Abstract
Background
In this study we are using source localized neurofeedback to moderate tinnitus related distress by influencing neural activity of the target region as well as the connectivity within the default network.
Hypothesis
We hypothesize that up-training alpha and down-training beta and gamma activity in the posterior cingulate cortex has a moderating effect on tinnitus related distress by influencing neural activity of the target region as well as the connectivity within the default network and other functionally connected brain areas.
Methods
Fifty-eight patients with chronic tinnitus were included in the study. Twenty-three tinnitus patients received neurofeedback training of the posterior cingulate cortex with the aim of up-training alpha and down-training beta and gamma activity, while 17 patients underwent training of the lingual gyrus as a control situation. A second control group consisted of 18 tinnitus patients on a waiting list for future tinnitus treatment.
Results
This study revealed that neurofeedback training of the posterior cingulate cortex results in a significant decrease of tinnitus related distress. No significant effect on neural activity of the target region could be obtained. However, functional and effectivity connectivity changes were demonstrated between remote brain regions or functional networks as well as by altering cross frequency coupling of the posterior cingulate cortex.
Conclusion
This suggests that neurofeedback could remove the information, processed in beta and gamma, from the carrier wave, alpha, which transports the high frequency information and influences the salience attributed to the tinnitus sound. Based on the observation that much pathology is the result of an abnormal functional connectivity within and between neural networks various pathologies should be considered eligible candidates for the application of source localized EEG based neurofeedback training.
Keywords: Posterior cingulate cortex, Effective connectivity, Cross-frequency coupling, Distress
Graphical abstract
1. Introduction
Neurofeedback is a brain-computer interface method that makes it possible for users to gain voluntary control of their cortical oscillations by receiving direct feedback from their EEG (Congedo et al., 2004). In other words, humans can learn how to shape their brain electrical activity in a desired direction (Congedo et al., 2004) through operant conditioning (Sterman et al., 1970). Neurofeedback is considered efficacious for ADHD (Micoulaud-Franchi et al., 2014) and medically intractable epilepsy (Tan et al., 2009). In classical neurofeedback, usually only one recording electrode is used, recording electrical activity from widespread cortical areas. This has been successfully used in tinnitus in pilot studies (Dohrmann et al, 2007, Hartmann et al, 2014, Kahlbrock and Weisz, 2008). However, the development of source localized neurofeedback (Congedo et al., 2004, Cannon et al, 2009) permits to train specific targets in the brain, such as the anterior cingulate cortex and possibly the posterior cingulate cortex, potentially changing activity in the trained area and connectivity to/from the trained area (Cannon et al., 2009).
The posterior cingulate cortex is the most densely connected brain area (Tomasi and Volkow, 2010), part of the self-referential (Buckner et al., 2008, Svoboda et al., 2006) default mode network (Raichle et al., 2001) and its overall function is postulated to permit adaptation of the self to a changing internal and external environment (Pearson et al., 2011). This requires a predictive capacity, based on memory and related to the self. And indeed the default mode network is involved in remembering the past to predict the future (Schacter et al., 2007). It has 4 subregions, each with a specific function to permit this formidable task (Leech and Sharp, 2014): the precuneus is associated with attention, the ventral posterior cingulate cortex processes information from the internal world, the dorsal posterior cingulate cortex processes information from the external world, and the retrosplenial cortex which is connected to the ant thalamus and (para)hippocampus, is associated with memory processes (Leech and Sharp, 2014). The posterior cingulate cortex is also involved in tinnitus (De Ridder et al., 2011a, De Ridder et al., 2011b; Maudoux et al., 2012a, Maudoux et al., 2012b; Moazami-Goudarzi et al, 2010, Schecklmann et al, 2013, Silchenko et al, 2013, Vanneste and De Ridder, 2012a, Vanneste and De Ridder, 2012b, Vanneste and De Ridder, 2015a, Vanneste et al., 2012, Vanneste et al, 2010a, Weisz et al, 2014) and especially in tinnitus distress (De Ridder et al., 2011a, De Ridder et al., 2011b, Maudoux et al., 2012a, Maudoux et al., 2012b, Schecklmann et al, 2013, Silchenko et al, 2013, Vanneste and De Ridder, 2012a, Vanneste and De Ridder, 2012b, Vanneste and De Ridder, 2015a, Vanneste et al., 2012, Vanneste et al, 2010a), in other words, in how the self-adapts to an internally generated sound that is perceived as coming from the external world (Cannon et al., 2009).
Tinnitus, i.e. the perception of a sound in the absence of an external sound source, has been considered as an emergent property of multiple parallel networks, whereas at least two distinct neural networks have been involved, underpinning tinnitus related loudness and distress (De Ridder et al., 2011a, De Ridder et al., 2011b). Tinnitus is proposed to be a filling-in mechanism of reduced auditory input in order to reduce auditory sensory uncertainty (De Ridder et al., 2014). The loudness and distress networks intercommunicate only in distressed tinnitus patients via a specific and discrete functional connection, i.e. the connection between the parahippocampal area and the subgenual anterior cingulate cortex/ventromedial prefrontal cortex (Vanneste et al., 2013). The posterior cingulate cortex is not only a major hub of the default network but is as well involved in the tinnitus related distress network, in which distressed tinnitus patients have a decrease in alpha activity (Vanneste et al, 2010a, Vanneste et al., 2013), a frequency range that normally correlates positively with activity in the default network (Mantini et al., 2007). However, reduction of tinnitus related distress has been correlated with increased synchronization of alpha activity in the posterior cingulate cortex combined with decreased beta and gamma activity within the precuneus/posterior cingulate cortex (Vanneste and De Ridder, 2012b). Additionally, desynchronization of alpha activity seems to be related to cognitive processing (Nunez et al., 2001), which might suggest that distressed tinnitus patients are continuously actively engaged in processing the tinnitus sound resulting in a constant state of attentional processing. Based on these observations, we hypothesize that up-training alpha and down-training beta and gamma activity in the posterior cingulate cortex has a moderating effect on tinnitus related distress by influencing neural activity of the target region as well as the connectivity within the default network and other functionally connected brain areas.
2. Methodology
2.1. Subjects
Fifty-eight patients with chronic tinnitus were included in the study from the Tinnitus Research Initiative (TRI) clinic, Antwerp, Belgium. The mean age of the patients was 45.36 years (Sd = 9.54) and the mean tinnitus duration was 3.56 years (Sd = 4.23). Of these 58 patients, 23 tinnitus patients received NF training of the posterior cingulate cortex with the aim of up-training alpha and down-training beta and gamma activity, while 17 patients underwent training of the lingual gyrus as a control situation. The lingual gyrus is adjacent to the posterior cingulate cortex and the parahippocampal gyrus, but not involved in tinnitus distress processing. Its main function is related to visual rather than auditory processing. A second control group consisted of 18 tinnitus patients on a waiting list for future tinnitus treatment.
Individuals with pulsatile tinnitus, Ménière disease, otosclerosis, chronic headache, neurological disorders such as brain tumors, and individuals being treated for mental disorders were not included in the study in order to obtain a homogeneous sample. All patients included for this study first underwent a complete audiological, ENT, and neurological investigation. In addition, several technical investigations were performed, including MRI of the brain. Collection of the data was under approval of IRB UZA OGA85. All patients gave an informed consent.
2.2. Healthy control group
Group age and gender matched EEG data of a healthy control group (N = 22; M = 45.2 years; Sd = 10.02) was collected. None of these subjects were known to suffer from tinnitus. Exclusion criteria were known psychiatric or neurological illness, psychiatric history or drug/alcohol abuse, history of head injury (with loss of consciousness) or seizures, headache, or physical disability.
2.3. Questionnaires
All patients filled out a numeric rating scale (NRS) before and after the NF training measuring tinnitus loudness (‘How loud do you perceive your tinnitus?’: 0 = no tinnitus and 10 = as loud as imaginable). Moreover, to assess tinnitus severity all patients filled in the validated Dutch version of the Tinnitus Questionnaire (TQ) (Meeus et al., 2007; Vanneste et al., 2010b), originally published by Goebel and Hiller (1994), and shown to be a reliable measure for tinnitus-related distress (Vanneste et al., 2010b). The global TQ score can be computed to measure the general level of psychological and psychosomatic distress, with a further subdivision made to measure emotional and cognitive distress, intrusiveness, auditory perceptual difficulties, sleep disturbances, and somatic complaints. A 3-point scale is given for all 52 items, ranging from “true” (2 points) to “partly true” (1 point) and “not true” (0 points). The total score (from 0 to 84) was computed according to standard criteria published in previous work (Hiller and Goebel, 1992, Hiller et al., 1994, Meeus et al., 2007). Based on the total score on the TQ, participants were assigned to a distress category: slight (0–30 points; grade 1), moderate (31–46; grade 2), severe (47–59; grade 3), and very severe (60–84; grade 4). Furthermore, Goebel and Hiller (1994) stated that grade 4 tinnitus patients are psychologically decompensated, indicating that patients categorized into this group cannot cope with their tinnitus.
2.4. Neurofeedback training
The neurofeedback training was performed using Brain Tuner Version 1.4 (http://www.mitsar-medical.com) from Mitsar. The EEG mitsar has 19 electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1 O2) in the standard 10–20 International placement, and impedances were checked to remain below 5 kΩ. We used a bipolar montage and the ground electrode on the skull, which is optimal for artifact rejection. Training protocols were individually selected, combining behavioral information and data from the QEEG. LORETA source localization permits the selection of any region of the brain for feedback of the current density, using 5 voxels as ROI, which are selected based on MNI coordinates (see Table 1). The 5 voxels are chosen with one central voxel in addition to an inferior, superior, medial and lateral voxel in the form of a cross. The current densities for the chosen voxels are computed continuously using Fast Fourier Transformation and the inverse solution LORETA software. The current density signal is computed as a ratio of powers in two bands and can be fed back by changing the height of a bar on the computer screen. More specifically, the protocol was designed to up-regulate alpha activity (8 Hz–12 Hz) and suppress beta and gamma activity (12.5 Hz–44 Hz) in a region corresponding to the posterior cingulate cortex in 23 patients or the lingual gyrus in 17 patients. This feedback parameter is proportionally presented as a visual beam on a screen in front of the patients.
Table 1.
