Abstract
Clinical studies indicate that Alzheimer's disease (AD) disproportionately affects women in both disease prevalence and severity, but the mechanisms underlying this sex divergence are unknown. Though some have suggested this difference in risk is a reflection of known differences in longevity between men and women, mounting clinical and preclinical evidence supports women also having intrinsic susceptibilities towards the disease. While a number of potential risk factors have been hypothesized to affect these differences in risks, none have been definitively verified. In this review, we discuss a novel hypothesis whereby women's susceptibility to chronic stress also mediates increased risk for AD. As stress is a risk factor for AD, and women are twice as likely to develop mood disorders where stress is a major etiology, it is possible that sex dimorphisms in stress responses contribute to the increase in women with AD. In line with this, sex divergence in biochemical responses to stress have been noted along the hypothalamic-pituitary-adrenal (HPA) axis and among known molecular effectors of AD, with crosstalk between these processes also being likely. In addition, activation of the cortical corticotrophin-releasing factor receptor 1 (CRF1) signaling pathway leads to distinct female-biased increases in molecules associated with AD pathogenesis. Therefore, the different biochemical responses to stress between women and men may represent an intrinsic, sex-dependent risk factor for AD.
Keywords: Stress, Sex difference, HPA axis, Corticotrophin-releasing factor receptor 1 signaling, Alzheimer's disease
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease that affects 5.4 million Americans and is the fifth leading cause of death among Americans aged 65 or older (http://www.alz.org/facts). The neuropathological basis of the disease involves production of pathogenic amyloid-β (Aβ) oligomers from amyloid precursor protein (APP), hyperphosphorylated tau, and synapse loss resulting in a “dying back” neuropathy and ultimately neuron death in both cortical and sub-cortical regions (Katzman, 1986; Arnold et al., 1991). Although 3–5% of AD cases are caused by distinct mutations in APP, Presenillin 1 (PS1), and Presenillin 2 (PS2) genes, the vast majority is sporadic, depending on a complex interplay of genomic and environmental factors.
One intriguing statistic is that, of the estimated 5.4 million Americans with AD, 3.3 million (nearly 70%) are women (Alzheimer's Association, 2015; Hebert et al., 2001). In attempts to explain this striking difference in prevalence, scientists and physicians have investigated both epidemiological and biological hypotheses (Lin and Doraiswamy, 2014; Mielke et al., 2014; Riedel et al., 2016). One prevailing hypothesis is that in most populations around the world, women tend to outlive men, and, as the gap between the number of men and women in a population widens with advancing age, relatively more women age to the point where AD symptoms begin to present (Brookmeyer et al., 1998; Hebert et al., 2001; Plassman et al., 2007; Seshadri et al., 1997). In general, epidemiological evidence is split, with some studies showing no increased incidence for women (Bachman et al., 1993; Edland et al., 2002; Hebert et al., 2001; Rocca et al., 1998), which supports the longevity hypothesis, while others have more recently detected an increase in incidence for women (Li et al., 2017; Koran et al., 2016; Pirskanen et al., 2005; Rasmuson et al 2001, 2011; Gallart-Palau et al., 2016; Ardekani et al., 2016; Damoiseaux et al., 2012), supporting intrinsic, biological differences in susceptibility.
Though previously there were few studies that supported female-specific biological mechanisms for increased AD risk, a growing number of studies in recent years have provided evidence for such mechanisms. Most notably, these include sex-specific genetic interactions (Altmann et al., 2014; Janicki et al., 2014; Ungar et al., 2014), hormones and associated endocrinological changes with age (Morrison et al., 2006; Rocca et al., 2011), sex dimorphism in brain structures (Elbejjani et al., 2015; Sampedro et al., 2015), and female-specific alterations in central inflammation and microglial function (Hanamsagar and Bilbo, 2016).
In addition to these potential mechanisms, one intriguing hypothesis is based on how women respond differently to chronic stress on a cellular and molecular level. The risk of AD in general is increased with chronic stress, which in pre-clinical models is often defined as daily stressors applied for 3 weeks or greater (Catania et al., 2009; Dong and Csernansky, 2009; Khalsa, 2015; Machado et al., 2014; Bao et al., 2008; Pardon and Rattray, 2008; Swaab et al., 2005; Wilson et al., 2007). Notably, women are twice as likely to develop other disorders where stress is a central etiology, such as mood disorders (Verma et al., 2011), prompting the possibility that stress and sex may also interact for AD risk. In this review, we summarize the specific evidence suggesting stress increases AD risk and severity in both sexes and then discuss possible mechanisms where stress and sex interact and lead to greater disease burden for women. In particular, we describe both corticosteroid-driven and central Corticotrophin releasing factor receptor 1 (CRF1)-driven signaling mechanisms whereby women are more greatly affected by chronic stress and develop increased activity along known pro-AD pathways.
2. Chronic stress, glucocorticoids, and AD
In mammals, the HPA axis, sympathetic nervous system, and centrally active stress hormone signaling pathways are activated in response to stress. The HPA axis is the most often described response mechanism (Bao et al., 2008; Johnson et al., 1992), and includes CRF release from the periventricular nucleus (PVN) of the hypothalamus, downstream facilitation of pituitary secretion of adrenal corticotrophin hormone (ACTH), final activation of the adrenal cortex to induce release of glucocorticoids (GCs), and lastly negative feedback onto CNS regions to limit harmfully high somatic stress responses. In addition, GCs have profound effects on neuronal function in many cognitive and limbic brain regions (Gray et al., 2017; Miller and O'Callaghan, 2003). Intertwined with HPA axis activation, the sympathetic nervous system produces the quickest somatic response to acute stress, while central CRF signaling through non-pituitary CRF receptor activation leads to some of the most salient effects of stress on cognition (McEwen, 1998; McEwen and Gianaros, 2010).
