Abstract
Early-life adversity increases the risk for emotional disorders such as depression and schizophrenia. Anhedonia, thought to be a core feature of these disorders, is provoked by our naturalistic rodent model of childhood adversity (i.e., rearing pups for one week in cages with limited bedding and nesting, LBN). Drug use and addiction are highly comorbid with psychiatric disorders featuring anhedonia, yet effects of LBN on drug-seeking behavior and the reward and stress-related circuits that underlie it remain unknown. Here we examined the effects of LBN on cocaine intake and seeking, using a battery of behavioral tests measuring distinct aspects of cocaine reward, and for comparison, chocolate intake. We also examined activation of neurons within the pleasure/reward and stress circuits following cocaine in LBN and control rats. Early-life adversity reduced spontaneous intake of palatable chocolate, extending prior reports of sucrose and social-play anhedonia. In a within-session cocaine behavioral economic test, LBN rats self-administered lower dosages of cocaine under low-effort conditions, consistent with a reduced hedonic set-point for cocaine, and potentially anhedonia. In contrast, cocaine demand elasticity was not consistently affected, indicating no major changes in motivation to maintain preferred cocaine blood levels. These changes were selective, as LBN did not cause an overt anxiety-like phenotype, nor did it affect sensitivity to self-administered cocaine dose, responding for cocaine under extinction conditions, cocaine- or cue-induced reinstatement of cocaine seeking, or locomotor response to acute cocaine. However, high Fos expression was seen after cocaine in both reward- and stress-related brain regions of LBN rats, including nucleus accumbens core, central amygdala, and lateral habenula. In contrast, hypothalamic orexin neuron activation after cocaine was significantly attenuated in LBN rats. Together, these findings demonstrate enduring effects of early-life adversity on both reward- and fear/anxiety-related neural circuits, as well as anhedonia-like reductions in consumption of natural and drug rewards.
Keywords: Plasticity, Circuits, Early-life stress/adversity, Limited bedding, Addiction, Anhedonia
Highlights
-
•
Adversity during a sensitive developmental period provokes persistent anhedonia.
-
•
This adversity reduces cocaine hedonic set point, but not motivation.
-
•
Cocaine-associated Fos is altered in reward- and anxiety/fear circuits.
-
•
Cocaine-dose sensitivity, reinstatement, and locomotion are unchanged.
-
•
Effects are selective, as anxiety-related behaviors were unaltered.
Nonstandard abbreviations
- ABC
avidin-biotin complex
- Α
motivation, reflecting slope of the decay in responding at higher effort requirements
- BLA
basolateral amygdala
- CeA
central amygdala
- CRH
corticotropin releasing hormone
- Ctrl
Control
- DAB
diaminobenzidine
- DMH
dorsomedial region of hypothalamus
- FR1
fixed ratio 1 schedule
- ILC
infralimbic medial prefrontal cortex
- LBN
limited bedding and nesting
- LH
lateral region of hypothalamus
- LHb
lateral subregion of habenula
- MHb
medial subregion of habenula
- mPFC
medial prefrontal cortex
- NAcC
nucleus accumbens core
- NAcSh
nucleus accumbens shell
- PD
postnatal day
- PFA
perifornical region of hypothalamus
- PLC
prelimbic medial prefrontal cortex
- Q0
hedonic set point, reflecting extrapolated value of cocaine at price 0
- RDoC
Research Domain Criterion
- SA
self-administration
1. Introduction
Early-life adversity in humans is associated with increased risk for emotional disorders such as depression and schizophrenia (Heim et al., 2008, Klengel and Binder, 2015, Pratchett and Yehuda, 2011, Sharma et al., 2016). An early or predictive sign for these disorders is anhedonia, defined as a lack of pleasure derived from otherwise enjoyable activities. Indeed, anhedonia has recently been recognized as an important Research Domain Criterion (RDoC) by the National Institute of Mental Health (Insel, 2014). In agreement with these findings in humans, we have identified anhedonia for natural rewards as a long-term consequence of early-life adversity in our naturalistic model, in which an impoverished early-life environment (limited bedding and nesting: LBN) induces aberrant maternal care (Molet et al., 2016a). LBN-induced anhedonia, evidenced by decreased preference for sucrose and reduced social play, was reversed by knockdown of corticotropin releasing hormone (CRH) in the central nucleus of the amygdala (CeA) (Bolton et al., 2018). In addition, social play induced aberrant Fos activation of CeA CRH neurons in LBN rats, and neuroimaging suggested increased connectivity between amygdala and medial prefrontal cortex (mPFC). These findings suggest that early-life adversity disrupts the normal maturation of interconnected anxiety/fear-and reward circuits. However, the extent to which early-life adversity influences reward circuitry remains poorly understood.
A potential consequence of LBN-induced changes in reward circuits is altered sensitivity to drug rewards, and risk for addiction (Enoch, 2011). The neural circuits underlying pleasure and motivation for natural and drug rewards heavily overlap (Kelley and Berridge, 2002), but animal studies using intermittent early-life stress caused by repeated maternal separation have shown complex and sometimes conflicting effects on addiction-related behaviors (Lehmann and Feldon, 2000, Moffett et al., 2007). For example, Meaney et al. (2002) showed that repeated maternal separation increases cocaine-induced locomotion, whereas O'Connor et al. (2015) showed that maternal separation decreases cocaine self-administration (SA). One possible explanation for these conflicting findings is the variability inherent to intermittent stress caused by maternal separation. Unlike human early-life adversity, maternal separation stress in rodents is intermittent and predictable and may not fully recapitulate the experiences of children receiving poor or distracted parental care (Davis et al., 2017). In contrast, the LBN paradigm provides both the chronicity and the fragmented and chaotic maternal care elements found in humans, which seem to contribute to long-lasting anhedonia-like behaviors in adult rodents, as well as humans reared under these conditions (Baram et al., 2012, Bolton et al., 2017, Davis et al., 2017, Molet et al., 2016a). However, how the early-life adversity provoked by LBN influences drug and natural reward-seeking in rodents is unknown.
Therefore, we examined effects of early-life adversity on cocaine intake and seeking, using a suite of specific behavioral tests of preferred low-effort cocaine intake, economic demand elasticity, dose-responsivity, relapse-like behavior, and locomotor activation. With this approach, we determine the specific behavioral processes affecting cocaine reward that are influenced by early-life adversity. To identify the LBN-induced neural circuit alterations underlying changes in cocaine hedonics or motivation, we examined Fos expression in reward- and anxiety/fear-related brain regions after acute cocaine. Among these were hypothalamic orexin/hypocretin neurons, which play important roles in motivational activation associated with drug- and natural-reward seeking, as well as stress and arousal (Berridge et al., 2010, Mahler et al., 2014a). These studies, focusing on addiction- and anhedonia-related changes in neural circuits caused by LBN in early life, suggest potential targets for therapeutic interventions in those who experienced a chaotic and/or traumatic early childhood.
2. Materials and methods
2.1. Animals
Four primiparous, timed-pregnant rats were obtained from Envigo (Livermore, CA) on E15, and maintained in an uncrowded, quiet animal facility room on a 12 h light/dark cycle. Parturition was checked daily, and the day of birth was considered postnatal day (PD)0. Male offspring were used in these experiments (n = 16), and equal numbers from each dam were assigned to LBN and control groups. Rats were weaned at PD21, housed in pairs until PD49+, and single-housed in a reverse light cycle thereafter. Food and water were available ad libitum throughout experiments. All procedures were approved by the University of California Irvine Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals. Experimental timeline is shown in Fig. 1.
Fig. 1.
Experimental Timeline: Timeline of experimental procedures is shown. Manipulation or test is shown at top, postnatal test day or range shown at bottom. Rats were sacrificed 120 min after 10 mg/kg cocaine on postnatal day 120.
2.2. Limited bedding and nesting (LBN) procedures
On PD2, pups from four litters were gathered, and 12 pups (8 males) were assigned at random to each dam, to prevent the potential confounding effects of genetic variables or litter size. Dams and pups assigned to the LBN group were transferred to cages fitted with a plastic-coated aluminum mesh platform sitting ∼2.5 cm above the cage floor. Bedding only sparsely covered the cage floor under the platform, and one-half of a 24.2 cm × 23.5 cm paper towel was provided for nesting material. Control group (Ctrl) dams and pups were placed in cages containing a standard amount of bedding (∼0.33 cubic feet of corn cob) without a platform, and one full paper towel. Ctrl and LBN cages remained undisturbed during PD2-9, during which maternal behaviors were monitored as previously described (Ivy et al., 2008). The LBN paradigm provokes significant chronic stress in the pups, as measured by increased corticosterone levels and adrenal hyperplasia, which return to normal by adulthood (Brunson et al., 2005). The stress likely arises because of an abnormal, fragmented pattern of maternal behaviors provoked by the limited nesting material (Molet et al., 2014, Molet et al., 2016a).