MNI-coordinates for neurofeedback stimulation for the posterior cingulate cortex and the lingual gyrus.
| X | Y | Z | |
|---|---|---|---|
| Posterior cingulate cortex | 0 | −50 | 25 |
| 0 | −15 | 35 | |
| 0 | −33 | 31 | |
| 1 | −15 | 35 | |
| −1 | −15 | 35 | |
| Lingual gyrus | −28 | −60 | −4 |
| −25 | −60 | −5 | |
| −21 | −59 | −1 | |
| −19 | −67 | −2 | |
| −32 | −64 | −3 |
Patients undergoing source localized neurofeedback participated in 15 sessions of neurofeedback training, in which they were asked to focus on increasing the visual beam height, representing the ratio of alpha and beta/gamma activity. Each session lasted 45 min, with 30–35 min of training. A 2 min' baseline was recorded before the training procedure to determine the level of threshold for reinforcement. This baseline was sometimes adjusted during the session, however, to regulate the percentage of time above threshold between 70% and 85%. After 5 min of training, there was a 1 min pause. During baseline and pauses, patients were asked to relax; to breathe deeply, not focusing on anything special; and to ‘‘lower their shoulders.’’. Every training session started with simple visual feedback: a green bar on a grey screen following the dynamics of the feedback parameters and a horizontal threshold line in the middle of the screen. It was explained to patients that this was a method of increasing their attention skills, and that the feedback was controlled by their brainwaves reflecting levels of attention. They were asked to raise the bar above the threshold without tightening their muscles and to notice their own mental state when they succeeded. The percentage of time above threshold was shown at the top of the screen.
2.5. EEG recording: pre -post treatment
Resting state EEG was collected immediately before treatment and immediately after the last treatment (15 sessions). EEGs were obtained in a fully lighted room with each participant sitting upright in a comfortable chair. The EEG was sampled with 19 electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1 O2) in the standard 10–20 International placement referenced to linked ears and impedances were checked to remain below 5 kΩ. Data were collected with the eyes closed during 5 min, from which we removed the artifacts. Subsequently, we collected the first 100 2-s epochs of the remaining EEG (sampling rate = 1024 Hz, band passed 0.15–200 Hz). Data were resampled to 128 Hz, band-pass filtered (fast Fourier transform filter) to 2–44 Hz. These data were transposed into Eureka! Software (Congedo, 2002), plotted and carefully inspected for manual and ICA dependent artifact-rejection. All episodic artifacts including eye blinks, eye movements, teeth clenching, body movement, or ECG artifacts were removed from the stream of the EEG. Average Fourier cross-spectral matrices were computed for the frequency bands delta (2–3.5 Hz), theta (4–7.5 Hz), alpha (8–12 Hz), beta (12.5–30 Hz) and gamma (30.5–44 Hz).
2.5.1. Source localization
Standardized low-resolution brain electromagnetic tomography (sLORETA) (Pascual-Marqui, 2002) was used to estimate the intracerebral electrical sources that generated the scalp-recorded activity in each of the eight frequency bands. sLORETA computes electric neuronal activity as current density (A/m2) without assuming a predefined number of active sources. The sLORETA solution space consists of 6239 voxels (voxel size: 5-5-5 mm) and is restricted to cortical grey matter and hippocampi, as defined by digitized MNI 152 template (Fuchs et al., 2002). Scalp electrode coordinates on the MNI brain are derived from the international 10–20 system (Jurcak et al., 2007). The tomography sLORETA has received considerable validation from studies combining LORETA with other more established localization methods, such as fMRI (Mulert et al, 2004, Vitacco et al, 2002), structural MRI (Worrell et al., 2000) and PET (Dierks et al, 2000, Pizzagalli et al, 2004, Zumsteg et al, 2005). Further sLORETA validation has been based on accepting as ground truth the localization findings obtained from invasive, implanted depth electrodes, in which case there are several studies in epilepsy (Zumsteg et al, 2006, Zumsteg et al., 2006a) and cognitive event-related potentials (Volpe et al., 2007).
2.6. Region of interest analysis
Furthermore, the log-transformed electric current density was averaged across all voxels belonging to the region of interest (ROI), for the posterior cingulate cortex and lingual gyrus for delta (2–3.5 Hz), theta (4–7.5 Hz), alpha (8–12 Hz), beta (12.5–30 Hz), and gamma (30.5–44 Hz) frequency band.
2.7. Power to phase nesting
It has been proposed that alpha-beta and alpha-gamma coupling, e.g. by nesting, is an effective way of communication between cortically distant areas (Canolty et al, 2006, Roux and Uhlhaas, 2014). To verify whether a difference in alpha-beta and alpha-gamma nesting was present at the posterior cingulate cortex or lingual gyrus in any of the three groups before and after neurofeedback, alpha-beta and alpha-gamma nesting was computed as follows: first the time-series for the x, y, z component of the sLORETA current for each ROI was obtained, filtered in the alpha (8–12 Hz), beta (12.5–30 Hz) and gamma (30.5–44 Hz) frequency band-pass regions, representing the time series of the current in the three orthogonal directions in space. In each frequency band and for each ROI (i.e. posterior cingulate cortex and lingual gyrus) a principal component analysis was computed and the first component was retained for theta and gamma. The Hilbert Transform was then computed on the beta or gamma component and the signal envelope retained. Finally, the Pearson correlation between the alpha component and the envelope of the beta/gamma envelope was computed on the total sample.
2.8. Functional connectivity
Brain connectivity can refer to a pattern of anatomical links (i.e. structural connectivity), statistical dependencies (i.e. functional connectivity), or causal interactions (i.e. effective connectivity) between distinct regions within the brain. In this study we focused on both functional and effective connectivity of various brain regions. Coherence and phase synchronization between time series corresponding to different spatial locations are usually interpreted as indicators of the ‘‘functional connectivity’’. However, any measure of dependence is highly contaminated with an instantaneous, non-physiological contribution due to volume conduction and low spatial resolution (Pascual-Marqui, 2007a). Therefore Pascual-Marqui introduced a new technique (i.e. Hermitian covariance matrices) that removes this confounding factor considerably (Pascual-Marqui, 2007b). As such, this measure of dependence can be applied to any number of brain areas jointly, i.e. distributed cortical networks, whose activity can be estimated with sLORETA. Measures of linear dependence (coherence) between the multivariate time series are defined. The measures are expressed as the sum of lagged dependence and instantaneous dependence. The measures are non-negative, and take the value zero only when there is independence of the pertinent type and are defined in the frequency domains: delta (2–3.5 Hz), theta (4–7.5 Hz), alpha (8–12 Hz), beta (12.5–30 Hz), and gamma (30.5–44 Hz). Based on this principle, lagged linear connectivity was calculated. Regions of interest were defined based on previous brain research on tinnitus and included the posterior cingulate cortex, dorsal anterior cingulate cortex, subgenual anterior cingulate cortex and parahippocampal area.
2.9. Effective connectivity
Granger causality reflects the strength of effective connectivity (i.e. causal interactions) from one region to another by quantifying how much the signal in the seed region is able to predict the signal in the target region (Geweke, 1982, Granger, 1969). In other words, it can be considered as a directional functional connectivity. Granger causality is based on formulating a multivariate autoregressive model, and calculating the corresponding partial coherences after setting all irrelevant connections to zero. All technical details can be found in (Pascual-Marqui et al., 2014). In general, the autoregressive coefficients correspond to Granger causality (Granger, 1969, Valdes-Sosa et al, 2011). It is defined as the log-ratio between the error variance of a reduced model, which predicts one-time series based only on its own past values, and that of the full model, which in addition, includes the past values of another time series. It is important to note that Granger causality does not imply anatomical connectivity between regions but directional functional connectivity between two sources. As effective connectivity is a reflection of directional functional connectivity, only the frequency band that was significantly different in the functional connectivity analysis was selected for analyzing Granger causality.
2.10. Statistical analysis
Calculations were performed using SPSS 22 software package. A repeated measures analysis of variance (ANOVA) was performed with tinnitus loudness pre and post neurofeedback as within-subject variable and group (posterior cingulate cortex, lingual gyrus and waiting list) as between-subject variable. Likewise, a repeated measures ANOVA was performed with tinnitus distress pre- and post-treatment as within-subject variable and group (posterior cingulate cortex, lingual gyrus and waiting list) as between-subject variable.
In addition, we evaluated the effect of neurofeedback in tinnitus patients with low (i.e. grade I and II) and high (i.e. grade III and IV) levels of distress, according to the Tinnitus Questionnaire, separately. Therefore, we again performed a repeated measures ANOVA for both groups with tinnitus distress as within-subject variable and group (posterior cingulate cortex, lingual gyrus and waiting list) as between-subject variable. To further interpret the interaction effect, we conducted a simple contrast analysis.
Differences in brain electrical activity between pre and post neurofeedback for the three groups (posterior cingulate cortex, lingual gyrus and waiting list) was computed, as well as differences before and after the treatment for the PCC treated tinnitus group in comparison to healthy controls. sLORETA was used to perform voxel-by-voxel between-condition comparisons of the current density distribution. Non-parametric statistical analyses of sLORETA images (statistical non-parametric mapping; SnPM) were performed for each contrast using sLORETA-built-in voxel-wise randomization test (5000 permutations) and t-statistics for paired groups (p < 0.05). The SnPM methodology does not require any assumption of Gaussianity and corrects for all multiple comparisons.
To identify differences in the log-transformed current density between the 2 ROI's, i.e. the posterior cingulate cortex and lingual gyrus, we performed a repeated measures ANOVA for the 2 ROI's and all frequency bands before and after the neurofeedback. In addition, we also calculated the log-transformed current density for the posterior cingulate cortex for the healthy controls. We applied a MANOVA to compare the tinnitus group who received posterior cingulate cortex neurofeedback, before and after the treatment, with the healthy controls for all frequency bands. If significant, we applied a univariate ANOVA to compare the difference at a specific frequency band.
To evaluate coupling, Pearson autocorrelations were calculated for the three groups, i.e. posterior cingulate cortex, lingual gyrus and waiting list, before and after neurofeedback at the posterior cingulate cortex. Pearson correlations were considered significant if p < 0.05. Moreover, a repeated measures ANOVA was performed to assess the differences before and after neurofeedback training in power to phase coupling for alpha-beta and alpha-gamma nesting. If significant, we applied a paired t-test to look at the specific differences for alpha-beta and alpha-gamma nesting respectively. In addition, we also calculated the alpha-beta and alpha-gamma nesting for the healthy control group. A comparison was made between the PCC neurofeedback treated tinnitus group and the healthy controls respectively before and after neurofeedback using a MANOVA including alpha-beta and alpha-gamma nesting. If significant, we applied a post hoc test to look at the specific differences for alpha-beta and alpha-gamma coupling respectively.
