Abstract
Opioid peptides and their receptors re-organize within hippocampal neurons of female, but not male, rats following chronic immobilization stress (CIS) in a manner that promotes drug-related learning. This study was conducted to determine if there are also sex differences in gene expression in the hippocampus following CIS. Adult female and male rats were subjected to CIS (30 min/day) for 10 days. Twenty-four hours after the last stressor, the rats were euthanized, the brains were harvested and the medial (dentate gyrus/CA1) and lateral (CA2/CA3) dorsal hippocampus were isolated. Following total RNA isolation, cDNA was prepared for gene expression analysis using a RT2 Profiler PCR expression array. This custom designed qPCR expression array contained genes for opioid peptides and receptors, as well as genes involved in stress-responses and candidate genes involved in synaptic plasticity, including those upregulated following oxycodone self-administration in mice. Few sex differences are seen in hippocampal gene expression in control (unstressed) rats. In response to CIS, gene expression in the hippocampus was altered in males but not females. In males, opioid, stress, plasticity and kinase/signaling genes were all down-regulated following CIS, except for the gene that codes for corticotropin releasing hormone, which was upregulated. Changes in opioid gene expression following chronic stress were limited to the CA2 and CA3 regions (lateral sample). In conclusion, modest sex- and regional-differences are seen in expression of the opioid receptor genes, as well as genes involved in stress and plasticity responses in the hippocampus following CIS.
Keywords: Delta opioid receptor, Neural plasticity, Corticotrophin releasing factor, Drug addiction
Highlights
-
•
Unstressed female rats have less Arc expression in hippocampus than males.
-
•
Chronic immobilization stress (CIS) down-regulates opioid gene expression in males.
-
•
CIS up-regulates Crh but down-regulates other stress genes in male hippocampi.
-
•
CIS down-regulates Arc and other plasticity genes in male hippocampi.
-
•
CIS down-regulates select kinases and signaling molecules in male hippocampi.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CIS
chronic immobilization stress
- CRF
corticotrophin releasing factor
- CRFR1
corticotrophin releasing factor receptor 1
- Ct
threshold cycle
- DOR
delta opioid receptor
- FKBP5
FK506 binding protein 5
- LTP
long-term potentiation
- MOR
mu opioid receptor
- PARV
parvalbumin
- PSD
post-synaptic density
- RIN
RNA integrity number
- ROI
region of interest
- RT
reverse transcription
1. Introduction
Over the last two decades opioid use and abuse has risen dramatically (Centers for Disease Control and Prevention, 2013, Centers for Disease Control and Prevention, 2015). Moreover, women are more apt than men to abuse opioids through initial prescription pain reliever use (Jones, 2012, Lee and Ho, 2013). Drug addiction, especially relapse, is often provoked by stress (reviewed by Bruchas et al., 2008, Shalev et al., 2000). Although stress can have powerful influences on the addictive processes in both sexes (Koob and Kreek, 2007, Koob, 2008, Kreek and Koob, 1998), females have a heightened sensitivity to stress (Becker et al., 2007). Moreover, unlike male rodents, female rodents have an enhanced cognitive performance following chronic stress (Luine et al., 2007) that may contribute to an accelerated course of addiction and/or reinstatement (Hu et al., 2004, Lynch et al., 2000, Robbins et al., 1999).
Associative learning and motivational incentives (Koob and Volkow, 2010) that critically involve the hippocampus (Luo et al., 2011, Vorel et al., 2001) are importantly involved in addictive processes. Notably, the hippocampus is a target of opioid receptor agonists, such as prescription morphine and methadone, as well as the drug of abuse heroin and various short acting opiates. Notably, the opioid system in the hippocampal CA3 region has been implicated in visual-spatial pattern completion (Kesner and Warthen, 2010), an important element of context associative learning which is essential for associating a drug of abuse with a particular place and set of events (i.e. drug-related learning) (Berke and Hyman, 2000, Kilts et al., 2001, Risinger and Oakes, 1995, Volkow et al., 2006).
Our previous anatomical and physiological studies have demonstrated sex differences in the opioid system in the rat dorsal hippocampus, especially in response to chronic immobilization stress (CIS) (McEwen and Milner, 2017). In particular, females at elevated estrogen states compared to low estrogen states and males, have: (1) elevated levels of enkephalin immunoreactivity in the mossy fibers, (2) greater densities of mu opioid receptor (MOR) immunolabeling on the plasma membrane of GABAergic hilar interneurons, (3) elevated densities of delta opioid receptor (DOR) immunolabeling near the plasma membrane in CA1 and CA3 pyramidal cell dendrites and (4) greater densities of DOR immunolabeling in CA3 dendritic spines contacted by mossy fibers (Harte-Hargrove et al., 2015, Mazid et al., 2016, Torres-Reveron et al., 2008, Torres-Reveron et al., 2009, Williams et al., 2011b). Such redistribution would enhance excitability and learning processes (McEwen and Milner, 2017). Additionally, female rats at high-estrogen states compared to females in low-estrogen states and males have a lower baseline transmission of the mossy fiber-CA3 pathway that is regulated by MORs and exhibit a DOR-mediated low-frequency form of long-term potentiation (LTP) (Harte-Hargrove et al., 2015). Following CIS, females regardless of estrogen state are “primed” for even greater excitation of CA3 pyramidal cells. Specifically, after CIS, females do not display the dendritic atrophy of CA3 pyramidal cell dendrites and the loss of GABAergic parvalbumin (PARV)-containing interneurons in the dorsal hippocampus observed in males (McEwen, 1999, Milner et al., 2013, Vyas et al., 2002). Rather, in CIS females, enkephalin levels in mossy fibers are elevated and the elevation of MORs and DORs near the plasma membrane in hippocampal pyramidal and interneurons resembles that seen in unstressed females at high estrogen states (Mazid et al., 2016, Milner et al., 2013, Pierce et al., 2014). Moreover, following CIS, DORs are elevated near the plasma membrane of GABAergic hilar interneurons and thus could promote associative learning processes in the hippocampus (Mazid et al., 2016).
This study was conducted to determine if there also are sex differences in opioid gene expression in the dorsal hippocampus prior to stress (baseline) or following a model of chronic stress. In addition to opioid and stress genes, we assessed synaptic plasticity genes that we previously have shown were up-regulated in the hippocampus of male mice after oxycodone self-administration (Zhang et al., 2015) and/or altered with sex or hormonal levels in the hippocampus (McEwen and Milner, 2007).
2. Materials and methods
2.1. Animals
All procedures were approved by the Rockefeller University Institutional Animal Care and Use Committee and were in accordance with the 2011 Eighth edition of the National Institutes of Health guidelines for the Care and Use of Laboratory Animals. Adult male (275–325 gm) and female (∼225–250 gm) Sprague-Dawley rats (N = 24; Charles River Laboratories, Wilmington, MA) were approximately 2 months old at the time of arrival. The rats were pair-housed with 12:12 light/dark cycles (Lights on 0600–1800) and had access to food and water ad libitum. All rats were kept in custom-built cabinets especially designed for stress experiments (Phenome Technologies, Inc., Skokie, IL). Each cabinet held two cages (25 cm × 46 cm and 20.5 cm tall). The cabinets are directly attached to the ventilation system. White LEDs are installed in the cabinets.
Estrous cycle stage was determined using vaginal smear cytology (Turner and Bagnara, 1971) at the termination of the experiment. To control for the effects of handling, mock estrous cycling was performed in males at the termination of the experiment. Previous studies in rats showed that CIS had no effect on the length of the estrous cycle or the duration of the individual estrous phases (Milner et al., 2013).
