Abstract
Background:
Guillain-Barre syndrome (GBS) is an immune-mediated peripheral neuropathy. We compared clinical, laboratory characteristics, and disease course of GBS subtypes in a large group of Iranian patients in Isfahan.
Materials and Methods:
We collected data from patients who were admitted to Alzahra referral university Hospital, Isfahan, Iran with a diagnosis of GBS. In this population-based cross-sectional research, characteristic of 388 cases with GBS between 2010 and 2015 were studied.
Results:
The current study recruited 388 patients with GBS including 241 males (62.1%) and 147 females (37.9%) with a mean age of 42.78 ± 21.34. Patients with polyradiculopathy had the highest mean age of 55.12 ± 20.59 years, whereas the least age was seen in acute motor axonal neuropathy (AMAN) with the mean of 36.30 ± 18.71 years. The frequency of GBS witnessed the highest frequency in spring with 113 cases (29.1%) and winter with 101 cases (26%). Patients' electrodiagnostic findings indicated that the highest frequency pertained to AMSAN with 93 cases (24%), whereas the least frequent diagnosis was acute Polyradiculopathy with 8 cases (2.1%). Most of the patients did not have any infections (53.6%) and among patients with infections, AMSAN had the highest frequency (22.9%) and finally, patients with AMSAN and AMAN had a higher length of stay.
Conclusion:
The study demonstrated incidence, sex distribution, preceding infection, and surgery similar to previous studies. However, our data differs from a study in Tehran that showed acute inflammatory demyelinating polyradiculoneuropathy is more prevalent than other types and we found a seasonal preponderance in cold months, particularly in axonal types.
Keywords: Electrodiagnosis, epidemiology, Guillain-Barre syndrome, subtypes
Introduction
Guillain-Barre syndrome (GBS) is a peripheral neuropathy with acute onset and characterized by rapidly progressive motor weakness.[1,2,3]
The disease triggered by a preceding infection in two-third of cases and thought to be autoimmune mediated.[1,3] GBS occurs worldwide with an annual incidence of 0.34–1.34 cases per 0.16-4/100,000 persons aged 18 years or less [4,5,6] and 0.16 4/100,000/year in individuals of all ages.[1,3] The incidence increases by approximately 20% with every 10-year increase in an age beyond the first decade of life.[7,8]
GBS can be divided into at least 4 main subtypes of disease: acute inflammatory demyelinating polyradiculoneuropathy (AIDP), the axonal subtypes, for example, acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN) and Miller Fisher syndrome that are different among different geographical states.[9,10]
Insight into the incidence of the disease is important for the identification of patient characteristics such as age, geographic location and any changes in incidence following changes in environmental factors.
This study was conducted to determine the frequency of GBS subtypes in a large group of Iranian patients in Isfahan, and we wanted to compare clinical, laboratory characteristics, disease course, and seasonal frequency among the different subtypes.
Materials and Methods
We collected data from patients who were admitted to Alzahra referral university Hospital, Isfahan, Iran with a diagnosis of GBS.
In this population-based cross-sectional research medical history, clinical features, electrodiagnosis, disability scale, and prognosis of 388 cases with GBS between 2010 and 2015 were studied.
Diagnosis of GBS was made based on diagnostic criteria from the National Institute of Neurological Disorders and Stroke FROM 1990.[1]
Patients were excluded when the diagnosis of chronic inflammatory demyelinating polyneuropathy was suggested during follow-up, and the patients who have any underlying disease such as diabetes, neoplasia, hypothyroidism, renal failure, vasculitis, or history of intoxication were excluded from the study.
Information on clinical presentation (upper and lower weakness), sensory loss, dysarthria, dysphagia, facial paresis, history of infection (common cold, diarrhea), history of surgery, seasonal onset, electrodiagnosis, and cerebrospinal fluid were extracted. Albumino-cytological dissociation that find in GBS patients was defined as elevated CSF proteins with a total cell count of fewer than 10/cubic millimeter.[11] In addition, the period between beginning of the symptom until admission, period of time until intubation and recurrence of GBS were investigated. Besides, the need of intensive care unit admission and type of treatment were evaluated.