In general, when stress is acute (usually <3 days), self-limited, and of moderate intensity, an organism's stress response is adaptive and activates the organism to resolve the stressful stimuli. However, when stress is prolonged (usually >3 weeks), i.e. chronic stress, it causes deleterious effects which are often opposite of those caused by the acute situation (McEwen, 1998; Schneiderman et al., 2005). In terms of cognition, chronic and high intensity stress lead to blunting of the HPA axis, synaptic plasticity changes induced through prolonged GC secretion, and alterations in CRF receptor signaling that lead to impaired memory and learning (McEwen, 1998; McEwen and Gianaros, 2010; Chen et al., 2010). This abnormal prolongation or repetitive activation of the stress response can lead to the development of neuropsychatric disorders, including Major Depressive Disorder (MDD) and Generalized Anxiety Disorder as well as worsening other chronic diseases, such as artherosclerotic cardiovascular disease (Salvagioni et al., 2017). Importantly, it has also been shown that chronic stress can increase AD risk, as seen in studies that evaluate AD patients that are more prone to stress (Wilson et al., 2003, 2006; Greenberg et al., 2014; Hasegawa, 2007; Machado et al., 2014).
The mechanism whereby stress increases AD risk has not been completely described, but there are numerous studies that suggest potential causative processes. Chronic stress is associated with degenerative processes in the hippocampus through GC-dependent mechanisms (Salvagioni et al., 2017), and it is possible that stress affects AD through increased GC signaling. In support of this, stress-related increases in plasma cortisol levels (Swanwick et al., 1998; Rehman, 2002; Umegaki et al., 2000) as well as correlations between increased cortisol levels and the severity of cognitive decline (Pedersen et al., 2001) have been reported in AD. Importantly, these changes in the HPA axis in AD patients do not appear to be secondary to MDD, as AD patients with and without MDD have higher cerebrospinal fluid cortisol levels compared to controls (Hoogendijk et al., 2006). Thus, the degenerative effect of high GC signaling may play a role in the overall loss of cognition during early AD pathogenesis.
Despite these examples linking AD and HPA axis dysregulation, human studies have not yet been helpful in elucidating the mechanisms by which stress might influence AD pathogenesis and the contribution of GCs to AD pathogenesis is still far from clear. Fortunately, transgenic mouse models can recapitulate at least some of the neuropathological and behavioral changes associated with AD and provide an opportunity to investigate how stress affects AD-like behavior and molecular signaling.
One important point of congruity between AD patients and AD mouse models is that increased production of pathological, soluble Aβ and Aβ plaques in response to behavioral stressors is ubiquitously seen (Dong et al., 2004; Jeong et al., 2006; Cuadrado-Tejedor et al., 2012). In terms of GC effects on AD pathogenesis, administration of the corticosteroid dexamethasone to APP/PS1/MAPT mice increases APP and Aβ levels as well as β-secretase (BACE) and the β-C-Termial Fragment (β-CTF) of APP, suggesting a direct role between GC signaling and AD pathogenesis (Green et al., 2006). In addition, it has been shown that co-administration of Aβ and GCs into the rat hippocampus increases hyperphosphorylated tau and worsens cognition (Catania et al., 2007; Sotiropoulos et al., 2011). Mechanistically, a recent paper has shown that the non-genomic effects of GCs through membrane bound GR-α cause an increase in Aβ through Gs-cAMP-PKA-dependent signaling, downstream pCREB transcriptional activation, and resultant increases in BACE1 (Choi et al., 2017). Thus, it is likely that there is a direct link between stress and AD pathogenesis, and the increased cortisol seen in AD patients may further influence the rate of AD pathology through GCs promoting pro-AD signaling.
3. CRF/CRF1 signaling pathway and AD
Outside of the HPA axis, increased CRF Receptor 1 (CRF1) density has been noted in the brain of AD patients compared to age-matched controls (Behan et al., 1995; De Souza, 1995), and CRF signaling through this receptor may also contribute to AD pathogenesis and severity. Again, data from animal models have shown that acute restraint stress increases hyperphosphorylated tau in a central CRF1-dependent manner in adrenalectomized mice (Rissman et al., 2007). In support of this, our group and others have shown that behavioral stressors can increase Aβ levels by increasing CRF transmission at CRF1 sites located outside of the HPA axis, implicating central CRF signaling as potentially causative of increased AD pathogenesis (Kang et al., 2007; Campbell et al., 2015; Carroll et al., 2011; Dong et al., 2008; Rissman et al., 2012).
Increased CRF1 signaling has been associated with multiple stages of APP proteolysis, regulation of Aβ generation, and Aβ-mediated toxicity (Thathiah and De Strooper, 2011; Thathiah et al., 2013). CRF overexpression in the forebrain can lead to accumulation of Aβ and hyperphosphorylated tau through CRF1-Gs-PKA, consistent with this pathway's role in influencing the amyloid production cascade through modulation of α-, β- and γ secretases (Park et al., 2015; Robert et al., 2001; Thathiah and De Strooper, 2011; Thathiah et al., 2013; Xu et al., 1996). Specifically, while transient activation of CRF1-Gs-PKA shifts APP metabolism towards the α-secretase-mediated pathway that results in non-pathogenic amyloids, chronic activation of these signaling cascades shifts APP metabolism to the β-, γ−secretase, and perhaps also η-secretase mediated pathways that result in increased pathogenic Aβ generation (da Cruz e Silva et al., 2009; Willem et al., 2015). Additionally, PKA signaling is associated with tau phosphorylation, another molecular pathway that is highly implicated in AD pathogenesis (Blanchard et al., 1994; Sanchez-Mut et al., 2014). Thus, there is supporting evidence implicating CRF1 signaling as the causative pathway facilitating the detrimental effects of psychosocial stress.