Stress-induced differences in gene expression (RNA levels) are highly sensitive to environmental factors, such as ambient noise, time of day and familiarity of the experimenter, because each can rapidly and significantly alter circulating corticosterone levels, thereby inducing changes in gene expression. Therefore tissues from all groups were collected concurrently to promote the rigor and reproducibility of the results. Importantly, the same individuals conducted the stress experiments and the tissue was harvested at the same time of day, between 9am and 1pm, to control for circadian surges in cortisol levels. Given these attempts to minimize the variation in environmental factors, 24 rats was the maximum number of animals that can be run in a single experiment.
2.2. Chronic immobilization stress
Rats (6 male and 6 female) were transported from their home-room into a procedure room between 9:00 a.m. and 1:00 p.m., and CIS was performed, as previously described (Mazid et al., 2016). Briefly, rats were placed in plastic cone-shaped polyethylene bags with a small breathing hole at the apex and a Kotex mini-pad was placed in the bag underneath them to collect urine. The rats were placed with their nose at the hole and the bag was sealed with tape; they then were left undisturbed on the countertop for 30 min per day for 10 days. Cages containing control rats were brought into the procedure room prior to the stressed rats. The control rats were picked up and then placed back in their cages for 30 min prior to returning them to their homeroom. Animals were euthanized 24 h after the final stress period.
2.3. RNA isolation and cDNA synthesis
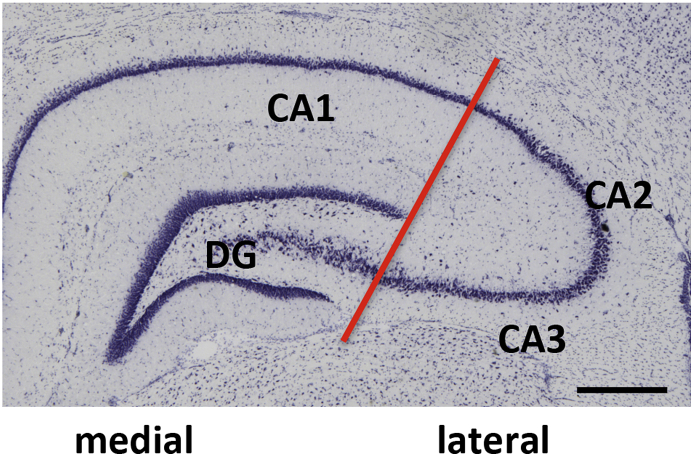
RNA was prepared as previously described (Zhang et al., 2014). The rats were anesthetized with CO2, decapitated with a guillotine and their brains were harvested. A coronal section of the dorsal hippocampus was dissected from the brain using a rat brain matrix (ASI Instruments, Warren MI). The medial (dentate gyrus/CA1) and lateral (CA2/CA3) dorsal hippocampus (Fig. 1) were isolated, homogenized in 700 μl of Qiazol (Qiagen, Inc., Germantown MD) and immediately frozen and stored at −80° C until the RNA was prepared.
Fig. 1.
Schema of hippocampal dissection. A Nissl-stained coronal section of the rat dorsal hippocampus denotes where the medial and lateral samples were dissected (red line). Bar = 0.5 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Total RNA was isolated from homogenates using the miRNeasy Kit (Qiagen Inc.), following manufacturer's recommended protocol. The quality and quantity of RNA from each sample was determined using the Agilent 2100 Bioanalyzer (Agilent Technologies, San Francisco, CA). Starting with 0.5 μg of RNA, complementary DNA (cDNA) was prepared using the RT2 First Strand Kit (Qiagen Inc.), which uses a proprietary procedure to eliminate contamination of genomic DNA from the RNA samples before reverse transcription (RT). Random hexamers and oligo-dT primers were used in the RT reaction in an unbiased manner. An external RNA control also was included to monitor the RT efficiency and tests for enzyme inhibitors that may contaminate the RNA samples during subsequent gene expression analysis.
2.4. RT2 profiler array
Gene expression levels were measured using RT2 SYBR® Green qPCR Mastermix and a custom RT2 PCR Array (both from Qiagen, Inc.) following the manufacturer's recommended protocol. The gene expression array contained 22 genes of interest (Table 1) and five reference genes. The genes of interest included; seven opioid peptide and receptor related-genes, six genes involved in stress-responses, four genes involved in synaptic plasticity and five kinase or signaling molecule genes important for opioid, stress and plasticity responses including those upregulated following oxycodone self-administration in mice (Zhang et al., 2014). The qPCR reactions were run on an ABI PRISM™ 7900HT Sequence Detection System (Applied Biosystems, Inc., Foster City, CA). The threshold cycle (Ct) values were used to calculate the mean (±SEM) normalized gene expression levels.
Table 1.
Genes used on RT Profiler PCR array.
| Group | Gene symbol | Gene name |
|---|---|---|
| Opioid | Oprm1 | Opioid receptor, mu 1 |
| Oprd1 | Opioid receptor, delta | |
| Oprk1 | Opioid receptor, kappa | |
| Oprl1 | Nociception receptor | |
| Pdyn | Prodynorphin | |
| Penk | Proenkephalin | |
| Pomc | Proopiomelanocortin | |
| Stress | Crh | Corticotrophin releasing factor |
| Crhr1 | Corticotrophin releasing factor receptor 1 | |
| Crhr2 | Corticotrophin releasing factor receptor 2 | |
| Avpr1a | Arginine vasopressin (AVP) receptor 1a | |
| Avpr1b | AVP receptor 1b | |
| Fkbp5 | FK506 binding protein 5 | |
| Plasticity | Arc | Activity regulated cytoskeletal-associated protein |
| Bdnf | Brain derived neurotrophin factor | |
| Ntrk2 | Neurotrophic tyrosine kinase, receptor, type 2 | |
| Cdh2 | Calcium dependent adhesion transmembrane protein | |
| Kinases and signaling molecules | Akt1 | Protein kinase B |
| Mapk1 | Mitogen-activated protein kinase | |
| Pim 1 | Pim-1, proto-oncogene, serine/threonine kinase | |
| Arrb1 | Beta arrestin 1 | |
| Arrb2 | Beta arrestin 2 |
2.5. Statistical analysis and figure preparation
A 2 × 2 between-subjects factorial design, in which the factors were Sex (male/female) and Condition (control/stress), was applied. Ct values were normalized by taking their differences to the most stable gene in the analyzed brain region and used as response variables in the statistical model. A two-way analysis of variance (ANOVA) with Sex and Condition as main effects, as well as their interaction was tested for significance at a 5% level. For genes that produce a significant ANOVA results (P < .05), either for main effects and/or interaction, Tukey-Kramer HSD post-hoc pairwise comparisons among the four studied groups were carried out. Post-hoc P-values < 0.05 reported as nominally significant and P-values < 0.1 reported, for exploratory purposes. Data are summarized by their mean (±SEM) normalized gene expression levels.
3. Results
At the termination of the CIS experiment, unstressed (control) female rats were in the following estrous cycle stages: proestrus/estrus (N = 1), estrus (N = 4) and diestrus (N = 1), and all CIS female rats were in estrus (N = 6). Statistical evaluation of gene expression was similar in the female rats when data from the proestrus/estrus and diestrus control rats were included and omitted. Thus, the data presented for the control females includes all 6 rats, regardless of estrous cycle stage. A complete listing of the statistical analysis for all genes is in Supplementary Tables 1 and 2.
All RNA samples were of high quality with a RNA Integrity Number (RIN) ranging from 8.0 to 9.0, as determined by the Agilent 2100 Bioanalyzer. Sex and stress affected gene expression in all three categories: opioid, plasticity and stress.
3.1. Opioid genes
Group differences in opioid gene expression were mainly limited to the lateral (CA2/CA3) hippocampal sample.
