Short abstract
Objective
To provide novel insights into the clinical treatment of adenomyosis.
Methods
Two hundred patients with adenomyosis were enrolled in this prospective, nonrandomized, parallel-controlled study with a 1-year follow-up in our hospital. Group 1 was treated with 3.75 mg leuprorelin acetate (LA) (n = 40), Group 2 was treated with 1.88 mg LA (n = 40), Group 3 underwent Mirena implantation (n = 40), Group 4 underwent Mirena implantation after treatment with 3.75 mg LA (n = 40), Group 5 underwent Mirena implantation after treatment with 1.88 mg LA (n = 20), and Group 6 received San-Jie-Zhen-Tong capsules alone (n = 20). Uterine volume, pain, cancer antigen 125 level, ovary function, adverse effects, and Mirena expulsion were evaluated.
Results
The uterine volume and pain scores were lower in the groups treated with 1.88 than 3.75 mg LA, but the lower dose was associated with significantly fewer hot flashes and sweating. The 1-year Mirena expulsion rate was higher in Group 3 than in Groups 4 and 5 (10.00% vs. 3.33%, respectively). Costs were significantly higher in Groups 1 and 4 than in Groups 2 and 5.
Conclusion
Administration of 1.88 mg LA may be an alternative therapy for Asian patients with adenomyosis. The combination of LA and Mirena could enhance the therapeutic effect.
Registration number: ChiCTR-IPR-15005971
Keywords: Adenomyosis, gonadotropin-releasing hormone analogues, half-dose, efficacy, adverse effect, Mirena
Introduction
Adenomyosis is a common benign gynecological disease characterized by the presence of ectopic glandular and stromal tissues in the myometrium. The pathogenesis of adenomyosis remains unclear. Two possible mechanisms of adenomyosis are invagination of the endometrium into the myometrium and development from embryologically misplaced pluripotent Müllerian remnants.1 The rate of histologically confirmed adenomyosis in previously reported surgical series ranges from 5% to 70%.2 Menorrhagia, dysmenorrhea, an enlarged uterine volume, and infertility are major clinical manifestations of adenomyosis. Dysmenorrhea is found in approximately 30.0% to 77.8% of patients with adenomyosis.1,3 It is the second most prevalent symptom after menorrhagia and the most debilitating symptom.4 Hysterectomy is the only radical treatment method currently available and is contraindicated for patients with a desire to keep their uterus. Conservative treatment involves the administration of drugs, including gonadotropin-releasing hormone analogue (GnRH-a), a levonorgestrel-releasing intrauterine system (LNG-IUS) (Mirena; Bayer Pharmaceuticals, Leverkusen, Germany), androgen derivatives, and progesterone. GnRH-a is widely used in clinical practice,5 but it is expensive and associated with adverse effects. Decreasing the adverse effects of this medication and reducing the economic burden on patients while ensuring treatment efficacy are problems requiring urgent resolution. San-Jie-Zhen-Tong (SJZT) capsule is a Chinese patent medicine that consists of Resina Draconis, pseudoginseng, Fritillaria thunbergii Miq, and coix seed. It has been widely used in China for the treatment of adenomyosis and endometriosis because its Chinese herbal ingredients can ameliorate patients’ pain. We analyzed the efficacy and adverse effects of different doses of leuprorelin acetate (Enantone; Takeda Pharmaceutical Company Limited, Osaka, Japan), Mirena, and SJZT capsules in this prospective, nonrandomized, parallel-controlled study with the aim of providing new ideas for the clinical treatment of adenomyosis.
Materials and methods
Patients
After obtaining approval from the in-hospital ethics committee (Ethics Committee of Obstetrics and Gynecology Hospital of Fudan University, 2013-42), patients diagnosed with adenomyosis were enrolled from the outpatient department of the Obstetrics and Gynecology Hospital of Fudan University from February 2015 to February 2016. All patients had dysmenorrhea, had no fertility requirements, were premenopausal, and were not pregnant. Their diagnosis was confirmed by transvaginal ultrasound,6 and they had no significant liver or kidney dysfunction or malignant tumors. The length of the uterus was <8.5 cm, and the cancer antigen 125 (CA-125) level was <200 U/mL. Patients who had received progesterone therapy in past 1 month, used GnRH-a, had undergone Mirena implantation in the past 3 months, or had undergone uterine surgery in the past 6 months were excluded from this study.
