Abstract
Background:
Approximately 20–30% of all intracranial metastases are located in the posterior fossa. The clinical evolution hinges on factors such as tumor growth dynamics, local topographic conditions, performance status, and prompt intervention. Fourth ventricle (V4) compression with secondary life-threatening obstructive hydrocephalus remains a major concern, often requiring acute surgical intervention. We have previously reported on the application of adaptive hypofractionated Gamma Knife Radiosurgery in the acute management of critically located metastases, a technique known to us as rapid rescue radiosurgery (3R). We report the results of 3R in the management of posterior fossa lesions and ensuing V4 decompression.
Case Descriptions:
Four patients with V4 compression due to posterior fossa metastases were treated with 3R by three separate gamma knife radiosurgical sessions (GKRS) over a period of seven days. Mean V4 volume was 1.02 cm3 at GKRS 1, 1.13 cm3 at GKRS 2, and 1.12 cm3 at GKRS 3. Mean tumor volume during the week of treatment was 10 cm3 at both GKRS 1 and 2 and 9 cm3 at GKRS 3. On average, we achieved a tumor volume reduction of 52% and a V4 size increase of 64% at the first follow-up (4 weeks after GKRS 3). Long-term follow-up showed continued local tumor control, stable V4 volume, and absence of hydrocephalus.
Conclusion:
For this series, 3R was effective in terms of rapid tumor ablation, V4 decompression, and limited radiation-induced toxicity. This surgical procedure may become an additional tool in the management of intractable posterior fossa metastasis with V4 compression.
Keywords: Adaptive hypofractionated gamma knife radiosurgery, fourth ventricle, posterior fossa metastases, rapid rescue radiosurgery, treatment of obstructive hydrocephalus
INTRODUCTION
The posterior fossa harbors 20–30% of all intracranial metastases.[10,22,44,47] These tumors are often neurologically impairing and potentially life-threatening. The most common primary origin is lung cancer, breast cancer, malignant melanoma, renal cell carcinoma, and colorectal and gynecological cancers.[44] Their clinical presentation and evolution is mainly dependent on the lesion's location and growth rate. Symptoms may arise from focal compression of the cerebellum and vital brainstem nuclei, tonsillar herniation, and increased intracranial pressure due to obstructive hydrocephalus. The latter conditions often require emergency surgical procedures aiming to remove the threat of acute neurological impairment and death. Similar to their supratentorial counterparts, the management of posterior fossa metastases include microsurgery, radiotherapy, and systemic treatment. In general, the last-mentioned treatments aim to extend the survival and retain the quality of life as the overall prognosis for patients with these lesions remains poor,[16,44] particularly in the case of intrinsic brainstem metastases.[40] We have previously reported the benefits of applying adaptive hypofractionated gamma knife radiosurgery (GKRS) in the acute management of aggressive/critically located metastases;[40,41] the application of this procedure in next-to emergency conditions and in strict structured settings has been coined rapid rescue radiosurgery (3R). The present case series describes the results of 3R in terms of prompt decompression of the V4, subsequent avoidance of hydrocephalus, and preservation of neurological function during treatment and follow-up.
MATERIALS AND METHODS
Patients
Four patients diagnosed with posterior fossa metastasis and underlying V4-compression underwent 3R-intervention between November 2013 and August 2016. The treatment's main goals were:
Achieve prompt V4 decompression and tumor ablation from the week of treatment to the first follow-up magnetic resonance imaging (MRI) (generally 4 weeks after treatment completion)
Analyze the sustained decompressing effects on the V4, including avoidance of hydrocephalus
Analyze eventual development of adverse radiation event (ARE) up to the last follow-up MRI.
With previous institutional and ethical approval, we conducted a retrospective analysis of all medical records, as well as corresponding imaging and Leksell Gamma Plan (LGP) volume data.
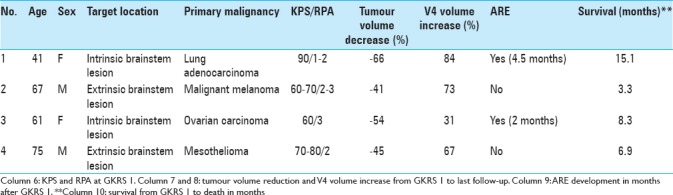
Table 1 summarizes the key general criteria for all reviewed and treated cases [age, gender, tumor histology, target location, and Karnofsky performance score (KPS)/recursive partitioning analysis (RPA)]. The average age of the patients was 61 years (range, 41–75 years).
Table 1.
Key clinical data at GKRS 1
Inclusion criteria according to field of treatment
-
Brainstem radiosurgery: Intrinsic and extrinsic brainstem metastases with radiological signs of V4 compression (with or without perilesional edema) and the following criteria:
- Patients who were not candidates for microsurgical removal, other forms of radiotherapy, or systemic treatment targeting the intracranial lesion at hand
- Metastases assessed as not suitable for single fraction GKRS (SF-GKRS): V10Gy>1 cm3 when applying a peripheral prescription dose of 16–18 Gy with prior radiotherapeutic focal impact (such as WBRT) or V10Gy>3 cm3 when applying a peripheral prescription dose of 16–18 Gy without previous radiotherapy
- KPS at least 70 and RPA of 1–2 when possible. However, exceptions were considered (KPS <70, RPA 3) in cases of V4 compression requiring acute salvage of neurological function and/or avoidance of neurological death (”compassionate” treatment).
-
Non-brainstem radiosurgery: infratentorial lesions nonadjacent to the brainstem but with compressive impact on V4 (with or without perifocal edema), with the following inclusion criteria:
- Patients not candidates for microsurgical removal, other form of radiotherapy, or systemic treatment targeting the intracranial lesion at hand
- Metastases requiring a peripheral dose of at least 18 Gy but not suitable for SF-GKRS due to volume-constrains (Gross Tumor Volume=GTV >8–10 cm3). Lesser volumes (GTV <8 cm3) were considered for 3R if local clinical conditions and radiobiological variables demanded it (such as previous radiotherapy, extensive perilesional edema, and the degree of regional eloquence)
- KPS at least 70 and RPA of 1–2 when possible, although exceptions could be considered (KPS <70, RPA 3) in cases aiming to avoid further neurological deterioration and subsequent neurological death (compassionate treatment).