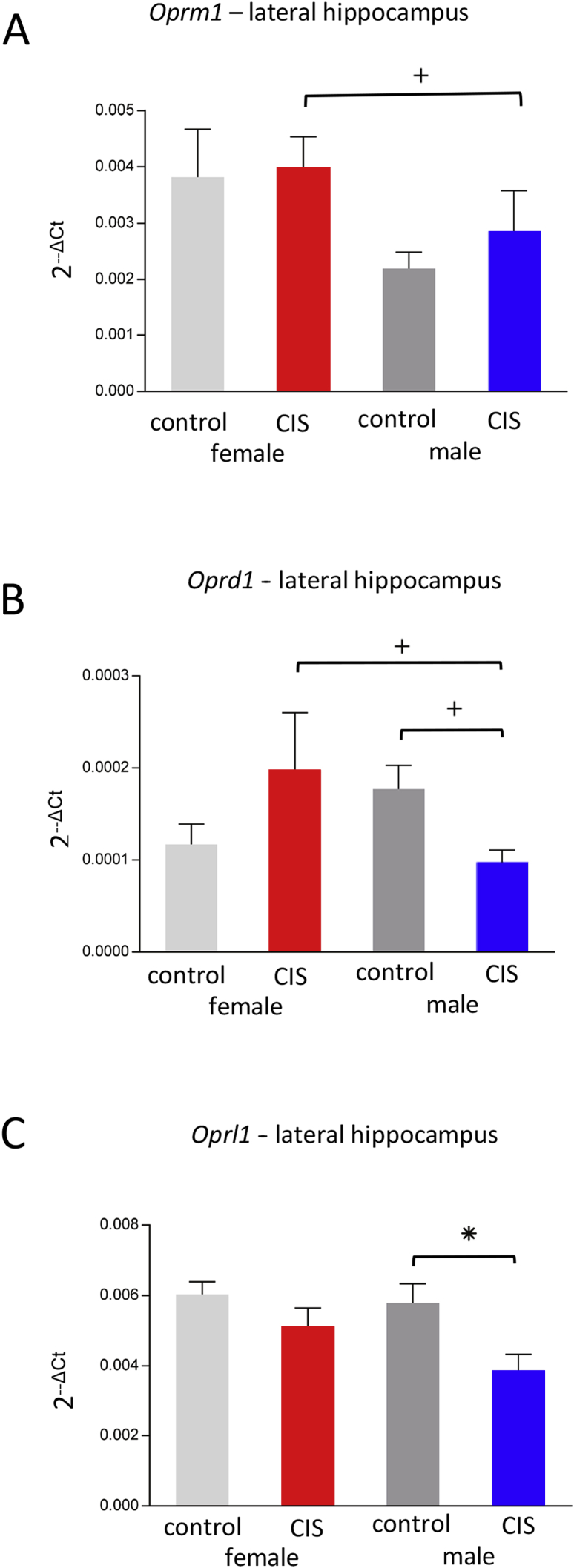
Two-way ANOVA demonstrated a significant main effect of Sex on Oprm1 expression in the lateral hippocampus [F(1,20) = 5.91, P = .025]. Post-hoc analysis showed that CIS females had a trend toward significantly more Oprm1 expression than CIS males (P = .09; Fig. 2A).
Fig. 2.
Sex differences in opioid gene expression after CIS. Significant group differences were limited to genes in the lateral hippocampal sample. (a) Oprm1 expression had a main effect of sex with CIS females showed a trend toward significantly more expression than CIS males (+p = .09). (b) Oprd1 expression had significant sex and condition interaction with CIS females trending toward greater expression than CIS males (+p = .069) and control males show a trend to have more expression than CIS males (+p = .060). (c) Oprl1 expression shows control males have significantly greater expression than CIS males (*p = .030).
There was a significant Sex x Condition interaction for Oprd1 expression [F(1,20) = 5.94, P = .024]. Post-hoc analysis showed CIS females tended to have greater Oprd1 expression than CIS males (P = .069) and that control males tended to have greater Oprd1 than CIS males (P = .060; Fig. 2B).
There was a significant main effect of Stress on Oprl1 expression [F(1,20) = 5.71, P = .028]. Post-hoc analysis showed that control males have significantly greater Oprl1 expression than CIS males (P = .030; Fig. 2C).
There was no significant effect of sex or stress on expression of Oprk1, Pdyn, Penk and Pomc in either hippocampal region.
3.2. Stress genes
Group differences in the expression of stress-related genes were observed in both the lateral and medial hippocampal samples.
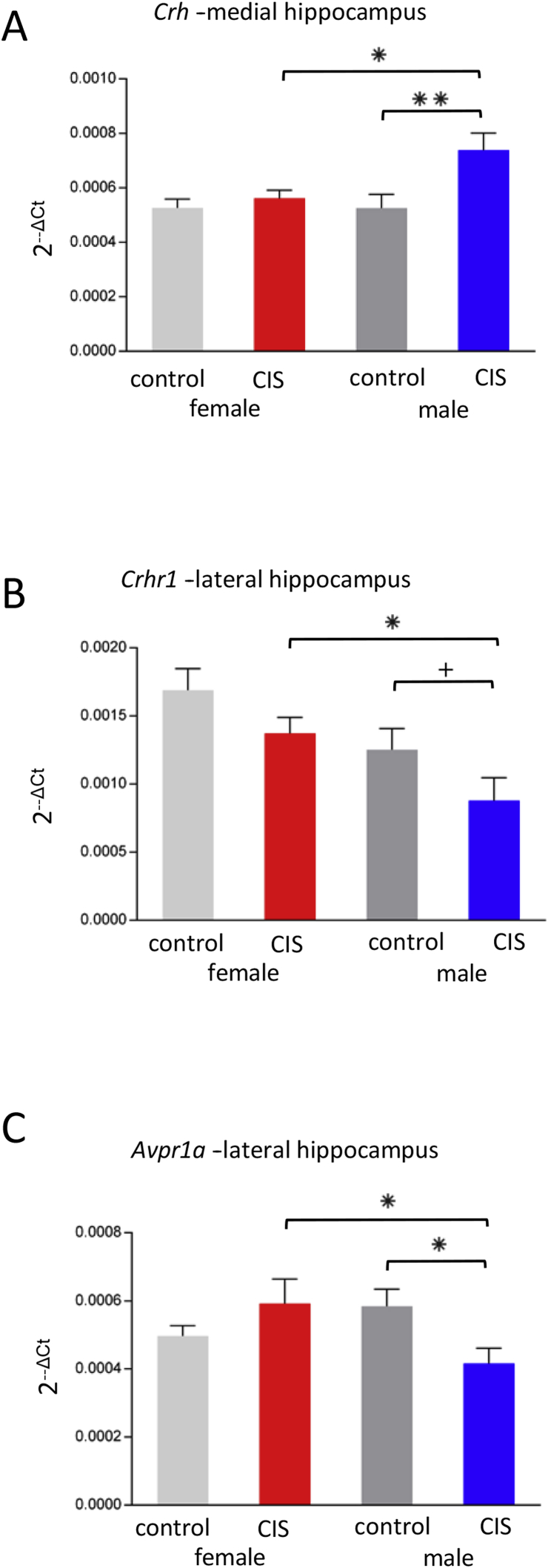
Two-way ANOVA showed a significant main effect of Condition on Crh expression [F(1,20) = 7.04, P = .015] in the medial hippocampus. Post-hoc analysis revealed that CIS males have significantly greater Crh than both control males (P = .006) and CIS females (P = .030; Fig. 3A).
Fig. 3.
Sex differences in stress gene expression after CIS. (a) In the medial hippocampal sample, CIS males have significantly greater Crh expression than both control males (**p = .0056) and CIS females (p = .0303). (b) CIS males had significantly less Crhr1 expression than CIS females (*p = .0238) and tended to have less than control males (+p = .068) in the lateral hippocampal sample. (c) In the lateral sample, both CIS females and male controls had significantly more Avpr1a expression than CIS males (*p = .0342 and *p = .0328, respectively).
Two-way ANOVA showed a significant main effect of Sex on Crhr1 expression [F(1,20) = 7.35, P = .013] in the lateral hippocampus. Post-hoc analysis revealed that CIS females have significantly more Crhr1 than CIS males (P = .024), further control males trended toward greater expression than CIS males (P = .068; Fig. 3B).