2.3. Intravenous catheter surgery
At PD48, rats were anesthetized prior to surgery using ketamine and xylazine (66/8 mg/kg, i. p.), and meloxicam for postsurgical analgesia (1 mg/kg, i. p.). Indwelling catheters were inserted into the right jugular vein, and exited 2 cm caudal to the scapulae. Beginning 7 d after surgery, catheters were flushed daily following SA training with cefazolin (10 mg/0.1 ml), then heparin lock solution (10 U/0.1 ml).
2.4. Drugs
Cocaine HCl, provided by the National Institute on Drug Abuse (Research Triangle Park, NC, USA), was dissolved in 0.9% saline. Cocaine was available for SA at 0.2 mg/infusion during SA acquisition (∼50 μg/kg in ∼250 g rats), and 0.1, 0.2, and 0.4 mg/infusion during cocaine SA dose-response testing (∼40, 80, and 160 μg/kg in ∼400 g rats). For behavioral economic tests, dosage of 2.73 mg/ml cocaine was determined by the duration of syringe pump activity after each lever press, which decreased in succeeding 10-min bins. Diazepam was purchased from Hospira (Lake Forest, IL).
2.5. Behavioral tests
2.5.1. Chocolate candy intake
Ad libitum-fed rats were habituated to M&M's brand chocolate candy pieces (2/rat) in their home cage on the night prior to a 1-hr intake test, also conducted in the home cage. Pre-weighed M&Ms were placed in the cage, and intake was measured by weight 1 h later.
2.5.2. Acquisition of cocaine self-administration
SA training and testing took place in Med Associates operant chambers within sound-attenuating boxes, each equipped with two retractable levers with white lights above them, a white house light, and a tone generator. Intravenous cocaine was administered via a pump located outside the sound-attenuating box. For the first five days of training, rats received daily 2-h SA sessions, when pressing on the available lever yielded a 3.6-sec cocaine infusion (0.2 mg/50 μL infusion; ∼50 μg/kg), and concurrent 2.9-kHz tone and lever light illumination (fixed ratio 1 schedule: FR1). A 20-sec timeout period (signaled by turning off the house light) followed each infusion/cue presentation, during which additional lever presses were not reinforced with cocaine but were recorded. On the 6th SA day, a second lever (inactive lever) was introduced, but pressing it had no consequences. Training continued for an additional 5 + days with both levers present.
2.5.3. Within-session behavioral economic thresholding procedure
The day after completion of FR1 training, rats were trained on a previously-described within-session economic threshold procedure (Bentzley et al., 2013, Oleson and Roberts, 2009). On each training day, presses on the active lever delivered cocaine + cue presentations as on FR1 training, except the duration of each cue/infusion was decreased every 10 min across the 110-min session (383.5, 215.6, 121.3, 68.2, 38.3, 21.6, 12.1, 6.8, 3.8, 2.2, and 1.2 μg/infusion, ∼320 g rats). In this task, rats can readily achieve their preferred blood levels of cocaine during the initial loading bin, but as the session progresses, increasing effort is required to maintain these blood levels, due to decreasing cocaine dose available upon each press (Oleson and Roberts, 2009). Consumption data was then modeled with an exponential demand equation as previously described (Bentzley et al., 2013), yielding variables corresponding to hedonic set point (Q0, reflecting extrapolated value of cocaine at price 0), and motivation (α, reflecting demand elasticity of cocaine). Rats were run daily on threshold procedure for 2 weeks, and behavior derived from the average of the last 7 days (after habituating to the protocol) was analyzed.
2.5.4. Extinction and reinstatement procedures
Following behavioral economic thresholding, rats received 10 consecutive extinction training days. On extinction, both levers were present, and presses on both were recorded, but did not deliver cocaine or cues. Subsequently, a single cue-induced reinstatement test was conducted, on which one cocaine cue (but no cocaine) was delivered 10 s after the start of the session, then presses on the active lever yielded additional cue presentations as in SA training, also without cocaine. Following cue-induced reinstatement, rats underwent 5 additional extinction training days, then a cocaine-primed reinstatement test. Immediately prior to this, they received a cocaine injection (10 mg/kg, i. p.), then were placed into operant boxes for a 2-hr session, during which lever presses were recorded, but did not yield additional cocaine or cues.
2.5.5. Cocaine dose differences
Following reinstatement testing, rats were retrained to SA 0.2 mg/50 μl cocaine (∼80 μg/kg) + cues on an FR1 schedule for 5 + days, until daily infusions varied by less than 10%. Then they received 3 days FR1 training at 0.4 mg cocaine/50 μl (∼160 μg/kg), followed by 3 days at 0.1 mg/50 μl (∼40 μg/kg).
2.5.6. Diazepam test
Next, rats were re-stabilized on the 0.2 mg/μl cocaine SA dose, and effects of diazepam (0, 1, 2 mg/kg, i. p., 30 min prior to session) on SA were tested. Following each diazepam test, rats received 1 day of drug-free training to re-stabilize behavior, and ensure diazepam washout.
2.5.7. Open field test
Following all SA testing, rats underwent one 10-min open field test. Testing was conducted in a quiet, dimly-lit room, where rats were allowed to acclimate in their home cages for 1 h before the test. The arena was a 100 cm × 100 cm, wooden open box, painted matte black, without bedding. In Noldus Ethovision, the arena was divided into 36 equivalent squares (16.7 cm2): the 16 center squares were designated as the “center” zone, and the 20 squares around the edges were designated as the “surround” zone. Time spent in the periphery is thought to be indicative of anxiety-like behavior (Ivy et al., 2008, Simon et al., 1994). The percentage of the session spent in the center and surrounds of the open field was analyzed, as was the number of entries into the center zone, and latency to first enter the center zone. Mean time in each of the 36 divisions was also plotted.
2.5.8. Cocaine-induced locomotion
Finally, rats underwent a single 2-hr test of cocaine-induced locomotion, and brains were collected immediately after the session for analysis of Fos immunoreactivity. The chamber was 43 × 43 × 30.5 cm Med Associates locomotor testing chamber without bedding or food/water. Rats were injected with cocaine (10 mg/kg, i. p.), then immediately placed in the chamber. Horizontal locomotor activity and rearing were determined via infrared beam break, recorded in 5-min bins.
2.6. Histology
2.6.1. Tissue preparation
Immediately after the 2-hr cocaine-induced locomotion test, rats were transcardially perfused with ice-cold 0.9% saline followed by 4% paraformaldehyde. Brains were removed and postfixed overnight, then cryoprotected in a 20% sucrose-azide solution. Frozen 30-μm coronal sections were collected on a cryostat, and every 12th section was processed for Fos immunoreactivity, and in hypothalamus, also immunoreactivity for orexinA.
2.6.2. Immunohistochemistry
An avidin-biotin complex (ABC)-amplified, diaminobenzidine (DAB) reaction was conducted to visualize Fos protein in medial prefrontal cortex (prelimbic: PLC, infralimbic: ILC subregions), nucleus accumbens (NAc, core and shell), amygdala (central, basolateral subregions: CeA, BLA), and epithalamus (lateral, medial habenula subregions: LHb, MHb) (Mahler and Aston-Jones, 2012, Mahler et al., 2014b). Endogenous peroxidase was blocked with 0.3% H2O2, then 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and 2% normal goat serum (Vector Laboratories, Burlingame, CA) in phosphate-buffered saline containing 0.3% Triton-X. Sections were incubated for 16 h at RT in Millipore (#ABE457) polyclonal rabbit anti-c-Fos (1:10,000), washed, then incubated 2 h in biotinylated goat anti-rabbit IgG (1:400, Vector Laboratories). After ABC amplification (3 h), Fos was visualized with DAB in Tris buffer with 0.01% H2O2 and Nickel Ammonium Chloride (0.04%; Vector Laboratories). Sections were mounted and coverslipped in Permount medium. Sections stained for orexinA were separately stained using a similar Fos protocol including ABC amplification and DAB visualization, except with normal donkey serum (2%) blocking, and donkey anti-rabbit secondary antibody (Jackson Laboratories); visualizing nuclear Fos in blue/black. These tissues were then processed for another DAB stain to visualize somatic orexinA in brown (without DAB nickel intensification). Santa Cruz (catalog #SC-8070, lot #A2915) goat anti-orexinA (1:2500) was reacted 16 h at RT, followed by donkey anti-goat (1:500), ABC amplification, and DAB reaction.