CRF and CRF receptors are widespread modulators of neuronal activity throughout the cortex and hippocampus (de Souza, 1988; Orozco-Cabal et al., 2006). In these areas, CRF exerts its cellular effects by activating G-protein coupled receptors (GPCRs) CRF1 and CRF receptor 2 (CRF2) (Gallagher et al., 2008). The function of CRF1 and CRF2 in stress regulation depends on the brain region, is cell type-specific, and can be influenced by the individual's prior experiences (Henckens et al., 2016). Additionally, at least one study has indicated that, in some specific regions such as the dorsal raphe, the receptor distribution of CRF1 and CRF2 can shift following stress, where CRF1 is internalized and CRF2 is recruited to the membrane, resulting in more CRF2 mediated effects in response to CRF stimulation (Wood et al., 2013). Even with these potential changes, however, CRF1 activation and downstream Gs/cAMP/PKA signaling is the more common result of stress (Wood and Woods, 2007; Grammatopoulos et al., 2001; Blank et al., 2003). Thus, one hypothesis could ascribe an increase in CRF1 activation in AD, which leads to overactive PKA pathways, might underlie changes in pro-AD signaling. It is furthermore important to note that membrane bound GR signaling (Choi et al., 2017) and CRF1 show converging activation of PKA pathways.
Indeed, multiple pre-clinical studies support elevated cAMP/PKA signaling in the prefrontal cortex and hippocampus of AD mouse models (Choi et al., 2006; da Cruz e Silva et al., 2009; de Barry et al., 2010; Kim et al., 2011; Lee et al., 2004; Nelson et al., 2009; Takashima, 2006; Thathiah and De Strooper, 2011; Thathiah et al., 2013). For example, PKA activation directly influences the amyloid cascade through modulation of α-, β- and γ−secretases, thereby affecting the proteolysis of APP (Amini et al., 2015; Robert et al., 2001; Thathiah and De Strooper, 2011; Thathiah et al., 2013; Xu et al., 1996). While non-pathogenic activation of the α-secretase-mediated pathway results in non-amyloidogenic cleavage of APP, long-lasting PKA activation shifts APP metabolism to the β-secretase-mediated pathway and increases Aβ generation (da Cruz e Silva et al., 2009). Whether CRF1 increases PKA signaling in the subcellular compartments that also regulate Aβ generation in humans remains to be determined (Thathiah and De Strooper, 2011); although, one report from our group suggests that CRF increased Aβ levels in cultured cortical neurons through cAMP-PKA signaling (Dong et al., 2014).
One last important aspect of CRF1 signaling is the indirect impact that CRF1-mediated PKA increases have on cognition, which may result in worsening symptoms in patients who have developed AD. It has been noted that increased PKA intracellular signaling leads to the reduction of prefrontal neuron firing and impairments in working memory (Birnbaum et al., 2004; Hains and Arnsten, 2008), and thus increases in CRF1 signaling may worsen the cognitive deficits that already plague AD patients.
Overall, the increase in GCs and CRF with chronic stress is likely to have quantifiable impacts on AD pathogenesis. Moreover, the central CRF1 signaling cascades could result in worsened cognition, earlier AD onset, and increased AD severity.
4. Sex differences in glucocorticoid responses to stress and AD
In addition to evidence suggesting that stress affects AD pathogenesis, sex dimorphism in stress responses likely lead to increased AD risk for women who suffer from chronic stress as compared to men. As there is evidence that both GCs and centrally active CRF play a role in this increased risk, understanding pro-AD signaling processes affected by both is important for delineating the mechanism behind this sex-specific risk increase. In this section, we discuss the influence of GCs on AD pathogenesis specifically in women.
In terms of circulating GCs in rodents, namely corticosterone, females show increased concentrations compared to males at baseline (Kitay, 1961; Handa et al., 1994; Bangasser and Wicks, 2017). However, similar comparisons in humans have yet to consistently find differences in baseline plasma cortisol, the peripheral human GC (Seeman et al., 2001; Stroud et al., 2002; Kudielka and Kirschbaum, 2005; Uhart et al., 2006). While the reason for this discepency is unknown, increased complexities concerning estrogen regulation of GC secretion (Bangasser and Valentino, 2014; Toufexis et al., 2014), diurnal rhythms, and the age of patients tested (Veldhuis et al., 2013) may need to be controlled more carefully, as such conditions may strongly influence the difference in GC secretion in women compared to men (Kudielka and Kirschbaum, 2005).
For GC release in response to stressors, female rodents showing consistently higher corticosterone levels than males after stress (Weinstock et al., 1998; Seale et al., 2004; Weathington et al., 2012; Bangasser and Valentino, 2014; Toufexis et al., 2014) while women and men have similar levels of peak cortisol with stress (Handa et al., 1994; Bangasser and Valentino, 2014). Interestingly, however, the duration of elevated cortisol is greater in women, though women also have a longer half-life for cortisol due to higher concentrations of proteins that bind cortisol in the blood -(Veldhuis et al., 2013; Bangasser and Valentino, 2014). Again, the reasons for these differences between animals and humans are unknown, perhaps stress-dependent GC release is augmented in humans only when neuropsychiatric dysfunction is present. For instance, it has been reproducibly shown that women with neuropsychological disorders such as depression display higher levels of cortisol at baseline and during stressful life events (Peeters et al., 2003; Cooper and Stroehla, 2003; Young and Korszun, 2010). As mentioned before, the risk for these diseases in women is twice as high as compared to men, and, in concert, female AD patients also show a similarly higher level of cortisol than male patients (Rasmuson et al., 2011).