There was a significant Sex x Condition interaction for Avpr1a expression [F(1,20) = 5.15, P = .034] in the lateral hippocampus. Post-hoc analysis revealed that both CIS females and male controls had significantly more Avpr1a expression than CIS males (P = .034 and P = .033, respectively; Fig. 3C).
There was no significant effect of sex or stress on expression of Avpr1b, Crhr2 and Fkbp5 in either hippocampal region.
3.3. Plasticity genes
Group differences in the expression of several plasticity genes were observed in both the lateral and medial hippocampal samples.
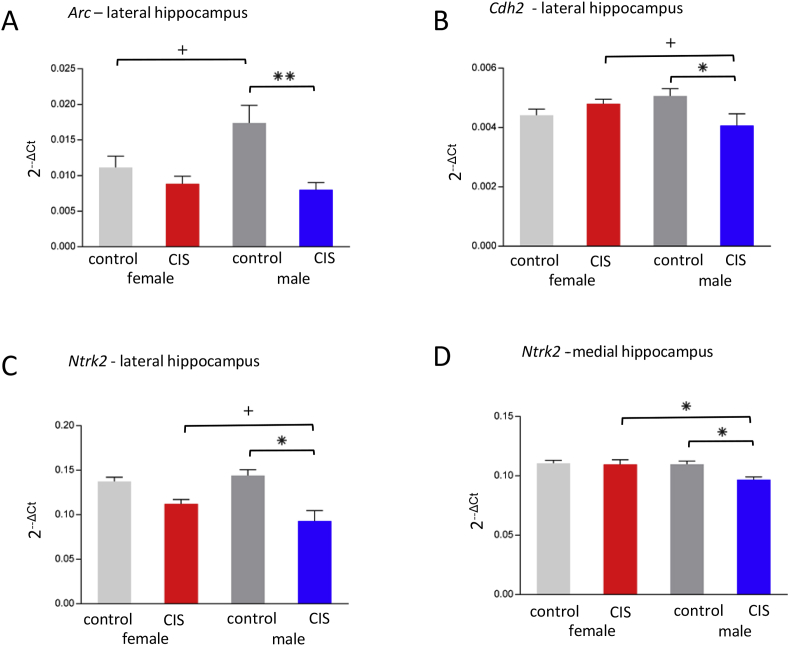
There was a significant main effect of Condition on Arc expression in the lateral hippocampus [F(1,20) = 8.70, P = .008]. Post-hoc analysis showed that control males have significantly more Arc expression than CIS males in the lateral (Fig. 4A) hippocampus (P = .004). Further, control females showed a trend toward significantly less Arc expression than control males in the lateral hippocampus (p = .077; Fig. 4A).
Fig. 4.
Sex differences in plasticity gene expression after CIS. (a) Control females tended (+p = .07) to have less Arc expression than control males in the lateral hippocampus. Control males had significantly higher Arc expression than CIS males in the lateral hippocampus (**p = .0039). (b) In the lateral hippocampus, CIS males had significantly less Cdh2 expression than control (*p = .027) and tended to have less Cdh2 expression than CIS females (+p = .071). (c, d) Control male rats had significantly more Ntrk2 expression than CIS males, in both the lateral and medial hippocampus (*p = .0021 and *p = .0070, respectively). CIS females have significantly more Ntrk2 expression than CIS males (*p = .0079) in the medial hippocampus and trend toward significance in the lateral hippocampus. (+p = .088).
There was a significant effect of Sex on the expression of Bdnf [F(1,20) = 5.27, P = .032] in the lateral hippocampal region. Post-hoc analysis showed that female control rats had a trend toward significantly less Bdnf (P = .08) expression than control males (not shown).
Two way ANOVA showed a significant Sex x Condition interaction for Cdh2 expression [F(1,20) = 5.28, P = .032] in the lateral hippocampus (Fig. 4B). Post-hoc analysis revealed that control males had significantly greater Cdh2 expression than CIS males (P = .027) while CIS females showed a trend to greater Cdh2 expression than CIS males (P = .071).
There was a significant main effect of Condition on Ntrk2 expression in both the lateral hippocampus [F(1,20) = 12.25, P = .002] (Fig. 4C) and in the medial hippocampus [F(1,20) = 5.24, P = .033] (Fig. 4D). Further, the expression of Ntrk2 in the medial hippocampus demonstrated a significant main effect of Sex [F(1,20) = 4.88, P = .039]. Post-hoc analysis showed that males in the control group had significantly more Ntrk2 expression than the CIS males, in both the lateral and medial hippocampus (P = .002 and P = .007, respectively). In addition, CIS females had significantly more Ntrk2 expression than CIS males in the medial hippocampus (P = .008) and trended toward significantly more in the lateral hippocampus (P = .088).
3.4. Kinases and signaling molecules
Group differences in the expression of kinase and signaling molecule genes associated with opioids, stress and synaptic plasticity were observed in both the lateral and medial hippocampal samples.
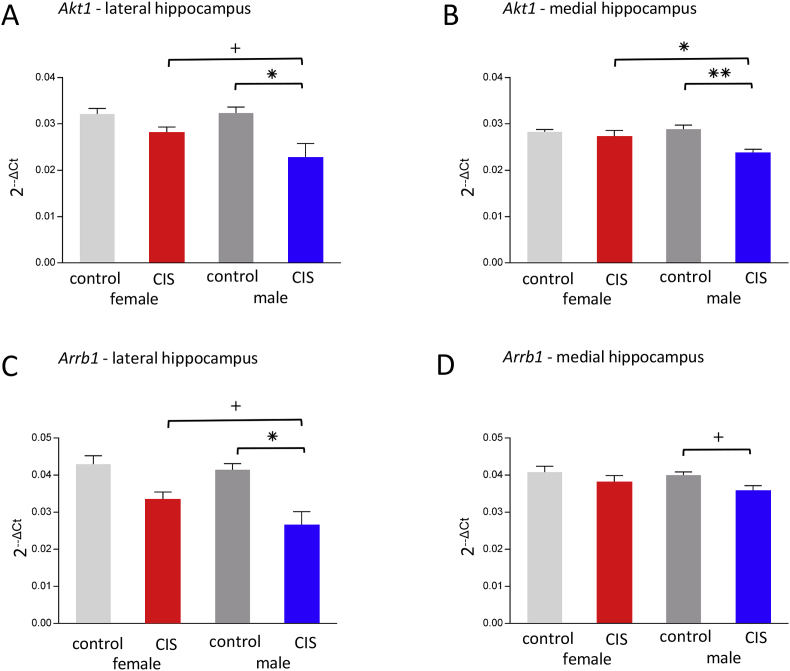
Two-way ANOVA demonstrated significant main effect of Condition on Akt1 gene expression in the lateral hippocampus [F(1,20) = 6.76, P = .017] (Fig. 5A), as well as in the medial hippocampus [F(1,20) = 10.41, P = .004] (Fig. 5B). Post-hoc analysis revealed that control male rats had significantly more Akt1 expression than CIS males in both the lateral and medial hippocampus (P = .011 and P = .001, respectively). In addition, CIS female rats have significantly more Akt1 expression compared to CIS males (P = .018) in the medial hippocampus and trend toward significance in the lateral hippocampus. (P = .071; Fig. 5A and B).
Fig. 5.
Sex differences in kinases and signaling molecule gene expression after CIS. (a,b) The male controls had significantly more Akt1 expression than CIS males in the lateral and medial hippocampus (*p = .0109 and **p = .0013, respectively). CIS females had significantly more Akt1 expression compared to CIS males in the medial hippocampus (*p = .018) with a trend toward significance in the lateral hippocampus (+p = .071). (c,d) Control males had significantly more Arrb1 expression than CIS males (*p = .0037) in the lateral hippocampus and trended toward significantly greater than CIS males (+p = .056) in the medial hippocampus. Additionally, CIS females showed a trend toward greater expression of Arrb1 than CIS males (+p = .069) in the lateral region.