Treatment protocol
Treatment settings were structured on three separate GKRS delivered during a period of 7 days (every 60–72 hours). Prior to each GKRS, the Leksell G-frame (Elekta AB, Stockholm) was affixed to the head using local anesthesia (lidocaine + adrenaline). MRI was thereafter acquired on a 1.5-T GE Discovery 450 MR camera (General Electric) with the fiducial box applied to the respective G-frame before each GKRS. The stereotactic MRI protocol for each patient included sagittal T1-weighted spin echo, axial T2-weighted propeller and post-gadolinium (0.4 ml/kg of Dotarem 279.3 mg/ml, range 20–35 ml) contrast-enhanced (CE) axial T1-weighted fast spin echo, and three-dimensional (3D) T1-weighted FSPGR MR sequences. The stereotactic images at each fraction had the main purpose of (1) acquiring a fiducial-based stereotactic space, (2) delineating the GTV, V4 boundaries and the brainstem on postcontrast T1-weighted 3D volume sequences, (3) identifying the extension of underlying perilesional edema (T2-weighted series), and (4) creating a highly conformed dose plan.
All images were carefully reviewed by experienced neuroradiologists and gamma knife surgeons prior to treatment planning (LGP treatment planning system, version 10.1.1, Elekta instruments AB, Stockholm). No margins were added to the GTV. Each GKRS treatment plan was carefully conceived and approved by a team of experienced gamma knife surgeons and medical physicists. Prescription doses at each GKRS were based on a set of treatment-feasibility variables including clinical/performance status, tumor histology, radiosensitivity-surrogate properties corresponding to the treated tumor and adjacent organs at risk (OAR), tumor volume, prior intracranial radiotherapy with local impact on the GTV, and degree of response to previous intra/extracranial radiotherapy. For each GKRS, prescription doses were set at a low isodose line (35% line) to maximize/boost dose distributions inside the tumor while keeping dose increment to the surrounding healthy tissue as steady or limited as possible. Prescription doses were dynamically adapted to tumor volume changes during treatment course. First follow-up MRI with corresponding clinical follow-up was set at 4 weeks after treatment completion.
Clinical summaries
Case 1
A 41-year-old, nonsmoking female was initially diagnosed with metastatic TTF-1 positive lung adenocarcinoma in 2011. She underwent radiotherapy for her primary lesion as well as several lines of systemic treatments with initially positive but later mixed response. In March 2013, the patient reported symmetric sensory symptoms with numbness and tingling around her lips and limbs which eventually caused impaired balance. Brain MRI revealed more than 20 supra and infratentorial metastases including one to the left in the pons (M1) and signs of leptomeningeal carcinomatosis. The lesion in the pons (M1) had central necrosis and measured 25 × 30 × 26 mm at the time of diagnosis. The patient started high-dose systemic corticosteroid therapy and was treated the same month with whole brain radiation therapy (WBRT) with a dose fractionation of 4 Gy delivered over 5 consecutive days (4 Gy × 5). A follow-up MRI in July 2013 revealed volume reduction of all intracranial metastases including the pontine lesion (M1). The ensuing follow-up MRI in October 2013 demonstrated progression of M1 from 19 mm to 27 mm and slight V4 floor compression without hydrocephalus. A previously known small metastasis in the left lateral occipital lobe (M2) showed signs of progression as well. All other metastases were deemed under control at this point. To avoid further neurological impairment and V4 obstruction due to tumor growth and/or posttreatment ARE, M1 was treated by 3R in November 2013 [Table 2].
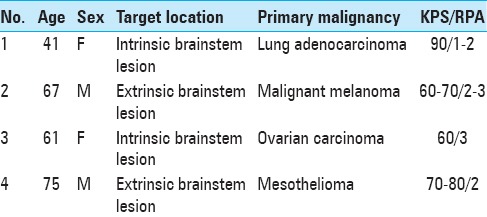
Table 2.
Treatment data for patient 1
At the time of GKRS 1, M1 proved larger (9.2 cm3) with further perilesional edema and more apparent V4 floor compression, though still without signs of hydrocephalus. M2 had also grown slightly (from 10 to 12 mm in diameter) and was treated by SF-GKRS with 21 Gy to the 50% isodose prescription line. Apart from slight fatigue, left-sided facial numbness, and tingling the patient reported no other symptoms at the time of GKRS 1; at this stage, her KPS was estimated to be 90 and the RPA to be 1–2.
Despite ongoing cortisone treatment, the perilesional edema remained nearly unchanged between GKRS 1 and GKRS 3; cortisone treatment was discontinued shortly after completion of GKRS 3. Nevertheless, V4 proved slightly wider at the time of GKRS 3 (from 0.9 cm3 at GKRS 1 to 1.2 cm3 at GKRS 3) and the tumor had decreased approximately 18% in volume (from GKRS 1 to GKRS 3). At 1-month follow-up, the patient was almost asymptomatic (KPS 90–100); the corresponding MRI revealed a significant reduction of tumor volume of approximately 67% (from 9.2 cm3 at GKRS 1 to 3.1 cm3 at the first follow-up) as well as renormalization of V4 boundaries (0.9 cm3 to 1.7 cm3) [Table 2; Figure 1].
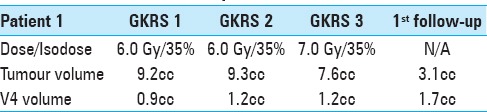
Figure 1.
Patient 1, MRI Contrast enhanced (CE) 3D T1: Axial, coronal and sagittal images (left to right) illustrate decreasing tumour volume and V4 decompression from GKRS 1 (above) to first follow up 4 weeks after GKRS 3 (below)
M1 developed a local ARE radiologically verified by MRI (follow-ups between March and November 2014) as well as 18F-FDG PET-CT (April and December 2014), which subsequently yielded without cortisone 6 months later (October 2014). Despite the above, the patient remained next to asymptomatic from her treated lesion during the follow-up. Unfortunately, systemic treatment eventually failed; the patient underwent SF-GKRS for several metastases (distant to M1 and M2 boundaries) from April to November 2014; her condition deteriorated steadily in December 2014, and she subsequently died in February 2015 (15 months after 3R) mainly due to intrathoracic tumor progression. Her quality of life was overall good from the time of 3R treatment and 12 months onwards.[18]
Case 2
A 67-year-old previously healthy male presented in September 2011 with a 3-week history of fatigue, dizziness, balance impairment, left-sided motor/sensory deficits, and transient mainly right-sided headache. Brain CT and MRI demonstrated a 25-mm large metastasis in the superior left cerebellar hemisphere (M1) with slight compression of the brainstem in addition to three smaller metastases in the right frontal lobe and one in the left occipital lobe. Further investigations revealed a small pulmonary lesion which was deemed unlikely to be the primary tumor. A stereotactic biopsy was, therefore, performed on one of the frontal lesions to establish a definitive diagnosis; the microscopic evaluation demonstrated a metastasis from malignant melanoma with underlying BRAF-mutation.