While the requirement for a neuropsychological state is plausible, cross-reactivity between stress and sex hormones suggests that the synergism between these hormones and GCs may be of greater importance than disease presentation. One of the most striking examples is from a modified version of the dexamethasone suppression test. In this modified version, when CRF administration follows dexamethasone suppression, the cortisol response is higher in women than in men (Heuser et al., 1994; Kunugi et al., 2006). In addition, it has been shown that the phase of the menstrual cycle affects corticosterone and cortisol levels (Bangasser and Valentino, 2014; Toufexis et al., 2014), and combinatorial changes in transcription occur with activation of the estrogen receptor (ER) and GR that do not occur with either in isolation (Whirledge and Cidlowski, 2013; Whirledge et al., 2013; Deak et al., 2015). All in all, women may have higher levels of GCs in specific but important situations that predisopose them to stress-related disease. Ultimately, the elevation in GCs during these time periods could have a significant effect on AD pathogenesis and represent a potential mechanism whereby women are at greater risk for AD than men.
In addition to absolute circulating corticosterone, the response through GRs may differ between the sexes. Though the female and male responses to GCs at the level of altered transcription are incompletely detailed in the CNS, one study looking at dexamethasone signaling in hepatic cells suggested sex divergence in downstream transcriptional patterns. In this study, GR response resulted in female-specific activation in inflammatory pathways, such as IL-6 signaling, that were not seen as strongly in males (Duma et al., 2010). As neuroinflammation may greatly affect AD status (Hanamsagar and Bilbo, 2016), this divergence in GR signaling may be especially important, though replication in neural cells or brain tissue would be warranted. Regardless, this suggests that there is also a degree of sex dimorphism in GC signaling, which may affect the risk of AD in men and women differently. In addition, the retraction of CA3 dendrites in the hippocampus with chronic stress, a process which is primarily GC-dependent (McEwen et al., 2015), is found to occur primarily in male rats but to a much lesser degree in females. Subsequent research has shown that the typical hippocampal memory impairment seen in male rats with chronic stress is not found in female rats (Galea et al., 1997; Bowman et al., 2001; Kitraki et al., 2004; McLaughlin et al., 2010). By contrast, female rats showed greater morphological change with chronic stress along PFC-amgydala pathways, a process that was not observed in males (Shansky et al., 2009, 2010).
Though the reason for these differences between the sexes in these chronic stress paradigms are still unclear, it is likely that differences in GC signaling are important contributing factors. In terms of GCs directly interacting with sex in promoting AD, the data is alarmingly scarce. In fact, nearly all the pre-clinical studies on GC-dependent increases in pro-AD signaling were done in males, and so it is unknown if these same processes cause a larger change in females (Catania et al., 2007; Sotiropoulos et al., 2015; Choi et al., 2017). Still, given the high likelihood that females exhibit higher GC burdens with chronic stress, it is likely that GCs represent one mechanism whereby AD risk and severity is increased in women.
5. Sex differences in CRF1 signaling and AD
Though GC signaling makes up an important component of the chronic stress response, there is increasing emphasis on signaling pathways outside of the HPA axis that predispose individuals to certain neuropsychiatric diseases. Of these different signaling pathways, central CRF/CRF1 signaling in areas like the amygdala, hippocampus, prefronal cortex, locus coeruleus (LC), and dorsal raphe (DR) have been shown to have a major impact on behavior (Blank et al., 2002, 2003; Gallagher et al., 2008; Orozco-Cabal et al., 2006). Perhaps unsurprisingly, CRF signaling has also been shown to be particularly divergent based on the sex of the organism (Bangasser and Valentino, 2014; Bangasser and Wicks, 2017).
As mentioned in the previous section, women seem to have increased sensitivity to CRF in terms of HPA axis activation (Heuser et al., 1994; Kunugi et al., 2006) and increased CRF concentrations have been noted in the portal venous system and hypothalamus of aged female rats over males (Veldhuis et al., 2013). Further research has shown that this increased sensitivity extends beyond hypothalamic and pituitary sites to other important limbic structures. One strong example of this increased female sensitivity to CRF comes from studies in the LC, where the activity of these norepinephrine secreting neurons showed higher activation at lower doses of CRF in female rats compared to males (Curtis et al., 2006). In addition, males show greater activation of serotonergic neurons in the DR with CRF administration, and these changes have been shown to cause an indirect reduction in corticosterone release (Howerton et al., 2014).
Interestingly, the differences in both of these areas have been ascribed to sex dimorphism of CRF1 activation. For female LC neurons, there is greater activation of CRF1-Gs-PKA pathways during prolonged CRF administration, but in male LC neurons, CRF1-β-arrestin-2 signaling predominates (Bangasser et al., 2010; Valentino et al., 2013). The activation of this alternative pathway in males leads to CRF1 internalization and downregulation, facilitating desensitization of central CRF signaling, while females continue to show high levels of Gs-cAMP-PKA signaling (Bangasser et al., 2010; Curtis et al., 2006). In contrast, DR neurons tend to show higher overall CRF1 expression in males compared to females, suggesting that a difference in expression leads to sex dimorphism in this region (Bangasser et al., 2010). Thus, there may be multiple mechanisms whereby sex differences in CRF1 signaling are appreciated.