2.6.3. Imaging and Fos analysis
Images of whole sections containing quantified structures were taken at 5× on a Leica DM4000B microscope with stage automation, and stitched using StereoInvestigator software (MicroBrightfield). Two slices per structure were quantified bilaterally in each animal from near the rostrocaudal center of each sampled region. Brain region borders were delineated with comparison to a brain atlas (Paxinos and Watson, 2006), and area of the delineated region on each sample was quantified in mm2. Fos + neurons were identified using the Stereoinvestigator particle counter tool, with thresholding parameters incorporating particle size (2–200 μm2, average size 100 μm2) and contrast with background, optimized for this experiment. Accuracy of Fos quantified using this strategy is >90% accordant with trained human observers. For analysis, Fos density (Fos/mm2) was computed for each unilateral structure, and average of the 4 samples/rat (2 slices/region, 2 hemispheres/slice) were analyzed. All structure delineation and quantification was done blind to experimental condition, and thresholding was consistent across animals.
2.6.4. Orexin neuron Fos analysis
For analysis of Fos expression in orexin neurons, two sections ∼3.0 mm caudal of bregma were quantified, near the center of the orexin-containing region of hypothalamus. OrexinA + neurons in both hemispheres of each section were manually counted by a blinded observer, and assigned localization to the lateral, perifornical, or dorsomedial regions [LH = lateral of fornix, PFA = 0–1.4 mm medial to LH, DMH = PFA to midline (Harris et al., 2005)]. The number of these orexin neurons expressing Fos was also quantified, as was Fos in non-orexin cells within the same zones. The average number of orexin + neurons/hemisphere/rat was calculated, as was the average percentage of orexin neurons/sample that also expressed Fos, and the number of Fos + neurons without orexinA.
2.7. Analysis and statistics
For analyses containing within-subjects variables (e.g. LBN group x early vs. late SA training behavior, extinction learning, active vs. inactive lever, orexin subfield), mixed model ANOVAs were used, with group (LBN/Control) as a between-subjects factor. Following significant interactions in ANOVAs, Tukey posthoc tests were used to further distinguish among groups. Independent samples t-tests were used to determine effects of LBN on M&M intake and Fos expression in each measured region. Pearson correlations determined correlation coefficients for Fos expression across brain regions. A criterion of 2 standard deviations from group mean was used to determine statistical outliers (n = 1 LBN rat removed from M&M intake test). Two control group rats were excluded from analysis of open field data, due to camera malfunction.
3. Results
3.1. Early-life adversity reduces intake of highly palatable food
Ad libitum-fed LBN rats ate fewer highly-palatable chocolate M&Ms in a 1-hr free access test (conducted in their home cage with available water, but no chow) than controls (t12 = 2.2, p = .048; Fig. 2). This is consistent with other measures of “anhedonia” found in these rats, including diminished sucrose preference and social play (Bolton et al., 2018, Molet et al., 2016a). These effects were evident without visible changes in rodent well-being. Accordingly, the early-life LBN experience did not influence body weight as seen in multiple prior cohorts (Brunson et al., 2005, Molet et al., 2016a) [average body weight during behavioral economic training: Control mean(SEM) = 315.17(5.9), LBN mean = 315.48(4.6)]. Notably, others have found no LBN-induced change in overall food intake (Yam et al., 2017).
Fig. 2.
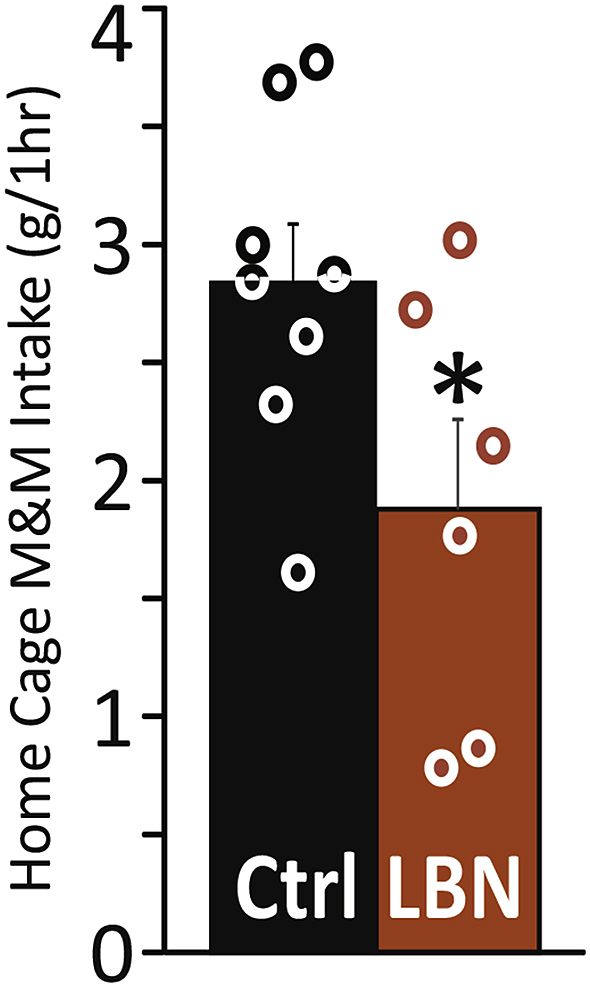
LBN Reduces Voluntary Chocolate Intake: Early-life limited bedding and nesting (LBN) reduced 1-hr home cage intake (grams) of M&M's brand chocolate candy, relative to control rats (Ctrl), tested in adulthood. Individual rats' data shown with circles. *p < .05.
3.2. Early-life adversity influences parameters of cocaine addiction in a complex manner
The patency of catheters employed for cocaine administration was tested twice for all rats using the ultrashort-acting barbiturate methohexital (Brevital; 0.1–0.2 ml, 5 mg/mL), once at the end of initial FR1 SA training, and once prior to SA dose-response testing. Catheter failure in one LBN rat at the first test resulted in its exclusion from all SA analyses, and failure in one control rat at the second test caused it to be excluded from dose-response and diazepam effects analyses.
3.2.1. Acquisition of cocaine self-administration
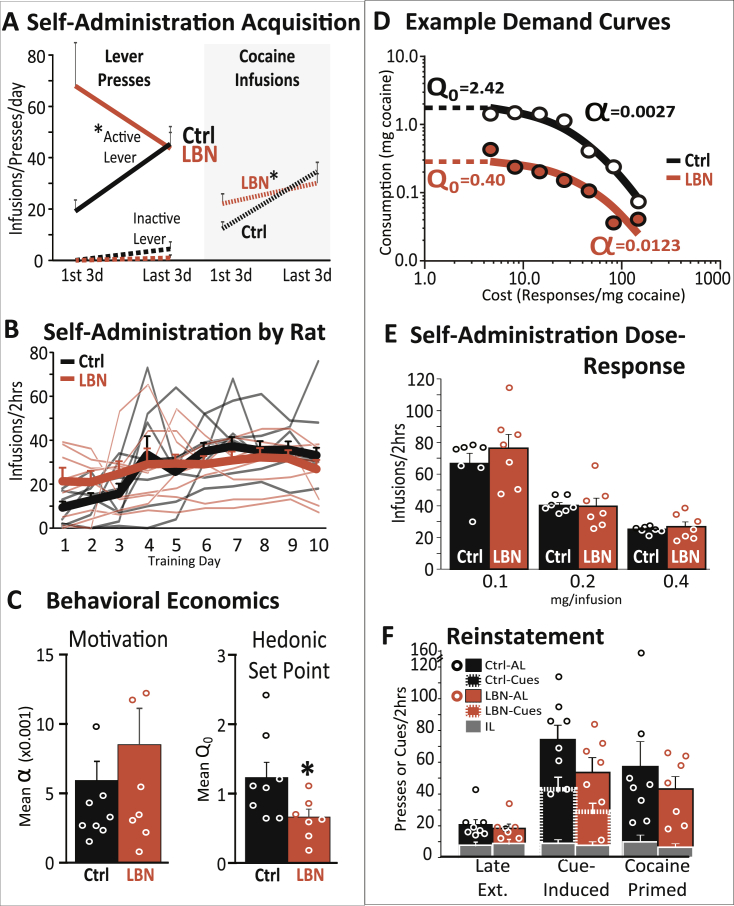
LBN rats acquired cocaine SA faster than controls, though both groups ended up self-administering similar daily levels of cocaine by the last 3 days of training (group x early/late SA period interaction; infusions/day: F1,13 = 7.14, p = .019, day 1: t13 = 2.15, p = .051; Fig. 3A–B). A similar pattern was seen on active, but not inactive lever pressing from early to late training (group x lever × period interaction: F1,13 = 8.4, p = .012; day 1 active lever: t13 = 3.0, p = .010; inactive lever: mean = 0(0) in both groups Fig. 3A). Across all 10 days of cocaine self-administration there was a group × day interaction on infusions (F9,117 = 2.1, p = .037; individual rat data shown in Fig. 3B), but no significant interaction for active (F9,117 = 1.6, p = .139) or inactive lever presses was observed (F9,117 = 0.12, n.s.). Overall, LBN and control rats experienced a similar total number of cocaine/cue pairings during training [control group total cues/infusions presented: mean = 271.5(39.4), LBN group: mean = 240.1(44.5)] and both groups took similar amounts of cocaine over the 10 days of training [controls: mean = 53.2(6.3) mg; LBN: mean = 54.9(6.6) mg; t13 = 0.064, n.s.].