Patients who received an injection of a half-dose of leuprorelin acetate (1.88 mg) provided written informed consent for inclusion in the study. If the patient was willing and the medical experts approved, the patient also underwent Mirena implantation. Informed consent was not needed for the control group, which comprised patients undergoing conventional clinical therapies during the study period (injection of 3.75 mg of leuprorelin acetate, Mirena implantation, both injection of 3.75 mg of leuprorelin acetate and Mirena implantation, or administration of SJZT capsules).
All 200 patients in this study were divided into 6 different treatment groups:
Group 1 (LA3.75 group): Subcutaneous injection of 3.75 mg of leuprorelin acetate on one of the first 3 days of the menstrual cycle, once every 4 weeks, three times
Group 2 (LA1.88 group): Subcutaneous injection of 1.88 mg of leuprorelin acetate on one of the first 3 days of the menstrual cycle, once every 4 weeks, three times
Group 3 (M group): Implantation of Mirena during the first half of the menstrual cycle
Group 4 (M + LA3.75 group): Implantation of Mirena after three injections of 3.75 mg of leuprorelin acetate
Group 5 (M + LA1.88 group): Implantation of Mirena after three injections of 1.88 mg of leuprorelin acetate
Group 6 (SJZT group): Treatment with oral SJZT capsules alone (4 capsules 3 times daily) starting on the first day of menstruation and continuing for 3 months
Clinical follow-up
Before the initial treatment, the patients’ age, height, and weight were recorded. Before treatment and at 3, 6, and 12 months after the initial treatment, the menstrual cycle, menstrual volume, degree of dysmenorrhea (10-point visual analogue scale (VAS)7), size of the uterus, CA-125 level, hemoglobin level, and platelet count were recorded. Additionally, the serum levels of follicle-stimulating hormone, luteinizing hormone, and estradiol were measured during days 3 to 5 of the menstrual cycle before and after 3 months of treatment. The adverse effects of the drugs were assessed 12 months after the initial treatment using the domestically improved Kupperman Index (KI) score, which is used to identify menopausal symptoms and has been modified for use in Chinese women.8 We calculated the body mass index (BMI) in kg/m2 (i.e., weight/height2) and the uterine volume in cm3 (i.e., 4/3 × π × r3, where r = (length + width + height)/6). The fees for drugs and tests were also recorded.
Statistical analysis
All data were statistically analyzed using chi-square tests (categorical variables) or one-way analysis of variance (continuous variables) using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Differences were considered significant at a P-value of <0.05.
Results
Patient characteristics
In total, 200 patients were enrolled in this study. Of these 200 patients, 60 provided informed consent for treatment (Group 2 (LA1.88), n = 40; Group 5 (M + LA1.88), n = 20). The control group (those receiving conventional clinical therapies during the study period) comprised 140 patients (Group 1 (LA3.75), n = 40; Group 3 (M), n = 40; Group 4 (M + LA3.75), n = 40; Group 6 (SJZT), n = 20).
The general characteristics of the patients in the six groups are shown in Table 1. The patients ranged in age from 25 to 50 years with a mean age ranging from 36.28 to 40.45 years among the six groups. The mean weight and BMI among the six groups were 53.50 to 60.93 kg and 21.88 to 23.56 kg/m2, respectively. No statistically significant differences in age, weight, or BMI were found among the six groups.
Table 1.
Patient age, weight, and BMI in the six treatment groups.
| Group | n | Age (years) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| 1 | 40 | 37.55 ± 6.07 | 57.60 ± 5.81 | 22.82 ± 1.65 |
| 2 | 40 | 36.28 ± 10.39 | 59.42 ± 8.39 | 22.70 ± 2.00 |
| 3 | 40 | 38.63 ± 7.96 | 53.50 ± 9.19 | 21.88 ± 1.40 |
| 4 | 40 | 39.40 ± 8.21 | 60.93 ± 7.23 | 23.56 ± 2.10 |
| 5 | 20 | 39.25 ± 10.33 | 60.75 ± 9.81 | 23.20 ± 3.52 |
| 6 | 20 | 40.45 ± 10.44 | 57.29 ± 3.77 | 22.25 ± 1.13 |
| P | NS | NS | NS |
Data are presented as mean ± standard deviation.