Two-way ANOVA demonstrated a significant main effect of Condition on Arrb1 expression in the lateral hippocampus [F(1,20) = 11.80, P = .003] and in the medial hippocampus [F(1,20) = 5.22, P = .033]. Post-hoc analysis revealed that control males have significantly more Arrb1 expression than CIS males (P = .004) and CIS females show a trend toward significantly more expression than CIS males (P = .069) in the lateral hippocampus (Fig. 5C). While, in the medial hippocampus, Arrb1 expression in the control males had a trend toward significantly higher levels than the CIS males (P = .056; Fig. 5D).
There was no significant effect of sex or stress on expression of Mapk1, Pim1 and Arrb2 in either hippocampal region.
4. Discussion
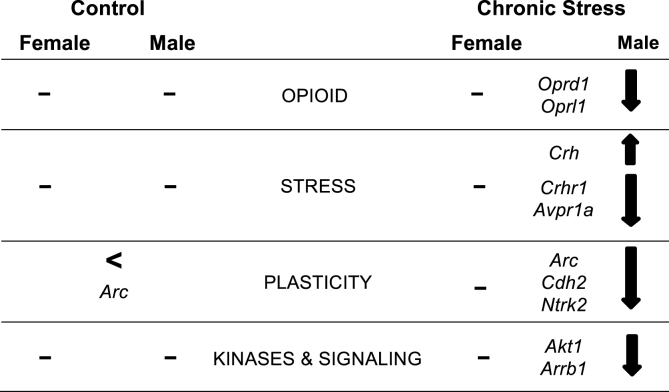
These studies demonstrate that sex and CIS impact the expression of opioid, stress, plasticity, as well as kinases and signaling genes associated with these processes in the rat hippocampus (Fig. 6). Few sex differences were seen in hippocampal gene expression in control (unstressed) rats. However, following CIS, gene expression in the hippocampus was altered in males but not females. In males, opioid, stress and plasticity genes were almost exclusively down-regulated following CIS. The one exception was Crh, which was up-regulated. These findings are consistent with our previous anatomical and physiological findings (McEwen and Milner, 2017) that, unlike males, the female rodent hippocampus is resistant to the deleterious effects of chronic stress.
Fig. 6.
Summary. Schematic diagram of sex differences in the changes in gene expression in the dorsal hippocampus at baseline (control) and following chronic immobilization stress. Arrows indicate direction of change. For simplicity, results from lateral and medial hippocampus are not distinguished.
4.1. Methodological considerations
As we could not consistently separate the CA1 region from the dentate gyrus, we choose to process these regions together (i.e., the medial sample). Our rationale for this was that our primary interest was the opioid system. In hippocampus, granule cells produce most of the dynorphin and enkephalin (Drake et al., 2007) and thus changes in expression for these genes in the medial sample would correlate with changes in these cells. Moreover, interneurons containing MORs and DORs are sparsely distributed in the CA1 and dentate gyrus so Oprm1 and Oprd1 mRNA would be easier to detect if the samples were combined. However, as pyramidal and granule cell neurons in rats have low levels of Oprd1 expression, our medial sample would not be able distinguish between these. Thus, these technical considerations could have contributed to the lack of sex and stress effects on the expression of opioid genes, as well as some stress and plasticity genes in the medial sample. As the CA1 and dentate gyrus have different functions and connections, future studies would explore gene expression in the CA1 and dentate gyrus separately.
In the present study, we chose to study the effect of CIS on gene expression one day after discontinuing the last stressor. We selected this time point because we wanted to correlate gene expression changes with our previous studies examining changes in enkephalin levels and opioid receptor trafficking changes in the hippocampus after CIS (Mazid et al., 2016, Milner et al., 2013, Pierce et al., 2014). One day after CIS, previous studies have demonstrated that corticosterone levels are elevated in male rats (Lakshminarasimhan and Chattarji, 2012).
4.2. Baseline sex differences in hippocampal gene expression
Of the opioid, synaptic and stress genes analyzed in the hippocampal samples, the present study found that Arc expression tended to be less in female rats compared to male rats in the CA3 region (lateral sample). Arc (activity-regulated cytoskeleton protein, also known as Arg3.1) is an immediate early gene that is targeted to synapses and is an important “master regulator” of protein synthesis-dependent forms of synaptic plasticity (reviewed in Bramham et al. (2010)). In particular, Arc is up-regulated during brain-derived neurotrophic factor (BDNF)-stimulated long-term potentiation (Wibrand et al., 2006) and following enhancement of TrkB signaling by antidepressants (Larsen et al., 2007, Molteni et al., 2008). Notably, hormone levels influence Arc expression (Christensen et al., 2015) as well as BDNF levels in the mossy fiber pathway (Scharfman and MacLusky, 2014) and the number and types of cellular profiles containing phosphorylated TrkB in the hippocampus (Spencer-Segal et al., 2011). The majority of rats in the present study were in estrus (i.e., the phase when estrogen levels are declining and progesterone levels are rising), therefore, it is possible that Arc expression in females may differ in other estrous cycle phases. Additionally, it is also possible that Arc is involved in different synaptic plasticity processes (e.g., LTP vs neurogenesis) in females and males.
Few papers have looked at gene expression differences in the hippocampi of adult male and females rats at basal conditions. In adult male Wistar rats compared to females, genes involved in exocytosis, glutamate signaling and synaptic transmission were elevated in the hippocampus (Biala et al., 2011). Moreover, in this same study genes involved in negative regulation of apoptosis and in programmed cell death were upregulated in the hippocampi of adult female rats compared to male rats (Biala et al., 2011).
4.3. Sex differences in opioid gene expression after CIS
Together with our previous studies (reviewed in McEwen and Milner (2017)), the present study reveals opposing effects of CIS on hippocampal opioid system in female and male rats. Notably, expression of three of the seven opioid genes examined (Oprm1, Oprd1 and Oprl1) were down-regulated in the CIS males compared to control males and/or females consistent with the idea that CIS essentially shuts down the opioid system in males. Changes in opioid gene expression following CIS were limited to the CA2/3 region (lateral sample) which harbors DOR-containing pyramidal cells (Mazid et al., 2016), and scattered DOR-, MOR- and enkephalin-containing interneurons (reviewed in Drake et al. (2007)). Previous studies have shown that the CA3 opioid system is important in visual-spatial pattern completion, a component of context learning (Kesner and Warthen, 2010). We have shown that DORs are important for low frequency opioid-dependent LTP at the mossy fiber-CA3 pyramidal cell synapse that is unique to females (Harte-Hargrove et al., 2015). At the cellular level, females at high estrogen states compared to males have about three times more DORs in CA3 dendritic spines contacted by mossy fibers (Harte-Hargrove et al., 2015) and about twice as many DORs near the plasma membrane of CA3 pyramidal cell dendrites (Mazid et al., 2016). Moreover, high estrogen-state females compared to males contain about twice as many enkephalin-containing mossy fibers (Pierce et al., 2014) and enkephalin expressing interneurons (Bryson, Milner, Gray unpublished). Thus, down regulation of hippocampal opioid genes in CA2/3 in males following CIS would severely hamper the already limited opioid mediated synaptic plasticity in this region.
4.4. Sex differences in stress gene expression after CIS
The only gene to be up-regulated following CIS was Crh and this occurred exclusively in the males. This finding is consistent with other studies demonstrating an increase in hippocampal Crh mRNA and protein levels in adult rats (sex unspecified) following maternal separation stress (Wang et al., 2014). Crhr1 and Avpr1a expression were down-regulated in males, but not females, after CIS. A similar decrease in hippocampal Avpr1a mRNA has been reported in adult male mice subjected to forced-swim stress (Lesse et al., 2017). Moreover, in humans specific variants in CRHR1, AVPR1A and FKBP5 are associated with heroin addiction (Levran et al., 2014).
