Abstract
Objective
To investigate the role of quantitative analysis of T2 relaxation time in the magnetic resonance imaging (MRI) diagnosis of breast cancer.
Methods
The study enrolled patients with clinical breast masses who were examined using MRI at eight different echo times. The differences in T2 relaxation time of benign and malignant breast lesions were analysed.
Results
A total of 67 patients (67 breast lesions: 46 malignant, 21 benign) were examined. The mean ± SD T2 relaxation time was significantly lower in the 46 malignant lesions compared with the 21 benign lesions (82.69 ± 15.37 ms versus 95.48 ± 26.51 ms, respectively). The area under the curve was 0.731. Using 79.52 ms as the cut-off between benign and malignant breast lesions, a sensitivity of 85.7% and a specificity of 58.7% were obtained.
Conclusions
There was a significant difference in T2 relaxation time between benign and malignant breast lesions. The specificity of using T2 relaxation time alone for the differentiation of benign from malignant lesions was not high, but it could constitute a new adjunct in the MRI diagnosis of breast cancer.
Keywords: Breast cancer, magnetic resonance imaging (MRI), T2 relaxation time
Introduction
Breast cancer is a common cancer.1 Obtaining an accurate diagnosis is of considerable importance as it informs the choice of treatment and the prognostic outcomes of the disease.2 Magnetic resonance imaging (MRI) has a higher sensitivity for the diagnosis of breast cancer than mammography and ultrasound.2 Using a high-field, dedicated breast coil and contrast agent, both the spatial and temporal resolutions of breast MRI have been greatly improved, with a sensitivity of up to 88–100%.2 However, the background parenchymal enhancement may produce a high false-positive rate with breast dynamic contrast-enhanced (DCE) imaging.2,3 The ‘breast imaging and reporting data system’ classification provides a detailed set of diagnostic criteria that are primarily based on morphology, enhancement pattern, dynamic curve, peripheral involvement, and distribution of lesions.4 However, these criteria are somewhat subjective; thus, a correct diagnosis relies on an observer’s experience. Therefore, quantitative diagnostic criteria for breast cancer have been of interest to researchers.
Functional imaging has been widely employed in clinical practice. Diffusion-weighted imaging, spectroscopy, and perfusion are being increasingly used in breast MRI.5–7 These methods can provide higher specificity than DCE-MRI.5–7 However, spectroscopy and perfusion have greater scanning requirements.5,7 Breast apparent diffusion coefficient measurement is simple, but its specificity is controversial due to significant overlap between benign and malignant lesions.6
A study using T2-fast spin echo (FSE) sequences, showed that 87% of breast cancers were hypointense relative to the mammary glands and that 71% of fibroadenomas were hyperintense.8 Thus, differentiation between breast cancer and fibroadenoma could be accomplished using T2-weighted imaging (T2-WI).8 However, this differentiation relied on visual interpretation and it lacked quantitative standards.8 Differences in the relaxation time of different tissues constitute the basis for MRI image contrast. MRI can measure the T2 relaxation time (transverse relaxation time) and the T1 relaxation time (longitudinal relaxation time). T2 maps the graphs that are based on the T2 relaxation time. In general, T2 maps and tissue T2 relaxation times are obtained by scans with long times of repetition (TR) and two or more times of echo (TE). Currently, T2 relaxation time plays an important role in imaging of the articular cartilage, prostate, liver and the nervous system.9–12
This study used the T2 mapping method to measure T2 relaxation time in benign and malignant breast lesions to determine the potential role of this method in the differentiation of benign from malignant breast lesions.
Patients and methods
Patient population
This prospective study included patients with clinical breast masses who presented at the Department of Radiology, Huashan Hospital, Fudan University, Shanghai, China between July 2014 and October 2015. Patients with previous aspiration, chemotherapy, radiotherapy, or hormone replacement therapy and lactating patients were excluded. All of the patients underwent MRI examinations before surgery. All of the cases were confirmed by surgery and pathology.
This study was approved by the Institutional Review Board of Huashan Hospital, Fudan University, Shanghai, China (no. 2011-133). Verbal informed consent was obtained from all study participants.