Throughout this review, evidence for CRF1 promoting AD and increased CRF1 signaling in females has been presented. However, our recent unbiased, proteomic study found a direct link between female-predominant CRF1 signaling and pro-AD pathway activation that is not seen in males (Bangasser et al., 2016). In this study, CRF overexpression in the frontal cortex of mice led to sex divergent phosphoproteomic patterns with males showing sex-specific Rho signaling activation and females showing sex-specific activation of amyloidogenesis pathways. When CRF was overexpressed in the forebrain of Tg2576 mice, cortical tissue demonstrated higher BACE1 activation and greater presence of amyloid plaques. These changes in amyloidogenesis further impacted behavior, as female Tg2576 mice with CRF overexpression showed greater deficits in prefrontal cortex-dependent working memory than male genotype- and age-matched controls and both male and female Tg2576-only mice (Bangasser et al., 2016).
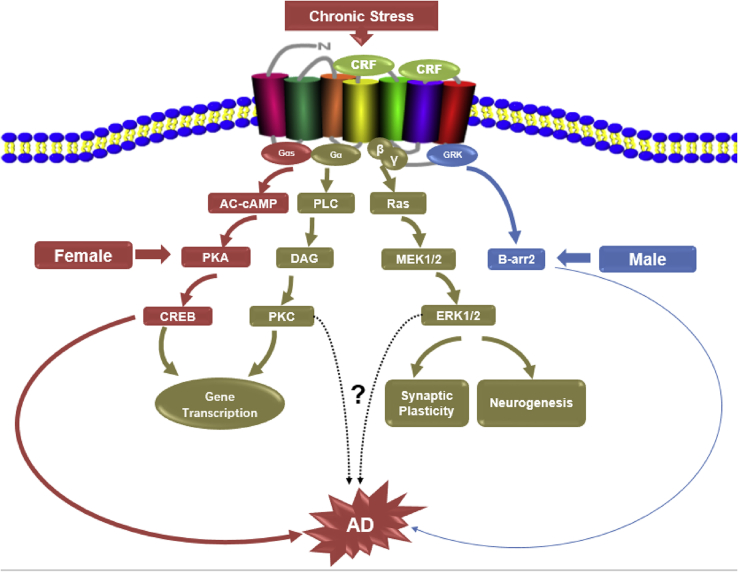
In all, it seems that sex divergent CRF1 signaling plays a significant role in promoting AD-like pathogenesis in female rodents (Fig. 1), and while human studies of HPA axis response to CRF in women further support different downstream signaling pathways to CRF (Bangasser and Valentino, 2014), confirmation of the existence of these sex differences at the second messenger level are essential for understanding how sex affects AD in humans. Future research into sex divergent phosphoproteomic patterns in post-mortem samples would be especially informative, especially from aged and AD patients.
Fig. 1.
Schematic illustration of sex biased CRF/CRF1 signaling in response to stress can result in sex differentiation of the cortical phospho-proteome and translate to sex distinct impact on neuropathology of AD (red-female, blue-male). Whether other signaling pathways of CRF/CRF1 downstreams (brown) also display sex difference after stress is unknown. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
6. Conclusion and future direction
Across the literature presented in this review, there are three major points of convergence that should be emphasized: First, while an explanation for the apparent difference in the rate of AD diagnoses between men and women remains elusive, more and more evidence is beginning to suggest that a true increase in risk is partly due to sex dimorphism in chronic stress responses. As clinical epidemiological studies indicate that neuropsychiatric diseases with stress as a major etiology have a higher incidence in women, such as MDD, PTSD, and GAD, perhaps AD should be similarly thought of as a disease with an etiology based on stress, though probably to a lesser degree than for mood disorders. At the very least, both sex and stress should be accounted for as covariates in future human and animal studies of AD.
The second important point of convergence is the obvious impact that Gs-cAMP-PKA signaling has on pro-AD pathways with stress. Multiple lines of evidence detail increased signaling along this pathway with both GC- and CRF-mediated signaling. Though therapeutic targeting of pervasive PKA signaling is unlikely to be a viable treatment strategy, incorporation of PKA signaling into other hypotheses centered on AD risk and treatment are certain to benefit inclusion of this pro-AD mechanism. Perhaps a future treatment strategy that indirectly alters PKA signaling could be used in patients with high AD risk, such as in those with significant family history or pro-AD genetic markers.
The last and probably most speculative point of convergence is that altered CRF1 signaling in females is likely to be a more important point of sex divergence than altered GC signaling in terms of AD risk and severity. Though sparse reports have mentioned divergence in GC signaling by sex (Deak et al., 2015), other studies suggest that, in response to stress in rats, pathways mediated by GR transcriptional control are the most convergent pathways between males and females (Daskalakis et al., 2014). As most of the evidence in terms of female-specific risk of AD and GCs centers around differences in GC expression, either with disease or in response to CRF, it is likely that the most impactful point of sex divergence is in the differing sensitivities of women to CRF during stress. Though this would be the most parsimonious explanation, the possibility that GCs may augment CRF1 downstream signaling remains. It is also important to note that women may still preferentially benefit from therapies targeted against GR signaling even if CRF is the major upstream effector. The reason for this benefit, however, may have more to do with a higher presence of GR-dependent pathways subsequent to higher circulating GCs than downstream sex dimorphism in GR-dependent signaling or transcription patterns.
Though the current hypotheses presented in this review concerning stress, sex, and AD are promising, there are still a number of questions to be answered. First, while GCs and CRF influence many of the central and somatic responses to chronic stress, it's still possible that other non-endocrinological pathways are altered in a way that makes AD pathogenesis more likely in women. Some of these mechanisms could include direct changes in plasticity or connectivity between brain regions in response to stressful situations, altered immune and inflammatory reactions due to stress, and differences in the balance of the parasympathetic and sympathetic arms of the autonomic nervous system. We hope that future investigations will examine these mechanisms in terms of AD risk.