Group 1, 3.75 mg leuprorelin acetate; Group 2, 1.88 mg leuprorelin acetate; Group 3, Mirena; Group 4, Mirena + 3.75 mg leuprorelin acetate; Group 5, Mirena + 1.88 mg leuprorelin acetate; Group 6, San-Jie-Zhen-Tong capsules
BMI, body mass index; NS, not statistically significant
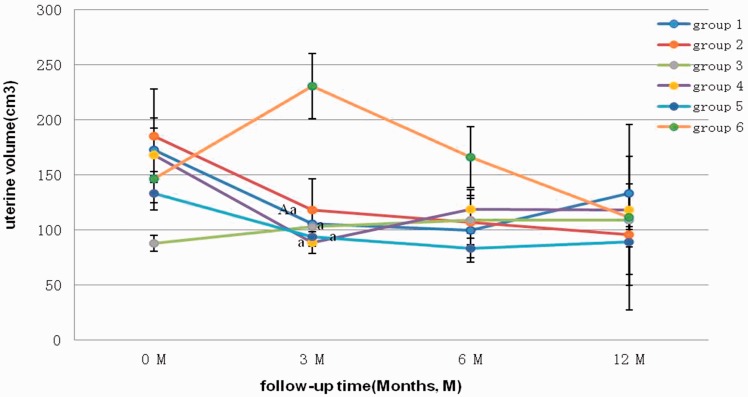
The changes in the uterine volume in the six groups are shown in Figure 1. As shown in the line graph, no significant differences in the uterine volume were found between the pretreatment period and the 6- or 12-month post-treatment period among the six groups. However, 3 months after the initial treatment, the uterine volumes in Group 1 (LA3.75), Group 3 (M), Group 4 (M + LA3.75), and Group 5 (M + LA1.88) were significantly lower than those in Group 6 (SJZT) (all P < 0.05). The uterine volume of patients in Group 1 (LA3.75) was significantly lower after 3 months of follow-up than at the initial treatment (P < 0.05). At the 3-month follow-up, the uterine volume was larger in Group 3 (M) and Group 6 (SJZT), but in the other groups, the volume was generally lower and stabilized across time. After 12 months of follow-up, the volume fluctuated near 100 cm3. The uterine volume was lower in Group 2 (LA1.88), while no significant differences were found among the groups that received a half-dose versus regular dose of leuprorelin acetate.
Figure 1.
Mean uterine volume in each group before and after treatment. Group 1, 3.75 mg leuprorelin acetate; Group 2, 1.88 mg leuprorelin acetate; Group 3, Mirena; Group 4, Mirena + 3.75 mg leuprorelin acetate; Group 5, Mirena + 1.88 mg leuprorelin acetate; Group 6, San-Jie-Zhen-Tong capsules
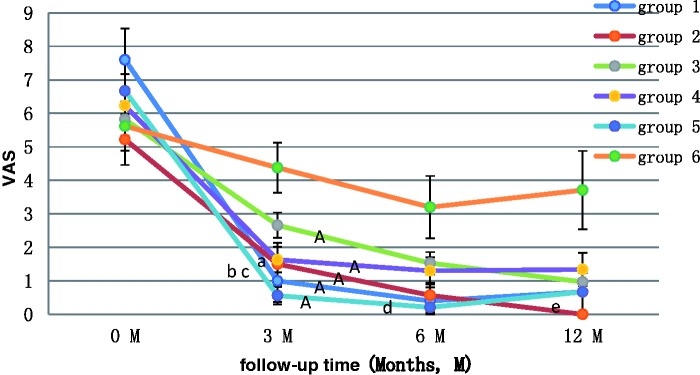
The changes in the VAS scores in the six groups are shown in Figure 2. The VAS scores were significantly lower after treatment in all groups except Group 6 (SJZT) (all P < 0.01). Patients in Group 2 (LA1.88) had significantly lower pain scores at 3 months (P < 0.1), 6 months (P < 0.01), and 12 months (P < 0.01) after treatment. No significant differences in VAS scores were found among the groups using 1.88 versus 3.75 mg of leuprorelin acetate. At the 3-month follow-up, the VAS scores in Group 1 (LA3.75) and Group 5 (M + LA1.88) were significantly lower than those in Group 6 (SJZT) (P < 0.05 and P < 0.01, respectively), and the VAS scores in Group 5 (M + LA1.88) were significantly lower than those in Group 3 (M) (P < 0.001). At the 6-month follow-up, the VAS scores in Group 5 (M + LA1.88) were significantly lower than those in Group 6 (SJZT) (P < 0.05). At the 12-month follow-up, the VAS scores in Group 2 (LA1.88) were significantly lower than those in Group 3 (M) (P < 0.05).
Figure 2.