MRI examinations
All MRI examinations were undertaken prior to the biopsy procedure. MRI was performed using a 1.5 T MRI scanner (SIGNA™ Infinity; GE Medical Systems, Milwaukee, WI, USA). The patient was in the prone position, and both breasts were examined with a 4-channel phased-array breast surface coil. The MRI included the following sequences: axial FSE T1-weighted image; sagittal fat-suppressed T2-WI; axial short time inversion recovery (STIR); axial diffusion weighted imaging (DWI); b values obtained at 0 and 1000. After reviewing the STIR and DWI images, four slices of the largest part of the lesion were selected. T2 mapping was performed using the parameters of these four slices (location, thickness, spacing, field of view). T2 mapping was performed with the following parameters: FSE sequence; 8 echoes; TE: 12.6-189.4 ms; TR: 1000 ms; matrix 256 × 256; and one cycle of excitation.
Image analysis
The images were analysed by two experienced radiologists (B.Y. and L.L.) who were blinded to the pathology results. Post-processing of the images was performed using the FuncTool T2 mapping software on an Advantage Workstation 4.3 (GE Medical Systems). If the lesion was clearly visualized on the T2 map, measurements were obtained directly from the image; otherwise, a fusion of the DWI images with the T2 map was performed. Combining the region of interest (ROI) with STIR avoided necrotic and cystic tumour areas. The ROIs ranged from 1 to 9 cm2 according to size of the tumour. Measurements were obtained at three different regions of the lesions, and the mean value was calculated.
The T2 maps were generated from images using a command line program by fitting the logarithm of the signal intensity of each pixel (STE) at each TE value to the linear transformation of the T2 relaxation equation: log (STE) = log (S0) , where S0 is the signal intensity before any T2 decay occurs.
The T2 maps were viewed and hand-drawn ROIs of the lesion were sampled to calculate the mean T2 time of the lesion.
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 11.5 (SPSS Inc., Chicago, IL, USA) for Windows®. All of the data were recorded as mean ± SD. T2 relaxation times of the benign and malignant lesions were compared using the independent sample’s t-test. The differences in the mean T2 relaxation time among the subgroups of benign and malignant lesions were compared using a one-way analysis of variance when the number of groups was greater than two, and post-hoc analysis was performed using the least significant difference method. To calculate the receiver operating characteristic (ROC) curve, the maximum of Youden’s index = (sensitivity + specificity) −1 was used as a critical point to identify benign and malignant breast lesions. A P-value <0.05 was considered statistically significant.
Results
The study included 67 patients with a mean ± SD age of 50.7 ± 17.3 years (all female; age range 26–74 years). Breast disease was confirmed by surgery and pathology in all patients: 21 were benign, including 10 patients with fibroadenoma, seven patients with intraductal papilloma, three patients with fibrocystic adenosis and one patient with phyllodes tumour; and 46 patients had malignant disease, including 28 patients with invasive ductal carcinoma, eight patients with invasive lobular carcinoma, two patients with medullary carcinoma, six patients with ductal carcinoma in situ and two patients with malignant phyllodes tumour.
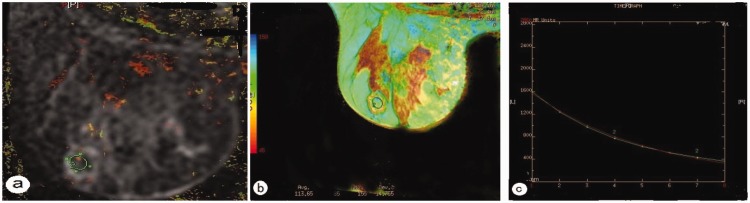
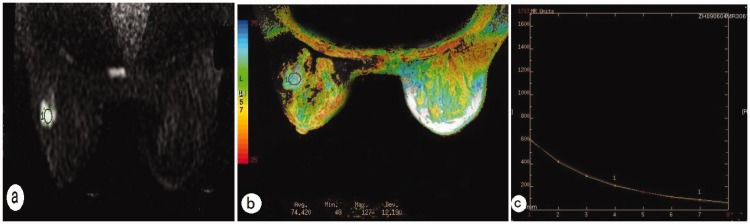
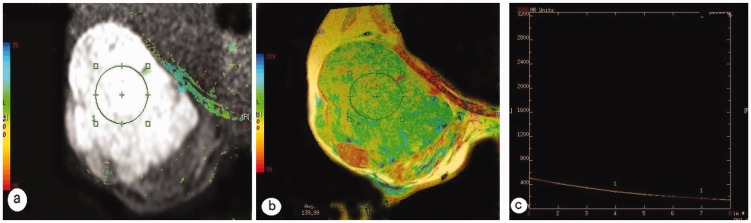
In the group of 21 benign lesions, the mean ± SD T2 relaxation time of the fibroadenomas was 92.53 ± 22.76 ms, that of the intraductal papillomas was 84.36 ±14.69 ms, that of the fibrocystic adenosis was 103.56 ± 4.17 ms (Figure 1), and that of the one benign was phyllodes tumour was 178.64 ms. The benign phyllodes tumour had a longer T2 relaxation time than the other benign lesions, but no statistical analysis was undertaken due to the small number of patients. There were no significant differences in the T2 relaxation times between the other types of benign lesions. In the group of 46 malignant lesions, the mean ± SD T2 relaxation time of invasive ductal carcinomas was 80.64 ± 10.16 ms (Figure 2), that of the invasive lobular carcinomas was 76.87 ± 14.01 ms, that of the ductal carcinomas in situ was 82.29 ±12.51 ms, that of the medullary carcinomas was 84.64 ± 3.75 ms, and that of the malignant phyllodes tumours was 133.81 ±7.35 ms (Figure 3). The T2 relaxation time of the malignant phyllodes tumours was longer than that of the other types of malignant lesions, but no statistical analysis was performed due to the small number of patients. No statistically significant differences existed in the T2 relaxation times between the various types of malignant lesions.
