patient, WPW syndrome was known since the age of 50 years and had not yet manifested clinically. Nonetheless, cardiologists proposed ablation to prevent future complications, such as ventricular arrhythmias or sudden cardiac death (SCD). SCD in association with WPW syndrome has been previously described,5 particularly in children.6 A further reason was the nonsustained ventricular tachycardia on telemetry. A third indication for RFA in the index case was the family history. His brother who also had LHON and LVHT had died suddenly, and it was suspected that it was SCD. Whether ablation of a WPW syndrome in general is truly justified or simply an over-reaction is under debate, since the significance of WPW syndrome is also a matter of debate.7 However, since the conduction system was not accessible to RFA in the present patient, cardiologists suggested ICD implantation.
Whether WPW syndrome is a complication of LVHT or a manifestation of LHON remains unknown. WPW syndrome may not only be a cardiac manifestation of primary LHON mutations but also a complication of LVHT.8 WPW syndrome has been repeatedly reported in association with LVHT (Table 1).8–23 It can be speculated that WPW syndrome was a manifestation of the underlying genetic defect since LVHT may be associated with the genetic defect as well.
Table 1.
Patients in whom left ventricular hypertrabeculation/noncompaction and Wolff-Parkinson-White syndrome have been reported.8–22
| Age | Sex | Manifestations/diagnosis | Additional cardiac abnormalities | Reference |
|---|---|---|---|---|
| 20 years | F | Fabry disease | None | Alper et al. 20169 |
| 14 years | M | None | Ebstein's anomaly, BAV | Malagoli et al. 201410 |
| 30 years | F | Mitochondrial disorder | hCMP | García-Díaz et al. 201311 |
| 14 years | M | None | None | Ho et al. 201012 |
| 6 months | M | None | Bradycardia | Salerno et al. 20088 |
| 24 years | F | Pregnant | None | Munehisa et al. 200713 |
| 18 years | F | None | Fibrillation | Fichet et al. 200714 |
| NM | NM | NM | None | El-Menyar et al. 200715 |
| 7 years | M | NM | SVT, FS ↓ | Celiker et al. 200516 |
| NA | NA | NA | NA | Attenhofer Jost et al. 200517 |
| 6 months | F | None | FS ↓ | Nihei et al. 200418 |
| 9 months | F | None | Sudden death | Ozkutlu et al. 200219 |
| 9 years | F | None | Tachycardia | Ozkutlu et al. 200219 |
| NA | NA | NA | NA | Ozkutlu et al. 200219 |
| 9 months | F | None | Sudden death, ICD | Yasukawa et al. 200120 |
| 5.5 years | F | None | VEB | Hussein et al. 199921 |
| 2 months | F | None | FS ↓, PH | Ichida et al. 199922 |
| 2 months | F | None | SVT | Ichida et al. 199922 |
| 3 years | M | None | FS ↓ | Ichida et al. 199922 |
| 4 years | M | None | None | Ichida et al. 199922 |
| 28 months | M | None | FS ↓, SVT | Yoshinaga et al. 201323 |
F, female; M, male; BAV, bicuspid aortic valve; hCMP, hypertrophic cardiomyopathy; NM, not mentioned; SVT, supraventricular tachycardia; FS, fractional shortening; NA, not accessible; ICD, implantable cardioverter defibrillator; VEB, ventricular ectopic beats; PH, pulmonary hypertension.
Whether the ischaemic stroke at age 61 years was due to an embolism originating from either the intertrabecular spaces, carotid artery dissection, macroangiopathy, a rhythm abnormality, or from temporary systolic dysfunction remains speculative. Due to the unusual distribution of the lesion not being confined to a vascular territory it can be speculated that the putative ischaemic stroke was indeed a stroke-like episode, but the particular cerebral magnetic resonance image was no longer available for review. Most likely, however, atherosclerosis was the cause.
In conclusion, LHON may be associated with cardiac disease manifesting as noncompaction, myocardial thickening, reduced systolic function, enlarged atria, RBBB, WPW syndrome, and nonsustained ventricular tachycardias. Potential complications of WPW syndrome in LHON may be prevented by RFA or implantation of an ICD.