Changes in VAS scores in each group before and after treatment. VAS, visual analogue scale. Group 1, 3.75 mg leuprorelin acetate; Group 2, 1.88 mg leuprorelin acetate; Group 3, Mirena; Group 4, Mirena + 3.75 mg leuprorelin acetate; Group 5, Mirena + 1.88 mg leuprorelin acetate; Group 6, San-Jie-Zhen-Tong capsules
As shown in Table 2, laboratory examinations were conducted before treatment and at the 3-month follow-up. The serum levels of CA-125 and estradiol were decreased after all treatments. The levels of follicle-stimulating hormone and luteinizing hormone were differently altered within the groups. Statistical analysis revealed no significant differences in any indexes among the groups. No significant changes in laboratory results were observed in any group before and after treatment.
Table 2.
Laboratory indexes before and after treatment.
| Group | CA-1250 | CA-1253 | FSH0 | FSH3 | LH0 | LH3 | E20 | E23 |
|---|---|---|---|---|---|---|---|---|
| 1 | 122.53 ± 49.82 | 99.42 ± 23.98 | 4.54 ± 0.87 | 12.19 ± 4.40 | 2.67 ± 0.38 | 10.64 ± 4.71 | 136.30 ± 31.78 | 117.38 ± 74.59 |
| 2 | 82.86 ± 14.65 | 62.70 ± 15.90 | 6.46 ± 0.64 | 5.59 ± 1.20 | 7.09 ± 0.75 | 4.22 ± 2.31 | 137.21 ± 22.79 | 45.43 ± 19.08 |
| 3 | 83.15 ± 26.25 | 32.00 ± 7.58 | 10.07 ± 3.46 | 14.58 ± 3.48 | 5.97 ± 0.83 | 8.20 ± 3.20 | 53.60 ± 17.21 | 43.50 ± 4.50 |
| 4 | 67.32 ± 15.24 | 52.06 ± 11.11 | 6.84 ± 0.80 | 6.59 ± 0.64 | 5.02 ± 0.87 | 3.33 ± 0.88 | 94.67 ± 17.69 | 56.86 ± 17.22 |
| 5 | 134.13 ± 41.82 | 84.60 ± 36.46 | 7.30 ± 0.97 | 5.37 ± 1.09 | 8.39 ± 1.45 | 2.05 ± 0.78 | 149.67 ± 30.60 | 57.17 ± 42.14 |
| 6 | 66.70 ± 19.03 | 40.44 ± 19.56 | 10.61 ± 2.95 | 7.60 ± 2.60 | 5.77 ± 1.22 | 6.87 ± 0.14 | 113.40 ± 41.28 | 55.00 ± 5.00 |
Data are presented as mean ± standard deviation.
Group 1, 3.75 mg leuprorelin acetate; Group 2, 1.88 mg leuprorelin acetate; Group 3, Mirena; Group 4, Mirena + 3.75 mg leuprorelin acetate; Group 5, Mirena + 1.88 mg leuprorelin acetate; Group 6, San-Jie-Zhen-Tong capsules
0: Before treatment
3: At the 3-month follow-up
CA-125, cancer antigen 125; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol
Adverse effects
The KI scores in the six groups of patients are shown in Table 3. No significant differences were found among the groups. We compared different items from the KI scale, and the results are shown in Table 4. The incidence of sweating significantly differed among the groups (P = 0.023). Patients in Group 1 (LA3.75) and Group 4 (M + LA3.75) were more likely to develop sweating than those in Group 2 (LA1.88), Group 5 (M + LA1.88), and Group 6 (SJZT). Other symptoms were not significantly different. In Table 5, we further analyzed the adverse effects in patients treated with 3.75 mg of leuprorelin acetate (Groups 1 and 4) and those treated with 1.88 mg (Groups 2 and 5). We found that patients treated with 3.75 mg had more adverse effects. The rates of hot flashes and sweating (P = 0.045), hot flashes alone (P = 0.029), and sweating alone (P = 0.004) among patients treated with 3.75 mg of leuprorelin acetate were significantly higher than those among patients treated with 1.88 mg (23.75%, 21.25%, and 20.00% vs. 10.00%, 6.67%, and 3.34%, respectively). Table 6 shows the rate of Mirena expulsion after implantation. The expulsion rate in patients treated with Mirena implantation alone was 10.00%, whereas the rate in patients who underwent Mirena implantation after leuprorelin acetate injection was 3.33%. However, Fisher’s exact test revealed no statistically significant difference between the two groups.
Table 3.