Finally, to identify differences in functional connectivity pre and post neurofeedback, connectivity contrast maps were calculated through multiple ROI-by-ROI comparisons using t-statistics. The significance threshold was based on a permutation test with 5000 permutations. A repeated measures ANOVA was calculated between the Granger causality outcome measure per patient of the posterior cingulate cortex, lingual gyrus and waiting list group. In addition, we also calculated the Granger causality for the healthy control group for the alpha frequency band. A comparison was made between the healthy control group and before and after neurofeedback treatment in the tinnitus group who received PCC neurofeedback using a MANOVA including. If significant we applied a post-hoc test to look at the specific differences for functional connections.
3. Results
3.1. Behavioral measures
3.1.1. Loudness
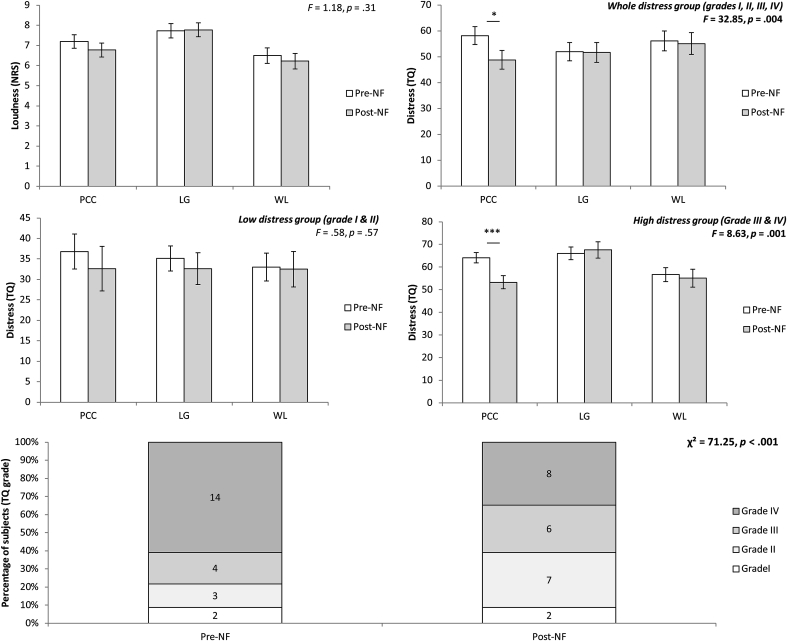
A repeated measures ANOVA revealed no significant main effect of treatment (F = 2.48, p = 0.12) for tinnitus loudness and no significant interaction effect could be demonstrated for the group (posterior cingulate cortex, lingual gyrus or waiting list) (F = 0.43, p = 0.65). Fig. 1 gives an overview.
Fig. 1.
A comparison between pre and post loudness for the NF group targeting the posterior cingulate cortex, lingual gyrus and the waiting list group revealed no significant effects. A comparison between pre and post distress for the NF group targeting the posterior cingulate cortex, the lingual gyrus and the waiting list group including all levels of distress (A), low distress (B) and high distress (C). An additional comparison demonstrating the distribution of distress grades before and after treatment for the posterior cingulate cortex, lingual gyrus and the waiting list group.
3.1.2. Distress
A repeated measures ANOVA revealed a significant main effect (F = 6.49, p = 0.014), indicating that the distress level was decreased after treatment (M = 46.16, Sd = 17.85) in comparison to the distress level before treatment (M = 51.12, Sd = 17.86). This effect was further mediated by the group the patient was assigned to (F = 5.69, p = 0.006). That is, neurofeedback training of the posterior cingulate cortex revealed a significant effect (F = 22.40, p = 0.00002), demonstrating that the distress level post neurofeedback (M = 47.78, Sd = 15.22) was significantly decreased compared to pre neurofeedback (M = 58.17, Sd = 14.36). No significant effect was obtained when the lingual gyrus was targeted with neurofeedback (F = 0.29, p = 0.59) or in the waiting list group (F = 0.0089, p = 0.93). An overview can been found in Fig. 1.
When we only focus on tinnitus patients with low levels of distress (grade I and II) no significant effect could be obtained comparing pre-versus post neurofeedback (F = 0.88, p = 0.36) as well as for the interaction effect with group (F = 1.20, p = 0.33) (Fig. 2B). When we apply the same analysis for patients with high levels of distress (grade III and IV), no significant main effect could be obtained (F = 2.66, p = 0.11). However, a significant interaction effect between treatment and group exits (F = 4.81, p = 0.016). A simple contrast analysis revealed that a significant effect could be obtained for the patient group undergoing neurofeedback training of the posterior cingulate cortex (F = 22.63, p = 0.00005) indicating that after neurofeedback (M = 52.00, Sd = 13.43) a decrease was obtained for the distress level in comparison to pre neurofeedback (M = 64.11, Sd = 8.07). No significant effect could be obtained when targeting the lingual gyrus (F = 0.21, p = 0.65) or if patients were in the waiting list group (F = 0.22, p = 0.64). An overview is demonstrated in Fig. 1.
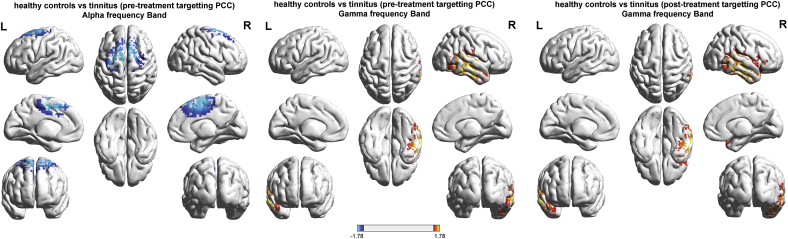
Fig. 2.
Comparison between healthy controls subjects and tinnitus patients before and after neurofeedback treatment targeting the posterior cingulate cortex (PCC). Left and mid panel show decreased (blue) activity in the posterior cingulate cortex for tinnitus patients in comparison to healthy controls subjects for the alpha frequency band and increased (yellow) activity at the auditory cortex for tinnitus patients in comparison to healthy controls subjects before neurofeedback training. The right panel shows increased (yellow) activity at the auditory cortex for tinnitus patients after the neurofeedback training in comparison to healthy controls subjects for the gamma frequency band. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To further explore the data, we sought to determine if patients changed in grade after treatment. For neurofeedback targeting the posterior cingulate cortex, we see that 38.46% of the patients who were a grade 4 go to a lower grade, while the number of patients who became a grade 2 after treatment is 57.14% (Fig. 2D). These results are not present for neurofeedback targeting the LC or the waiting list group. See Fig. 1.
3.2. Neuropsychological measures
3.2.1. Source localization
Source analysis comparing pre-versus post-treatment for the three groups, i.e. posterior cingulate cortex, lingual gyrus and waiting list group, did not reveal significant effects for the delta, theta, alpha, beta and gamma frequency bands.
A comparison between a control group and the posterior cingulate cortex neurofeedback tinnitus group pre-treatment showed a significant decrease at the posterior cingulate cortex for the alpha frequency band and a significant increase at the auditory cortex for the gamma frequency band for the posterior cingulate cortex treated neurofeedback tinnitus group in comparison to healthy controls (Fig. 2). No significant effects were obtained for the delta, theta and beta frequency band.
After neurofeedback, a comparison between a control group and the posterior cingulate cortex group showed a significant increase at the auditory cortex for the gamma frequency band for the posterior cingulate cortex neurofeedback treated tinnitus group in comparison to healthy controls (Fig. 2). No significant effects were obtained for the delta, theta, alpha and beta frequency band.
3.2.2. Region of interest
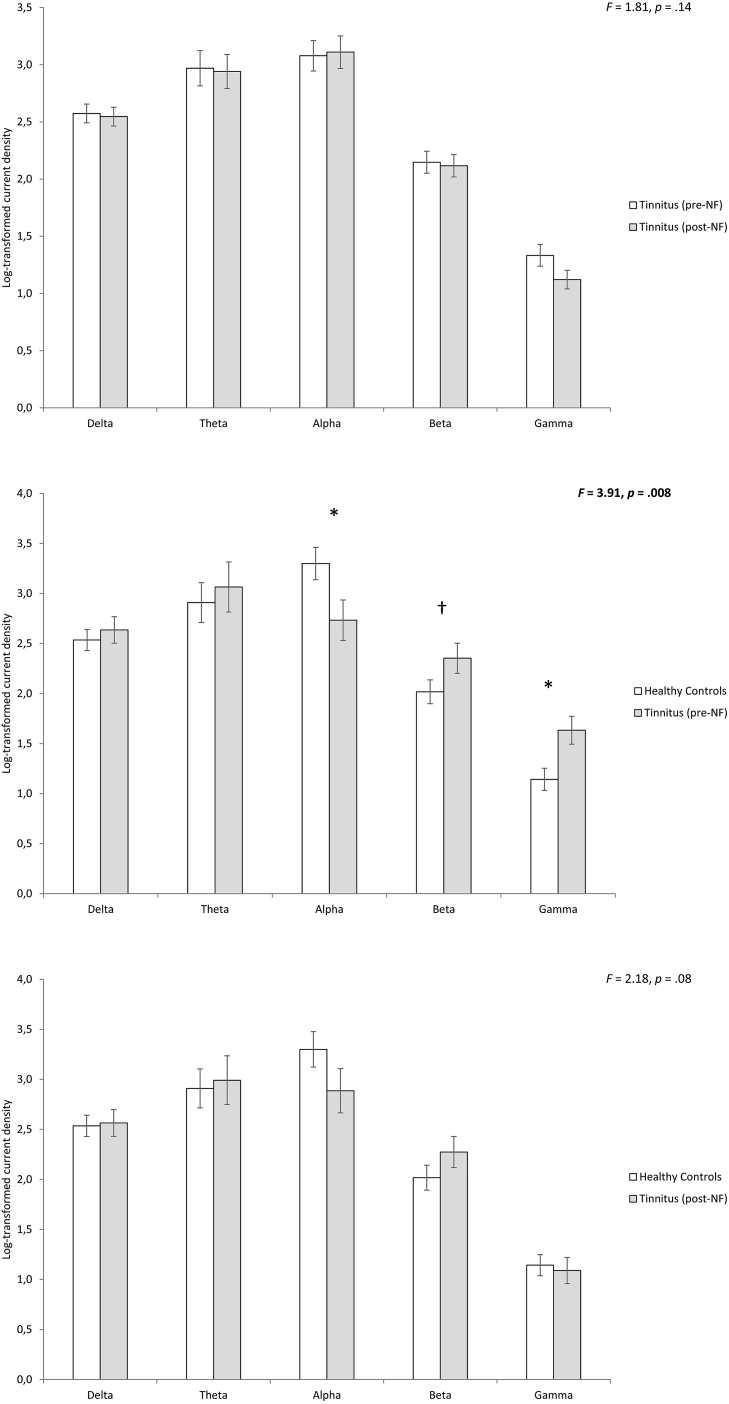
A region interest analysis of the activity (= current density) in the posterior cingulate cortex and the lingual gyrus could not reveal a significant effect for the posterior cingulate cortex, lingual gyrus and waiting list group in any of the frequency bands, including delta, theta, alpha, beta and gamma (Fig. 3).