Fig. 3.
LBN Decreases Cocaine Hedonic Set Point, without Affecting Other Cocaine-Seeking Behaviors: A) Self-administration acquisition data is shown, averaged across the first three days of FR1 self-administration training, and the last three days of training. Control rats are represented with black lines, LBN animals in orange. At left, active lever presses are shown with solid lines, inactive lever presses are represented with dashed lines. At right, number of cocaine infusions/session are shown with dotted lines. *p < .05, interaction of training block (first three vs. last three) and LBN/control group, group mean ± SEM is represented. B) Self-administration by rat: Number of cocaine infusions self-administered by individual rats (thin lines) on each of the first 10 days of training. Group means and SEM are shown with thick lines. Black = control, orange = LBN. C) Behavioral economics: Motivation to maintain preferred cocaine levels (demand elasticity; α) is represented for control (black) and LBN (orange) rats. Hedonic set point (Low-effort preferred intake level, extrapolated for zero cost: Q0) is also shown for control and LBN rats (Bentzley et al., 2013). *p < .05, control vs. LBN. D) Example demand curves: Effects of cost (lever pressing required to obtain 1 mg cocaine; x axis) on consumption (average cocaine self-administered per cost, binned over 7 days of stable economic testing; y axis) are shown for individual control (black circles and curves) and LBN rats (orange circles/curves). Q0 and α values are shown for these representative rats. E) Self-administration dose-response: Number of cocaine infusions self-administered by control and LBN rats are shown for low, medium, or high doses. F) Reinstatement: Active lever presses (AL; solid bars, and circles representing individual rats), cue presentations (dashed border bars), and inactive lever presses (grey bars) are shown for control and LBN rats in late extinction training (late ext), cue-induced reinstatement, and cocaine-primed reinstatement tests. In panels C, E, & F, bars represent mean (±SEM), and individual rats' data are represented with circles. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2.2. Cocaine economic demand
LBN and control rats stabilized on the within-session cocaine economic demand procedure similarly [days to stable Q0: controls mean = 7.63(1.4); LBN mean = 6.29(1.01); t13 = 0.75). After performance had stabilized (training days 8–14), we compared groups on demand elasticity (α; reflecting slope of the decay in responding at higher effort requirements) and hedonic set point (Q0, reflecting extrapolated demand at zero effort) (Bentzley et al., 2013, Hursh, 1993, Oleson et al., 2011). The average daily hedonic set point (Q0) was decreased in LBN rats relative to controls (t13 = 2.21, p = .046; Fig. 3C), while average demand elasticity (α) did not differ between groups on these days (Fig. 3C, t13 = 0.91, n.s.). Example demand curves from individual control and LBN rats are shown in Fig. 3D.
3.2.3. Cocaine dose sensitivity
LBN and control rats were tested to determine dose-sensitivity of cocaine SA. Low (0.1 mg/infusion), medium (0.2 mg/infusion), or high (0.4 mg/infusion) dose i. v. cocaine (∼40, 80, and 160 μg/kg) were available on three consecutive sessions per dose. All rats adjusted their intake similarly based on dose, and LBN and controls did not differ at any dosage (main effect of dose: F2,24 = 87.1, p < .0001; no main effect of group: F1,12 = 0.36, n.s.; or interaction of group x dose: F2,24 = 1.17; n.s.; Fig. 3E).
3.2.4. Extinction and reinstatement
Following behavioral economic testing (prior to re-acquisition for dose-response testing reported above), rats underwent extinction training, followed by cue-induced and cocaine-primed reinstatement. Performance during the first 2-hr extinction training session did not differ between LBN and control rats [no main effect of group: F1,13 = 0.001, n.s.; or interaction of group and time in session (active lever pressing in 30-min bins); F3,39 = 2.0, n.s.]. Likewise, pressing was similar in LBN and controls across the 7 d of extinction training (no effect of group: F1,13 = 0.8, n.s.; or interaction of group with training day or lever F9,117 = 1.3, n.s.). LBN did not significantly affect cue-induced reinstatement (group: F1,13 = 2.4, n.s.; group x lever: F1,13 = 2.32, n.s.), though LBN rats trended toward self-administering fewer cues during this session (t13 = 2.0, p = .070; Fig. 3F). Cocaine-primed reinstatement (10 mg/kg) was not affected by early-life adversity (group: F1,12 = 0.9, group x lever: F1,12 = 0.9; Fig. 3F).
3.3. Early-life adversity does not alter cocaine-induced anxiety-like behaviors or locomotion
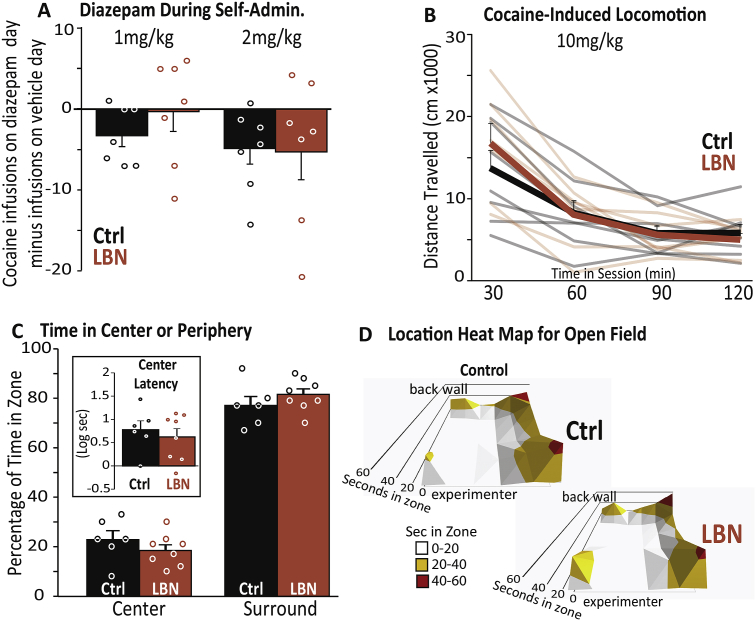
3.3.1. Diazepam effects on cocaine self-administration
After re-stabilizing rats on SA of the 0.2 mg/infusion cocaine dosage, we examined effects of diazepam (1 or 2 mg/kg, i. p.) on cocaine SA. We found a generally sedative effect of high dose diazepam (main effect of dose: F2,24 = 4.5, p = .022; veh versus 2 mg/kg: t13 = 2.7, p = .019; Fig. 4A), but no main effect of LBN group (F1,12 = 0.1, n.s.) or group × dose interaction (F2,24 = 0.6, n.s.).
Fig. 4.
LBN Does Not Affect Anxiety or Cocaine-Induced Locomotion: A) Diazepam during self-administration: Effects of diazepam (1 or 2 mg/kg) on self-administered cocaine infusions in control (black bars) and LBN rats (orange bars). Bars show mean (±SEM) for each group. B) Cocaine-induced locomotion: Distance travelled (cm X 1000) during 30-min bins of a 2-hr cocaine-induced locomotion test. Thick lines represent mean (±SEM), and semi-transparent lines show individual rats' data. C) Time in center or periphery: Time (sec) spent in center or surround zones of an open-field anxiety test. Inset shows latency (Log10 s) to first enter the center zone. Bars represent mean (±SEM). Individual rats' data are represented with circles in panels A & C. D) Location heat map for open field: Mean seconds spent in each of the 36 regions (6 × 6, 16.7 cm each) of the open field for control (top) and LBN (bottom) groups (600-sec test). Height and colors of peaks indicate greater time spent in the corresponding zones (scale bar shown at left, white = 0–20 s, orange = 20–40 s, red = 40–60 s). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3.2. Open field behavior
In an open field test for anxiety-like behavior, performed ∼72 h after completion of all cocaine SA tests, rats in both groups spent the great majority of session time in the surround (Fig. 4C). LBN rats did not differ from controls in time spent in the center zone (t12 = 1.08, n.s.), number of entries into the center zone (t12 = 1.29, n.s.), or latency to first entry to the center of the arena (t12 = 0.58, n.s.). Notably, prior studies have extensively examined anxiety, and have not found evidence for augmented anxiety-like behaviors in LBN rats (Brunson et al., 2005, Molet et al., 2016a, Molet et al., 2016b, Bolton et al., 2017). Mean time in each of the 36 regions of the open field for control and LBN rats is represented in Fig. 4D.