Another important question is how age factors into the stress-by-sex interaction that potentially drives AD risk. As of yet, there are not studies that have rigorously tested the impact that stress on AD pathogenesis at different points of the life cycle. Therefore, it is unknown if early life stress, mid-life stress, or late-life stress of similar durations and intensities would cause differences in AD risk based solely on timing. This represents another area of investigation that will be important in characterizing how stress may affect AD risk.
As we move towards “personalized medicine,” improving our understanding of sex-specific disease mechanisms will be one of the first areas to yield substantial improvements in prevention and treatment outcomes across all branches of medicine. Still, much remains to be described before adopting any of these potential explanations as drivers of disease, and certainly a greater understanding of the biology underlying these mechanisms is warranted before therapeutic development should be undertaken.
It is also important to note that investigations of sex-specific risk are just as likely to reveal central mechanisms of disease pathogenesis that are independent of sex. By careful comparison and inclusion of both sexes in preclinical and clinical research, upstream and downstream pathways that converge in both sexes will point the way towards shared mechanisms, such as Gs-cAMP-PKA signaling. Exploiting these commonalities is more likely to lead to successful interventions and treatments than ignoring sex as a potential risk factor, as careful consideration of disease pathogenesis by sex will reduce sex-specific motifs that are mistaken as being causative but are, in truth, epiphenomenal. So, while improving our understanding of underlying sex dimorphism in AD risk will be critical for developing treatments that are especially effective in women, this line of research also has the potential to reveal new targets for the treatment of AD and other neurodegenerative disorders in both sexes.
Declarations of interest
None.
Acknowledgments
This work was supported by the National Institutes of Health (1R56AG053491-01, Dong, H) and (1 R01 AG057884-01 Dong, H).
References
- Altmann A. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini E. 2015. Paradoxical Role of PKA Inhibitor on Amyloidbeta-induced Memory Deficit. [DOI] [PubMed] [Google Scholar]
- Ardekani B.A. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J. Alzheime Dis. 2016;50:847–857. doi: 10.3233/JAD-150780. [DOI] [PubMed] [Google Scholar]
- Arnold S.E. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cerebr. Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Association, A. s., 2015. <facts_figures_2015.pdf>.
- Bachman D.L. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham study. Neurology. 1993;43:515–519. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatr. 2010;15(877):896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A. Corticotropin-releasing factor overexpression gives rise to sex differences in Alzheimer's disease-related signaling. Mol. Psychiatr. 2016;185 doi: 10.1038/mp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Wicks B. Sex-specific mechanisms for responding to stress. J. Neurosci. Res. 2017;95:75–82. doi: 10.1002/jnr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao A.M. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res. Rev. 2008;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Behan D.P. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum S.G. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Blanchard B.J. Hyperphosphorylation of human TAU by brain kinase PK40erk beyond phosphorylation by cAMP-dependent PKA: relation to Alzheimer's disease. Biochem. Biophys. Res. Commun. 1994;200:187–194. doi: 10.1006/bbrc.1994.1432. [DOI] [PubMed] [Google Scholar]
- Blank T. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J. Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J. Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman R.E. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am. J. Publ. Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S.N. Impact of CRFR1 ablation on amyloid-beta production and accumulation in a mouse model of Alzheimer's disease. J. Alzheime Dis. 2015;45:1175–1184. doi: 10.3233/JAD-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.C. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania C. The amyloidogenic potential and behavioral correlates of stress. Mol. Psychiatr. 2009;14:95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- Catania C. The amyloidogenic potential and behavioral correlates of stress. Mol. Psychiatr. 2007;14:95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- Chen Y. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.S. PKCepsilon increases endothelin converting enzyme activity and reduces amyloid plaque pathology in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8215–8220. doi: 10.1073/pnas.0509725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G.E. Membrane-associated effects of glucocorticoid on BACE1 upregulation and Aβ generation: involvement of lipid raft-mediated CREB activation. J. Neurosci. 2017;37:8459–8476. doi: 10.1523/JNEUROSCI.0074-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G.S., Stroehla B.C. The epidemiology of autoimmune diseases. Autoimmun. Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M. Chronic mild stress accelerates the onset and progression of the Alzheimer's disease phenotype in Tg2576 mice. J. Alzheime Dis. 2012;28:567–578. doi: 10.3233/JAD-2011-110572. [DOI] [PubMed] [Google Scholar]
- Curtis A.L. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva O.A. Enhanced generation of Alzheimer's amyloid-beta following chronic exposure to phorbol ester correlates with differential effects on alpha and epsilon isozymes of protein kinase C. J. Neurochem. 2009;108:319–330. doi: 10.1111/j.1471-4159.2008.05770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J. Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc. Natl. Acad. Sci. U.S.A. 2014;111:13529–13534. doi: 10.1073/pnas.1401660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barry J. Protein kinase C as a peripheral biomarker for Alzheimer's disease. Exp. Gerontol. 2010;45:64–69. doi: 10.1016/j.exger.2009.10.015. [DOI] [PubMed] [Google Scholar]
- de Souza E.B. CRH defects in Alzheimer's and other neurologic diseases. Hosp. Pract. 1988;23:59–71. doi: 10.1080/21548331.1988.11703535. [DOI] [PubMed] [Google Scholar]
- De Souza E.B. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Deak T. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress (Amsterdam, Netherlands) 2015;18:367. doi: 10.3109/10253890.2015.1053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Csernansky J.G. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J. Alzheime Dis. 2009;18:459–469. doi: 10.3233/JAD-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dong H. Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid-beta and behavior in Tg2576 mice. Psychopharmacology (Berlin) 2014;231:4711–4722. doi: 10.1007/s00213-014-3629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma D. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010;3 doi: 10.1126/scisignal.2001077. ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edland S.D. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch. Neurol. 2002;59:1589–1593. doi: 10.1001/archneur.59.10.1589. [DOI] [PubMed] [Google Scholar]