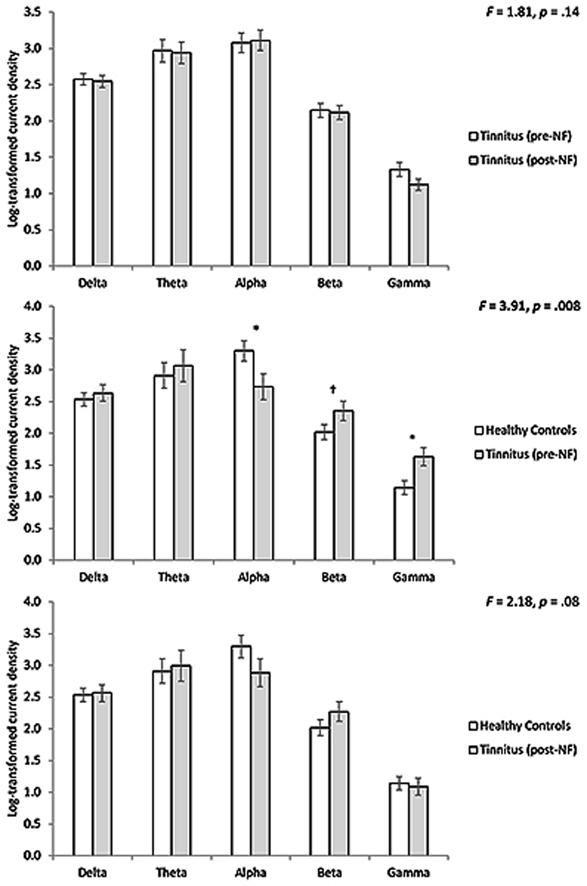
Fig. 3.
A region of interest analysis for the posterior cingulate cortex shows between pre- and post-treatment targeting the posterior cingulate cortex no significant effects for the tinnitus patients (top panel). A region of interest analysis for the posterior cingulate cortex between the tinnitus patients pre neurofeedback treatment in comparison to healthy controls shows a significant different for the alpha, beta and gamma frequency bands (Mid panel). A region of interest analysis for the posterior cingulate cortex between the tinnitus patients post neurofeedback treatment in comparison to healthy controls shows no significant differences (Bottom panel).
A comparison for the posterior cingulate cortex neurofeedback treated tinnitus group pre-treatment in comparison with the healthy control subjects revealed a significant effect (F = 3.91, p = 0.008) (Fig. 3). A univariate test shows that the effect is for alpha (F = 4.77, p = 0.036) and gamma (F = 7.59, p = 0.009) frequency bands. For alpha we noted a reduced log-transformed current density for the healthy controls in comparison to the tinnitus group, while for beta and gamma we found an increased log-transformed current density for the tinnitus groups in comparison to the controls. For beta, no significant effect was obtained although the effect was close to significance (F = 3.06, p = 0.089), showing a similar pattern as for the gamma frequency band. No significant effects were obtained for the delta and theta frequency bands.
A comparison for the posterior cingulate cortex neurofeedback treated tinnitus group post-treatment in comparison with the healthy control subjects revealed no significant effects for the delta, theta, alpha, beta and gamma frequency bands (Fig. 3).
3.2.3. Phase-amplitude nesting
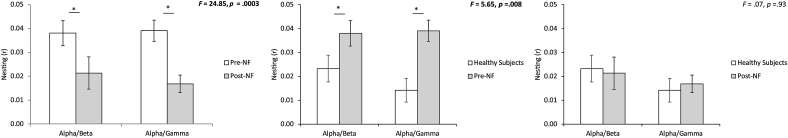
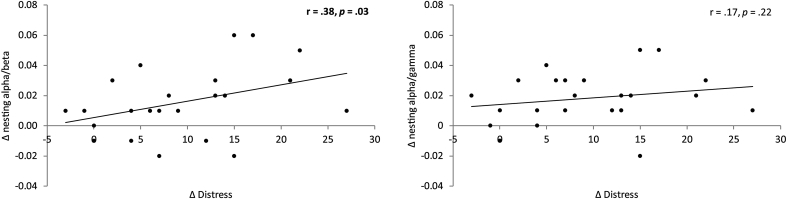
Phase-amplitude cross-frequency coupling (= nesting) analysis revealed when comparing pre- and post-neurofeedback for the posterior cingulate cortex a general significant effect (F = 16.41, p = 0.0003). Looking at the alpha-beta (F = 4.67, p = 0.04) and the alpha-gamma nesting (F = 24.85, p = 0.0002) revealed a decrease in nesting at the posterior cingulate cortex after neurofeedback training targeting the posterior cingulate cortex compared to before the neurofeedback training (see Fig. 4). In addition, a significant correlation was obtained between the difference between pre - post neurofeedback for the posterior cingulate cortex treated neurofeedback tinnitus group and the reduction in distress score (pre –post TQ) for the alpha-beta nesting (r = 0.38, p = 0.03). This indicates the higher the difference obtained for distress, the more a reduction in nesting was obtained (Fig. 5). A similar analysis for the alpha-gamma nesting revealed no significant effect (r = 0.17, p = 0.22) (Fig. 5). For loudness no correlation could be obtained for both alpha-beta and alpha-gamma nesting.
Fig. 4.
Phase to power nesting for alpha/beta and alpha/gamma decreases at the level of the posterior cingulate cortex when comparing pre and post neurofeedback (Left panel). Phase to power nesting for alpha/beta and alpha/gamma between healthy controls and tinnitus group pre-treatment shows a significant increased coupling for the tinnitus patients in comparison (Mid panel). After the treatment targeting the posterior cingulate cortex no significant effect for alpha-beta and alpha-gamma nesting was obtained between the tinnitus group and the healthy controls (Right Panel).
Fig. 5.
A correlation between the difference between pre - post neurofeedback for the posterior cingulate cortex and the reduction in distress score (pre –post TQ) for the alpha-beta nesting (left panel) and alpha-gamma nesting (right panel).
A comparison for the posterior cingulate cortex tinnitus group pre-treatment in comparison with the healthy control subjects for alpha-beta and alpha-gamma nesting revealed a significant effect (F = 5.62, p = 0.008). A univariate test shows that the effect is for both alpha-beta nesting (F = 7.74, p = 0.013) and alpha-gamma (F = 11.04, p = 0.002) nesting, indicating that the nesting is higher in the tinnitus group than for the healthy controls (Fig. 4).
After the treatment targeting the posterior cingulate cortex no significant difference for alpha-beta and alpha-gamma nesting was found between the tinnitus group and the healthy controls (F = 0.07, p = 0.93) (Fig. 4).
No significant effects were obtained for the group targeting the lingual gyrus and the waiting list group. A similar analysis using the lingual gyrus as the region of interest could not reveal a significant effect in one of the three groups.
3.2.4. Functional connectivity (lagged phase synchronization)
3.2.4.1. Loudness network
A comparison for the functional connectivity between the auditory cortex and the posterior cingulate cortex for the neurofeedback group targeting the posterior cingulate cortex revealed no significant effects for the delta, theta, alpha, beta and gamma frequency bands. In addition, no significant effect could be demonstrated when looking at the functional connectivity between the posterior cingulate cortex and auditory cortex.
A comparison between healthy controls and the tinnitus group pre- and post-treatment, respectively, targeting the posterior cingulate cortex revealed no significant effects for functional connectivity between the auditory cortex and the posterior cingulate cortex for the delta, theta, alpha, beta and gamma frequency bands.
An identical analysis looking at the functional connectivity between the auditory cortex and the lingual gyrus, as well as the posterior cingulate cortex for the neurofeedback group targeting the lingual gyrus revealed no significant effects for the delta, theta, alpha, beta, and gamma frequency bands.
For the waiting list, no significant effect was obtained between lingual gyrus and posterior cingulate cortex and respectively the auditory cortex for the delta, theta, alpha, beta and gamma frequency bands.
3.2.4.2. Distress network
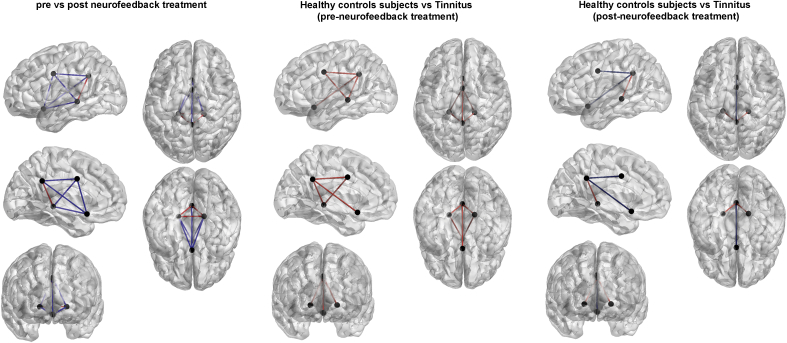
For posterior cingulate cortex neurofeedback, a comparison for the functional connectivity between the different areas involved in distress (subgenual anterior cingulate cortex, dorsal anterior cingulate cortex, parahippocampus) (Vanneste et al., 2010a) revealed a significant effect (t = 4.28, p = 0.047) for the alpha frequency band (Fig. 6). Decreased functional connectivity was observed between the posterior cingulate cortex and dorsal anterior cingulate cortex, the posterior cingulate cortex and subgenual anterior cingulate cortex, the subgenual anterior cingulate cortex and dorsal anterior cingulate cortex, the parahippocampus and subgenual anterior cingulate cortex, while an increased connectivity between the posterior cingulate cortex and the parahippocampus was revealed. No significant effects were obtained for the delta, theta, beta and gamma frequency bands.