Severity of weakness was graded using the Medical Research Council Scale where 5: normal, 4: opposes resistance, 3: opposes gravity, 2: moves joint, 1: flicker and 0: absent.[12,13]
Clinical course was described by using the GBS disabling scale adapted from Hughes was graded as 0: healthy state, 1: minor symptoms and capable of running, 2: able to walk 10 m or move without assistance but able to run, 3: able to walk 10 m across an open space with help, 4: bedridden or chairbound, 5: respiratory assisted ventilation for at least part of the day, 6: dead.[14]
After that data were analyzed using the Statistical Package for the Social Sciences version 15.(SPSS, Chicago, Il, USA) Association between qualitative and quantitative variables was tested using the likelihood Chi-squared test and t-test, respectively. P < 0.05 was considered statistically significant. This study was approved by the Ethics Committee of the Esfahan University of Medical Sciences.
Results
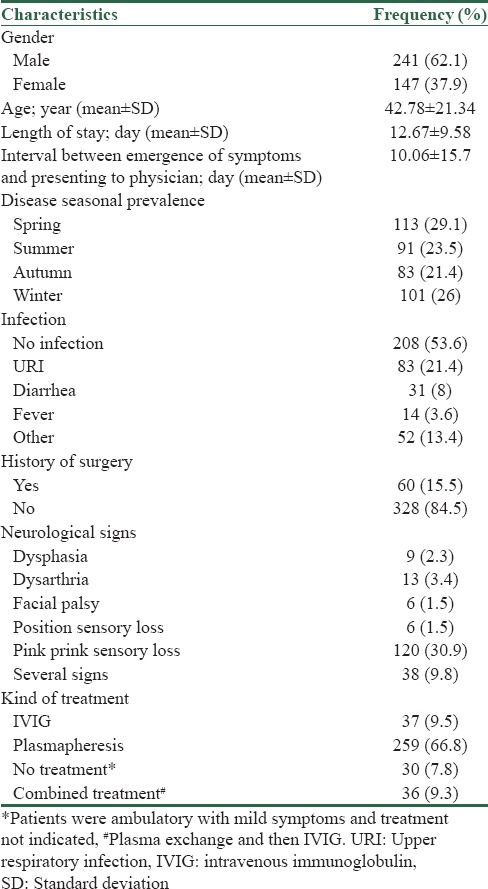
The current study recruited 388 patients with GBS including 241 males (62.1%) and 147 females (37.9%) with a mean age of 42.78 ± 21.34. The frequency of GBS witnessed the highest frequency in spring with 113 cases (29.1%) and winter with 101 cases (26%), whereas it had the lowest frequency in fall with 83 cases (21.4%). Two hundred and eight patients did not have any preceding infection (53.6%), but 83 people (21.4%) suffered upper respiratory tract infection, 31 people (8%) diarrhea and vomiting, 14 people (3.6%) fever and 52 people (13.4%) other infections [Table 1].
Table 1.
Frequency distribution of clinical and demographic characteristics of the study population
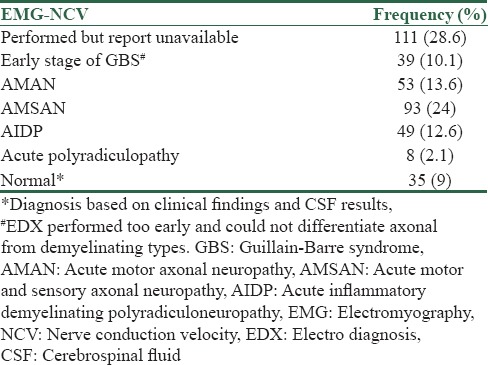
The electrodiagnosis (EDX) was done for all of the patients and diagnosis of GBS has been confirmed by EDX and clinical findings, but we had no access to details of EDX in the 28.6% of patients due to retrospective nature of our study. Patients' EDX findings indicated that 35 patients (9%) had normal EDX due to doing it early in the course of disease, and the highest frequency pertained to AMSAN with 93 cases (24%), whereas the least frequent diagnosis was acute polyradiculopathy with 8 cases (2.1%) [Table 2]. In patient with normal EXD, diagnosis confirmed by clinical findings and results of CSF analysis.
Table 2.
Frequency distribution of electromyography-nerve conduction study results of the study population
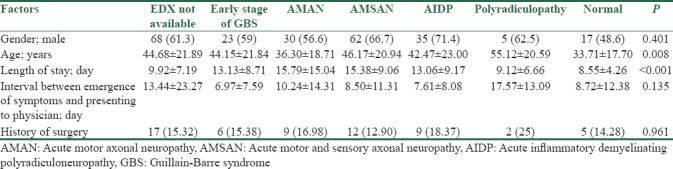
Moreover, electro diagnostic (EDX) findings revealed significant differences by factors such as age and length of stay (P < 0.05): patients with polyradiculopathy had the highest mean age of 55.12 ± 20.59 years, whereas the least age was seen in AMAN patients with the mean of 36.30 ± 18.71 years. On the other hand, patients with AMSAN and AMAN had a higher length of stay. However, gender, history of surgery, and the interval between the emergence of symptoms and presenting to a physician did not have significant differences over EDX findings (P > 0.05) [Table 3].