Three weeks after the biopsy, the patient underwent GKRS for all metastases. At the time of treatment, his neurological condition had deteriorated with increasing paresis of the left leg requiring assisted mobilization by wheelchair (KPS 60–70; RPA 2–3). The stereotactic MRI at GKRS 1 revealed a short-interval significant size increase of M1 to 37 mm (approximately 15 cm3) with central necrosis and slight surrounding edema despite ongoing corticosteroid therapy. V4 proved now deformed and partially compressed though without radiological evidence of hydrocephalus. The lesion was assessed as inaccessible for microsurgical decompression. Due to the size of the lesion and high risk for ARE in the brainstem, M1 was adaptively treated by means of 3R [Table 3]; the remaining four lesions were treated with SF-GKRS. During the three-part treatment time M1 experienced a tumor reduction of almost 10% (from 15.1 at GKRS 1 to 13.8 cm3 at GKRS 3); V4 volume increased slightly (from 0.83 cm3 at GKRS 1 to 0.93 cm3 at GKRS 3). The dose fractionation schedule is described in Table 3. The first follow-up MRI was planned 4 weeks after the third GKRS; because of increased headache/dizziness and to assess the need for post-GKRS systemic treatment the examination was performed 10 days earlier than planned (MRI at 3 weeks).
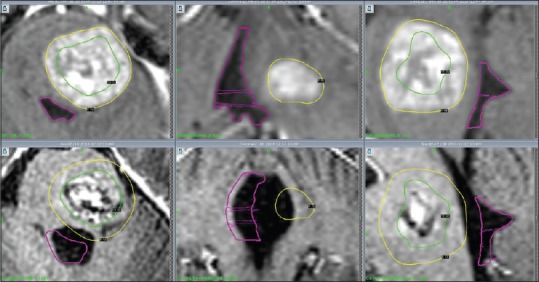
Table 3.
Treatment data for patient 2
Although volume assessments by LGP were technically not possible on this particular MRI due to image artefacts, M1 had higher signal on T2 with a more necrotic appearance whereas V4 remained almost unchanged. However, with supportive/ongoing corticosteroid therapy, the patient recovered some balance and strength in his left leg shortly after his examination; systemic antitumoral treatment (BRAF inhibitor dabrafenib) was started at this stage. A new follow-up MRI at 8 weeks revealed decreased volume of M1 (41% compared to MRI at GKRS 1) and diminished compression of V4 (volume of V4 increased from 0.83 cm3 at GKRS 1 to 1.43 cm3 at 8 weeks) [Figure 2]. Local edema had diminished. The remaining treated lesions were under control. However, a CT scan at 10 weeks was performed due to general deterioration over the previous few weeks; the examination demonstrated new areas of hemorrhage and surrounding edema adjacent to two frontal metastases which were treated with SF-GKRS, and slightly increased edema surrounding M1 although with stable V4 conditions. He died 2 weeks later (10 weeks after GKRS) in palliative care. No autopsy was performed. According to the available medical data, the patient might have died of extracranial tumor activity and possible intracranial complications.
Figure 2.
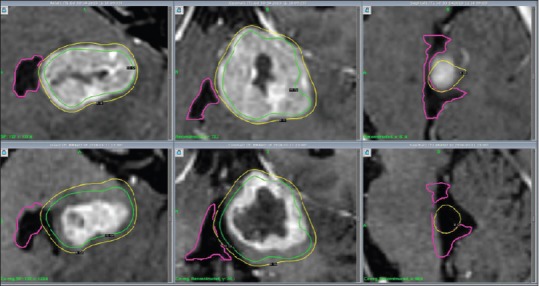
Patient 2, MRI (CE 3D T1): Axial, coronal and sagittal cross-sections (left to right) illustrate decreasing tumour volume and V4 decompression from GKRS 1 (above) to second follow up 8 weeks after GKRS 3 (below)
Case 3
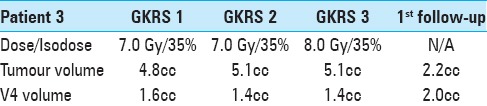
A 62-year-old female patient was diagnosed in 2005 with ovarian carcinoma. She underwent surgery, several lines of antitumoral treatment, and regional fractionated radiotherapy prior to developing metastases to regional lymph nodes as well as peritoneal carcinomatosis (2008). In January 2016, the patient presented with 2-week history of nausea, vomiting, impaired balance and speech, severe swallowing difficulties, and sensory symptoms. The brain MRI demonstrated a large, solitary, 20-mm metastasis in the upper part of the medulla oblongata (M1) without significant perilesional edema, yet compressing the foramina of Luschka and Magendie with signs of mild hydrocephalus [Figure 3]. Corticosteroid therapy was initiated shortly thereafter. Microsurgical removal/debulking of the metastasis were deemed not feasible and 3R was offered after multidisciplinary in-hospital discussion. At the time of GKRS 1 in February 2016, KPS was estimated to be 60 and RPA 3. The corresponding dose fractionation schedule is described in Table 4. No tumor volume reduction or V4 decompression was noted between GKRS 1 and GKRS 3. A follow-up MRI at 4 weeks revealed a 54% decrease in the size of M1 (4.8 cm3 at GKRS 1 to 2.2 cm3 at follow-up). V4 volume increased (1.56 cm3 at GKRS 1 to 2 cm3 at follow-up), and the foramina of Luschka and Magendie were less compressed [Figure 3]. There was no evidence of hydrocephalus. The patient's neurological condition also improved dramatically at this time and KPS was estimated to be 70. The dysphagia, nausea, and vomiting disappeared, and she could articulate much better. Nevertheless, no considerable improvement in balance was reported. The patient continued systemic antitumoral treatment. Consecutive, initially monthly and later bimonthly radiological follow-ups until September 2016 (7 months after 3R) showed further volume decrease of M1. However, the patient developed mild radiation-induced perilesional edema (assessed as ARE) 5 months after treatment which slowly progressed until her last MRI at 7 months (September 2016) though without evidence of hydrocephalus. At this time, the patient was experiencing mild facial numbness almost solely limited to her right cheek and the right side of the jaw. The patient was also experiencing some more fatigue and balance impairment than usual although it was difficult to assess whether it was caused by the lesion itself and/or other factors such as aggressive systemic tumor progression, ongoing systemic treatment, and steroids. Nevertheless, despite the presence of perilesional edema, we assessed her neurological status and quality of life in relation to M1 as stable. She died 7 months after completion of 3R-treatment due to aggressive extracranial tumor disease progression.
Figure 3.