KI scores in each group.
| Group | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Mean KI score | 2.93 | 1.45 | 1.65 | 2.45 | 1.65 | 0.80 |
| Maximum KI score | 16 | 10 | 14 | 15 | 10 | 10 |
| Minimum KI score | 0 | 0 | 0 | 0 | 0 | 0 |
| P | 0.338 | |||||
KI, Kupperman Index
Group 1, 3.75 mg leuprorelin acetate; Group 2, 1.88 mg leuprorelin acetate; Group 3, Mirena; Group 4, Mirena + 3.75 mg leuprorelin acetate; Group 5, Mirena + 1.88 mg leuprorelin acetate; Group 6, San-Jie-Zhen-Tong capsules
Table 4.
Adverse effects in each group.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | χ2 (F) | P |
|---|---|---|---|---|---|---|---|---|
| Patients (n) | 40 | 40 | 40 | 40 | 20 | 20 | ||
| Hot flashes and sweating | 11 | 3 | 5 | 8 | 3 | 1 | 8.232 | 0.130 |
| Hot flashes | 9 | 2 | 3 | 8 | 2 | 1 | 8.835 | 0.097 |
| Sweating | 8 | 1 | 4 | 8 | 1 | 0 | 11.920 | 0.023 |
| Paresthesia | 0 | 0 | 0 | 0 | 0 | 0 | NS | NS |
| Insomnia | 7 | 6 | 5 | 9 | 2 | 2 | 2.492 | 0.792 |
| Irritability | 10 | 5 | 4 | 6 | 3 | 2 | 4.013 | 0.547 |
| Depression | 1 | 0 | 0 | 0 | 0 | 0 | 5.079 | 1.000 |
| Vertigo | 6 | 4 | 2 | 6 | 3 | 2 | 3.287 | 0.661 |
| Fatigue | 2 | 2 | 0 | 1 | 0 | 0 | 3.225 | 0.604 |
| Muscle and joint pain | 3 | 1 | 1 | 3 | 2 | 1 | 2.941 | 0.779 |
| Headache | 2 | 1 | 1 | 2 | 1 | 1 | 1.565 | 1.000 |
| Palpitation | 2 | 2 | 2 | 3 | 1 | 0 | 1.554 | 0.942 |
| Formication | 0 | 0 | 0 | 1 | 0 | 0 | 5.097 | 1.000 |
| Algopareunia | 5 | 2 | 1 | 1 | 2 | 0 | 5.783 | 0.277 |
| Urinary system symptoms | 1 | 1 | 4 | 1 | 0 | 0 | 4.237 | 0.400 |
(F), Fisher’s exact test; NS, not statistically significant
Group 1, 3.75 mg leuprorelin acetate; Group 2, 1.88 mg leuprorelin acetate; Group 3, Mirena; Group 4, Mirena + 3.75 mg leuprorelin acetate; Group 5, Mirena + 1.88 mg leuprorelin acetate; Group 6, San-Jie-Zhen-Tong capsules
Table 5.
Adverse effects in patients treated with 3.75 and 1.88 mg of leuprorelin acetate.
| Group | 1, 4 (3.75 mg) | 2, 5 (1.88 mg) | χ2 | P |
|---|---|---|---|---|
| Patients (n) | 80 | 60 | ||
| Hot flashes and sweating | 19 | 6 | 4.419 | 0.045 |
| Hot flashes | 17 | 4 | 5.719 | 0.029 |
| Sweating | 16 | 2 | 8.500 | 0.004 |
| Paresthesia | 0 | 0 | NS | NS |
| Insomnia | 16 | 8 | 1.073 | 0.368 |
| Irritability | 16 | 8 | 1.073 | 0.368 |
| Depression | 1 | 0 | 0.750 (F) | 0.386 (F) |
| Vertigo | 12 | 7 | 0.325 | 0.569 |
| Fatigue | 3 | 2 | 0.017 (F) | 1.000 (F) |
| Muscle and joint pain | 5 | 3 | 0.099 (F) | 1.000 (F) |
| Headache | 4 | 2 | 0.231 (F) | 0.700 (F) |
| Palpitation | 5 | 3 | 0.099 (F) | 1.000 (F) |
| Formication | 1 | 0 | 0.750 (F) | 0.386 (F) |
| Algopareunia | 6 | 4 | 0.036 (F) | 1.000 (F) |
| Urinary system symptoms | 2 | 1 | 0.113 (F) | 0.737 (F) |
(F), Fisher’s exact test; NS, not statistically significant
Table 6.