Fig. 6.
Functional connectivity for the alpha frequency band changes for tinnitus patients receiving neurofeedback targeting the posterior cingulate cortex when comparing pre-versus post-treatment (Left panel). Functional connectivity for the alpha frequency band between healthy controls and tinnitus patients before neurofeedback training targeting the posterior cingulate cortex. (Mid panel). Functional connectivity for the alpha frequency band between healthy controls and tinnitus patients after neurofeedback training targeting the posterior cingulate cortex. (Right panel). Red: increased connectivity, Blue: decreased connectivity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A comparison between the healthy control subjects and the tinnitus group before the treatment targeting the posterior cingulate cortex for the alpha frequency band (Fig. 6) showed increased connectivity between the posterior cingulate cortex and respectively dorsal anterior cingulate cortex, the subgenual anterior cingulate cortex and the parahippocampus for the tinnitus group. In addition, also increased connectivity was identified between the dorsal anterior cingulate cortex and the parahippocampus for the tinnitus group in comparison to the healthy controls for the alpha frequency band. No significant effects were obtained for the delta, theta, beta and gamma frequency bands.
A comparison between the healthy control subjects and the tinnitus group after the treatment targeting the posterior cingulate cortex for the alpha frequency band (Fig. 6) identified increased connectivity between the posterior cingulate cortex and the parahippocampus. Also, decreased connectivity was found for the alpha frequency band between the posterior cingulate cortex and respectively dorsal anterior cingulate cortex as well as the subgenual anterior cingulate cortex. Furthermore, decreased connectivity between the subgenual anterior cingulate cortex and the parahippocampus was identified for the tinnitus group in comparison to the healthy controls. No significant effects were obtained for the delta, theta, beta and gamma frequency bands.
When performing the same analysis for the distress network for neurofeedback of the lingual gyrus, no significant effects for the delta, theta, alpha, beta and gamma frequency bands were revealed. For the waiting list group, no significant effects could be obtained for the delta, theta, alpha, beta and gamma frequency band within the distress network.
3.2.5. Effective connectivity
3.2.5.1. Loudness network
When comparing the effective connectivity between the auditory cortex and the posterior cingulate cortex and the auditory cortex and the lingual gyrus for both the neurofeedback groups targeting the posterior cingulate cortex and the lingual gyrus, no significant effects of the delta, theta, alpha, beta, and gamma frequency bands could be obtained. Similarly, no significant results were present in the waiting list group.
A comparison between healthy controls and the tinnitus group targeting the posterior cingulate cortex revealed no significant effects for effective connectivity in the posterior cingulate cortex, respectively pre and -post-treatment, for the delta, theta, alpha, beta and gamma frequency bands.
3.2.5.2. Distress network
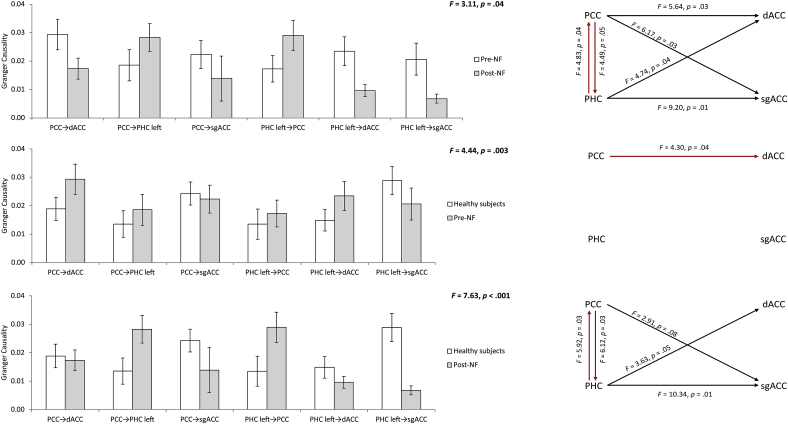
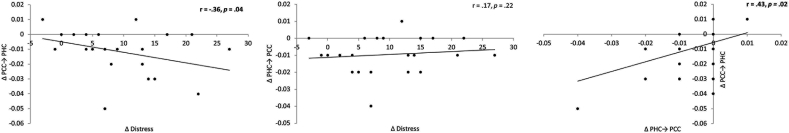
Comparing effective connectivity before and after neurofeedback training targeting the posterior cingulate cortex revealed a significant effect (F = 3.11, p = 0.04) (Fig. 7). A post hoc analysis showed a decrease for the alpha frequency from the posterior cingulate cortex to the dorsal anterior cingulate cortex (F = 5.64, p = 0.03), from the posterior cingulate cortex to the subgenual anterior cingulate cortex (F = 6.17, p = 0.03), from the parahippocampus to the subgenual anterior cingulate cortex (F = 9.20, p = 0.01) and an increase from the posterior cingulate cortex and parahippocampus (F = 4.49, p = 0.05) as well as from the parahippocampus to posterior cingulate cortex (F = 4.83, p = 0.04). No significant effects were obtained for the delta, theta, beta, and gamma frequency band. In addition, a significant correlation was obtained between the difference between pre - post effective connectivity from the posterior cingulate cortex to the parahippocampus and the reduction in distress score (pre –post TQ) (r = −0.36, p = 0.04) (Fig. 8). This indicates the stronger the effective connectivity differences (pre-post) from the posterior cingulate cortex to the parahippocampus, the stronger the reduction is distress is. A similar analysis between the difference pre - post effective connectivity from the parahipocampus to the posterior cingulate cortex and the reduction in distress score (pre –post TQ) was not significant (r = 0.17, p = 0.22) (Fig. 7). However, a correlation between pre - post effective connectivity from the posterior cingulate cortex to the parahippocampus and from the posterior cingulate cortex to the parahippocampus showed a significant positive correlation (r = 0.43, p = 0.02) (Fig. 8). A similar analysis for other connections and distress revealed no significant effects. In addition, a similar analysis for loudness revealed no significant effect.
Fig. 7.
Effective connectivity for the distress network for the alpha frequency band. Comparing effective connectivity before and after neurofeedback training targeting the posterior cingulate cortex revealed a significant effect (Top panel). A comparison between healthy controls and the tinnitus group targeting the posterior cingulate cortex before treatment showed a significant effect (Mid panel). A comparison between healthy controls and the tinnitus group targeting the posterior cingulate cortex after treatment indicated a significant effect (Bottom panel).
Fig. 8.
Correlation between the reduction in distress score (pre –post TQ and changes in effective connectivity from the posterior cingulate cortex (PCC) to the parahippocampus (PHC) for the distress network (pre-post) for the alpha frequency band shows a negative correlation (Left panel). Correlation between the reduction in distress score (pre –post TQ and changes in effective connectivity from the parahippocampus (PHC) to the posterior cingulate cortex (PCC) to for the distress network (pre-post) for the alpha frequency band shows no significant effect correlation (Mid panel). Correlation between the effective connectivity from the posterior cingulate cortex (PCC) to the parahippocampus (PHC) and the parahippocampus (PHC) to the posterior cingulate cortex (PCC) (pre-post) for the alpha frequency band shows positive correlation (Right panel).
A comparison between healthy controls and the tinnitus group targeting the posterior cingulate cortex before treatment showed a significant effect (F = 5.64, p = 0.03) (Fig. 7). An increase was demonstrated for the alpha frequency from the parahippocampus to the dorsal anterior cingulate cortex (F = 4.30, p = 0.04) for the tinnitus group. No other effects were obtained.
A comparison between healthy controls and the tinnitus group targeting the posterior cingulate cortex after treatment indicated a significant effect (F = 7.63, p < 0.001) (Fig. 8). We found an increase in granger causality from the posterior cingulate cortex to the parahippocampus (F = 6.12, p = 0.03) and from the parahippocampus to the posterior cingulate cortex (F = 5.92, p = 0.03). Also reduced Granger causality was noted from the parahippocampus to the subgenual anterior cingulate cortex (F = 10.34, p = 0.01), from posterior cingulate cortex to the subgenual anterior cingulate cortex (F = 2.91, p = 0.08), and from parahippocampus to the dorsal anterior cingulate cortex (F = 3.63, p = 0.05).
A paired t-test to compare the effective connectivity pre- and post-treatment when targeting the lingual gyrus with neurofeedback training or when patients were in the waiting list group could not reveal a significant effect for the delta, theta, alpha, beta, and gamma frequency band.
4. Discussion
The main goal of this study was to apply neurofeedback training of the posterior cingulate cortex in patients with chronic tinnitus in an attempt to reduce tinnitus related distress via normalization of brain activity and connectivity. This was pursued by up-regulation of alpha activity, i.e. the normal resting state activity of the posterior cingulate cortex, which is decreased in tinnitus distress (Vanneste et al., 2010a) and down-regulation of beta and gamma activity and by assessing the effect of neurofeedback training on neural activity of the target region as well as on functional and effective connectivity and on cross-frequency coupling. This study revealed that neurofeedback training of the posterior cingulate cortex results in a significant decrease of tinnitus related distress, mainly due to its impact on highly distressed patients, i.e. grade III (very severely distressed) and IV (extremely distressed with psychological decompensation). An unexpected (see (Cannon et al., 2007)) but very important finding is that there was no significant effect on neural activity of the target region, i.e. the alpha current density did not increase and the beta current density did not decrease, even though there was a clinical improvement, suggesting that activity of the posterior cingulate cortex is not the determining factor in the clinical phenomenology per se. However, a comparison with control subjects showed that before the treatment the tinnitus patients were characterized by a decrease in alpha activity, which disappeared after the neurofeedback treatment. The observed changes of distress modulation without loudness modulation have been described after cingulotomies, in which a lesion is made in the dorsal part of the anterior cingulate cortex (Beard, 1965). Previous studies suggest that gamma activity in the auditory cortex is related to tinnitus loudness (De Ridder et al., 2015, van der Loo et al, 2009). In comparison to healthy controls, tinnitus patients showed increased gamma band activity in the auditory cortex both pre and post-treatment. These findings could explain why the loudness is not modified by neurofeedback training targeting the posterior cingulate cortex, as the gamma activity within the auditory cortex is not modulated. In this study, the functional connectivity between posterior cingulate cortex and dorsal anterior cingulate cortex and effective connectivity from the posterior cingulate cortex to the dorsal anterior cingulate cortex is decreased, which could exert a similar suppressing effect on the dorsal anterior cingulate cortex. This finding was further confirmed, showing that in comparison to healthy controls the functional and effective connectivity from the posterior cingulate cortex to the dorsal anterior cingulate cortex is decreased.