Table 3.
Patient's electrodiagnostic results by clinical and demographic factors
An investigation into the seasonal prevalence of this illness and electromyography-nerve conduction study (EMG-NCV) findings illustrated that frequency distribution of EMG-NCV results had significant seasonal changes (P < 0.05), such that in winter and fall, AMSAN diagnosis had the highest frequency of over 20%, Fall and summer witnessed the highest frequency of AIDP (14.4%) [Figure 1].
Figure 1.
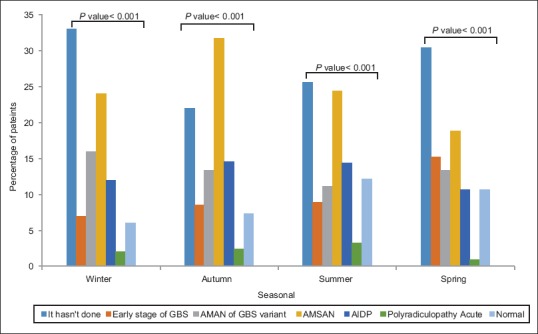
Comparison of electromyography-nerve conduction studyresults with seasonal frequency percentage
Finally, most patients did not have any preceding infections, and among patients with infections, AMSAN had the highest frequency (22.9%). Furthermore, preceding diarrhea was found to have the highest frequency in AMAN and AMSAN with 25.8% and 35.5%, respectively. Statistically, fever or other infections were shown to have no impact on EMG-NCV results (P > 0.05).
Discussion
Incidence
Annual incidence of GBS has varied from 0.34 to 1.34 cases/100/000 persons aged 18 years or less.[4,5,6] the incidence is lower in children than in adults. Males are affected 1.5 times more often than females in all age groups.[9,15,16]
In our study, we have evaluated 388 patients: 62/1% of male (241 patients) and 37/9% female (147 patients) with mean age of 42/78 ± 21/34 years. Men were more affected than women, but mean age of our patients was not very high. In North America and Europe, the incidence of GBS increases with age.[9]
Patients with polyradiculopathy have more mean age than other forms and AMAN types have less mean age than other types as well.
One proposed mechanism for GBS is that infection evokes an immune response which cross-react with peripheral nerve (molecular mimicry). This process can be directed against myelin or axon. Campylobacter infection is the most commonly identified microorganism in GBS patients in up to 30% of cases.[17] Other infections include cytomegalovirus, Epstein-Barr virus, mycoplasma pneumonia, Influenza-like illness, HIV, and Zika virus [18,19,20,21] Up to two-thirds of patients report an antecedent infections before the onset of neurological symptoms.[1,3] Respiratory infections are most frequently reported, followed by gastrointestinal.[22] Moreover, in small group of patients GBS develops after immunization, surgery, trauma, or bone-marrow transplantation.
In our study, 208 patients did not have any preceding infection (53.6%), 83 patients (21.4%) upper respiratory infection (URI), 31 patients (8%) had gastroenteritis, 14 patients (3.6%), had fever and 52 patients (13.4%) had another infections. Lower incidence of infection in our patients could be due to missing data in patients' dossier.
Like other articles, in our study, AMSAN type of GBS had more links to infection than other types. For example, URI had more correlation with AMSAN (22.9%) and less correlation with polyradiculopathy (1.2%). AMAN and AMSAN types of GBS had more correlation with gastroenteritis 35.5% and 25.8%, respectively.
Most cases of GBS are sporadic, but in north China fallowing campylobacter jejuni infection [17] clustering of GBS occurred in summer. On the other hand, in Hung Kong, chines patients did not show any seasonal preponderance.[23]
In a study from Saudi Arabia, 40% of the cases occurred in the cold seasons with the highest peak in February.[24]
In our study, interestingly, we had clustering of patients in winter and autumn with AMSAN type of GBS (more than 20%) that could be related to the high frequency of URI during these seasons. AIDP variant of GBS was more frequent in autumn and summer (14.6%–14.4%, respectively).