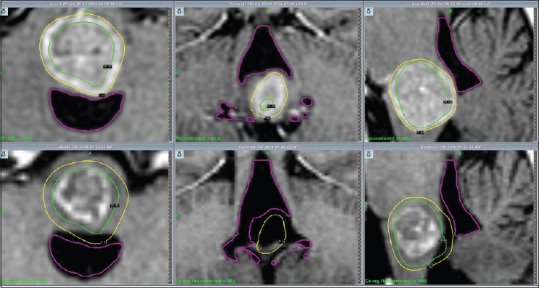
Patient 3, MRI (CE 3D T1): Axial, coronal and sagittal images (left to right) illustrate decreasing tumour volume and V4 decompression from GKRS 1 (above) to first follow up 4 weeks after GKRS 3 (below)
Table 4.
Treatment data for patient 3
Case 4
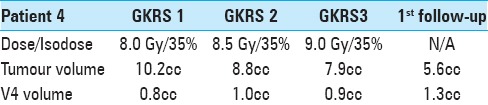
A 75-year-old man was diagnosed in June 2016 with right-sided pleural desmoplastic mesothelioma. He had a previous history of smoking and exposure to asbestos as well as substantial underlying cardiovascular comorbidity. Curative treatment was deemed not feasible. While awaiting palliative antitumoral treatment, the patient experienced impaired balance, severe rotational vertigo, nausea, and vomiting. Brain CT and MRI in July 2016 revealed a solitary 20-mm metastasis with slight surrounding edema in the left middle cerebellar peduncle and extending along the tentorium (M1). V4 was minimally compressed without hydrocephalus. Corticosteroid therapy was initiated but microsurgery was deemed not feasible due to previous cardiovascular morbidity. Systemic palliative chemotherapy was initiated and he was referred for GKRS as no other treatment options remained available for M1. The patient underwent 3R-intervention for his solitary lesion in August 2016. KPS was estimated as 70–80 and RPA as 2 at GKRS 1 during steroid treatment de-escalation. KPS subsequently improved to 80 at GKRS 2 and 90–100 at GKRS 3 despite decreased steroid dose. M1 underwent a volume reduction of 22% between GKRS 1 and GKRS 3 (10.2 cm3 to 7.9 cm3) while V4 volume increased from 0.79 cm3 to 0.93 cm3 (21%). One-month follow-up with clinical assessment and MRI revealed mild fatigue presumed due to ongoing systemic antitumoral treatment (KPS 90) though without major neurological impairment, and substantial tumor volume reduction of almost 50% (10.2 cm3 at GKRS 1 to 5.6 cm3 at 4 weeks after GKRS 3). V4 volume had also increased significantly (from 0.79 cm3 at GKRS 1 to 1.31 cm3 at 4 weeks) [Figure 4]. A follow-up MRI at 3 months showed a small suspected recurrence with mild surrounding edema at the inferior aspect of the previous radiation field which was successfully treated in December 2016 along with ten new metastases; all eleven lesions were treated with SF-GKRS. No clinical signs of neurological deficits or radiological evidence of ARE were reported. The patient died 6 months after completion of 3R due to an aggressive, drug-resistant pneumonia.
Figure 4.
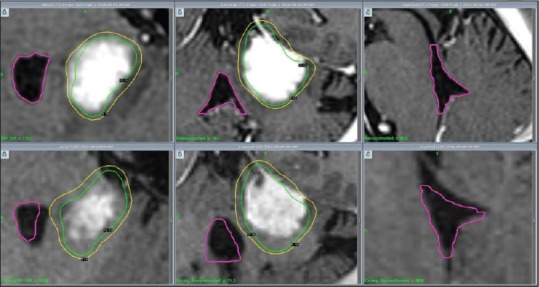
Patient 4, MRI (T1-GAD): Axial, coronal and sagittal cross-sections (left to right) illustrate decreasing tumour volume and V4 decompression from GKRS 1 (above) to first follow up 4 weeks after GKRS 3 (below)
RESULTS
Figures 5 and 6 illustrate V4 and tumor volume dynamics throughout treatment until first follow-up MRI for all cases. V4 volume increased between GKRS 1 and 2 for cases 1 and 4 and remained essentially unchanged for the other two patients. V4 volumes remained constant between GKRS 2 and 3 for all patients. V4 volumes increased between 30% and 84% at 1st follow-up MRI compared to GKRS 1 [Figure 5]; the average V4 volume increased at 1st follow-up MRI was 61%. The largest increase of V4 volume was observed in the patient with lung cancer metastasis (case 1) whereas the least V4 volume increase was found in the patient with ovarian cancer metastasis (case 3). V4 volume estimates between the first follow-up MRI and the last follow-up MRI remained stable, indicating that the decompressive effects on V4 (due to tumor regression) occur time wise within 4 weeks after treatment completion [Tables 5 and 6].
Figure 5.
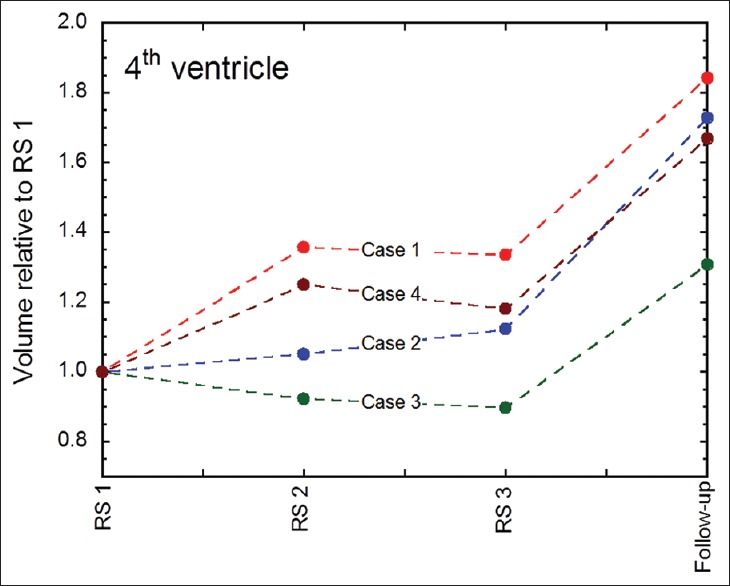
V4 volume dynamics at each fraction and at 1st follow-up MRI (usually 4 weeks after GKRS3) for all patients. Case 1 = red line, Case 2 = blue line, Case 3 = green line, and Case 4 = brown line
Figure 6.