3.3.3. cocaine-induced locomotion
On the final day of the experimental series, rats were tested for their locomotor response to cocaine in a novel locomotor testing chamber. Early-life experience did not influence cocaine-induced locomotion during the 2-hr session (effect of group: F1,12 = 0.02, n.s.; group × time interaction: F3,36 = 2.0, n.s.; Fig. 4B).
Together, these data suggest that early-life adversity primarily alters the hedonic set point for cocaine and/or cocaine satiety mechanisms, rather than highly motivated cocaine-seeking (demand elasticity, responding in early extinction, reinstatement), sensitivity to cocaine dosage (self-administration or cocaine-induced locomotion), or anxiety-like behavior. The fact that cocaine SA was not increased by the anxiolytic diazepam in either group further discounts group-specific anxiety as a likely explanation for the LBN group's anhedonia-like effects.
3.4. Activity in reward- and stress-related brain circuits
3.4.1. Fos expression in forebrain reward/stress circuits
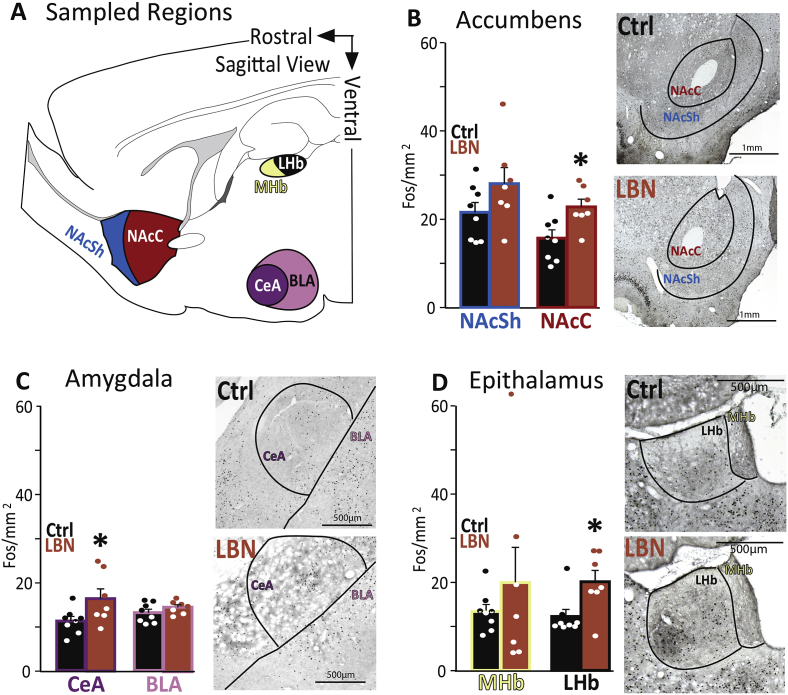
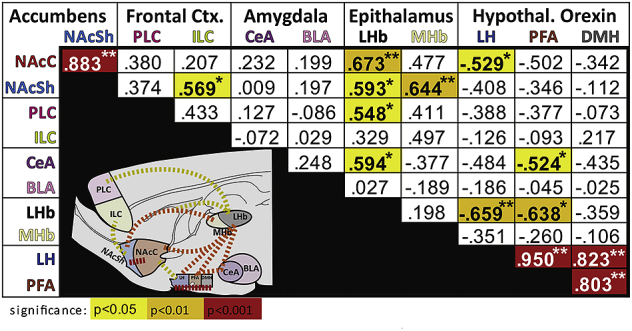
To probe the underlying neural substrates of the cocaine anhedonia-like effects, we quantified neuronal activity following acute cocaine (10 mg/kg) in LBN and control rats. Patterns of Fos expression, indicative of neuronal activation, diverged in the two groups. Specifically, greater Fos expression after cocaine was observed in nucleus accumbens core (NAcC: t12 = 2.3, p = .038; Fig. 5B), central nucleus of amygdala (CeA: t12 = 2.4, p = .035; Fig. 5C), and lateral habenula (LHb: t12 = 2.6, p = .020; Fig. 5D) of LBN rats (Fig. 5). These augmented patterns of activation were selective, in that Fos was similar between groups in prelimbic and infralimbic medial prefrontal cortex (group effect on prefrontal cortex overall: F1,12 = 0.64, n.s., PLC: t12 = 1.2, n.s.; ILC: t12 = 0.2, n.s.), nucleus accumbens shell (NAcSh: t12 = 1.3, n.s.; accumbens overall: F1,12 = 3.04, p = .107), basolateral amygdala (BLA: t12 = 1.4, n.s.; amygdala overall: F1,12 = 7.2, p = .02), and medial habenula (MHb: t12 = 0.4, n.s.; epithalamus overall: F1,12 = 2.7, p = .126). We also examined correlations between Fos expression in measured brain regions including hypothalamic orexin neurons, and found co-activation within nucleus accumbens, and orexin neuron subregions (Pearson r values and significance level shown in Table 1). We also saw evidence of co-activation across regions with known anatomical connections (Table 1). Notably, LHb activity was correlated with both NAc and CeA, as well as PLC. Together, these data suggest that post-cocaine activity in both pleasure- (accumbens) and anxiety/aversion-related circuits (amygdala, habenula) is significantly altered as a result of early-life adversity.
Fig. 5.
LBN Alters Neuronal Activity after Cocaine in Reward- and Stress-Related Regions: A) Sampled regions: Regions for which Fos immunoreactivity are shown are represented in a sagittal view. NAcSh: nucleus accumbens shell, NAcC: nucleus accumbens core, MHb: medial habenula, LHb: lateral habenula, CeA: amygdala central nucleus, BLA: basolateral amygdala. Fos expression in B) Accumbens: Nucleus accumbens shell and core, C) Amygdala: Central and basolateral amygdala, and D) Epithalamus: Medial and lateral habenula is shown, along with representative photomicrographs from control and LBN rats. Density of Fos + cells (mean ± SEM Fos/mm2) is shown for each sampled brain region in control (Ctrl: black bars) and LBN rats (orange bars). In panels B-D, bars represent control (black) and LBN (orange) group means ± SEM, and dots represent data from individual rats. *p < .05. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Network Co-Activity Observed After Cocaine: Pearson r values reflect the strength of co-activation of Fos expression amongst measured brain regions and subregions: Nucleus Accumbens (core, shell), frontal cortex (PLC, ILC), amygdala (CeA, BLA), epithalamus (LHb, MHb), and hypothalamic orexin neuron subregions (LH, PFA, DMH). Significance level is shown with stars and cell color: *p < .05, yellow; **p < .01, orange; ***p < .001, red. Inset represents measured brain regions, connected with dashed lines between co-activated regions.
3.4.2. Fos expression in orexin/hypocretin neurons
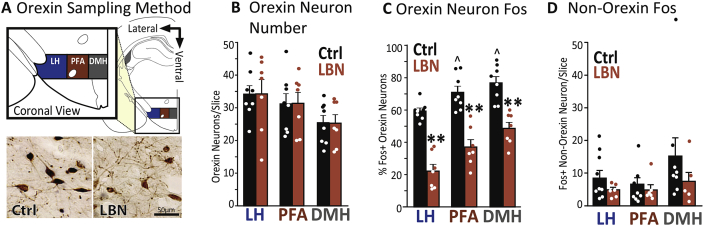
To further probe the nature of the apparent circuit dysfunction provoked by LBN, we examined post-cocaine activity in hypothalamic orexin neurons, which are activated by psychostimulants (Estabrooke et al., 2001, Johnson et al., 2012), and are crucial for drug and natural reward-seeking and intake, as well as for stress-like responses to aversive stimuli (Berridge et al., 2010, Boutrel et al., 2005, Harris et al., 2005, Muschamp et al., 2014, Winsky-Sommerer et al., 2005). Fos expression in orexin neurons following cocaine was greatly reduced in the LBN group. These changes were found in the LH, PFA, and DMH cell groups (Harris et al., 2005) (percent Fos + orexin neurons: main effect of group: F1,12 = 60.2, p < .001; LH: t12 = 9.3, p < .001; PFA: t12 = 6.6, p < .001; DMH: t12 = 4.6, p = .001, Fig. 6C). Notably, these differences were not a result of the number of orexinA + cells, which was equivalent in LBN and controls (F1,12 = 0.4, n.s.; Fig. 6B). Fos expression was unchanged in non-orexin neurons in these areas (no main effect of group: F1,12 = 0.96, n.s., Fig. 6D).