Interestingly, training of a brain area not related to tinnitus (distress), i.e. the lingual gyrus, could neither induce a reduction of tinnitus loudness nor a reduction of tinnitus related distress, suggesting that nonspecific training does not result in a therapeutic effect, and that the clinical beneficial effect is not an aspecific effect, but that neurofeedback training has to access the network involved in the clinical picture.
It has been previously demonstrated that tinnitus loudness and distress are related to distinct and separable neural networks in that tinnitus related distress correlates with alpha and beta activity in the dorsal anterior cingulate while the amount of perceived distress is related to increased alpha activity in a network comprising the amygdala - subgenual anterior cingulate cortex – insula - parahippocampus area and decreased alpha activity in the posterior cingulate cortex, precuneus and dorsolateral prefrontal cortex (Vanneste et al., 2010a). An independent component analysis revealed that distress correlates with increased alpha activity in the subgenual anterior cingulate cortex/ventromedial prefrontal cortex and increased beta activity in the dorsal anterior cingulate cortex, in addition to decreased alpha and beta activity in the posterior cingulate cortex (De Ridder et al., 2011a, De Ridder et al., 2011b, Vanneste et al., 2013). This is possibly causally related as moderating tinnitus related distress by the intake of alcohol was associated with decreased beta and gamma activity and increased alpha activity in the posterior cingulate cortex extending into the precuneus (Vanneste and De Ridder, 2012b).
Communication between cortically distant areas has been proposed to be coordinated via cross-frequency coupling, that is, where properties of two different frequency signals become correlated (Singer, 2009, Salazar et al, 2012, Havenith et al, 2011). Importantly, it has been suggested that low-frequencies modulate activity over large spatial regions in long temporal windows, whereas high frequencies modulate activity over small spatial regions and short temporal windows (von Stein and Sarnthein, 2000). In other words, low frequencies (delta, theta, alpha) can be considered as carrier waves (Freeman and Rogers, 2002), and higher frequencies (beta, gamma) as information waves, and the higher frequencies are nested or carried by the lower frequencies. In this study we looked at cross-frequency coupling, i.e. power to phase nesting at the posterior cingulate cortex and lingual gyrus for all three groups. When observing phase to power coupling, neurofeedback training decreased alpha-beta and alpha-gamma nesting. This was further confirmed by comparison to healthy controls, demonstrating that before neurofeedback treatment targeting the posterior cingulate cortex tinnitus patients have increased alpha-beta and alpha-gamma nesting, while after neurofeedback training no differences were shown in comparison to healthy controls, i.e. the abnormal nesting was normalized. This suggests that neurofeedback could remove the information, processed in beta and gamma, from the carrier wave, alpha, which carries the high frequency information (Song et al., 2013). Moreover, current models of perception posit that the brain actively looks for information it predicts to be present in the environment based on an intention or goal (Freeman, 2003), in which predicting “when” predominantly involves low-frequency oscillations, predicting “what” points to a combined role of beta and gamma oscillations. In addition, there is a growing body of evidence that implicates rhythmic activity in the alpha band in cortical communication and cognition (Jensen and Mazaheri, 2010, Klimesch et al., 2007) and according to the inhibition timing hypothesis (Klimesch et al., 2007), alpha oscillations may play an active role during cognitive processes through the inhibition of task-irrelevant brain regions (Roux and Uhlhaas, 2014). By decoupling and normalizing the alpha-beta and alpha-gamma nesting this process might inhibit irrelevant information (i.e. stress associated with tinnitus) or reduce the information transmission.
As a normal working brain requires the concerted action of multiple brain networks, it is not surprising that abnormal brain states, as in several neurological and psychiatric disorders, are thought to arise from the impaired functional coupling of remote brain areas (Barttfeld et al, 2014, Friston and Frith, 1995, Honey et al, 2005, Just et al, 2007, Uddin et al., 2013, Wang et al, 2007, Zhang et al, 2010). The default mode network is one of the most consistently identified functional brain networks, which becomes most active when individuals are left to mind wander, i.e. to engage in stimulus-independent thought (Mason et al., 2007), especially with regards to mental explorations referenced to one-self, including remembering, considering hypothetical social interactions, and thinking about one's own future (Buckner et al., 2008), while it becomes deactivated during task related or stimulus dependent cognitive processing (Shulman et al, 1997, Mazoyer et al, 2001). Attenuation of the default mode network is a prerequisite for effective performance when switching to externally oriented processing (Sidlauskaite et al., 2014). A major hub of the default mode network is the posterior cingulate cortex, a brain area representing a sub-component cognitive process of self-reference, i.e. getting caught up in one's experience (Brewer et al., 2013). In normal circumstances, the default network correlates positively with alpha activity (Mantini et al, 2007, Jann et al, 2009), while in distressed tinnitus patients decreased alpha activity is consistently identified at the posterior cingulate cortex (Vanneste et al, 2010a, Vanneste et al., 2013), possibly reflecting the continuously active processing and pathologic awareness of a non-existing sound in the environment, inhibiting a person to find himself into a conscious resting or default mode state. Moreover, the posterior cingulate cortex has been proposed to exert a salience-based cognitive auditory comparator function (Laufer et al., 2009). This could be related to its flip-flop mechanism involved in encoding items (sound) into memory or retrieving items (sound) from memory (Huijbers et al, 2011, Kahn et al, 2008). When the posterior cingulate cortex component is deficient or less active, such as in distressed tinnitus patients, this might reflect the incapability of the posterior cingulate cortex to exert its salience based comparator function, pulling irrelevant auditory information, i.e. tinnitus, from the (para)hippocampal memory (De Ridder et al., 2006). Activity in the posterior cingulate cortex has been linked to successful retrieval from auditory (and visual) memory (Shannon and Buckner, 2004, Sadaghiani et al., 2009), while axonal tracing studies (Suzuki and Amaral, 1994, Morris et al., 1999) have demonstrated neural connectivity between the posterior cingulate cortex and medial temporal lobe regions, including the parahippocampal gyrus, a region known to be the link between the default mode network and memory (Ward et al., 2014). In addition to the decreased activity in the posterior cingulate cortex an increased activity of the dorsal anterior cingulate cortex, which forms a core region of the salience network, has been revealed in distressed tinnitus patients. The salience network is considered a switch between the default network and the dorsal attention or stimulus dependent network in the presence of relevant stimuli (Seeley et al., 2007). Hence, influencing the connectivity between the dorsal anterior cingulate cortex and posterior cingulate cortex/parahippocampus region may have an impact on the salience attributed to the tinnitus sound. In other words, the increased functional connectivity between the default mode network and the salience network, which signals and processes behaviorally relevant information, impedes normal anti-correlated activity (Daniels et al., 2010). Moreover, a very specific connection between the subgenual anterior cingulate cortex/ventromedial prefrontal cortex and parahippocampal region at 10 and 11.5 Hz has been highlighted as the crucial link between the tinnitus loudness and distress network (Vanneste et al., 2013) and decreasing the functional and/or effective connectivity between the subgenual anterior cingulate cortex and the parahippocampus region in the alpha range may have the ability of disrupting the communication between the parahippocampus and the subgenual anterior cingulate cortex/ventromedial prefrontal cortex, i.e. disconnecting the tinnitus loudness and distress network. Furthermore, the parahippocampus and subgenual anterior cingulate cortex are part of a non-specific aversive distress network (Moulton et al., 2011), and disrupting the distress network might block its emergent property, distress to arise.
The promising results of this neurofeedback study on tinnitus related distress may be applicable for other pathologies as well. First of all, the tinnitus distress network is an aspecific network, known as a multimodal salience network (Legrain et al, 2011, Mouraux et al, 2011), activated by sound, somatosensory, nociceptive, and visual stimuli (Legrain et al, 2011, Mouraux et al, 2011). Patients with tinnitus and chronic pain share many similarities clinically (Moller, 1997), pathophysiologically (De Ridder et al., 2011a, De Ridder et al., 2011b, De Ridder and Van de Heyning, 2007, Tonndorf, 1987) and treatment-wise (De Ridder and Van de Heyning, 2007). Moreover both have a sensory/discriminative and affective dimension related to distinct neural networks (De Ridder et al., 2011a, De Ridder et al., 2011b, De Ridder et al, 2012), whereas a sensory-discriminative aspect of chronic pain is related to the primary and secondary cortices, the affective component comprises the medial thalamic nuclei, anterior cingulate cortex, insula, prefrontal cortices as well as the amygdala (Treede et al, 1999, Albe-Fessard et al, 1985, Avenanti et al, 2005). Hence, demonstrating an overlap with the brain areas of the tinnitus related distress network. Moreover, areas involved in the distress network of tinnitus and chronic pain are similar to those in dyspnea (von Leupoldt et al., 2009), social rejection (Masten et al., 2009) and somatoform disorders (Landgrebe et al., 2008), supporting the statement that the distress network is rather aspecific. In addition, various pathologies have been related to a dysfunctional default network including autism (Kennedy et al., 2006, Cherkassky et al, 2006), Alzheimer's disease (Zhang et al, 2010, Rombouts et al, 2005, Sorg et al, 2007), depression (Greicius et al., 2007), schizophrenia (Garrity et al, 2007, Harrison et al, 2007), ADHD (Tian et al., 2006), and even chronic pain (Baliki et al., 2008). Currently, limited studies have applied source localized EEG neurofeedback, however some promising results concerning its therapeutic abilities have been demonstrated. One study, performing source localized neurofeedback training of the precuneus in both healthy subject and adults with heterogeneous psychiatric disorders reported an improvement in executive functions in all participants as well as significantly less psychopathology (Cannon et al., 2014), moreover resting state fMRI of the insula in schizophrenia patients could reveal a learning effect in self-regulation which led to changes in the perception of emotions and this in the presence of modulation of brain network connectivity (Ruiz et al., 2013). Based on the observation that much pathology is the result of an abnormal functional connectivity within and between neural networks (Fornito and Bullmore, 2015), e.g. the default network, various pathologies should be considered eligible candidates for the application of source localized EEG based neurofeedback training.