- Elbejjani M. Depression, depressive symptoms, and rate of hippocampal atrophy in a longitudinal cohort of older men and women. Psychol. Med. 2015;45:1931–1944. doi: 10.1017/S0033291714003055. [DOI] [PubMed] [Google Scholar]
- Galea L.A. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gallagher J.P. Synaptic physiology of central CRH system. Eur. J. Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallart-Palau X. Gender differences in white matter pathology and mitochondrial dysfunction in Alzheimer's disease with cerebrovascular disease. Mol. Brain. 2016;9:27. doi: 10.1186/s13041-016-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos D.K. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J. Neurochem. 2001;76:509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Gray J.D. 2017. Genomic and Epigenomic Mechanisms of Glucocorticoids in the Brain. [DOI] [PubMed] [Google Scholar]
- Green K.N. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J. Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.S. 2014. Stress, PTSD, and Dementia. [DOI] [PubMed] [Google Scholar]
- Hains A.B., Arnsten A.F. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn. Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R., Bilbo S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016;160:127. doi: 10.1016/j.jsbmb.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R.J. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hasegawa T. Prolonged stress will induce Alzheimer's disease in elderly people by increased release of homocysteic acid. Med. Hypotheses. 2007;69:1135–1139. doi: 10.1016/j.mehy.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Hebert L.E. Is the risk of developing Alzheimer's disease greater for women than for men? Am. J. Epidemiol. 2001;153:132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- Henckens M.J. Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat. Rev. Neurosci. 2016;10:636–651. doi: 10.1038/nrn.2016.94. [DOI] [PubMed] [Google Scholar]
- Heuser I.J. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol. Aging. 1994;15:227–231. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hoogendijk W.J. Increased cerebrospinal fluid cortisol level in Alzheimer's disease is not related to depression. Neurobiol. Aging. 2006;27 doi: 10.1016/j.neurobiolaging.2005.07.017. 780 e1-780 e2. [DOI] [PubMed] [Google Scholar]
- Howerton A.R. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol. Psychiatr. 2014;75:873–883. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki S.C. Estrogen receptor alpha variants affect age at onset of Alzheimer's disease in a multiethnic female cohort. Dement. Geriatr. Cognit. Disord. 2014;38:200–213. doi: 10.1159/000355559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y.H. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer's disease model. Faseb. J. 2006;20:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- Johnson E.O. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kang J.E. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R. Alzheimer's disease. N. Engl. J. Med. 1986;314:964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Khalsa D.S. 2015. Stress, Meditation, and Alzheimer's Disease Prevention: Where the Evidence Stands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. Protein kinase C-regulated abeta production and clearance. Int. J. Alzheimer's Dis. 2011;2011:857368. doi: 10.4061/2011/857368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitay J.I. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Kitraki E. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Koran M.E. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imag. Behav. 2016;3 doi: 10.1007/s11682-016-9523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kunugi H. Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a Multicenter Study. Neuropsychopharmacology: Offic. Publ. Am. Coll. Neuropsychopharmacol. 2006;31:212–220. doi: 10.1038/sj.npp.1300868. [DOI] [PubMed] [Google Scholar]
- Lee W. Amyloid beta peptide directly inhibits PKC activation. Mol. Cell. Neurosci. 2004;26:222–231. doi: 10.1016/j.mcn.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Li G. Cerebrospinal fluid biomarkers for Alzheimer's and vascular disease vary by age, gender, and APOE genotype in cognitively normal adults. Alzheimer's Res. Ther. 2017;9:48. doi: 10.1186/s13195-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.A., Doraiswamy P.M. When Mars versus Venus is not a cliche: gender differences in the neurobiology of Alzheimer's disease. Front. Neurol. 2014;5:288. doi: 10.3389/fneur.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A. Chronic stress as a risk factor for Alzheimer's disease. Rev. Neurosci. 2014;25:785–804. doi: 10.1515/revneuro-2014-0035. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J. Endocrinol. 2015;226:T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.J. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M.M. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin. Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.B., O'Callaghan J.P. 2003. Effects of Aging and Stress on Hippocampal Structure and Function. [DOI] [PubMed] [Google Scholar]
- Morrison J.H. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J. Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T.J. Reduction of beta-amyloid levels by novel protein kinase C(epsilon) activators. J. Biol. Chem. 2009;284:34514–34521. doi: 10.1074/jbc.M109.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L. Regulation of synaptic transmission by CRF receptors. Rev. Neurosci. 2006;17:279–307. doi: 10.1515/revneuro.2006.17.3.279. [DOI] [PubMed] [Google Scholar]
- Pardon M.C., Rattray I. What do we know about the long-term consequences of stress on ageing and the progression of age-related neurodegenerative disorders? Neurosci. Biobehav. Rev. 2008;32:1103–1120. doi: 10.1016/j.neubiorev.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Park H.J. The stress response neuropeptide CRF increases amyloid-beta production by regulating gamma-secretase activity. EMBO J. 2015;34:1674–1686. doi: 10.15252/embj.201488795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W.A. Impact of aging on stress-responsive neuroendocrine systems. Mech. Ageing Dev. 2001;122:963–983. doi: 10.1016/s0047-6374(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Peeters F. Cortisol responses to daily events in major depressive disorder. Psychosom. Med. 2003;65:836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- Pirskanen M. 2005. Estrogen Receptor Beta Gene Variants Are Associated with Increased Risk of Alzheimer's Disease in Women. [DOI] [PubMed] [Google Scholar]