Fig. 6.
LBN Reduces Fos in Orexin Neurons After Cocaine: A) Orexin sampling method: Subregions of the hypothalamic orexinA-containing region are shown in a coronal view, inset shows enlarged view (sampled from 2.8 to 3.2 mm caudal of bregma. LH: lateral hypothalamus, PFA: perifornical area, DMH: dorsomedial hypothalamus. Photomicrographs at bottom show representative examples of orexin neurons (brown soma) with Fos + nuclei in Ctrl (left) and LBN (right) rats. B) Orexin neuron number: Mean (±SEM) orexin + cells in each hypothalamic subregion is shown for Ctrl (black) and LBN rats (orange). C) Orexin neuron Fos: Percentage of orexin neurons per hemisphere/subregion that were Fos+, and D) Non-Orexin Fos: Number of non-orexin Fos + neurons in each hemisphere in control and LBN rats. In panels B–D, dots represent data from individual rats. *p < .05: Ctrl vs. LBN; **p < .001: Ctrl vs. LBN; ˆp < .05: In control rats, difference in % Fos + Orexin neurons in LH vs. DMH or PFA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Here we demonstrate that early-life adversity in rats causes an anhedonia-like phenotype for food and cocaine, consistent with a robust alteration of both natural- and drug-reward processing in these animals. Early-life adversity reduced spontaneous intake of a highly palatable chocolate reward in young-adult rats compared to controls, and provoked a decrease in the preferred amount of cocaine that was self-administered under free-access conditions in a within-subjects behavioral economic test. These effects were selective: sensitivity to self-administered cocaine dose, motivation to maintain preferred cocaine blood levels, and locomotor response to noncontingent cocaine were unchanged. In addition, overt signs of anxiety were absent, and anxiolysis with 1 or 2 mg/kg diazepam did not influence cocaine SA. When the underlying circuit aberrations driving this behavioral phenotype were probed by examining Fos expression after acute cocaine injection, LBN rats had more Fos in several brain regions involved in reward and stress processing. Notably, early-life adversity instead attenuated activity of hypothalamic orexin neurons following acute cocaine. Together, these findings demonstrate that early-life adversity, potentially via the fragmented, unpredictable maternal care, causes long-lasting dysregulation of neural and behavioral responses to a psychostimulant reward, consistent with an anhedonic phenotype.
4.1. Early-life adversity promotes cocaine anhedonia
We previously reported that early-life adversity induced natural reward anhedonia—as operationalized by decreased voluntary intake of a weak sucrose solution, and decreased voluntary social play (Molet et al., 2016a). These behaviors have previously been linked with anhedonia, but neither behavioral task specifically measures reward-related behaviors such as hedonic set point or incentive motivation—distinct processes that are differentially mediated by brain regions like nucleus accumbens, frontal cortex, amygdala, and epithalamus (Berridge and Robinson, 2003, Kalivas and Volkow, 2005, Lecca et al., 2014, Nieh et al., 2013, Sparta et al., 2013). In addition, effects of early-life adversity with LBN on drug-seeking behaviors have not previously been tested. Here, we found specific effects of early adversity on cocaine intake and seeking. For example, initial acquisition of cocaine SA was facilitated in the LBN group, a behavior that has been linked to increased novelty-seeking or decreased risk avoidance—factors associated with propensity to try drugs, or to engage in other risky behaviors (Beckmann et al., 2011, Belin et al., 2016, Piazza et al., 1989). However, while most LBN rats learned to acquire cocaine SA earlier than controls, rats in both groups came to SA cocaine at similar levels within 10 days of FR1 self-administration training.
The within-subject behavioral economic cocaine demand test allows concurrent analysis of preferred cocaine intake levels (hedonic set point) (Ahmed and Koob, 1998) and motivation to maintain these levels as response requirements increase (economic demand elasticity) (Bentzley et al., 2013, Oleson et al., 2011), and thereby enabled precise probing of changes in cocaine reward provoked by early-life adversity. Specifically, LBN rats preferred to SA lower doses of cocaine than controls under low effort requirements (decreased Q0), despite similar levels of motivation to defend these decreased levels in the face of increasing effort requirements. Although LBN-induced changes in cocaine satiety mechanisms could result in reduced low-effort cocaine self-administration (Gerber and Wise, 1989, Tsibulsky and Norman, 1999, Zimmer et al., 2013), these data are most consistent with a decreased hedonic set point in LBN rats, and a specific deficit in hedonic properties of cocaine rather than motivation for the drug. This conclusion is supported by the lack of concurrent decreases in cue-induced and cocaine-primed reinstatement—measures previously shown to correlate with high-effort cocaine seeking (Bentzley and Aston-Jones, 2015). Importantly, rats in both groups successfully titrated their intake of cocaine when the available dose was altered, suggesting that they were similarly responsive to the rewarding or subjective effects of the drug. These data also imply that the cocaine satiety mechanisms largely determining behavior during 2-hr FR1 self-administration sessions (Gerber and Wise, 1989, Tsibulsky and Norman, 1999, Zimmer et al., 2013) were unlikely to have been affected by LBN.
Follow-up studies should replicate and extend these findings in larger samples, across species (e.g. mice), and in both sexes (only males were tested here). Further studies should also examine dose-responsivity of cocaine-induced reinstatement (Deroche et al., 1999), as well as potential effects of a saline injection on cocaine seeking, to further characterize the priming effects of cocaine on LBN and control rats. In addition, examining escalation of cocaine intake over extended training [thought to model addiction-like changes occurring with extended use (Koob et al., 2004)] would help determine effects of LBN on transition to cocaine addiction from recreational use. Determining effects of LBN on cocaine withdrawal responses would further characterize psychostimulant addiction liability of LBN rats, and by extension, humans receiving chaotic early-life parental care or other childhood adversity.
4.2. Cocaine anhedonia after early-life adversity likely does not result from augmented anxiety
Early-life adversity did not directly cause anxiety-like responses in rats tested in adulthood, as we have previously seen (Molet et al., 2016a). Instead, LBN rats are prone to exacerbated cognitive deficits from acute stressors, suggesting vulnerability to a “second hit” later in life (Molet et al., 2016b). Because cocaine has anxiogenic as well as rewarding effects (Ettenberg, 2004), we entertained the hypothesis that the reduced low-effort self-administered cocaine intake preferred by LBN rats was a result of enhanced anxiogenic responses to the drug, opposing cocaine's rewarding effects. We reasoned that diazepam might then increase cocaine intake in LBN but not control rats. Instead, we found that high-dose diazepam caused expected mild sedation in both groups, but did not increase intake in either group, inconsistent with the notion that adversity promotes the anxiogenic effects of cocaine (but consistent with prior reports that benzodiazepines dose-dependently decrease cocaine self-administration (Goeders et al., 1989, Goeders et al., 1993)). In addition, when cocaine was given non-contingently at a set dose (10 mg/kg), LBN and control rats had similar behavioral responses in both cocaine-primed reinstatement and cocaine-induced locomotion tasks. This further demonstrates normal responses to the acute motivational and locomotor-activating effects of cocaine (though other dosages of cocaine should be tested (Deroche et al., 1999) to verify no changes in cocaine-induced locomotion, rearing, or behavioral stereotypies). It remains possible that cocaine-induced anxiety could be present, but not manifest on the specific tasks we employed here—a possibility that should be explored in future studies. In addition, we note that anxiety-like behavior in the open field task was fairly high in both groups here, potentially due to cocaine withdrawal or other consequences of cocaine exposure or repeated behavioral testing conducted in these animals prior to the test. This said, our prior work has found no differences in anxiety-like behaviors on open field or elevated-plus maze tasks (Molet et al., 2016a), discounting the likelihood that anxiety alone accounts for the altered reward intake behaviors observed here in LBN rats.