Our anatomical studies in hippocampus have shown that unstressed male and female rats contain similar levels of CRF1 receptor-immunoreactivity in CA1 dendrites and that CRF1 receptor often co-localizes with DORs in these dendrites (Williams et al., 2011a). Moreover, DOR agonists can inhibit CRF-induced cAMP accumulation in NG108-15 cells (Williams et al., 2011a) suggesting that DORs regulate CRF receptor signaling. We have recently found that CIS decreases the levels of cytoplasmic CRF1 receptor in CA3 pyramidal cell dendrites in males but not females (McAlinin et al., 2016) and that these cellular changes are the opposite as those seen for DORs following CIS (Mazid et al., 2016). Thus, the observed decrease in Crh1 expression in males following CIS is consistent with these findings.
Arginine vasopressin (AVP) containing neurons in the hypothalamus innervate the hippocampal CA2 region (Zhang and Hernandez, 2013) which also contains high levels of phosphorylated DORs (Burstein et al., 2013). Activation of AVP receptors has been shown to facilitate hippocampal LTP (Chepkova et al., 2001, Dubrovsky et al., 2003). Thus, reduction of Avpr1a expression would likely negatively impact hippocampal LTP and thus learning processes. However, as AVP elicits anxiogenic and depressive responses (Engelmann et al., 2004, Neumann and Landgraf, 2012), down-regulation of Avpr1a in males following CIS could help reduce these behaviors.
4.5. Sex differences in plasticity gene expression after CIS
Of the plasticity genes examined, Arc, Cdh2 and Ntk2 were significantly reduced in the hippocampi of CIS males compared to control males and/or females. Moreover, Cdh2 in CIS females tended to be reduced compared to control females. As discussed above, Arc is important for BDNF-induced LTP (reviewed in Bramham et al. (2010)). Thus, down-regulation of both Arc and Ntrk2 following CIS may compromise BDNF-mediated neuroplasticity in males. This may be especially important for antidepressant treatment in which TrkB signaling is thought to promote or restore plasticity through gene expression regulation (Castren et al., 2007, Dagestad et al., 2006). The finding that Arc expression is disrupted by stress has been shown in other studies. For instance, male rats whose anxiety-like behaviors are minimally disrupted by predator odors have up-regulated Arc expression in the hippocampus whereas rats who exhibit anxiety-like behaviors after predator odors show no up-regulation in Arc (Kozlovsky et al., 2008). Moreover, following prenatal stress, both adult male and female rats exhibit a down-regulation of Arc in the hippocampus (Biala et al., 2011).
Cdh2 is part of a class of genes encoding synaptic adhesion molecules that not only play roles in adhering presynaptic and postsynaptic membranes together but also neuron-neuron recognition and in generating scaffolds onto which additional proteins can bind (reviewed in Rudenko, 2017, Seong et al., 2015). In line with the present findings in males, chronic restraint stress reduces N-Cadherin protein levels in the hippocampus of male rats (Castaneda et al., 2015).
The findings that genes involved in synaptic plasticity are down-regulated in males following CIS supports previous studies that chronic stress negatively impact synaptic structure and function (for reviews see (McEwen, 2016, McEwen and Chattarji, 2007)). In particular, chronic stress results in reduced spine density as well as synaptic turnover in the hippocampus. Moreover, numerous studies in male hippocampi have shown disruption of LTP and calcium currents as well as diminished responses to neurotransmitters following chronic stress (Alfarez et al., 2002, Kamal et al., 2014, Kim et al., 1996, Krugers et al., 2010).
4.6. Sex differences in kinase and signaling molecule gene expression after CIS
AKT (protein kinase B) is a key signal transduction intermediate that plays a critical role in cell growth and survival and in synaptic protein translation including post-synaptic density (PSD)-95 (Akama and McEwen, 2003, Brunet et al., 2001, Chong et al., 2005). Our previous anatomical studies in rat hippocampus demonstrated that phosphorylated AKT protein levels are significantly decreased in the CA1 pyramidal cell dendrites of males compared to females, regardless of estrous cycle (Znamensky et al., 2003). The presence of phosphorylated AKT-immunoreactivity in CA1 pyramidal cell synapses fluctuates over the estrous cycle with females at high estrogen states having about twice the levels as those seen in males (Yildirim et al., 2011, Znamensky et al., 2003). Additionally, the presence of AKT-immunoreactivity in hippocampal synapses diminishes in aged female rats (Yildirim et al., 2011). The lack of sex differences in the Akt1 expression in the control rats suggests that these changes in phosphorylated AKT protein levels are post-translational. The reduction of Akt1 expression in the CIS males in both hippocampal regions could contribute to the dendritic remodeling and synaptic loss of hippocampal neurons seen in males after chronic stress (McEwen, 2016).
Β-arrestins are important regulators of the desensitization and internalization of G-protein-coupled receptors as well as signal transducers (DeWire et al., 2007). Β-arrestins are not only important for opioid receptor signal transduction (Al-Hasani and Bruchas, 2011, Fan et al., 2003) but regulating the kinetics and transduction pathway selectivity of CRF1 receptor signaling (Oakley et al., 2007). Thus, the observed down-regulation of Arrb1 in CIS males could impact both opioid and CRF1 receptor signaling in hippocampal neurons.
4.7. Functional considerations
The observed sex differences in hippocampal opioid, stress, plasticity, kinases and signaling molecules gene expression in response to CIS are consistent with our previous anatomical findings (McEwen and Milner, 2017). Moreover, these studies add to the growing body of literature demonstrating sex differences in hippocampal gene expression in response to stress (Anacker et al., 2016, Bagot et al., 2016, de Azeredo et al., 2017, Labonte et al., 2017, Mychasiuk et al., 2016, Wang et al., 2014). Importantly, the studies to date examining hippocampal gene expression following stress also have shown sexual dimorphisms in the specific genes that are up- or down-regulated depend on the stress model (CIS vs chronic social defeat stress), strain (rats vs. mice) and age of the stress (neonatal vs adult).
The down-regulation of opioid peptide and receptor genes, synaptic plasticity genes and the majority of stress genes in males following CIS would severely impact learning processes. This is consistent with behavioral observations that males, but not females, display impaired cognitive performance after chronic stress (Conrad et al., 2003, Kitraki et al., 2004, Luine et al., 2007, Weiss et al., 2005). In particular, sexual dimorphism in response to stress is important in context learning (Bangasser and Shors, 2010, Beck and Luine, 2010, Becker and Koob, 2016) which is a critical component of drug acquisition and relapse (Crombag et al., 2008, Koob and Volkow, 2010).
Acknowledgements
Study design: MJK, BSM, TAM, JDG; Data collection: SM, SCO, JCR, MR, YZ; Writing of report: MJK, BSM, TAM, MR, JDG. Decision to submit for publication: BSM, TAM, MR, JDG. Supported by NIH grant DA08259 (TAM, MJK, BSM) and Hope for Depression Research Foundation (BSM) and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (MJK).
Acknowledgments
Conflicts of interest
The authors declare no competing financial interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.01.001.
Contributor Information
Matthew Randesi, Email: randesm@mail.rockefeller.edu.
Teresa A. Milner, Email: tmilner@med.cornell.edu.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Two-way ANOVA - lateral hippocampus.
Two-way ANOVA - medial hippocampus.
Post-hoc tests - lateral hippocampus.
Post-hoc tests - medial hippocampus.