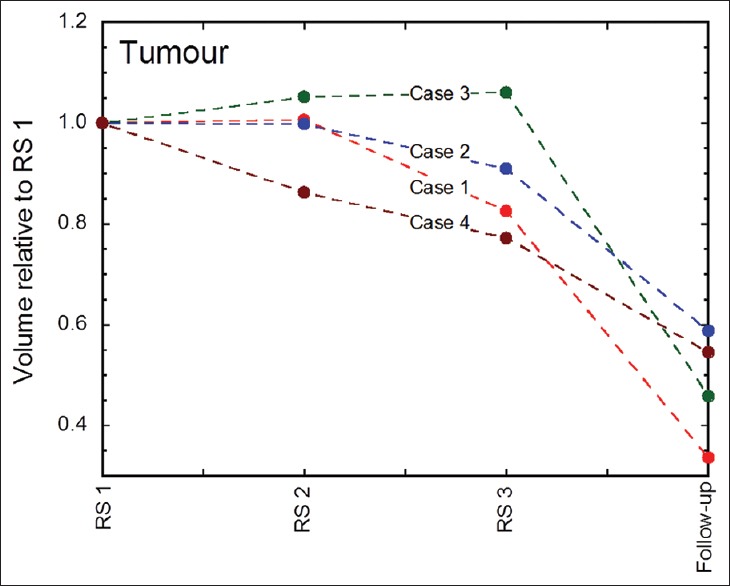
Tumor volume dynamics at each fraction and at 1st follow-up MRI (usually 4 weeks after GKRS 3) for all patients. Case 1 = red line, Case 2 = blue line, Case 3 = green line, and Case 4 = brown line
Table 5.
Summary of V4 volume-evolution at GKRS 1, first follow-up MRI and last follow-up MRI
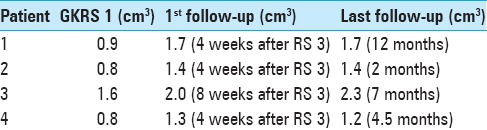
Table 6.
Summary of key clinical data for each patient
Tumor volume estimates between GKRS 1 and GKRS 2 remained practically constant for cases 1 and 2, increased slightly for case 3, and decreased significantly in case 4. Further volume reduction for cases 1, 2, and 4 was demonstrated between GKRS 2 and GKRS 3, and tumor volume remained stable for case 3. All four cases presented significant tumor volume reduction at 1st follow-up MRI [Tables 2-4 and 7]; average tumor volume reduction between GKRS 1 and 1st follow-up MRI was 52% [Figure 6]. The largest tumor volume reduction was observed in case 1 whereas the smallest reduction was in case 2.
Table 7.
Treatment data for patient 4
Both cases with intrinsic brainstem lesions (case 1 and case 3) developed an ARE, though without radiological impact on the V4 or major clinical impairment up to last follow-up MRI. The above mentioned ARE was identified 4.5 months after treatment completion in case 1 and 2.0 months in case 3. Median survival from GKRS 1 to death was 7.6 months. Median V4 volume increase and tumor volume decrease from GKRS 1 to last follow-up was 70% and −50%, respectively.
DISCUSSION
General aspects
Despite modern medical technology, the treatment of aggressive and/or large posterior fossa metastases remains a major challenge, particularly in acute/subacute settings. These neoplasms often lead to serious neurological impairment due the contentious nature of tumor kinetics involved at local and distal sites. V4 compression and subsequent obstructive hydrocephalus is a common mass effect-dependent complication requiring prompt intervention to relieve rapidly increasing and potentially fatal intracranial pressure.[44] Damage to cranial nerve nuclei and circumscribing motor and sensory fibers remains an issue. Serious disseminative complications such as leptomeningeal carcinomatosis and seeding/drop-metastases to the spinal canal resulting from infratentorial metastatic activity have also been described.[4,43] Consequently, posterior fossa metastases (with or without hydrocephalus) often require aggressive, tailored intervention, including microsurgery, preoperative CSF-diversion due to life-threatening/rapidly developing obstructive hydrocephalus prior to microsurgery, radiotherapy, and systemic treatment such as chemotherapy and targeted therapy.[4,16,32,44]
Microsurgery: Decisive factors
From a general perspective, the management of infratentorial brain metastases has significantly improved over the last decades due to a range of strategic factors including the development of high-performance imaging, safer microsurgical techniques,[12,23,28,23] advanced intraoperative neurologic monitoring,[18,46] optimized patient selection, and modern postsurgical care. Microsurgery followed by adjunctive radiotherapy to the surgical site is widely regarded as the ideal upfront management of single large brain metastases.[1,6,13,24,31,44] However, as in our series, microsurgery in the posterior fossa may not always be feasible for a number of reasons, including aggressive tumor infiltration, local anatomical conditions, degree of regional eloquence (such as the brainstem), synchronous metastatic activity, comorbidity, and RPA-surrogate factors.[15,40,41] In general, posterior fossa location remains associated with poorer survival, particularly in the presence of metastasis-induced hydrocephalus; this is likely related to the fact that posterior fossa lesions differ from supratentorial metastases in terms of symptoms, clinical outcome, radiological evolution, and complications.[4,16] Reviewing 708 cases that underwent resection of brain metastases, Chaichana et al. identified 140 patients subjected to posterior fossa surgery. Cerebellar location was associated with poor survival and increased spinal recurrence; in this study, radiation was reported to be the common denominator in prolonged local and distal progression-free survival.[4]
Post-microsurgery complications requiring CSF-diversion procedures
Even if microsurgery is deemed feasible in many cases, complications are not uncommon. The complex postoperative evolution intrinsic to posterior fossa tumors includes the potential impairment of normal CSF flow dynamics and consequent hydrocephalus development; postoperative CSF leakage (with or without hydrocephalus) and its subsequent risk of deep infections (meningitis, ventriculitis) are also potential complication after surgical procedures involving the posterior fossa.[2,44] As confirmed by a number of studies, postoperative CSF diversion procedures such as shunt-based interventions and endoscopic third ventriculostomy (ETV) are often required for the above cases. Ferguson et al. conducted a retrospective review of 55 cases having undergone surgery for tumors of V4 (1993–2010); 22% of patients required CSF-diversion following resection.[7] The group concluded that patients having required intraoperative ventriculostomy, transvermian approach, and <18 years of age were more likely to require postoperative CSF diversion. A retrospective study including 92 patients operated for posterior fossa metastases reported 14 patients (15%) developing hydrocephalus after surgery, 9 of which required CSF diversion.[44] Another retrospective analysis of 22 resected V4-ependymomas reported a shunt-dependent hydrocephalus rate of 18%. Six patients (27%) developed cranial nerve palsy, 2 of which resolved in time.[46]
Although clinically effective in the management of elevated intracranial pressure, CSF-diversion procedures are not free from complications. The rate of shunt failure after ventriculoperitoneal (VP) shunt placement remains a concern and shunt revisions can be required within the first 6 months after shunt placement.[17,35,37] Some studies have reported the overall VP-shunt failure rate to be around 15%.[17,34] The main etiologies of shunt dysfunction include shunt blockage (due to the presence of blood or high levels of protein in CSF), shunt infection, shunt migration, and CSF ascites.[17] The latter may lead to more complicated situations including further obstruction and/or the development of serious infectious processes in the CNS.[44] Different groups have suggested that factors such as age (pediatric and older age group), CSF-diversion prior to shunt-placement and previous neurosurgery (among others) are associated with shorter shunt survival and shunt revisions.[34,35,36] ETV is widely regarded as a good alternative to shunting; while the overall efficacy of ETV procedures seem comparable to VP-shunting, complication rates (during and after ETV-interventions) remain significantly lower.[3,11,15,48] However, this method requires experience and skill, optimal pre-operative planning, and suitable postoperative care due to complex anatomical conditions.[3,15,48] Reported complications include neurovascular injuries (including basilar artery rupture), endocrinologic abnormalities, electrolyte imbalances, CSF-leakage, and deep infection.[15] Although most complications are transient and relatively infrequent, the risk of severe and permanent ETV-induced morbidity is a variable that cannot be disregarded.[3,15,48]