A limitation of this study is the low resolution of the source localization inherently resulting from a limited number of sensors (19 electrodes) and a lack of subject-specific anatomical forward models. This is sufficient for source reconstruction but results in greater uncertainty in source localization and decreased anatomical precision, and thus the spatial precision of the present study is considerably lower than that of functional MRI. Nevertheless, sLORETA has received considerable validation from studies combining LORETA with other more established localization methods, such as functional Magnetic Resonance Imaging (fMRI) (Mulert et al, 2004, Vitacco et al, 2002), structural MRI (Worrell et al., 2000), Positron Emission Tomography (PET) (Dierks et al, 2000, Pizzagalli et al, 2004, Zumsteg et al, 2005) and was used in previous studies to detect for example activity in the auditory cortex (Zaehle et al., 2007, Vanneste et al, 2010c, Vanneste et al., 2011a, Vanneste et al., 2011b). Further sLORETA validation has been based on accepting as ground truth the localization findings obtained from invasive, implanted depth electrodes, in which case there are several studies in epilepsy (Zumsteg et al, 2006, Zumsteg et al., 2006a) and cognitive ERPs (Volpe et al., 2007). It is worth emphasizing that deep structures such as the anterior cingulate cortex (Pizzagalli et al., 2001), and mesial temporal lobes (Zumsteg et al., 2006b) can be correctly localized with these methods. The involvement of the parahippocampus was already illustrated in previous research using sLORTEA (Moazami-Goudarzi et al, 2010, Vanneste and De Ridder, 2012a, Vanneste and De Ridder, 2012b, Vanneste et al., 2011a, Vanneste et al., 2011b; Vanneste and De Ridder, 2015b), and was confirmed subsequently by PET (Song et al., 2012) and MRI (Maudoux et al., 2012a, Maudoux et al., 2012b, Schmidt et al, 2013) studies, suggesting the reliability of activity changes in deeper cortical structures in tinnitus. Our data further illustrate that source reconstruction can clearly make a difference between the hippocampus and the parahippocampus as the lagged phase synchronization is relatively small. However, further research could improve spatial precision, and higher spatial accuracy could be achieved using high-density EEG (e.g., 128 or 256 electrodes) and subject-specific head models, as well as by confirmation by magnetoencephalography (MEG) recordings.
In summary, EEG based neurofeedback training of a target region may have specific and selective therapeutic consequences, not necessarily by influencing neural activity of the target region but rather by influencing the functional and effectivity connectivity between remote brain regions or functional networks as well as by altering cross frequency coupling of the target region. In distressed tinnitus patients, the posterior cingulate cortex is a considerable candidate for further application of neurofeedback training as it is a consistently identified brain area of the tinnitus related distress network and a major hub of the default mode network with a wide range of connections to remote brain areas. Further research needs to be done about the alterations of effective connectivity underlying various pathologies and the optimal parameters used for neurofeedback training, including the target region and frequency.
References
- Albe-Fessard D. Diencephalic mechanisms of pain sensation. Brain Res. 1985;356(3):217–296. doi: 10.1016/0165-0173(85)90013-x. [DOI] [PubMed] [Google Scholar]
- Avenanti A. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat. Neurosci. 2005;8(7):955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Baliki M.N. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P. Functional connectivity and temporal variability of brain connections in adults with attention deficit/hyperactivity disorder and bipolar disorder. Neuropsychobiology. 2014;69(2):65–75. doi: 10.1159/000356964. [DOI] [PubMed] [Google Scholar]
- Beard A.W. Results of leucotomy operations for tinnitus. J. Psychosom. Res. 1965;9(1):29–32. doi: 10.1016/0022-3999(65)90008-5. [DOI] [PubMed] [Google Scholar]
- Brewer J.A., Garrison K.A., Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Front. Hum. Neurosci. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cannon R. The effects of neurofeedback training in the cognitive division of the anterior cingulate gyrus. Int. J. Neurosci. 2007;117(3):337–357. doi: 10.1080/00207450500514003. [DOI] [PubMed] [Google Scholar]
- Cannon R. Differentiating a network of executive attention: LORETA neurofeedback in anterior cingulate and dorsolateral prefrontal cortices. Int. J. Neurosci. 2009;119(3):404–441. doi: 10.1080/00207450802480325. [DOI] [PubMed] [Google Scholar]
- Cannon R.L. Loreta neurofeedback in the precuneus: operant conditioning in basic mechanisms of self-regulation. Clin. EEG Neurosci. 2014;45(4):238–248. doi: 10.1177/1550059413512796. [DOI] [PubMed] [Google Scholar]
- Canolty R.T. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313(5793):1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky V.L. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Congedo M. NovaTech EEG Inc; Knoxville, TN: 2002. EureKa! (Version 3.0) [Computer Software] Freeware avaible at www.NovatechEEG. [Google Scholar]
- Congedo M., Lubar J.F., Joffe D. Low-resolution electromagnetic tomography neurofeedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2004;12(4):387–397. doi: 10.1109/TNSRE.2004.840492. [DOI] [PubMed] [Google Scholar]
- Daniels J.K. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J. Psychiatry Neurosci. 2010;35(4):258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Van de Heyning P. The Darwinian plasticity hypothesis for tinnitus and pain. Prog. Brain Res. 2007;166:55–60. doi: 10.1016/S0079-6123(07)66005-1. [DOI] [PubMed] [Google Scholar]
- De Ridder D. Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Otolaryngol. Suppl. 2006;(556):50–53. doi: 10.1080/03655230600895580. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Congedo M. The distressed brain: a group blind source separation analysis on tinnitus. PLoS One. 2011;6(10):e24273. doi: 10.1371/journal.pone.0024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. U. S. A. 2011;108(20):8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D. Surgical brain modulation for tinnitus: the past, present and future. J. Neurosurg. Sci. 2012;56(4):323–340. [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Freeman W. The Bayesian brain: phantom percepts resolve sensory uncertainty. Neurosci. Biobehav Rev. 2014;44C:4–15. doi: 10.1016/j.neubiorev.2012.04.001. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Congedo M., Vanneste S. The neural correlates of subjectively perceived and passively matched loudness perception in auditory phantom perception. Brain Behav. 2015:e00331. doi: 10.1002/brb3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T. Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG-generators in Alzheimer's disease. Clin. Neurophysiol. 2000;111(10):1817–1824. doi: 10.1016/s1388-2457(00)00427-2. [DOI] [PubMed] [Google Scholar]
- Dohrmann K. Neurofeedback for treating tinnitus. Prog. Brain Res. 2007;166:473–485. doi: 10.1016/S0079-6123(07)66046-4. [DOI] [PubMed] [Google Scholar]
- Fornito A., Bullmore E.T. Reconciling abnormalities of brain network structure and function in schizophrenia. Curr. Opin. Neurobiol. 2015;30:44–50. doi: 10.1016/j.conb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Freeman W.J. Neurodynamic models of brain in psychiatry. Neuropsychopharmacology. 2003;28(Suppl. 1):S54–S63. doi: 10.1038/sj.npp.1300147. [DOI] [PubMed] [Google Scholar]
- Freeman W.J., Rogers L.J. Fine temporal resolution of analytic phase reveals episodic synchronization by state transitions in gamma EEGs. J. Neurophysiol. 2002;87(2):937–945. doi: 10.1152/jn.00254.2001. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Fuchs M. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002;113(5):702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Garrity A.G. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Geweke J. Measurement of linear dependence and feedback between multiple time series. J. Am. Stat. Assoc. 1982;77:304. [Google Scholar]
- Goebel G., Hiller W. The tinnitus questionnaire (tq) - a standardized instrument for grading the severity of tinnitus - results of A Multicenter study using the tq. Hno. 1994;42(3):166–172. [PubMed] [Google Scholar]
- Granger C.W.J. Investigating causal relations by econometrics models and crosss-spectral methods. Econometrica. 1969;37:424. [Google Scholar]
- Greicius M.D. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr. Res. 2007;91(1–3):82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Hartmann T. The effects of neurofeedback on oscillatory processes related to tinnitus. Brain Topogr. 2014;27(1):149–157. doi: 10.1007/s10548-013-0295-9. [DOI] [PubMed] [Google Scholar]
- Havenith M.N. Synchrony makes neurons fire in sequence, and stimulus properties determine who is ahead. J. Neurosci. 2011;31(23):8570–8584. doi: 10.1523/JNEUROSCI.2817-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller W., Goebel G. A psychometric study of complaints in chronic tinnitus. J. Psychosom. Res. 1992;36(4):337–348. doi: 10.1016/0022-3999(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Hiller W., Goebel G., Rief W. Reliability of self-rated tinnitus distress and association with psychological symptom patterns. Br. J. Clin. Psychol. 1994;33(Pt 2):231–239. doi: 10.1111/j.2044-8260.1994.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Honey G.D. Functional disconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128(Pt 11):2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W. Imagery and retrieval of auditory and visual information: neural correlates of successful and unsuccessful performance. Neuropsychologia. 2011;49(7):1730–1740. doi: 10.1016/j.neuropsychologia.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Jann K. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. Neuroimage. 2009;45(3):903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcak V., Tsuzuki D., Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34(4):1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Just M.A. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlbrock N., Weisz N. Transient reduction of tinnitus intensity is marked by concomitant reductions of delta band power. BMC Biol. 2008;6:4. doi: 10.1186/1741-7007-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(1):129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Redcay E., Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc. Natl. Acad. Sci. U. S. A. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Landgrebe M. Neuronal correlates of symptom formation in functional somatic syndromes: a fMRI study. Neuroimage. 2008;41(4):1336–1344. doi: 10.1016/j.neuroimage.2008.04.171. [DOI] [PubMed] [Google Scholar]
- Laufer I., Negishi M., Constable R.T. Comparator and non-comparator mechanisms of change detection in the context of speech–an ERP study. Neuroimage. 2009;44(2):546–562. doi: 10.1016/j.neuroimage.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V. The pain matrix reloaded: a salience detection system for the body. Prog. Neurobiol. 2011;93(1):111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Mantini D. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M.F. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect Neurosci. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudoux A. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 2012;1485:10–21. doi: 10.1016/j.brainres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Maudoux A. Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS One. 2012;7(5):e36222. doi: 10.1371/journal.pone.0036222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res. Bull. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Meeus O., Blaivie C., Van de Heyning P. Validation of the Dutch and the french version of the tinnitus questionnaire. B-Ent. 2007;3(Suppl. 7):11–17. [PubMed] [Google Scholar]