Figure 1.
Representative magnetic resonance imaging of a patient with fibrocystic adenosis: (a) a diffusion weighted image superimposed on a T2 map for the selection of the region of interest (circle); (b) T2 colour map shows a T2 relaxation time of 113.65 ms; (c) T2 relaxation time curve. The colour version of this figure is available at: http://imr.sagepub.com.
Figure 2.
Representative magnetic resonance imaging of a patient with an invasive ductal carcinoma: (a) diffusion weighted imaging shows a high signal of the lesion and regions of interest in the lesion (circle); (b) T2 colour map shows T2 relaxation time of 74.42 ms; (c) T2 relaxation time curve. The colour version of this figure is available at: http://imr.sagepub.com.
Figure 3.
Representative magnetic resonance imaging of a patient with a malignant phyllodes tumour: (a) diffusion weighted imaging shows a high signal of the lesion and regions of interest in the lesion (circle); (b) T2 colour map shows a T2 relaxation time of 139.99 ms; (c) T2 relaxation time curve. The colour version of this figure is available at: http://imr.sagepub.com.
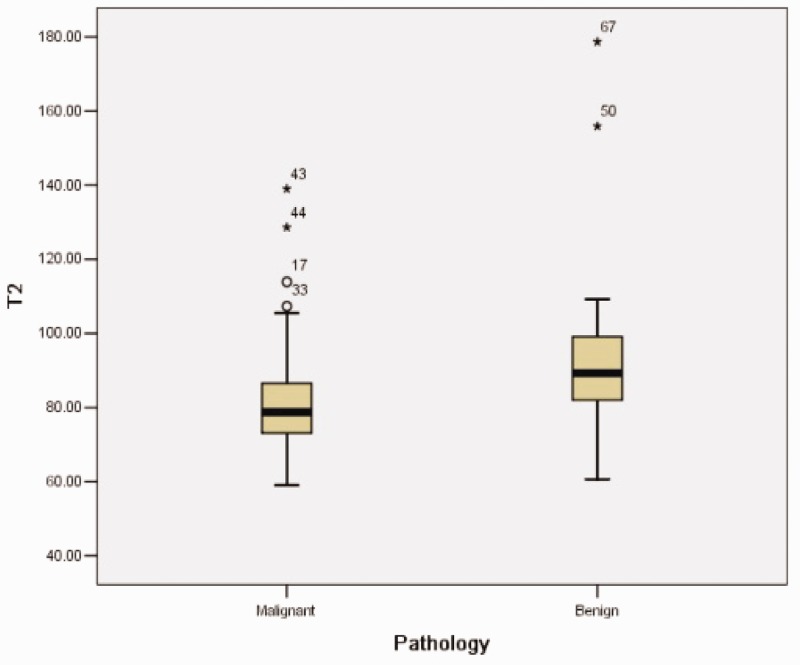
The mean ± SD T2 relaxation time of the 46 malignant lesions (82.69 ± 15.37 ms) was significantly lower compared with the 21 benign lesions (95.48 ± 26.51 ms; P = 0.015) (Figure 4). The area under the curve was 0.731 and the maximum Youden’s index was 0.444. Using a relaxation time of 79.52 ms as a cut-off between the benign and malignant breast lesions, a sensitivity of 85.7% and a specificity of 58.7% were obtained.
Figure 4.