GBS is a heterogeneous syndrome with several variant forms.[4,25] In Europe and North America, AIDP is more common [26] in the Middle East, demyelinating pattern has been reported in Kuwait,[27] Pakistan,[28] and India.[29]
In our study, AMSAN variant of GBS is more frequent than other types (93 patients, 24%). Hence, frequency of axonal GBS in our province is similar to Japan and Mexico and this finding is in contrast to report from the capital of Tehran, but of course, axonal type is not uncommon in GBS patients in Tehran.[30] Preceding infections may have a role in this distribution.[31] A comparison of clinical presentation between the subtypes of GBS shown not any difference [16,32,33] that is similar to our study.
Like other study,[30] the mean duration of hospitalization was more is AMSAN and AMAN types of GBS patients in our study and this finding showed that AMAN and AMSAN are associated with worse prognosis.
The association with antecedent URI and gastroenteritis before onset of GBS has been known for over 100 years and potential links have been reported between Campylobacter Jejuni and axonal forms,[34] so determining this subtypes has important role for predicting prognosis and also determination of infection and seasonal distribution help us to predict the types of GBS.
Conclusion
The study demonstrated frequency, sex distribution, preceding infection, and surgery similar to other previous studies. However, our data differs from the study in Tehran that showed AIDP is more prevalence than other types. Ages of patients in our study are less than other studies and we found a seasonal preponderance in cold months, particularly in axonal types.
In future studies, we could evaluate Gangliosid antibodies for prediction of disease prognosis and differentiation between GBS variants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hughes RA, Rees JH. Clinical and epidemiologic features of Guillain-Barré syndrome. J Infect Dis. 1997;176(Suppl 2):S92–8. doi: 10.1086/513793. [DOI] [PubMed] [Google Scholar]
- 2.Asbury AK, Arnason BG, Karp HR, McFarlin DE. Criteria for diagnosis of Guillain Barre syndrome. Ann Neurol. 1978;3:565–6. doi: 10.1002/ana.410030628. [DOI] [PubMed] [Google Scholar]
- 3.Govoni V, Granieri E. Epidemiology of the Guillain-Barre syndrome. Curr Opin Neurol. 2001;14:605–13. doi: 10.1097/00019052-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 5.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: A systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–33. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris AM, Elliott EJ, D'Souza RM, Antony J, Kennett M, Longbottom H, et al. Acute flaccid paralysis in Australian children. J Paediatr Child Health. 2003;39:22–6. doi: 10.1046/j.1440-1754.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 7.Carroll JE, Jedziniak M, Guggenheim MA. Guillain-Barré syndrome. Another cause of the “floppy infant”. Am J Dis Child. 1977;131:699–700. doi: 10.1001/archpedi.1977.02120190093022. [DOI] [PubMed] [Google Scholar]
- 8.Buchwald B, de Baets M, Luijckx GJ, Toyka KV. Neonatal Guillain-Barré syndrome: Blocking antibodies transmitted from mother to child. Neurology. 1999;53:1246–53. doi: 10.1212/wnl.53.6.1246. [DOI] [PubMed] [Google Scholar]
- 9.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–66. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 10.Burns TM. Guillain-Barré syndrome. Semin Neurol. 2008;28:152–67. doi: 10.1055/s-2008-1062261. [DOI] [PubMed] [Google Scholar]
- 11.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27:S21–4. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 12.Kleyweg RP, Van der Meche FG. Treatment related fluctuations in Guillain Barré syndrome after high dose immunoglobulins or plasma exchange. J Neurol Neurosurg Psychiatry. 1991;54:957–60. doi: 10.1136/jnnp.54.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT. Evidence-based guideline: Intravenous immunoglobulin in the treatment of neuromuscular disorders: Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2012;78:1009–15. doi: 10.1212/WNL.0b013e31824de293. [DOI] [PubMed] [Google Scholar]