- Micoulaud-Franchi J.A. EEG neurofeedback treatments in children with ADHD: an updated meta-analysis of randomized controlled trials. Front. Hum. Neurosci. 2014;8:906. doi: 10.3389/fnhum.2014.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazami-Goudarzi M. Temporo-insular enhancement of EEG low and high frequencies in patients with chronic tinnitus. QEEG study chronic tinnitus patients. 2010;11:40. doi: 10.1186/1471-2202-11-40. BMC Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller A.R. Similarities between chronic pain and tinnitus. Am. J. Otol. 1997;18(5):577–585. [PubMed] [Google Scholar]
- Morris R., Petrides M., Pandya D.N. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur. J. Neurosci. 1999;11(7):2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Moulton E.A. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J. Neurosci. 2011;31(10):3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. 2011;54(3):2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Mulert C. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22(1):83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Nunez P.L., Wingeier B.M., Silberstein R.B. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum. Brain Mapp. 2001;13(3):125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24(Suppl. D):5–12. [PubMed] [Google Scholar]
- Pascual-Marqui R. 2007. Instantaneous and Lagged Measurement of Linear and Nonlinear Dependence between Groups of Multivariate Time Series: Frequency Decomposition.http://arxiv.org/abs/0711.1455 Available from: [Google Scholar]
- Pascual-Marqui R. Discrete 3D distributed, linear imaging methods of electric neuronal activity. Part 1 Exact. Zero Error Localization. 2007 http://arxiv.org/abs/0710.3341 Available from: [Google Scholar]
- Pascual-Marqui R.D. Assessing direct paths of intracortical causal information flow of oscillatory activity with the isolated effective coherence (iCoh) Front. Hum. Neurosci. 2014;8:448. doi: 10.3389/fnhum.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.M. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn. Sci. 2011;15(4):143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am. J. Psychiatry. 2001;158(3):405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol. Psychiatry. 2004;9(4):325. doi: 10.1038/sj.mp.4001501. 393-405. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts S.A. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp. 2005;26(4):231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F., Uhlhaas P.J. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn. Sci. 2014;18(1):16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Ruiz S. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum. Brain Mapp. 2013;34(1):200–212. doi: 10.1002/hbm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Hesselmann G., Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J. Neurosci. 2009;29(42):13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar R.F. Content-specific fronto-parietal synchronization during visual working memory. Science. 2012;338(6110):1097–1100. doi: 10.1126/science.1224000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R., Buckner R.L. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schecklmann M. Neural correlates of tinnitus duration and distress: a positron emission tomography study. Hum. Brain Mapp. 2013;34(1):233–240. doi: 10.1002/hbm.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S.A. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS One. 2013;8(10):e76488. doi: 10.1371/journal.pone.0076488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon B.J., Buckner R.L. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 2004;24(45):10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sidlauskaite J. Anticipatory processes in brain state switching - evidence from a novel cued-switching task implicating default mode and salience networks. Neuroimage. 2014;98(9):359–365. doi: 10.1016/j.neuroimage.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Silchenko A.N. Impact of acoustic coordinated reset neuromodulation on effective connectivity in a neural network of phantom sound. Neuroimage. 2013;77:133–147. doi: 10.1016/j.neuroimage.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Singer W. Distributed processing and temporal codes in neuronal networks. Cogn. Neurodyn. 2009;3(3):189–196. doi: 10.1007/s11571-009-9087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.J. Mapping tinnitus-related brain activation: an activation-likelihood estimation metaanalysis of PET studies. J. Nucl. Med. 2012;53(10):1550–1557. doi: 10.2967/jnumed.112.102939. [DOI] [PubMed] [Google Scholar]
- Song J.J. Neural substrates predicting improvement of tinnitus after cochlear implantation in patients with single-sided deafness. Hear Res. 2013;299:1–9. doi: 10.1016/j.heares.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Sorg C. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterman M.B., Howe R.C., Macdonald L.R. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science. 1970;167(3921):1146–1148. doi: 10.1126/science.167.3921.1146. [DOI] [PubMed] [Google Scholar]
- Suzuki W.A., Amaral D.G. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J. Comp. Neurol. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Svoboda E., McKinnon M.C., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G. Meta-analysis of EEG biofeedback in treating epilepsy. Clin. EEG Neurosci. 2009;40(3):173–179. doi: 10.1177/155005940904000310. [DOI] [PubMed] [Google Scholar]
- Tian L. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci. Lett. 2006;400(1–2):39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Functional connectivity density mapping. Proc. Natl. Acad. Sci. U. S. A. 2010;107(21):9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonndorf J. The analogy between tinnitus and pain: a suggestion for a physiological basis of chronic tinnitus. Hear Res. 1987;28(2–3):271–275. doi: 10.1016/0378-5955(87)90054-2. [DOI] [PubMed] [Google Scholar]
- Treede R.D. The cortical representation of pain. Pain. 1999;79(2–3):105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Sosa P.A. Effective connectivity: influence, causality and biophysical modeling. Neuroimage. 2011;58(2):339–361. doi: 10.1016/j.neuroimage.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo E. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS ONE. 2009;4(10):e7396. doi: 10.1371/journal.pone.0007396. 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., De Ridder D. The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front. Syst. Neurosci. 2012;6:31. doi: 10.3389/fnsys.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., De Ridder D. The use of alcohol as a moderator for tinnitus-related distress. Brain Topogr. 2012;25(1):97–105. doi: 10.1007/s10548-011-0191-0. [DOI] [PubMed] [Google Scholar]
- Vanneste S., De Ridder D. Stress-related functional connectivity changes between auditory cortex and cingulate in tinnitus. Brain Connect. 2015;5(6):371–383. doi: 10.1089/brain.2014.0255. [DOI] [PubMed] [Google Scholar]
- Vanneste S., De Ridder D. Deafferentation-based pathophysiological differences in phantom sound: tinnitus with and without hearing loss. Neuroimage. 2015;129:80–94. doi: 10.1016/j.neuroimage.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Vanneste S. The neural correlates of tinnitus-related distress. Neuroimage. 2010;52(2):470–480. doi: 10.1016/j.neuroimage.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Vanneste S. Validation of the Mini-TQ in a Dutch-speaking population. A rapid assessment for tinnitus-related distress. B-Ent. 2010;7(1):31–36. [PubMed] [Google Scholar]
- Vanneste S. The difference between uni- and bilateral auditory phantom percept. Clin. Neurophysiol. 2010;122(3):578–587. doi: 10.1016/j.clinph.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Vanneste S. The difference between uni- and bilateral auditory phantom percept. Clin. Neurophysiol. 2011;122(3):578–587. doi: 10.1016/j.clinph.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Vanneste S., de Heyning P.V., Ridder D.D. Contralateral parahippocampal gamma-band activity determines noise-like tinnitus laterality: a region of interest analysis. Neuroscience. 2011;119:481–490. doi: 10.1016/j.neuroscience.2011.07.067. [DOI] [PubMed] [Google Scholar]
- Vanneste S., Joos K., De Ridder D. Prefrontal cortex based sex differences in tinnitus perception: same tinnitus intensity, same tinnitus distress, different mood. PLoS One. 2012;7(2):e31182. doi: 10.1371/journal.pone.0031182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., Congedo M., De Ridder D. Pinpointing a highly specific pathological functional connection that turns phantom sound into distress. Cereb. Cortex. 2013;24(9):2268–2282. doi: 10.1093/cercor/bht068. [DOI] [PubMed] [Google Scholar]
- Vitacco D. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum. Brain Mapp. 2002;17(1):4–12. doi: 10.1002/hbm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe U. The cortical generators of P3a and P3b: a LORETA study. Brain Res. Bull. 2007;73(4–6):220–230. doi: 10.1016/j.brainresbull.2007.03.003. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48(1):200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- von Stein A., Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Wang K. Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum. Brain Mapp. 2007;28(10):967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A.M. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum. Brain Mapp. 2014;35(3):1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N. Effects of individual alpha rTMS applied to the auditory cortex and its implications for the treatment of chronic tinnitus. Hum. Brain Mapp. 2014;35(1):14–29. doi: 10.1002/hbm.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell G.A. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr. 2000;12(4):273–282. doi: 10.1023/a:1023407521772. [DOI] [PubMed] [Google Scholar]
- Zaehle T., Jancke L., Meyer M. Electrical brain imaging evidences left auditory cortex involvement in speech and non-speech discrimination based on temporal features. Behav. Brain Funct. 2007;3:63. doi: 10.1186/1744-9081-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 2010;256(2):598–606. doi: 10.1148/radiol.10091701. [DOI] [PubMed] [Google Scholar]
- Zumsteg D. H2(15)O or 13NH3 PET and electromagnetic tomography (LORETA) during partial status epilepticus. Neurology. 2005;65(10):1657–1660. doi: 10.1212/01.wnl.0000184516.32369.1a. [DOI] [PubMed] [Google Scholar]
- Zumsteg D. Cortical activation with deep brain stimulation of the anterior thalamus for epilepsy. Clin. Neurophysiol. 2006;117(1):192–207. doi: 10.1016/j.clinph.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Zumsteg D., Lozano A.M., Wennberg R.A. Depth electrode recorded cerebral responses with deep brain stimulation of the anterior thalamus for epilepsy. Clin. Neurophysiol. 2006;117(7):1602–1609. doi: 10.1016/j.clinph.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Zumsteg D., Lozano A.M., Wennberg R.A. Mesial temporal inhibition in a patient with deep brain stimulation of the anterior thalamus for epilepsy. Epilepsia. 2006;47(11):1958–1962. doi: 10.1111/j.1528-1167.2006.00824.x. [DOI] [PubMed] [Google Scholar]