References
- Akama K.T., McEwen B.S. Estrogen stimulates PSD-95 rapid protein synthesis via the Akt/PKB pathway. J. Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R., Bruchas M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarez D.N., Wiegert O., Joels M., Krugers H.J. Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation. Neuroscience. 2002;115:1119–1126. doi: 10.1016/s0306-4522(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Anacker C., Scholz J., O'Donnell K.J., Allemang-Grand R., Diorio J., Bagot R.C., Nestler E.J., Hen R., Lerch J.P., Meaney M.J. Neuroanatomic differences associated with stress susceptibility and resilience. Biol. Psychiatr. 2016;79:840–849. doi: 10.1016/j.biopsych.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot R.C., Cates H.M., Purushothaman I., Lorsch Z.S., Walker D.M., Wang J., Huang X., Schluter O.M., Maze I., Pena C.J., Heller E.A., Issler O., Wang M., Song W.M., Stein J.L., Liu X., Doyle M.A., Scobie K.N., Sun H.S., Neve R.L., Geschwind D., Dong Y., Shen L., Zhang B., Nestler E.J. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron. 2016;90:969–983. doi: 10.1016/j.neuron.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Shors T.J. Critical brain circuits at the intersection between stress and learning. Neurosci. Biobehav. Rev. 2010;34:1223–1233. doi: 10.1016/j.neubiorev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K.D., Luine V.N. Evidence for sex-specific shifting of neural processes underlying learning and memory following stress. Physiol. Behav. 2010;99:204–211. doi: 10.1016/j.physbeh.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Koob G.F. Sex differences in animal models: focus on addiction. Pharmacol. Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Monteggia L.M., Perrot-Sinal T.S., Romeo R.D., Taylor J.R., Yehuda R., Bale T.L. Stress and disease: is being female a predisposing factor? J. Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke J.D., Hyman S.E. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Biala Y.N., Bogoch Y., Bejar C., Linial M., Weinstock M. Prenatal stress diminishes gender differences in behavior and in expression of hippocampal synaptic genes and proteins in rats. Hippocampus. 2011;21:1114–1125. doi: 10.1002/hipo.20825. [DOI] [PubMed] [Google Scholar]
- Bramham C.R., Alme M.N., Bittins M., Kuipers S.D., Nair R.R., Pai B., Panja D., Schubert M., Soule J., Tiron A., Wibrand K. The Arc of synaptic memory. Exp. Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas M.R., Xu M., Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A., Datta S.R., Greenberg M.E. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Burstein S.R., Williams T.J., Lane D.A., Knudsen M.G., Pickel V.M., McEwen B.S., Waters E.M., Milner T.A. The influences of reproductive status and acute stress on the levels of phosphorylated delta opioid receptor immunoreactivity in rat hippocampus. Brain Res. 2013;1518:71–81. doi: 10.1016/j.brainres.2013.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda P., Munoz M., Garcia-Rojo G., Ulloa J.L., Bravo J.A., Marquez R., Garcia-Perez M.A., Arancibia D., Araneda K., Rojas P.S., Mondaca-Ruff D., Diaz-Veliz G., Mora S., Aliaga E., Fiedler J.L. Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J. Neurosci. Res. 2015;93:1476–1491. doi: 10.1002/jnr.23602. [DOI] [PubMed] [Google Scholar]
- Castren E., Voikar V., Rantamaki T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prescription Painkiller Overdoses. A growing epidemic especially among women. Vital Signs. 2013;1 [Google Scholar]
- Centers for Disease Control and Prevention Drug overdose deaths hit record numbers in 2014. CDC Online Newsroom. 2015 p1218. [Google Scholar]
- Chepkova A.N., Kapai N.A., Skrebitskii V.G. Arginine vasopressin fragment AVP(4-9)facilitates induction of long-term potentiation in the hippocampus. Bull. Exp. Biol. Med. 2001;131:136–138. doi: 10.1023/a:1017583626625. [DOI] [PubMed] [Google Scholar]
- Chong Z.Z., Li F., Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol. Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A., Dewing P., Micevych P. Immediate early gene activity-regulated cytoskeletal-associated protein regulates estradiol-induced lordosis behavior in female rats. J. Neurosci. Res. 2015;93:67–74. doi: 10.1002/jnr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C.D., Grote K.A., Hobbs R.J., Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol. Learn. Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Crombag H.S., Bossert J.M., Koya E., Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagestad G., Kuipers S.D., Messaoudi E., Bramham C.R. Chronic fluoxetine induces region-specific changes in translation factor eIF4E and eEF2 activity in the rat brain. Eur. J. Neurosci. 2006;23:2814–2818. doi: 10.1111/j.1460-9568.2006.04817.x. [DOI] [PubMed] [Google Scholar]
- de Azeredo L.A., Wearick-Silva L.E., Viola T.W., Tractenberg S.G., Centeno-Silva A., Orso R., Schroder N., Bredy T.W., Grassi-Oliveira R. Maternal separation induces hippocampal changes in cadherin-1 (CDH-1) mRNA and recognition memory impairment in adolescent mice. Neurobiol. Learn. Mem. 2017;141:157–167. doi: 10.1016/j.nlm.2017.04.006. [DOI] [PubMed] [Google Scholar]
- DeWire S.M., Ahn S., Lefkowitz R.J., Shenoy S.K. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Drake C.T., Chavkin C., Milner T.A. Opioid systems in the dentate gyrus. Prog. Brain Res. 2007;163:245–814. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B., Tatarinov A., Gijsbers K., Harris J., Tsiodras A. Effects of arginine-vasopressin (AVP) on long-term potentiation in intact anesthetized rats. Brain Res. Bull. 2003;59:467–472. doi: 10.1016/s0361-9230(02)00961-9. [DOI] [PubMed] [Google Scholar]
- Engelmann M., Landgraf R., Wotjak C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front. Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Fan X.L., Zhang J.S., Zhang X.Q., Yue W., Ma L. Differential regulation of beta-arrestin 1 and beta-arrestin 2 gene expression in rat brain by morphine. Neuroscience. 2003;117:383–389. doi: 10.1016/s0306-4522(02)00930-2. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove L.C., Varga-Wesson A., Duffy A.M., Milner T.A., Scharfman H.E. Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat. J. Neurosci. 2015;35:1723–1738. doi: 10.1523/JNEUROSCI.0820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Crombag H.S., Robinson T.E., Becker J.B. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jones C.M. Frequency of prescription pain reliever nonmedical use: 2002-2003 and 2009-2010. Arch. Intern. Med. 2012;172:1265–1267. doi: 10.1001/archinternmed.2012.2533. [DOI] [PubMed] [Google Scholar]
- Kamal A., Ramakers G.M., Altinbilek B., Kas M.J. Social isolation stress reduces hippocampal long-term potentiation: effect of animal strain and involvement of glucocorticoid receptors. Neuroscience. 2014;256:262–270. doi: 10.1016/j.neuroscience.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Kesner R.P., Warthen D.K. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–557. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- Kilts C.D., Schweitzer J.B., Quinn C.K., Gross R.E., Faber T.L., Muhammad F., Ely T.D., Hoffman J.M., Drexler K.P. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatr. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Foy M.R., Thompson R.F. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitraki E., Kremmyda O., Youlatos D., Alexis M.N., Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Koob G., Kreek M.J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatr. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N., Matar M.A., Kaplan Z., Kotler M., Zohar J., Cohen H. The immediate early gene Arc is associated with behavioral resilience to stress exposure in an animal model of posttraumatic stress disorder. Eur. Neuropsychopharmacol. 2008;18:107–116. doi: 10.1016/j.euroneuro.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Kreek M.J., Koob G.F. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Krugers H.J., Lucassen P.J., Karst H., Joels M. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front. Synaptic Neurosci. 2010;2:24. doi: 10.3389/fnsyn.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B., Engmann O., Purushothaman I., Menard C., Wang J., Tan C., Scarpa J.R., Moy G., Loh Y.E., Cahill M., Lorsch Z.S., Hamilton P.J., Calipari E.S., Hodes G.E., Issler O., Kronman H., Pfau M., Obradovic A.L.J., Dong Y., Neve R.L., Russo S., Kazarskis A., Tamminga C., Mechawar N., Turecki G., Zhang B., Shen L., Nestler E.J. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan H., Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M.H., Rosenbrock H., Sams-Dodd F., Mikkelsen J.D. Expression of brain derived neurotrophic factor, activity-regulated cytoskeleton protein mRNA, and enhancement of adult hippocampal neurogenesis in rats after sub-chronic and chronic treatment with the triple monoamine re-uptake inhibitor tesofensine. Eur. J. Pharmacol. 2007;555:115–121. doi: 10.1016/j.ejphar.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Ho I.K. Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors. Mol. Pain. 2013;9:45. doi: 10.1186/1744-8069-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse A., Rether K., Groger N., Braun K., Bock J. Chronic postnatal stress induces depressive-like behavior in male mice and programs second-hit stress-induced gene expression patterns of OxtR and AvpR1a in adulthood. Mol. Neurobiol. 2017;54:4813–4819. doi: 10.1007/s12035-016-0043-8. [DOI] [PubMed] [Google Scholar]