Expulsion of Mirena after implantation.
| Group | n | Normal position | Expulsion |
|---|---|---|---|
| 3 (Minerva) | 40 | 36 (90.00) | 4 (10.00) |
| 4 (Minerva + 3.75 mg leuprorelin acetate)5 (Minerva + 1.88 mg leuprorelin acetate) | 60 | 58 (96.67) | 2 (3.33) |
| χ2 (Fisher’s exact test) | 1.891 | ||
| P | 0.214 | ||
Data are presented as n (%) patients.
Cost
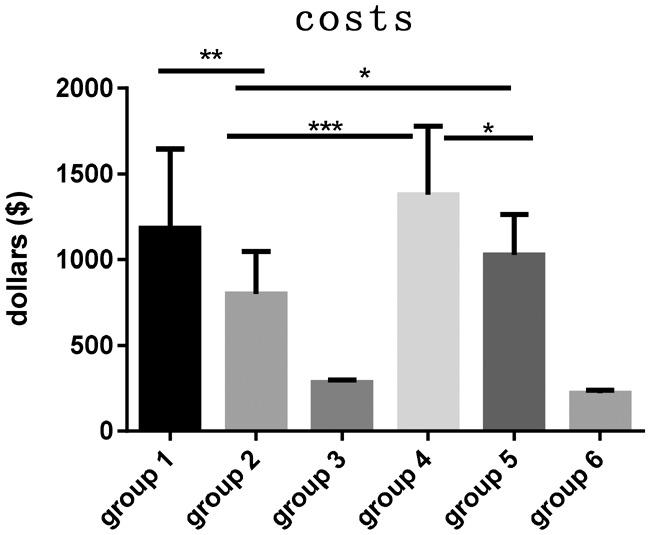
As shown in Table 7, the mean costs for patients in Groups 1, 2, 3, 4, 5, and 6 were $1167.58, $810.02, $315.66, $1380.57, $1074.48, and $229.04 USD, respectively. Patients in Group 3 (M) had significantly lower costs than those in Group 1 (LA3.75), Group 2 (LA1.88), Group 4 (M + LA3.75), and Group 5 (M + LA1.88) (all P < 0.001). However, patients in Group 3 (M) had significantly higher costs than those in Group 6 (SJZT) (P < 0.001). The costs for patients in Group 6 (SJZT) were much lower than those for patients in the other five groups. The costs for patients treated with 3.75 mg of leuprorelin acetate were much higher than those for patients treated with 1.88 mg. The patient cost in Group 1 (LA3.75) was $357.56 USD higher than that in Group 2 (LA1.88) (P < 0.01). The patient cost in Group 4 (M + LA3.75) was $306.09 USD higher than that in Group 5 (M + LA1.88) (P < 0.05).
Table 7.
Costs in each group.
|
Group |
Costs ($ USD) |
|
| 1 | 1167.58 ± 447.47 | |
| 2 | 810.02 ± 254.95 | |
| 3 | 315.66 ± 17.92a | |
| 4 | 1380.57 ± 399.97 | |
| 5 | 1074.48 ± 278.84 | |
| 6 | 229.04 ± 19.41b |
Data are presented as mean ± standard deviation.
Group 1, 3.75 mg leuprorelin acetate; Group 2, 1.88 mg leuprorelin acetate; Group 3, Mirena; Group 4, Mirena + 3.75 mg leuprorelin acetate; Group 5, Mirena + 1.88 mg leuprorelin acetate; Group 6, San-Jie-Zhen-Tong capsules.
aP < 0.001 when compared with Groups 1, 2, 4, 5, and 6; bP < 0.001 when compared with Groups 1 to 5; *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
GnRH-a, which is regulated by the hypothalamic–pituitary–gonadal axis, can effectively alleviate the chronic pelvic pain caused by adenomyosis, reduce menstrual capacity, and increase the probability of pregnancy. However, long-term application of GnRH-a predisposes women to symptoms of low estrogen, menopausal symptoms (such as hot flashes, sweating, vaginal dryness, and decreased sexual desire), decreased bone density, bone loss, and other adverse effects. Therefore, the estrogen level should be monitored during the course of treatment, and application of additional treatments should be conducted when necessary.