- 14.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC, et al. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 15.Frankle RT. Nutrition education in the medical school curriculum: A proposal for action: A curriculum design. Am J Clin Nutr. 1976;29:105–9. doi: 10.1093/ajcn/29.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Dourado ME, Félix RH, da Silva WK, Queiroz JW, Jeronimo SM. Clinical characteristics of Guillain-Barré syndrome in a tropical country: A Brazilian experience. Acta Neurol Scand. 2012;125:47–53. doi: 10.1111/j.1600-0404.2011.01503.x. [DOI] [PubMed] [Google Scholar]
- 17.Blaser MJ, Olivares A, Taylor DN, Cornblath DR, McKhann GM. Campylobacter serology in patients with Chinese paralytic syndrome. Lancet. 1991;338:308. doi: 10.1016/0140-6736(91)90447-w. [DOI] [PubMed] [Google Scholar]
- 18.Verity C, Stellitano L, Winstone AM, Andrews N, Stowe J, Miller E, et al. Guillain-Barré syndrome and H1N1 influenza vaccine in UK children. Lancet. 2011;378:1545–6. doi: 10.1016/S0140-6736(11)61665-6. [DOI] [PubMed] [Google Scholar]
- 19.Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F, et al. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: Population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956. doi: 10.1136/bmj.d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee GM, Greene SK, Weintraub ES, Baggs J, Kulldorff M, Fireman BH, et al. H1N1 and seasonal influenza vaccine safety in the vaccine safety Datalink project. Am J Prev Med. 2011;41:121–8. doi: 10.1016/j.amepre.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016;387:1531–9. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: A case-control study. doi: 10.1186/1756-0500-2-171. The epidemiology of Guillain-Barré syndrome in US military personnel: A case-control study BMC Res Notes 2009;2:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui AC, Chow KM, Tang AS, Fu M, Kay R, Wong KS, et al. Electrophysiological, clinical and epidemiological study of Guillain-Barre syndrome in Hong Kong Chinese. J Clin Neurosci. 2005;12:134–6. doi: 10.1016/j.jocn.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Bahou YG, Biary N, al Deeb S. Guillain-Barre syndrome: A series observed at Riyadh armed forces hospital January 1984 – January 1994. J Neurol. 1996;243:147–52. doi: 10.1007/BF02444006. [DOI] [PubMed] [Google Scholar]
- 25.Lin JJ, Hsia SH, Wang HS, Lyu RK, Chou ML, Hung PC, et al. Clinical variants of Guillain-Barré syndrome in children. Pediatr Neurol. 2012;47:91–6. doi: 10.1016/j.pediatrneurol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain-Barré syndrome: Clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann Neurol. 1998;44:780–8. doi: 10.1002/ana.410440512. [DOI] [PubMed] [Google Scholar]
- 27.Nagarajan V, Al-Shubaili A. Clinical and neurophysiological pattern of Guillain-Barré syndrome in Kuwait. Med Princ Pract. 2006;15:120–5. doi: 10.1159/000090916. [DOI] [PubMed] [Google Scholar]
- 28.Shafqat S, Khealani BA, Awan F, Abedin SE. Guillain-Barré syndrome in Pakistan: Similarity of demyelinating and axonal variants. Eur J Neurol. 2006;13:662–5. doi: 10.1111/j.1468-1331.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 29.Gupta D, Nair M, Baheti NN, Sarma PS, Kuruvilla A Diplomate-American Board. Electrodiagnostic and clinical aspects of Guillain-Barré syndrome: An analysis of 142 cases. J Clin Neuromuscul Dis. 2008;10:42–51. doi: 10.1097/CND.0b013e31818e9510. [DOI] [PubMed] [Google Scholar]
- 30.Yadegari S, Kazemi N, Nafissi S. Clinical and electrophysiological features of Guillain-Barré syndrome in Iran. J Clin Neurosci. 2014;21:1554–7. doi: 10.1016/j.jocn.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 31.Nagasawa K, Kuwabara S, Misawa S, Fujii K, Tanabe Y, Yuki N, et al. Electrophysiological subtypes and prognosis of childhood Guillain-Barré Syndrome in Japan. Muscle Nerve. 2006;33:766–70. doi: 10.1002/mus.20520. [DOI] [PubMed] [Google Scholar]
- 32.Ropper AH, Shahani BT. Pain in Guillain-Barré syndrome. Arch Neurol. 1984;41:511–4. doi: 10.1001/archneur.1984.04050170057018. [DOI] [PubMed] [Google Scholar]
- 33.Hao Q, Saida T, Yoshino H, Kuroki S, Nukina M, Saida K, et al. Anti-galNAc-GD1a antibody-associated Guillain-Barré syndrome with a predominantly distal weakness without cranial nerve impairment and sensory disturbance. Ann Neurol. 1999;45:758–68. [PubMed] [Google Scholar]
- 34.Nachamkin I, Allos BM, Ho T. Campylobacter species and Guillain-Barré syndrome. Clin Microbiol Rev. 1998;11:555–67. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]