Other complications associated with microsurgery
As described earlier, different groups have reported on the risk of leptomeningeal metastases/disease (LMD) after microsurgery.[43,45] Sucki et al. studied 379 patients with posterior fossa metastases having undergone resection (n = 260) or radiosurgery (n = 119); LMD was reported in 33 patients (26 in the resection group and 7 in the radiosurgery group). The authors concluded that there is an increased risk for LMD after piecemeal resection of posterior fossa metastasis but not with en-bloc removal.[43] Although most commonly seen in children after posterior fossa tumor resection, sporadic cases of cerebellar mutism in adults have also been reported; this syndrome include complete absence of speech without impaired consciousness, other cranial nerve deficits or long tract signs. Mean time of onset is approximately 48 hours and average duration is 7–8 weeks; recovery is usually spontaneous but long-term language/speech dysfunctions remains a comparable issue in children and adults alike.[38,49]
3R: A treatment option when microsurgery is deemed not indicated
From our viewpoint, microsurgery and subsequent radiation delivery to the cavity (with or without CSF-diversion procedures) remain the fundamental management of posterior fossa metastases. But as pointed out earlier, treatment alternatives need to be made available and employed in selected cases where microsurgery is deemed not be possible. For the latter cases, a more aggressive and custom-made radiotherapeutic approach may be considered. WBRT, once the frontline management of choice, is increasingly being avoided due to the risk of long-term complications including cognitive impairment[13,19,30,31,42] and its questionable efficacy in the face of more radio-resistant tumors. In terms of tumor control and radiation-induced local toxicity, SF-GKRS has proven effective in the treatment of small metastatic lesions[27,30,42] including those with radioresistant histologies.[8,33] However, in the case of larger unresectable metastases outside brainstem boundaries (generally >8–10 cm3) and dose-volume dependent intrinsic brainstem metastases, the effectiveness and safety of SF-GKRS remains a topic of vivid discussion, particularly in the face of ARE development.[20,21,25,27] Linear Accelerator (LINAC) and Gamma Knife-based hypofractionated regimens have proven more effective in balancing the need of local tumor control and limited/acceptable toxicity.[6,14,22,25,29,39,40,42,50]
The authors of this article have previously reported the use of gamma knife-based adaptive hypofractionation in the acute management of critically located, large, aggressive metastases,[40,41] a procedure known at our institution as Rapid Rescue Radiosurgery (3R). In general, 3R is a minimally invasive, MRI-guided procedure aiming to manage life-threatening, neurologically impairing neoplasms by achieving next to comparable debulking/decompressing surgery results within the course of treatment (days) and immediate follow-up time (weeks).[40,41] Within the confines of the posterior fossa, 3R covers two surgical fields: (i) brainstem radiosurgery (including both intrinsic and extrinsic lesions) and (ii) metastases outside brainstem boundaries, including metastatic meningeal lesions (nonbrainstem radiosurgery). The treatment's key element relies on dynamically adapting peripheral prescription doses and tumor bed dose distributions to ongoing tumor volume changes during the week of treatment. The latter allows the radiosurgeon to increase dose heterogeneity and achieve dose escalation within tumor bed limits with almost surgical precision while sparing circumscribing normal brain tissue.[40,41]
As described elsewhere, linear quadratic (LQ)-model based calculations are of utmost importance to determine tolerable biologically effective dose distributions in normal tissues in relation to necessary ablative peripheral dose prescriptions.[9,22,40,41] Based on our experience and previous reports,[11,26,33,40,41] tumor histopathology and corresponding radiosensitivity as well as RPA surrogate factors (particularly KPS and control over primary tumor) might have played a role in the outcome of 3R in terms of tumor volume reduction, subsequent increase in V4 volume, and hydrocephalus prevention. Finally, development of ARE occurred in both cases with intrinsic brainstem lesion (cases 1 and 3) within 6 months of treatment. The latter was expected, particularly in case 1 due to prior WBRT and subsequent radiation delivery constraints. Interestingly, both patients were clinically well despite the development of perilesional edema. For case 1, the edema yielded without cortisone treatment. For case 3, the edema remained but without major impact on her neurologic function. Yet, the development of perilesional edema despite dose hypofractionation might indicate that single fraction GKRS would not have been the best or safest option in terms of radiation-induced toxicity. The results of cases 1 and 3 concur with our experience regarding other intrinsic brainstem cases treated with 3R and will be the subject of a subsequent paper.
CONCLUSION
In our case series, 3R procedures proved effective in achieving rapid tumor volume reduction, long-lasting V4 decompression, and subsequent avoidance of hydrocephalus as well as preservation of neurological function. As expected, development of ARE after 3R intervention was seen with brainstem lesions but was well-tolerated as it did not cause serious neurological impairment. The tumor's histopathology and radiosensitivity might have played a determinant role in the outcome of 3R; yet, imaging dynamics during the week of treatment and throughout follow-up suggest that the concept of adapting marginal doses and corresponding dose distributions to tumor volumetric kinetics at each GKRS remains a crucial factor to achieve prompt tumor regression and subsequent V4-decompression. Algorithmically, we believe this procedure has the potential to become a complementary surgical tool in the acute/subacute decompressive management of unresectable/life-threatening V4 compressive brain metastases. Further prospective studies with focus on the role of 3R in the management of V4 compression due to brain metastases are warranted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