Author contribution
J.F. undertook the design, literature search, discussion, and prepared the first draft of the manuscript; C.S. and E.G. undertook the literature search, discussion, and provided critical comments.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Mashima Y, Kigasawa K, Hasegawa H, et al. High incidence of pre-excitation syndrome in Japanese families with Leber's hereditary optic neuropathy. Clin Genet 1996; 50: 535–537. [DOI] [PubMed] [Google Scholar]
- 2.Nikoskelainen EK, Savontaus ML, Huoponen K, et al. Pre-excitation syndrome in Leber's hereditary optic neuropathy. Lancet 1994; 344: 857–858. [DOI] [PubMed] [Google Scholar]
- 3.Finsterer J, Stöllberger C, Kopsa W, et al. Wolff-Parkinson-White syndrome and isolated left ventricular abnormal trabeculation as a manifestation of Leber's hereditary optic neuropathy. Can J Cardiol 2001; 17: 464–466. [PubMed] [Google Scholar]
- 4.Finsterer J Stöllberger C andMichaela J.. Familial left ventricular hypertrabeculation in two blind brothers. Cardiovasc Pathol 2002; 11: 146–148. [DOI] [PubMed] [Google Scholar]
- 5.Benson DW andCohen MI.. Wolff-Parkinson-White syndrome: lessons learnt and lessons remaining. Cardiol Young 2017; 27: S62–S67. [DOI] [PubMed] [Google Scholar]
- 6.Wöllner K Doberentz E andMadea B.. Sudden death of a 16-year-old girl with WPW syndrome: a case report. Arch Kriminol 2015; 235: 110–116 [in German, English abstract]. [PubMed] [Google Scholar]
- 7.Kim SS andKnight BP.. Long term risk of Wolff-Parkinson-White pattern and syndrome. Trends Cardiovasc Med 2017; 27: 260–268. [DOI] [PubMed] [Google Scholar]
- 8.Salerno JC Chun TU andRutledge JC.. Sinus bradycardia, Wolff Parkinson White, and left ventricular noncompaction: an embryologic connection? Pediatr Cardiol 2008; 29: 679–682. [DOI] [PubMed] [Google Scholar]
- 9.Alper AT, Kaya A, Tekkesin Aİ, et al. Wolff-Parkinson-White and left ventricular noncompaction in a Fabry patient: A case report. Turk Kardiyol Dern Ars 2016; 44: 248–250. [DOI] [PubMed] [Google Scholar]
- 10.Malagoli A, Rossi L, Mastrojanni C, et al. A perfect storm: Wolf Parkinson White syndrome, Ebstein's anomaly, biventricular non-compaction, and bicuspid aortic valve. Eur Heart J Cardiovasc Imaging 2014; 15: 827. [DOI] [PubMed] [Google Scholar]
- 11.García-Díaz L Coserria F andAntiñolo G.. Hypertrophic cardiomyopathy due to mitochondrial disease: prenatal diagnosis, management, and outcome. Case Rep Obstet Gynecol 2013; 2013: 472356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho TQ, Lenihan DJ, Kantharia BK, et al. Noncompacted ventricular myocardium: is syncope the only warning sign? Am J Med Sci 2010; 339: 497–500. [DOI] [PubMed] [Google Scholar]
- 13.Munehisa Y, Watanabe H, Kosaka T, et al. Successful outcome in a pregnant woman with isolated noncompaction of the left ventricular myocardium. Intern Med 2007; 46: 285–289. [DOI] [PubMed] [Google Scholar]
- 14.Fichet J, Legras A, Bernard A, et al. Aborted sudden cardiac death revealing isolated noncompaction of the left ventricle in a patient with Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol 2007; 30: 444–447. [DOI] [PubMed] [Google Scholar]
- 15.El-Menyar AA Gendi SM andNuman MT.. Noncompaction cardiomyopathy in the State of Qatar. Saudi Med J 2007; 28: 429–434. [PubMed] [Google Scholar]
- 16.Celiker A, Ozkutlu S, Dilber E, et al. Rhythm abnormalities in children with isolated ventricular noncompaction. Pacing Clin Electrophysiol 2005; 28: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 17.Attenhofer Jost CH, Connolly HM, O'Leary PW, et al. Left heart lesions in patients with Ebstein anomaly. Mayo Clin Proc 2005; 80: 361–368. [DOI] [PubMed] [Google Scholar]
- 18.Nihei K, Shinomiya N, Kabayama H, et al. Wolff-Parkinson-White (WPW) syndrome in isolated noncompaction of the ventricular myocardium (INVM). Circ J 2004; 68: 82–84. [DOI] [PubMed] [Google Scholar]
- 19.Ozkutlu S, Ayabakan C, Celiker A, et al. Noncompaction of ventricular myocardium: a study of twelve patients. J Am Soc Echocardiogr 2002; 15: 1523–1528. [DOI] [PubMed] [Google Scholar]
- 20.Yasukawa K, Terai M, Honda A, et al. Isolated noncompaction of ventricular myocardium associated with fatal ventricular fibrillation. Pediatr Cardiol 2001; 22: 512–514. [DOI] [PubMed] [Google Scholar]
- 21.Hussein A Schmaltz AA andTrowitzsch E.. Isolated abnormality (“noncompaction”) of the myocardium in 3 children. Klin Padiatr 1999; 211: 175–178 [in German, English abstract]. [DOI] [PubMed] [Google Scholar]
- 22.Ichida F, Hamamichi Y, Miyawaki T, et al. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 1999; 34: 233–240. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinaga M, Ushinohama H, Sato S, et al. Electrocardiographic screening of 1-month-old infants for identifying prolonged QT intervals. Circ Arrhythm Electrophysiol 2013; 6: 932–938. [DOI] [PubMed] [Google Scholar]