4.3. Neuronal activity after cocaine is influenced by early-life adversity, in a pattern distinct from that of natural rewards
Whereas acute behavioral responses to 10 mg/kg cocaine were not altered in the LBN group, neural responses after non-contingent cocaine were distinct, suggesting reorganization of brain reward- and/or stress-related circuits in these animals. After 10 mg/kg cocaine, an increased number of Fos-expressing cells was observed in CeA, a region involved in fear/anxiety, as well as reward seeking (Mahler and Berridge, 2009, Mahler and Berridge, 2012, Phelps and LeDoux, 2005). No significant changes in Fos after cocaine were seen in BLA or mPFC, but LBN potentiated Fos in LHb. This region is strongly associated with aversion and stress, and hyperactivity there has been linked to anhedonia- and depressive-like behaviors (Lecca et al., 2014, Li et al., 2013, Sartorius and Henn, 2007), as well as decreased SA of natural and drug rewards (Friedman et al., 2010, Friedman et al., 2011). Greater activity in the CeA and LHb of LBN rats after cocaine could indicate that cocaine exposure is perceived as more aversive by rats that experienced early-life adversity, in agreement with their previously observed anhedonia for natural rewards (Bolton et al., 2018). Intriguingly, the degree of LHb Fos expression across animals was related to Fos expression in other regions, including NAcC and NAcSh, PLC, CeA, and orexin populations—potentially indicating network connectivity of these regions revealed by cocaine administration. How such network activity relates to LBN-induced anhedonia or other effects should be explored further in the future.
Importantly, LBN did not only increase activity in stress-related brain regions after cocaine, but also in NAc core, a region well-established to participate in the acute and conditioned reinforcing properties of cocaine and cocaine cues (Augur et al., 2016, Ito et al., 2000, Kalivas and McFarland, 2003, Stefanik et al., 2013). Greater NAc activity in LBN rats following cocaine may help explain why cocaine is still reinforcing enough to maintain similar self-administration, reinstatement, and demand elasticity behaviors in LBN and control rats, despite the lower hedonic set point in LBN rats.
These findings only partially overlap with LBN-induced changes in neuronal activity after natural rewards such as social play: for example, we previously found that LBN potentiates activity specifically in CeA (but not BLA) CRH neurons, and in ILC parvalbumin-expressing interneurons in response to a normally pleasurable social play experience, without significant changes in neuronal activation in the habenula or nucleus accumbens. These distinct patterns of Fos activity after cocaine and natural rewards suggest that the consequences of early-life adversity on reward circuit function are reward type-specific (Hsu et al., 2015). Therefore, it would be interesting to determine if early-life adversity influences SA of, or addiction to, the drugs most commonly taken by those with depression or anxiety, such as opiates or alcohol (Markou et al., 1998).
4.4. Activity of orexin neurons after cocaine is decreased by early-life adversity
Probing the LBN-induced alterations in aversion- and reward-related circuits further, we discovered an attenuation of the ability of acute cocaine to elicit activity in hypothalamic orexin/hypocretin neurons of LBN rats. Notably, our results are consistent with decreased orexin neuron activity seen after restraint stress in adult rats previously exposed to early-life maternal separation stress (James et al., 2014). Orexin neurons have been linked to reward-seeking (including for cocaine) (Harris et al., 2005, Refojo et al., 2011, Smith et al., 2009) as well as to stress- and arousal-related functions (Berridge et al., 2010, Winsky-Sommerer et al., 2005). A medial-lateral gradient in orexin stress/arousal functions (linked to medial PFA and DMH populations) versus reward-related functions (linked to lateral LH populations) has been proposed (Harris et al., 2005), though here we found similar attenuation of Fos throughout the orexin field in LBN rats after cocaine. Of note, most orexin neurons also express glutamate, and nearly all express the aversion-related endogenous opioid peptide dynorphin (Chou et al., 2001, Mickelsen et al., 2017, Rosin et al., 2003). Investigation into the balance between peptide and amino acid neurotransmission in orexin target regions after early-life adversity should be informative for interpreting changes in orexin neurons caused by LBN.
Overall, these observations are consistent with LBN-induced reorganization of connectivity between and within reward and stress/aversion circuits, as we have previously suggested (Bolton et al., 2017, Davis et al., 2017). Therefore, we propose that LBN causes cocaine anhedonia by altering structural and functional connectivity of reward- and aversion-related brain circuits recruited by cocaine. However, we note that Fos expression observed here after acute cocaine was not necessarily induced by cocaine itself, since we did not compare results to Fos expression in LBN or control rats after a control condition (saline injection). Therefore, further studies should examine the relative roles of LBN alone, acute cocaine, injection stress, prior repeated behavioral testing, and other variables on Fos expression in reward- and stress-related circuits.
5. Conclusions
Together, these data provide evidence that early-life adverse experiences, perhaps mediated by chaotic parental care, have significant and enduring effects on reward- and aversion-related neural circuits, resulting in an anhedonic phenotype expressed for both natural and cocaine rewards. Greater activation of stress- and aversion-related, as well as reward-related, brain regions after cocaine exposure in LBN rats may underlie the delicate balance between LBN-induced anhedonia and preserved motivation for cocaine. It is likely that these effects do not apply to all types of rewards—indeed, we predict that LBN may actually facilitate reward for other classes of drugs with anxiolytic or overtly euphorigenic effects, such as opiates or alcohol. Notably, these are the types of drugs most commonly used by those with anhedonia (Hatzigiakoumis et al., 2011, Janiri et al., 2005, Schmidt et al., 2001), as occurs in depression and anxiety disorders (Markou et al., 1998). The current studies demonstrate that across reward classes, early-life adversity causes robust and consistent deficits in the hedonic processing of rewards.
Acknowledgements
We thank Brandon S Bentzley for providing analysis tools for behavioral economic data. This work was supported by the National Institutes of Health (grant numbers R00 DA035251 to SVM and P50 MH096889 to TZB), the UCI Office of Research, the Irvine Center for Addiction Neuroscience, and the George E. Hewitt Foundation for Medical Research (to JLB). No financial conflicts of interest exist for any of the authors.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.01.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Ahmed S.H., Koob G.F. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Augur I.F., Wyckoff A.R., Aston-Jones G., Kalivas P.W., Peters J. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J. Neurosci. 2016;36(39):10174–10180. doi: 10.1523/JNEUROSCI.0773-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram T.Z., Davis E.P., Obenaus A., Sandman C.A., Small S.L., Solodkin A., Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. Am. J. Psychiatr. 2012;169(9):907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann J.S., Marusich J.A., Gipson C.D., Bardo M.T. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav. Brain Res. 2011;216(1):159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Belin-Rauscent A., Everitt B.J., Dalley J.W. In search of predictive endophenotypes in addiction: insights from preclinical research. Gene Brain Behav. 2016;15(1):74–88. doi: 10.1111/gbb.12265. [DOI] [PubMed] [Google Scholar]
- Bentzley B.S., Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur. J. Neurosci. 2015;41(9):1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley B.S., Fender K.M., Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology. 2013;226(1):113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge C.W., España R.A., Vittoz N.M. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bolton J.L., Molet J., Ivy A., Baram T.Z. New insights into early-life stress and behavioral outcomes. Curr. Opin. Behav. Sci. 2017;14:133–139. doi: 10.1016/j.cobeha.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J.L., Molet J., Regev L., Chen Y., Rismanchi N., Haddad E., Yang D.Z., Obenaus A., Baram T.Z. Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biol. Psychiatr. 2018;83(2):137–147. doi: 10.1016/j.biopsych.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B., Kenny P.J., Specio S.E., Martin-Fardon R., Markou A., Koob G.F., de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102(52):19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K.L., Kramar E., Lin B., Chen Y., Colgin L.L., Yanagihara T.K., Lynch G., Baram T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25(41):9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.C., Lee C.E., Lu J., Elmquist J.K., Hara J., Willie J.T., Beuckmann C.T., Chemelli R.M., Sakurai T., Yanagisawa M., Saper C.B., Scammell T.E. Orexin (hypocretin) neurons contain dynorphin. J. Neurosci. 2001;21(19):RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E.P., Stout S.A., Molet J., Vegetabile B., Glynn L.M., Sandman C.A., Heins K., Stern H., Baram T.Z. Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proc. Natl. Acad. Sci. U. S. A. 2017;114(39):10390–10395. doi: 10.1073/pnas.1703444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V., Le Moal M., Piazza P.V. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur. J. Neurosci. 1999;11(8):2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Enoch M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke I.V., McCarthy M.T., Ko E., Chou T.C., Chemelli R.M., Yanagisawa M., Saper C.B., Scammell T.E. Fos expression in orexin neurons varies with behavioral state. J. Neurosci.: Off. J. Soc. Neurosci. 2001;21(5):1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci. Biobehav. Rev. 2004;27(8):721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Friedman A., Lax E., Dikshtein Y., Abraham L., Flaumenhaft Y., Sudai E., Ben-Tzion M., Ami-Ad L., Yaka R., Yadid G. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59(6):452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Lax E., Dikshtein Y., Abraham L., Flaumenhaft Y., Sudai E., Ben-Tzion M., Yadid G. Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology. 2011;60(2–3):381–387. doi: 10.1016/j.neuropharm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G.J., Wise R.A. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol. Biochem. Behav. 1989;32(2):527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Goeders N.E., McNulty M.A., Guerin G.F. Effects of alprazolam on intravenous cocaine self-administration in rats. Pharmacol. Biochem. Behav. 1993;44(2):471–474. doi: 10.1016/0091-3057(93)90493-d. [DOI] [PubMed] [Google Scholar]