Types of GnRH-a that are commonly used include leuprorelin acetate, goserelin acetate, and triptorelin. The typical dosage of leuprorelin acetate is 3.75 mg at 4-week intervals for a total of four to six cycles. Kang et al.9 analyzed 70 patients with either endometriosis or adenomyosis and found no significant differences in uterine volume or hormone levels between patients treated with GnRH-a at the normal interval (4 weeks) and those treated at a longer interval (6 weeks). The authors found that extending the interval of GnRH-a treatment did not affect its efficacy but reduced medical expenses by at least 33%.9 Because GnRH receptors are present in both the eutopic and ectopic endometrium, it is possible that a low dose of GnRH-a can be effective. In a patient with adenomyosis combined with deep vein thrombosis, Akira et al.10 found that a low dose of GnRH-a (450 µg/day buserelin acetate, nasally) was beneficial and had minor adverse effects, including a low estrogen level. In another study, Akira et al.11 compared the efficacy of a low dosage of GnRH-a (150–750 µg/day of buserelin acetate, nasally) in 12 patients with adenomyosis after using the regular dosage (900 µg/day of buserelin acetate, nasally). The authors found that a low dosage of GnRH-a can maintain plasma estradiol levels within the therapeutic window and suppress adverse events.11
We analyzed the clinical effects of and adverse reactions to different doses of leuprorelin acetate and found that both groups using the regular dose (Groups 1 and 4) and both groups using the half-dose (Groups 2 and 5) had a reduced uterine size and decreased VAS scores. However, the groups using the regular dose exhibited more adverse effects, such as hot flashes (P = 0.029) and sweating (P = 0.004). These groups also had higher costs than those using the half-dose. Patients in Group 1 spent an extra $357.56 USD compared with those in Group 2 (P < 0.01), and patients in Group 4 spent an extra $306.09 USD compared with those in Group 5 (P < 0.05). These results indicate that treatment with 1.88 mg of leuprorelin acetate has a similar efficacy, lower incidence of adverse effects, and lower cost than treatment with 3.75 mg of leuprorelin acetate. The instructions for leuprorelin acetate (Enantone) indicate that a half-dose can be used for patients weighing <50 kg. In the present study, no significant differences in weight or BMI were found among the six groups. We propose that weight should not limit the widespread use of a low dose of Enantone in patients with adenomyosis.
LNG-IUS (Mirena), a “T” type of IUD, contains a total of 52 mg of levonorgestrel and can release 20 g of levonorgestrel per day for 5 years.12,13 Mirena can induce endometrial glandular atrophy and regulate and control factors related to adenomyosis through its “progesterone effect” on the ectopic endometrium.13 Mirena implantation reportedly down-regulates the expression of nerve growth factor and nerve growth factor receptor (p75 and TrkA receptors) in the endometrium and myometrium, thus reducing pain.14 We found that implantation of Mirena significantly reduced the degree of dysmenorrhea. Youm et al.15 found that the 3-year expulsion rate of Mirena was 11.1% for patients with a large uterus and that it increased menstruation. Zhang et al.16 noted that for patients with adenomyosis with a large uterus (larger than that at 3 months of pregnancy), the combination of GnRH-a and LNG-IUS could significantly reduce the uterine volume, decrease the degree of pain, and reduce the rate of expulsion. We found that the 1-year expulsion rate of Mirena in Group 3 (M) was higher than that in Group 4 (M + LA3.75) and Group 5 (M + LA1.88) (10.00% vs. 3.33%, respectively). The combination of GnRH-a and Mirena could reduce missed doses and enhance the treatment effect.
CA-125 is a monoclonal antibody first described by Kabawat et al.17 in 1983. It is widely used as a tumor marker in the field of gynecologic oncology. Kil et al.18 found that the CA-125 level was significantly higher in patients with adenomyosis than in those with myoma. The authors used 19 U/mL as the cut-off value for diagnosis of the two diseases. Huang et al.19 found that patient pain was positively associated with the CA-125 level and reported that a CA-125 level of <10 U/mL may predict better pregnancy outcomes for patients with adenomyosis. Sheth and Ray20 found that the CA-125 level increased with uterine size. Consistent with the literature, we found that after 3 months of treatment, both the CA-125 level and severity of pain had decreased. However, no significant difference was observed among the six groups, probably because of the small sample size. Additionally, this study involved an Asian population of women with a lower BMI than that of women in Europe and America. Trials with larger and more diverse populations are needed to elucidate the full effects of these treatments and their mechanisms.