- Levran O., Peles E., Randesi M., Li Y., Rotrosen J., Ott J., Adelson M., Kreek M.J. Stress-related genes and heroin addiction: a role for a functional FKBP5 haplotype. Psychoneuroendocrinology. 2014;45:67–76. doi: 10.1016/j.psyneuen.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V.N., Beck K.D., Bowman R.E., Frankfurt M., MacLusky N.J. Chronic stress and neural function: accounting for sex and age. J. Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Luo A.H., Tahsili-Fahadan P., Wise R.A., Lupica C.R., Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W.J., Arizzi M.N., Carroll M.E. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Mazid S., Hall B.S., Odell S.C., Stafford K., Dyer A.D., Van Kempen T.A., Selegean J., McEwen B.S., Waters E.M., Milner T.A. Sex differences in subcellular distribution of delta opioid receptors in the rat hippocampus in response to acute and chronic stress. Neurobiol. Stress. 2016;5:37–53. doi: 10.1016/j.ynstr.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlinin H.R., Poulton Kamakura R., Dyer A.G., McEwen B.S., Waters E.M., Milner T., Milner T.A. Sex differences in the subcellular distribution of corticotrophin releasing factor 1 in the rat hippocampus in response to chronic stress. Soc. Neurosci. Abstr. 2016 Ref Type: Abstract. [Google Scholar]
- McEwen B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res. 2016;1645:50–54. doi: 10.1016/j.brainres.2015.12.043. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Chattarji S. Springer-Verlag; New York: 2007. Neuroendocrinology of stress., Handbook of Neurochemistry and Molecular Neurobiology; pp. 572–593. [Google Scholar]
- McEwen B.S., Milner T.A. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Milner T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2017;95:24–39. doi: 10.1002/jnr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner T.A., Burstein S.R., Marrone G.F., Khalid S., Gonzalez A.D., Williams T.J., Schierberl K.C., Torres-Reveron A., Gonzales K.L., McEwen B.S., Waters E.M. Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse. 2013;67:757–772. doi: 10.1002/syn.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R., Calabrese F., Mancini M., Racagni G., Riva M.A. Basal and stress-induced modulation of activity-regulated cytoskeletal associated protein (Arc) in the rat brain following duloxetine treatment. Psychopharmacology (Berl) 2008;201:285–292. doi: 10.1007/s00213-008-1276-7. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Muhammad A., Kolb B. Chronic stress induces persistent changes in global DNA methylation and gene expression in the medial prefrontal cortex, orbitofrontal cortex, and hippocampus. Neuroscience. 2016;322:489–499. doi: 10.1016/j.neuroscience.2016.02.053. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Oakley R.H., Olivares-Reyes J.A., Hudson C.C., Flores-Vega F., Dautzenberg F.M., Hauger R.L. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J.P., Kelter D.T., McEwen B.S., Waters E.M., Milner T.A. Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J. Chem. Neuroanat. 2014;55:9–17. doi: 10.1016/j.jchemneu.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger F.O., Oakes R.A. Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol. Biochem. Behav. 1995;51:457–461. doi: 10.1016/0091-3057(95)00007-j. [DOI] [PubMed] [Google Scholar]
- Robbins S.J., Ehrman R.N., Childress A.R., O'Brien C.P. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Rudenko G. Dynamic control of synaptic adhesion and organizing molecules in synaptic plasticity. Neural Plast. 2017;2017:6526151. doi: 10.1155/2017/6526151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H.E., MacLusky N.J. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology. 2014;76(Pt C):696–708. doi: 10.1016/j.neuropharm.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong E., Yuan L., Arikkath J. Cadherins and catenins in dendrite and synapse morphogenesis. Cell Adhes. Migrat. 2015;9:202–213. doi: 10.4161/19336918.2014.994919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U., Highfield D., Yap J., Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Spencer-Segal J.L., Waters E.M., Bath K.G., Chao M.V., McEwen B.S., Milner T.A. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J. Neurosci. 2011;31:6780–6790. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A., Khalid S., Williams T.J., Waters E.M., Drake C.T., McEwen B.S., Milner T.A. Ovarian steroids modulate leu-enkephalin levels and target leu-enkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Res. 2008;1232(70–84):70–84. doi: 10.1016/j.brainres.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A., Williams T.J., Chapleau J.D., Waters E.M., McEwen B.S., Drake C.T., Milner T.A. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp. Neurol. 2009;219:319–327. doi: 10.1016/j.expneurol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.D., Bagnara J.T. W.B. Saunders; Philadelphia: 1971. General Endocrinology. [Google Scholar]
- Volkow N.D., Wang G.J., Telang F., Fowler J.S., Logan J., Childress A.R., Jayne M., Ma Y., Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel S.R., Liu X., Hayes R.J., Spector J.A., Gardner E.L. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Shankaranarayana Rao B.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Nie W., Li H., Hou Y., Yu Z., Fan Q., Sun R. Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Sametsky E., Sasse A., Spiess J., Disterhoft J.F. Acute stress facilitates trace eyeblink conditioning in C57BL/6 male mice and increases the excitability of their CA1 pyramidal neurons. Learn. Mem. 2005;12:138–143. doi: 10.1101/lm.89005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibrand K., Messaoudi E., Havik B., Steenslid V., Lovlie R., Steen V.M., Bramham C.R. Identification of genes co-upregulated with Arc during BDNF-induced long-term potentiation in adult rat dentate gyrus in vivo. Eur. J. Neurosci. 2006;23:1501–1511. doi: 10.1111/j.1460-9568.2006.04687.x. [DOI] [PubMed] [Google Scholar]
- Williams T.J., Akama K.T., Knudsen M.G., McEwen B.S., Milner T.A. Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Exp. Neurol. 2011;230:186–196. doi: 10.1016/j.expneurol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.J., Torres-Reveron A., Chapleau J.D., Milner T.A. Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol. Learn. Mem. 2011;95:206–220. doi: 10.1016/j.nlm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M., Janssen W.G., Lou W.Y., Akama K.T., McEwen B.S., Milner T.A., Morrison J.H. Effects of estrogen and aging on the synaptic distribution of phosphorylated Akt-immunoreactivity in the CA1 region of the female rat hippocampus. Brain Res. 2011;1379:98–108. doi: 10.1016/j.brainres.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Hernandez V.S. Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience. 2013;228:139–162. doi: 10.1016/j.neuroscience.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brownstein A.J., Buonora M., Niikura K., Ho A., Correa da R.J., Kreek M.J., Ott J. Self-administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience. 2015;285:34–46. doi: 10.1016/j.neuroscience.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mayer-Blackwell B., Schlussman S.D., Randesi M., Butelman E.R., Ho A., Ott J., Kreek M.J. Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology (Berl) 2014;231:1277–1287. doi: 10.1007/s00213-013-3306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamensky V., Akama K.T., McEwen B.S., Milner T.A. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J. Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-way ANOVA - lateral hippocampus.
Two-way ANOVA - medial hippocampus.
Post-hoc tests - lateral hippocampus.
Post-hoc tests - medial hippocampus.