- Goeders N.E., McNulty M.A., Mirkis S., McAllister K.H. Chlordiazepoxide alters intravenous cocaine self-administration in rats. Pharmacol. Biochem. Behav. 1989;33(4):859–866. doi: 10.1016/0091-3057(89)90483-8. [DOI] [PubMed] [Google Scholar]
- Harris G.C., Wimmer M., Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hatzigiakoumis D.S., Martinotti G., Giannantonio M.D., Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front. Psychiatr. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Mletzko T., Miller A.H., Nemeroff C.B. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hsu D.T., Sanford B.J., Meyers K.K., Love T.M., Hazlett K.E., Walker S.J., Mickey B.J., Koeppe R.A., Langenecker S.A., Zubieta J.K. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol. Psychiatr. 2015;20(2):193–200. doi: 10.1038/mp.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh S.R. Behavioral economics of drug self-administration: an introduction. Drug Alcohol Depend. 1993;33(2):165–172. doi: 10.1016/0376-8716(93)90058-x. [DOI] [PubMed] [Google Scholar]
- Insel T.R. The NIMH research Domain criteria (RDoC) project: precision medicine for psychiatry. Am. J. Psychiatr. 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Ito R., Dalley J.W., Howes S.R., Robbins T.W., Everitt B.J. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J. Neurosci. 2000;20(19):7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A.S., Brunson K.L., Sandman C., Baram T.Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James Morgan H., Campbell Erin J., Walker Frederick R., Smith Doug W., Richardson Heather N., Hodgson Deborah M., Dayas Christopher V. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front. Behav. Neurosci. 2014 doi: 10.3389/fnbeh.2014.00244. https://doi.org/10.3389/fnbeh.2014.00244 23 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri L., Martinotti G., Dario T., Reina D., Paparello F., Pozzi G., Addolorato G., Di Giannantonio M., De Risio S. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. 2005;52(1):37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- Johnson P.L., Samuels B.C., Fitz S.D., Federici L.M., Hammes N., Early M.C., Truitt W., Lowry C.A., Shekhar A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol. Behav. 2012;107(5):733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P.W., McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Volkow N.D. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatr. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley A.E., Berridge K.C. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci.: Off. J. Soc. Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T., Binder E.B. Epigenetics of stress-related psychiatric disorders and gene x environment interactions. Neuron. 2015;86(6):1343–1357. doi: 10.1016/j.neuron.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Ahmed S.H., Boutrel B., Chen S.A., Kenny P.J., Markou A., O'Dell L.E., Parsons L.H., Sanna P.P. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci. Biobehav. Rev. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lecca S., Meye F.J., Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur. J. Neurosci. 2014;39(7):1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev. Neurosci. 2000;11(4):383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Li K., Zhou T., Liao L., Yang Z., Wong C., Henn F., Malinow R., Yates J.R., 3rd, Hu H. betaCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341(6149):1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Aston-Jones G.S. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J. Neurosci. 2012;32(38):13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Berridge K.C. Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J. Neurosci. 2009;29(20):6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Berridge K.C. What and when to "want"? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology. 2012;221(3):407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Moorman D.E., Smith R.J., James M.H., Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci. 2014;17(10):1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Vazey E.M., Beckley J.T., Keistler C.R., McGlinchey E.M., Kaufling J., Wilson S.P., Deisseroth K., Woodward J.J., Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat. Neurosci. 2014;17(4):577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A., Kosten T.R., Koob G.F. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18(3):135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Brake W., Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27(1–2):127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Mickelsen L.E., Kolling F.W.t., Chimileski B.R., Fujita A., Norris C., Chen K., Nelson C.E., Jackson A.C. Neurochemical heterogeneity among lateral hypothalamic hypocretin/orexin and melanin-concentrating hormone neurons identified through single-cell gene expression analysis. eNeuro. 2017;4(5) doi: 10.1523/ENEURO.0013-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett M.C., Vicentic A., Kozel M., Plotsky P., Francis D.D., Kuhar M.J. Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol. 2007;73(3):321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Heins K., Zhuo X., Mei Y.T., Regev L., Baram T.Z., Stern H. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl. Psychiatry. 2016;6:e702. doi: 10.1038/tp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Avishai-Eliner S., Baram T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014;56(8):1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Kinney-Lang E., Harris N.G., Rashid F., Ivy A.S., Solodkin A., Obenaus A., Baram T.Z. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016;26(12):1618–1632. doi: 10.1002/hipo.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp J.W., Hollander J.A., Thompson J.L., Voren G., Hassinger L.C., Onvani S., Kamenecka T.M., Borgland S.L., Kenny P.J., Carlezon W.A., Jr. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. U. S. A. 2014;111(16):E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh E.H., Kim S.Y., Namburi P., Tye K.M. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res. 2013;1511:73–92. doi: 10.1016/j.brainres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor R.M., Moloney R.D., Glennon J., Vlachou S., Cryan J.F. Enhancing glutamatergic transmission during adolescence reverses early-life stress-induced deficits in the rewarding effects of cocaine in rats. Neuropharmacology. 2015;99:168–176. doi: 10.1016/j.neuropharm.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Oleson E.B., Richardson J.M., Roberts D.C. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology. 2011;214(2):567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson E.B., Roberts D.C. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology: Off. Publ. Am. Coll. Neuropsychopharmacol. 2009;34(3):796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. sixth ed. Academic Press/Elsevier; Amsterdam; Boston: 2006. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Piazza P.V., Deminiere J.M., Le Moal M., Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Pratchett L.C., Yehuda R. Foundations of posttraumatic stress disorder: does early life trauma lead to adult posttraumatic stress disorder? Dev. Psychopathol. 2011;23(2):477–491. doi: 10.1017/S0954579411000186. [DOI] [PubMed] [Google Scholar]
- Refojo D., Schweizer M., Kuehne C., Ehrenberg S., Thoeringer C., Vogl A.M., Dedic N., Schumacher M., von Wolff G., Avrabos C., Touma C., Engblom D., Schutz G., Nave K.A., Eder M., Wotjak C.T., Sillaber I., Holsboer F., Wurst W., Deussing J.M. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333(6051):1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- Rosin D.L., Weston M.C., Sevigny C.P., Stornetta R.L., Guyenet P.G. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J. Comp. Neurol. 2003;465(4):593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Sartorius A., Henn F.A. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med. Hypotheses. 2007;69(6):1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Nolte-Zenker B., Patzer J., Bauer M., Schmidt L.G., Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry. 2001;34(2):66–72. doi: 10.1055/s-2001-15184. [DOI] [PubMed] [Google Scholar]
- Sharma S., Powers A., Bradley B., Ressler K.J. Gene x environment determinants of stress- and anxiety-related disorders. Annu. Rev. Psychol. 2016;67:239–261. doi: 10.1146/annurev-psych-122414-033408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P., Dupuis R., Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994;61(1):59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Smith R.J., See R.E., Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur. J. Neurosci. 2009;30(3):493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta D.R., Jennings J.H., Ung R.L., Stuber G.D. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav. Brain Res. 2013;255:19–25. doi: 10.1016/j.bbr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik M.T., Moussawi K., Kupchik Y.M., Smith K.C., Miller R.L., Huff M.L., Deisseroth K., Kalivas P.W., LaLumiere R.T. Optogenetic inhibition of cocaine seeking in rats. Addict. Biol. 2013;18(1):50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky V.L., Norman A.B. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839(1):85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R., Boutrel B., de Lecea L. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol. Neurobiol. 2005;32(3):285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- Yam K.Y., Naninck E.F.G., Abbink M.R., la Fleur S.E., Schipper L., van den Beukel J.C., Grefhorst A., Oosting A., van der Beek E.M., Lucassen P.J., Korosi A. Exposure to chronic early-life stress lastingly alters the adipose tissue, the leptin system and changes the vulnerability to western-style diet later in life in mice. Psychoneuroendocrinology. 2017;77:186–195. doi: 10.1016/j.psyneuen.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Zimmer B.A., Dobrin C.V., Roberts D.C. Examination of behavioral strategies regulating cocaine intake in rats. Psychopharmacology. 2013;225(4):935–944. doi: 10.1007/s00213-012-2877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.