Conclusions
The efficacy of a low dose (1.88 mg) of GnRH-a in patients with adenomyosis was similar to that of a regular dose (3.75 mg) but was associated with significantly fewer adverse effects. A half-dose (1.88 mg) of leuprorelin acetate may be a promising alternative therapy for Asian patients with adenomyosis. Additionally, the combination of GnRH-a and Mirena could reduce missed doses and enhance the curative effect.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Struble J Reid S andBedaiwy MA.. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol 2016; 23: 164–185. [DOI] [PubMed] [Google Scholar]
- 2.Graziano A, Lo MG, Piva I, et al. Diagnostic findings in adenomyosis: a pictorial review on the major concerns. Eur Rev Med Pharmacol Sci 2015; 19: 1146–1154. [PubMed] [Google Scholar]
- 3.Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol 2000; 95: 688–691. [DOI] [PubMed] [Google Scholar]
- 4.Guo SW, Mao X, Ma Q, et al. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril 2013; 99: 231–240. [DOI] [PubMed] [Google Scholar]
- 5.Morelli M, Rocca ML, Venturella R, et al. Improvement in chronic pelvic pain after gonadotropin releasing hormone analogue (GnRH-a) administration in premenopausal women suffering from adenomyosis or endometriosis: a retrospective study. Gynecol Endocrinol 2013; 29: 305–308. [DOI] [PubMed] [Google Scholar]
- 6.Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol 2017. 2017-08-30. [DOI] [PubMed]
- 7.Yang M, Chen X, Bo L, et al. Moxibustion for pain relief in patients with primary dysmenorrhea: A randomized controlled trial. Plos One 2017; 12: e170952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao M, Shao H, Li C, et al. Correlation between the modified Kupperman Index and the Menopause Rating Scale in Chinese women. Patient Prefer Adherence 2013; 7: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JL, Wang XX, Nie ML, et al. Efficacy of gonadotropin-releasing hormone agonist and an extended-interval dosing regimen in the treatment of patients with adenomyosis and endometriosis. Gynecol Obstet Invest 2010; 69: 73–77. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Iwasaki N, Ichikawa M, et al. Successful long-term management of adenomyosis associated with deep thrombosis by low-dose gonadotropin-releasing hormone agonist therapy. Clin Exp Obstet Gynecol 2009; 36: 123–125. [PubMed] [Google Scholar]
- 11.Akira S, Mine K, Kuwabara Y, et al. Efficacy of long-term, low-dose gonadotropin-releasing hormone agonist therapy (draw-back therapy) for adenomyosis. Med Sci Monit 2009; 15: R1–4. [PubMed] [Google Scholar]
- 12.Bahamondes L, Brache V, Meirik O, et al. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod 2015; 30: 2527–2538. [DOI] [PubMed] [Google Scholar]
- 13.Streuli I, Dubuisson J, Santulli P, et al. An update on the pharmacological management of adenomyosis. Expert Opin Pharmacother 2014; 15: 2347–2360. [DOI] [PubMed] [Google Scholar]
- 14.Choi YS, Cho S, Lim KJ, et al. Effects of LNG-IUS on nerve growth factor and its receptors expression in patients with adenomyosis. Growth Factors 2010; 28: 452–460. [DOI] [PubMed] [Google Scholar]
- 15.Youm J, Lee HJ, Kim SK, et al. Factors affecting the spontaneous expulsion of the levonorgestrel-releasing intrauterine system. Int J Gynaecol Obstet 2014; 126: 165–169. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Song K, Li L, et al. Efficacy of combined levonorgestrel-releasing intrauterine system with gonadotropin-releasing hormone analog for the treatment of adenomyosis. Med Princ Pract 2013; 22: 480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabawat SE, Bast RJ, Bhan AK, et al. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol 1983; 2: 275–285. [DOI] [PubMed] [Google Scholar]
- 18.Kil K, Chung JE, Pak HJ, et al. Usefulness of CA125 in the differential diagnosis of uterine adenomyosis and myoma. Eur J Obstet Gynecol Reprod Biol 2015; 185: 131–135. [DOI] [PubMed] [Google Scholar]
- 19.Huang BS, Seow KM, Tsui KH, et al. Fertility outcome of infertile women with adenomyosis treated with the combination of a conservative microsurgical technique and GnRH agonist: long-term follow-up in a series of nine patients. Taiwan J Obstet Gynecol 2012; 51: 212–216. [DOI] [PubMed] [Google Scholar]
- 20.Sheth SS andRay SS.. Severe adenomyosis and CA125. J Obstet Gynaecol 2014; 34: 79–81. [DOI] [PubMed] [Google Scholar]