Box-whisker plot showing the T2 relaxation times of benign and malignant breast lesions. The T2 relaxation times of the benign lesions were longer than those of the malignant lesions, but overlapping occurred. The black horizontal lines are medians, the extremities of the boxes are the 25th and 75th percentiles, the error bars represent the minimum and maximum outliers and the circles/asterisks above the boxes represent the extreme outlies.
Discussion
In addition to the effects of the main magnetic field strength, T2 relaxation time is affected by various factors depending on the intrinsic properties of the tissue and the environment, including tissue water content, random movement of water molecules and macromolecules, tissue fat content, the presence of paramagnetic particles and pH value.13,14 Among these factors, tissue water content is the most important. Water in the body exists in two states: free and bound water. Free water molecules are smaller in size, with a spin frequency greater than the Larmor frequency, so its T1 and T2 relaxation times are longer.13 In contrast, bound water is bound up with macromolecules, and its spin frequency is close to the Larmor frequency; thus, its T1 and T2 relaxation times are shorter than those of free water. In normal tissue, free water and bound water exist in dynamic equilibrium. However, in pathological conditions such as inflammation and oedema, bound water is released, and free water thus increases; as a result, the T1 and T2 relaxation times increase.13 There is a linear relationship between T2 relaxation time and water content.13 Prolongation of the T2 relaxation time indicates increased tissue water content.15,16
Due to the difference in tissue content, there is a difference in the relaxation time of the lesions. Many studies have used this characteristic to diagnose diseases in the musculoskeletal system, cardiovascular diseases, prostate and brain.9–12 As an important diagnostic tool in breast imaging, in addition to contrast enhancement and functional imaging, MRI currently uses contrast difference for lesion detection. This contrast difference is based on the different relaxation times of normal breast tissue and breast lesions. An early in vitro study found that there was a difference between the T2 relaxation times of benign and malignant breast lesions, which could be employed to differentiate them.17 A previous study found that the T2 relaxation time could predict the response to neoadjuvant chemotherapy in breast cancer.18
This present study found that the mean ±SD T2 relaxation time of the 46 malignant lesions was significantly lower than that of the 21 benign lesions (82.69 ± 15.37 ms versus 95.48 ± 26.51 ms, respectively; P =0.015). This difference could be attributed to increased tissue water content or interaction between alkaline metal cations and water in the pathological tissue.17,19 We speculate that benign breast lesions grow slowly, their cell density is relatively less, the extracellular interstitium is less than in malignant lesions, and tissue free water is thus present in greater volumes. In malignant lesions, particularly invasive ductal carcinoma, the cancer cells are atypical and are larger in size with abundant cytoplasm, the solid components are more abundant, and the interstitium is infiltrated by lymphocytes and plasma cells.20 Necrosis is also common in malignant lesions, and necrotic material is released into the intercellular fluid.20 The above factors lead to a reduction of extracellular space; tissue free water is then decreased, which might account for the shorter T2 relaxation times in malignant lesions.17
In this study, the two cases of malignant phyllodes tumours had a longer mean ± SD T2 relaxation time (133.81 ± 7.35 ms) than the overall mean ± SD of the malignant lesions (82.69 ± 15.37 ms). The T2 relaxation time of the one case of benign phyllodes tumour was 178.64 ms, which was longer than that of the other benign lesions. Phyllodes tumours are characterized by cystic changes and haemorrhage, which could be the reasons for the prolonged T2 relaxation times.21
Although there was a significant difference between the T2 relaxation times in the benign and malignant lesions, the overlap between the two groups was quite considerable. The area under the ROC curve was 0.731 and Youden’s index was 0.444. As a result, using the T2 relaxation time to differentiate benign from malignant lesions was difficult. However, in comparing the two groups, this present study found that benign lesions generally had a longer T2 relaxation time compared with malignant lesions, which could be employed as an adjunct in the MRI diagnosis of breast cancer. Because of this overlap in T2 relaxation times between benign and malignant breast lesions, we would recommend that physicians do not only rely on dynamic features but that they use these in combination with morphological findings.
This present study had several limitations. First, the number of benign lesions was small. Secondly, some of the lesions were small, leaving the possibility of a partial volume effect of adjacent adipose tissue leading to greater measurement error. Thirdly, the majority of the patients had invasive ductal carcinomas. The other malignant lesions, such as phyllodes tumour and ductal carcinoma in situ, were relatively less common, and because the T2 relaxation time was related to the composition of the lesions, this difference could have resulted in some bias in the measurement of the T2 relaxation time.