G. Sinclair, Email: georges.sinclair@sll.se.
H. Benmakhlouf, Email: hamza.benmakhlouf@sll.se.
H. Martin, Email: heather.martin@sll.se.
M. Brigui, Email: marina.brigui@gosh.nhs.uk.
M. Maeurer, Email: markus.maeurer@gmail.com.
E. Dodoo, Email: mailernest@me.com.
REFERENCES
- 1.Al-Omair A, Soliman H, Xu W, Karotki A, Mainprize T, Phan N, et al. Hypofractionated stereotactic radiotherapy in five daily fractions for post-operative surgical cavities in brain metastases patients with and without prior whole brain radiation. Technol Cancer Res Treat. 2013;12:493–9. doi: 10.7785/tcrt.2012.500336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altaf I, Vohra AH, Shams S. Management of Cerebrospinal Fluid Leak following Posterior Cranial Fossa Surgery. Pak J Med Sci. 2016;32:1439–43. doi: 10.12669/pjms.326.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouras T, Sgouros S. Complications of endoscopic third ventriculostomy: A systematic review. Acta Neurochir Suppl. 2012;113:149–53. doi: 10.1007/978-3-7091-0923-6_30. [DOI] [PubMed] [Google Scholar]
- 4.Chaichana KL, Rao K, Gadkaree S, Dangelmajer S, Bettegowda C, Rigamonti D, et al. Factors associated with survival and recurrence for patients undergoing surgery of cerebellar metastases. Neurol Res. 2014;36:13–25. doi: 10.1179/1743132813Y.0000000260. [DOI] [PubMed] [Google Scholar]
- 5.Dong P, Pérez-Andújar A, Pinnaduwage D. Dosimetric characterization of hypofractionated Gamma Knife radiosurgery of large or complex brain tumors versus linear accelerator–based treatments. J Neurosurg. 2016;125(Suppl 1):97–103. doi: 10.3171/2016.7.GKS16881. [DOI] [PubMed] [Google Scholar]
- 6.Eaton BR, Gebhardt B, Prabhu R, Shu HK, Curran WJ, Jr, Crocker I. Hypofractionated radiosurgery for intact or resected brain metastases: Defining the optimal dose and fractionation. Radiat Oncol. 2013;8:135. doi: 10.1186/1748-717X-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson SD, Levine NB, Suki D, Tsung AJ, Lang FF, Sawaya R, et al. The surgical treatment of tumors of the fourth ventricle: A single-institution experience. J Neurosurg. 2017 doi: 10.3171/2016.11.JNS161167. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Ferrel EA, Roehrig AT, Lamoreaux WT, Mackay AR, Fairbanks RK, Call JA, et al. Prolonged Survival following Repetitive Stereotactic Radiosurgery in a Patient with Intracranial Metastatic Renal Cell Carcinoma. Case Rep Neurol Med. 2015;2015:872915. doi: 10.1155/2015/872915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–68. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghia A, Tomé WA, Thomas S, Cannon G, Khuntia D, Kuo JS, et al. Distribution of brain metastases in relation to the hippocampus: Implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68:971–7. doi: 10.1016/j.ijrobp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Gonda DD, Kim TE, Warnke PC, Kasper EM, Carter BS, Chen CC. Ventriculoperitoneal shunting versus endoscopic third ventriculostomy in the treatment of patients with hydrocephalus related to metastasis. Surg Neurol Int. 2012;3:97. doi: 10.4103/2152-7806.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadanny A, Rozovski U, Nossek E, Shapira Y, Strauss I, Kanner AA, et al. Craniectomy Versus Craniotomy for Posterior Fossa Metastases: Complication Profile. World Neurosurg. 2016;89:193–8. doi: 10.1016/j.wneu.2016.01.076. [DOI] [PubMed] [Google Scholar]
- 13.Iorio-Morin C, Masson-Cote L, Ezahr Y, Blanchard J, Ebacher A, Mathieu D. Early gamma Knife stereotactic radiosurgery to the tumour bed of resected brain metastasis for improved local control. J Neurosurg. 2014;121(Suppl):69–74. doi: 10.3171/2014.7.GKS141488. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara T, Yamada K, Harada A, Isogai K, Tonosaki Y, Demizu Y, et al. Hypofractionated stereotactic radiotherapy for brain metastases from lung cancer: Evaluation of indications and predictors of local control. Strahlenther Onkol. 2016;192:386–93. doi: 10.1007/s00066-016-0963-2. [DOI] [PubMed] [Google Scholar]
- 15.Jung TY, Chong S, Kim IY, Lee JY, Phi JH, Kim SK, et al. Prevention of Complications in Endoscopic Third Ventriculostomy. J Korean Neurosurg Soc. 2017;60:282–8. doi: 10.3340/jkns.2017.0101.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanner AA, Suh JH, Siomin VE, Lee SY, Barnett GH, Vogelbaum MA. Posterior fossa metastases: Aggressive treatment improves survival. Stereotact Funct Neurosurg. 2003;81:18–23. doi: 10.1159/000075099. [DOI] [PubMed] [Google Scholar]
- 17.Khan F, Rehman A, Shamim MS, Bari ME. Factors affecting ventriculoperitoneal shunt survival in adult patients. Surg Neurol Int. 2015;6:25. doi: 10.4103/2152-7806.151388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim IY, Jung S, Moon KS, Jung TY, Kang SS. Neuronavigation-guided endoscopic surgery for pineal tumors with hydrocephalus. Minim Invasive Neurosurg. 2004;47:365–8. doi: 10.1055/s-2004-830150. [DOI] [PubMed] [Google Scholar]
- 19.Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014;190:521–32. doi: 10.1007/s00066-014-0648-7. [DOI] [PubMed] [Google Scholar]
- 20.Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. 2016;16:903–14. doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 21.Lippitz B, Lindquist C, Paddick I, Peterson D, O'Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: The current evidence. Cancer Treat Rev. 2014;40:48–59. doi: 10.1016/j.ctrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Martens B, Janssen S, Werner M, Fruhauf J, Christiansen H, Bremer M, et al. Hypofractionated stereotactic radiotherapy of limited brain metastases: A single-centre individualized treatment approach. BMC Cancer. 2012;12:497. doi: 10.1186/1471-2407-12-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maselli G1, De Paulis D, Ricci A, Galzio RJ. Posterior cranial fossa tumors: Results and prognostic factors in a consecutive series of 14 operated patients by occipital transtentorial approach. Surg Neurol Int. 2012;3:85. doi: 10.4103/2152-7806.99911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Métellus P, Faillot T, Guyotat J, Farah W, Bauchet L, Mornex F, et al. Place of surgery in brain metastases. Bull Cancer. 2013;100:51–6. doi: 10.1684/bdc.2012.1680. [DOI] [PubMed] [Google Scholar]