- Plassman B.L. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmuson S. 2001. Increased Glucocorticoid Production and Altered Cortisol Metabolism in Women with Mild to Moderate Alzheimer's Disease. [DOI] [PubMed] [Google Scholar]
- Rasmuson S. Increased serum levels of dehydroepiandrosterone (DHEA) and interleukin-6 (IL-6) in women with mild to moderate Alzheimer's disease. Int. Psychogeriatr. 2011;23:1386–1392. doi: 10.1017/S1041610211000810. [DOI] [PubMed] [Google Scholar]
- Rehman H.U. Role of CRH in the pathogenesis of dementia of Alzheimer's type and other dementias. Curr. Opin. Invest. Drugs. 2002;3:1637–1642. [PubMed] [Google Scholar]
- Riedel B.C. Age, APOE and sex: triad of risk of Alzheimer's disease. J. Steroid Biochem. Mol. Biol. 2016;012 doi: 10.1016/j.jsbmb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman R.A. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J. Neurosci. 2007;27:6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman R.A. Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6277–6282. doi: 10.1073/pnas.1203140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S.J. The human serotonin 5-HT4 receptor regulates secretion of non-amyloidogenic precursor protein. J. Biol. Chem. 2001;276:44881–44888. doi: 10.1074/jbc.M109008200. [DOI] [PubMed] [Google Scholar]
- Rocca W.A. Incidence of dementia and Alzheimer's disease: a reanalysis of data from Rochester, Minnesota, 1975-1984. Am. J. Epidemiol. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- Rocca W.A. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvagioni D.A.J. Physical, psychological and occupational consequences of job burnout: a systematic review of prospective studies. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185781. e0185781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro F. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. 2015;6:26663–26674. doi: 10.18632/oncotarget.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mut J.V. Promoter hypermethylation of the phosphatase DUSP22 mediates PKA-dependent TAU phosphorylation and CREB activation in Alzheimer's disease. Hippocampus. 2014;24:363–368. doi: 10.1002/hipo.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N. Stress and health: psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 2005;1:607. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale J.V. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J. Neuroendocrinol. 2004;16:989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- Seeman T.E. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Seshadri S. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- Shansky R.M. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebr. Cortex (New York, N.Y.: 1991) 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebr. Cortex (New York, N.Y.: 1991) 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos I. Stress acts cumulatively to precipitate Alzheimer's disease-like tau pathology and cognitive deficits. J. Neurosci. 2011;31:7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos I. Female hippocampus vulnerability to environmental stress, a precipitating factor in Tau aggregation pathology. J. Alzheime Dis. 2015;43:763–774. doi: 10.3233/JAD-140693. [DOI] [PubMed] [Google Scholar]
- Stroud L.R. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatr. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Swaab D.F. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Swanwick G.R. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer's disease: lack of association between longitudinal and cross-sectional findings. Am. J. Psychiatr. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J. Alzheime Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- Thathiah A., De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer's disease. Nat. Rev. Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- Thathiah A. beta-arrestin 2 regulates Abeta generation and gamma-secretase activity in Alzheimer's disease. Nat. Med. 2013;19:43–49. doi: 10.1038/nm.3023. [DOI] [PubMed] [Google Scholar]
- Toufexis D. Stress and the reproductive Axis. J. Neuroendocrinol. 2014;26:573–586. doi: 10.1111/jne.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M. Gender differences in hypothalamic–pituitary–adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Umegaki H. Plasma cortisol levels in elderly female subjects with Alzheimer's disease: a cross-sectional and longitudinal study. Brain Res. 2000;881:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- Ungar L. Apolipoprotein E, gender, and Alzheimer's disease: an overlooked, but potent and promising interaction. Brain Imag. Behav. 2014;8:262–273. doi: 10.1007/s11682-013-9272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R.J., Van Bockstaele E., Bangasser D. Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol. Sci. 2013;34:437–444. doi: 10.1016/j.tips.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis J.D. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab. Clin. N. Am. 2013;42:201–225. doi: 10.1016/j.ecl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R. 2011. Gender Differences in Stress Response: Role of Developmental and Biological Determinants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington J.M. Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Horm. Behav. 2012;61:91–99. doi: 10.1016/j.yhbeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int. J. Dev. Neurosci.: Offic. J. Int. Soc. Dev. Neurosci. 1998;16:289–295. doi: 10.1016/s0736-5748(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Whirledge S., Cidlowski J.A. Estradiol antagonism of glucocorticoid-induced GILZ expression in human uterine epithelial cells and murine uterus. Endocrinology. 2013;154:499–510. doi: 10.1210/en.2012-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirledge S. Global gene expression analysis in human uterine epithelial cells defines new targets of glucocorticoid and estradiol antagonism. Biol. Reprod. 2013;89:66. doi: 10.1095/biolreprod.113.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526:443–447. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.S. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wilson R.S. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom. Med. 2007;69:47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- Wilson R.S. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wood S.K., Woods J.H. Corticotropin-releasing factor receptor-1: a therapeutic target for cardiac autonomic disturbances. Expert Opin. Ther. Targets. 2007;11:1401–1413. doi: 10.1517/14728222.11.11.1401. [DOI] [PubMed] [Google Scholar]
- Wood S.K. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol. Psychiatr. 2013;73:1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. Metabolism of Alzheimer beta-amyloid precursor protein: regulation by protein kinase A in intact cells and in a cell-free system. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4081–4084. doi: 10.1073/pnas.93.9.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Korszun A. Sex, trauma, stress hormones and depression. Mol. Psychiatr. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]