In conclusion, there was a significant difference in the T2 relaxation times between benign and malignant breast lesions. The T2 relaxation times of benign lesions were longer than those of malignant lesions. However, differentiation between benign and malignant lesions based only on T2 relaxation times was difficult. Quantitative measurement of T2 relaxation times could provide an adjunct to MRI in the diagnosis of breast cancer.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
The project was supported by a grant from the National Natural Science Foundation of China (no. 81501435) awarded to Li Liu.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 2.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA 2004; 292: 2735–2742. [DOI] [PubMed] [Google Scholar]
- 3.Bignotti B, Signori A, Valdora F, et al. Evaluation of background parenchymal enhancement on breast MRI: a systematic review. Br J Radiol 2017; 90: 20160542–20160542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tardivon AA, Athanasiou A, Thibault F, et al. Breast imaging and reporting data system (BIRADS): magnetic resonance imaging. Eur J Radiol 2007; 61: 212–215. [DOI] [PubMed] [Google Scholar]
- 5.Baeka HM, Yu HJ, Chen JH, et al. Quantitative correlation between (1)H MRS and dynamic contrast-enhanced MRI of human breast cancer. Magn Reson Imaging 2008; 26: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marini C, Iacconi C, Giannelli M, et al. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol 2007; 17: 2646–2655. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang X, Zhang B, Zhu B, et al. Application of T2*-weighted first-pass perfusion imaging in the diagnosis of breast tumors. Chinese German J Clin Oncol 2007; 6: 357–360. [Google Scholar]
- 8.Kuhl CK, Klaschik S, Mielcarek P, et al. Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging 1999; 9: 187–196. [DOI] [PubMed] [Google Scholar]
- 9.Krepkin K, Bruno M, Raya JG, et al. Quantitative assessment of the supraspinatus tendon on MRI using T2/T2* mapping and shear-wave ultrasound elastography: a pilot study. Skeletal Radiol 2017; 46: 191–199. [DOI] [PubMed] [Google Scholar]
- 10.Kim PK, Hong YJ, Im DJ, et al. Myocardial T1 and T2 mapping: techniques and clinical applications. Korean J Radiol 2017; 18: 113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu LM, Yao QY, Zhu J, et al. T2* mapping combined with conventional T2-weighted image for prostate cancer detection at 3.0T MRI: a multi-observer study. Acta Radiol 2017; 58: 114–120. [DOI] [PubMed] [Google Scholar]
- 12.Knight MJ, McCann B, Tsivos D, et al. Quantitative T2 mapping of white matter: applications for ageing and cognitive decline. Phys Med Biol 2016; 61: 5587–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaland B, Mariette F, Marchal P, et al. 1H nuclear magnetic resonance relaxometric characterization of fat and water states in soft and hard cheese. J Dairy Res 2000; 67: 609–618. [DOI] [PubMed] [Google Scholar]
- 14.Gossuin Y, Roch A, Muller RN, et al. Relaxation induced by ferritin and ferritin-like magnetic particles: the role of proton exchange. Magn Reson Med 2000; 43: 237–243. [DOI] [PubMed] [Google Scholar]
- 15.Nissi MJ, Rieppo J, Töyräs J, et al. T(2) relaxation time mapping reveals age- and species- related diversity of collagen network architecture in articular cartilage. Osteoarthritis Cartilage 2006; 14: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 16.Lüsse S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging 2000; 18: 423–430. [DOI] [PubMed] [Google Scholar]
- 17.Merchant TE, Thelissen GR, de Graaf PW, et al. Application of a mixed imaging sequence for MR-imaging characterization of human breast disease. Acta Radiol 1993; 34: 356–361. [PubMed] [Google Scholar]
- 18.Tan PC, Pickles MD, Lowry M, et al. Lesion T2 relaxation times and volumes predict the response of malignant breast lesions to neoadjuvant chemotherapy. Magn Reson Imaging 2008; 26: 26–34. [DOI] [PubMed] [Google Scholar]
- 19.Edden RA, Smith SA, Barker PB. Longitudinal and multi-echo transverse relaxation times of normal breast tissue at 3 Tesla. J Magn Reson Imaging 2010; 32: 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO Classification of Tumours of the Breast, 4th edn Geneva: WHO Press, 2012. [Google Scholar]
- 21.Yabuuchi H, Soeda H, Matsuo Y, et al. Phyllodes tumor of the breast: correlation between MR findings and histologic grade. Radiology 2006; 241: 702–709. [DOI] [PubMed] [Google Scholar]