- 25.Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-Fraction Versus Multifraction (3×9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J Radiat Oncol Biol Phys. 2016;95:1142–8. doi: 10.1016/j.ijrobp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadi AM, Recinos PF, Barnett GH, Weil RJ, Vogelbaum MA, Chao ST, et al. Role of Gamma Knife surgery in patients with 5 or more brain metastases. J Neurosurg. 2012;117(Suppl):5–12. doi: 10.3171/2012.8.GKS12983. [DOI] [PubMed] [Google Scholar]
- 27.Mohammadi AM, Schroeder JL, Angelov L, Chao ST, Murphy ES, Yu JS, et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg. 2016:1–9. doi: 10.3171/2016.3.JNS153014. [DOI] [PubMed] [Google Scholar]
- 28.Moshel YA, Parker EC, Kelly PJ. Occipital transtentorial approach to the precentral cerebellar fissure and posterior incisural space. Neurosurgery. 2009;65:554–64. doi: 10.1227/01.NEU.0000350898.68212.AB. discussion 564. [DOI] [PubMed] [Google Scholar]
- 29.Navarria P, Pessina F, Cozzi L, Ascolese AM, De Rose F, Fogliata A, et al. Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol. 2016;11:76. doi: 10.1186/s13014-016-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niranjan A, Lunsford LD. Gamma Knife Radiosurgery for 5 to 10 Brain Metastases: A Good Option for Upfront Treatment. Oncology (Williston Park) 2016;30:314–5. 317. [PubMed] [Google Scholar]
- 31.Ojerholm E, Lee JY, Thawani JP, Miller D, O'Rourke DM, Dorsey JF, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121(Suppl):75–83. doi: 10.3171/2014.6.GKS14708. [DOI] [PubMed] [Google Scholar]
- 32.Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol. 2014;4:248. doi: 10.3389/fonc.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell JW, Chung CT, Shah HR, Canute GW, Hodge CJ, Bassano DA, et al. Gamma Knife surgery in the management of radioresistant brain metastases in high-risk patients with melanoma, renal cell carcinoma, and sarcoma. J Neurosurg. 2008;109(Suppl):122–8. doi: 10.3171/JNS/2008/109/12/S19. [DOI] [PubMed] [Google Scholar]
- 34.Reddy GK, Bollam P, Caldito G. Ventriculoperitoneal shunt surgery and the risk of shunt infection in patients with hydrocephalus: Long-term single institution experience. World Neurosurg. 2012;78:155–63. doi: 10.1016/j.wneu.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Reddy GK, Bollam P, Caldito G, Willis B, Guthikonda B, Nanda A. Ventriculoperitoneal shunt complications in hydrocephalus patients with intracranial tumors: An analysis of relevant risk factors. J Neurooncol. 2011;103:333–42. doi: 10.1007/s11060-010-0393-4. [DOI] [PubMed] [Google Scholar]
- 36.Reddy GK, Bollam P, Caldito G, Guthikonda B, Nanda A. Ventriculoperitoneal shunt surgery outcome in adult transition patients with pediatric-onset hydrocephalus. Neurosurgery. 2012;70:380. doi: 10.1227/NEU.0b013e318231d551. [DOI] [PubMed] [Google Scholar]
- 37.Reddy GK, Shi R, Nanda A, Guthikonda B. Obstructive hydrocephalus in adult patients: the Louisiana State University Health Sciences Center-Shreveport experience with ventriculoperitoneal shunts. World Neurosurg. 2011;76:176–82. doi: 10.1016/j.wneu.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 38.Sherman JH1, Sheehan JP, Elias WJ, Jane JA., Sr Cerebellar mutism in adults after posterior fossa surgery: A report of 2 cases. Surg Neurol. 2005;63:476–9. doi: 10.1016/j.surneu.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Shuryak I, Carlson DJ, Brown JM, Brenner DJ. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumour control data from 2965 patients. Radiother Oncol. 2015;115:327–34. doi: 10.1016/j.radonc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Sinclair G, Bartek J, Jr, Martin H, Barsoum P, Dodoo E. Adaptive hypofractionated gamma knife radiosurgery for a large brainstem metastasis. Surg Neurol Int. 2016;7(Suppl 4):S130–8. doi: 10.4103/2152-7806.176138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinclair G, Martin H, Fagerlund M, Samadi A, Benmakhlouf H, Doodo E. Adaptive hypofractionated gamma knife radiosurgery in the acute management of large thymic carcinoma brain metastases. Surg Neurol Int. 2017;8:95. doi: 10.4103/sni.sni_391_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7:12318–30. doi: 10.18632/oncotarget.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108:248–57. doi: 10.3171/JNS/2008/108/2/0248. [DOI] [PubMed] [Google Scholar]
- 44.Sunderland GJ, Jenkinson MD, Zakaria R. Surgical management of posterior fossa metastases J Neurooncol. 2016;130:535–42. doi: 10.1007/s11060-016-2254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Ree TC, Dippel DW, Avezaat CJ, Sillevis Smitt PA, Vecht CJ, van den Bent MJ. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999;66:225–7. doi: 10.1136/jnnp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler EA, Birk H, Safaee M, Yue JK, Burke JF, Viner JA, et al. Surgical resection of fourth ventricular ependymomas: Case series and technical nuances. J Neurooncol. 2016;130:341–9. doi: 10.1007/s11060-016-2198-6. [DOI] [PubMed] [Google Scholar]
- 47.Wu SG, Rao MY, Zhou J, Lin Q, Wang ZJ, Chen YX, et al. Distribution of metastatic disease in the brain in relation to the hippocampus: A retrospective single-center analysis of 6064 metastases in 632 patients. Oncotarget. 2015;6:44030–6. doi: 10.18632/oncotarget.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav YR, Parihar VS, Ratre S, Kher Y. Avoiding Complications in Endoscopic Third Ventriculostomy. J Neurol Surg. 2015;76:483–94. doi: 10.1055/s-0035-1551828. [DOI] [PubMed] [Google Scholar]
- 49.Yildiz O, Kabatas S, Yilmaz C, Altinors N, Agaoglu B. Cerebellar mutism syndrome and its relation to cerebellar cognitive and affective function: Review of the literature. Ann Indian Acad Neurol. 2010;13:23–7. doi: 10.4103/0972-2327.61272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zada G, Yu C, Pagnini PG, Khalessi AA, Zelman V, Apuzzo ML. Early decreased tumour volume following fractionated Gamma Knife Radiosurgery for metastatic melanoma and the role of “adaptive radiosurgery”: Case report. Neurosurgery. 2010;67:E512–3. doi: 10.1227/01.NEU.0000371984.18490.55. [DOI] [PubMed] [Google Scholar]


